Abstract
In adults with major depressive disorder (MDD), effective treatment has been associated with increases in both heart rate variability (HRV) and cortical thickness. However, the impact of treatment on these indices has not yet been examined in adolescents. Cortical thickness and HRV were measured in twelve adolescents with MDD before and after 8 weeks of treatment with a selective serotonin reuptake inhibitor (SSRI). We examined treatment-related changes in depression symptoms, HRV, heart rate (HR), and cortical thickness, and analyzed correlations among these change indices. At follow-up, patients showed significantly decreased depression severity, increased HRV and increased thickness of the left medial orbitofrontal cortex (OFC). Clinical improvement was associated with increased HRV and decreased HR. Increased HRV was associated with increased cortical thickness of left lateral OFC and superior frontal cortex. Due to the small sample size, results represent preliminary findings that need replication. Further, in the absence of a placebo arm, we cannot confirm that the observed effects are due solely to medication. These preliminary findings suggest that SSRI treatment in adolescents impacts both cortical thickness and autonomic functioning. Confirmation of these findings would support OFC thickness and HRV as neurobiological mediators of treatment outcome.
Keywords: Depression, Adolescent, Heart Rate, Heart Rate Variability, Cortical Thickness, Treatment, Longitudinal
1. Introduction
The vast majority of mental health disorders have their onset during adolescence (Kessler et al., 2007). About 75% of individuals with mental illness experience first symptoms before the age of 25 years (Kessler et al., 2007), with depression being one of the most common mental health problems in adolescents (Merikangas et al., 2009). The developmental neurobiology of major depressive disorder (MDD) is complex and not yet fully understood. MDD has wide-spread effects on neural circuits that mediate emotion and orchestrate a complex array of physiological systems (i.e., autonomic nervous system (ANS), endocrine, and immune systems) (Singh and Gotlib, 2014). Research in adolescents with MDD that integrates the assessment of these multiple intersecting systems will be critical to inform theories of MDD pathogenesis and guide both monitoring and development of treatment. Current knowledge about the neurobiology of MDD is limited as existing models are mostly grounded on cross-sectional evidence. Longitudinal research, such as studies examining treatment-related changes of neurobiological and psychological function in MDD, are essential for advancing knowledge about adolescent MDD.
Research examining heart rate variability (HRV), an index of ANS function that reflects vagal activity, has found that adults (Kemp et al., 2010) and adolescents (Koenig et al., 2016) with MDD tend to have lower HRV than controls (Sgoifo et al. 2015). Furthermore, several previous studies have shown that in adults with MDD, clinical improvement due to treatment is associated with increases in HRV (Balogh et al., 1993; Chambers and Allen, 2002; Glassman et al., 2007; Khaykin et al., 1998). Decrease in depression severity in adults following non-pharmacological treatments such as acupuncture (Chambers and Allen, 2002) or cognitive behavioral therapy (Jang et al., 2017) has been associated with an increase of cardiac vagal activity (i.e., indexed by respiratory sinus arrhythmia (RSA), high-frequency HRV (HF-HRV) or the mean squared difference of successive inter-beat-intervals (RMSSD)) and a decrease in heart rate (HR) (Chambers and Allen, 2002). However, findings from adult depression studies using pharmacological interventions are mixed, with some studies illustrating similar patterns of findings (Balogh et al., 1993; Khaykin et al., 1998) and others showing no significant effects of depression treatment on HRV measures reflecting cardiac vagal activity (Glassman et al., 2007).
Studies addressing the association between clinical improvement and changes in autonomic function in the context of clinical treatment of adolescents are scarce. In community based samples of adolescents, longitudinal associations between vagal activity and difficulties in emotion regulation have been reported, such that lower RSA was associated with and predicted greater difficulties in emotion regulation in annual follow-ups (Vasilev et al., 2009). One of the few longitudinal studies in adolescent patients recently showed that borderline personality disorder (BPD) symptom severity co-varies with HRV and HR in adolescents, such that improvements in BPD symptoms were associated with increases in HRV and decreases in HR (Koenig et al., 2017b). To the best of our knowledge, no prior studies have examined treatment-related changes in HR and HRV in adolescent patients with MDD. Further, the neural mechanisms underlying treatment-related changes in autonomic function are not well understood.
Similar to measures of ANS function, previous research in adults has shown that successful psychotherapy or pharmacological treatment can be accompanied by increases in cortical thickness. A study in adults with treatment-resistant MDD reported increases in cortical thickness of the middle frontal gyrus (MFG), orbitofrontal cortex (OFC), and inferior temporal gyrus following intensive pharmacotherapy in remitters over time (Phillips et al., 2015). Likewise, a study that examined brain structural differences in psychotherapy patients with late-life depression (~73 years) found that in comparison to responders, non-responders showed decreases in cortical thickness in the insular cortices, as well as the right medial OFC, among other regions of interest (ROI; Mackin et al., 2013). Changes in cortical thickness due to treatment may have high clinical significance since greater thickness may be a predictor of future outcomes. For example, in girls at heightened familial risk of depression, those with lower thickness of the OFC, precentral gyrus, anterior cingulate cortex (ACC), and insula were more likely to report depression onset in the subsequent 5 years (Foland-Ross et al., 2015).
While initial lines of research examining cortical thickness and HRV in MDD were conducted separately, more recent integrative studies in healthy subjects have begun to show that HRV is associated with cortical thickness of specific brain regions – suggesting that brain morphology is associated with ANS function (for a review see Carnevali et al. 2018). In healthy adults, greater vagally-mediated HRV was associated with greater thickness of prefrontal cortex (PFC) and ACC (Winkelmann et al., 2016; Wood et al., 2017; Yoo et al., 2017). In contrast, in a sample of healthy adolescents (Koenig et al., 2017a), vagally-mediated HRV was inversely associated with cortical thickness (particularly of the rostral ACC). These contrasting findings suggest that the association between cortical thickness and autonomic function may change as a function of age. However, no prior studies have examined the impact of treatment on autonomic function, cortical thickness and their association with depression severity in adolescents with MDD.
The present study aimed to investigate changes in autonomic function (indexed by resting state HR and time- and frequency-domain measures of HRV) and cortical thickness in adolescents with MDD following 8 weeks of treatment with a selective serotonin reuptake inhibitor (SSRI), and how these changes in neurobiology track with improvement in depression symptoms. In line with previous research, we hypothesized that clinical improvement would be associated with (a) increases in vagally-mediated HRV, (b) decreases in HR, and (c) increases in cortical thickness, predominately in parts of the frontal lobe (MFG and OFC). Further, we aimed to explore co-variance of changes in autonomic function with changes in cortical thickness. We hypothesized that increases in vagally-mediated HRV and decreases in HR would be associated with increases in cortical thickness.
2. Methods
2.1. General Procedures
Analyses for the present paper were conducted on data collected from a subsample of a larger study (“Fronto-Limbic Connectivity in Adolescent MDD”, PI Cullen). Data for the present study were analyzed using procedures previously described elsewhere; therefore, description of the methods largely overlaps with previous publications (Cullen et al., 2014, 2016; Hall et al., 2014; KLIMES-DOUGAN et al., 2014; Klimes-Dougan et al., 2018). The initial sample of these studies included 17 adolescents aged 12–19 years who were diagnosed with MDD and who completed pre- and post-treatment scans. Pulse oximetry data for six participants was not available (i.e., bad data quality), leaving a total of 12 participants with complete pre- and post-treatment scans and valid HRV data.
The study protocol was approved by the University of Minnesota Institutional Review Board. Recruitment methods included community postings and referrals from local mental health services. MDD participants were eligible to participate if they had a primary diagnosis of MDD and no intake of antidepressant medications in the past two months. Patients were excluded if they endorsed the presence of a neurological or chronic medical condition, mental retardation, pervasive developmental disorder, substance use disorder, bipolar disorder, schizophrenia, or if they had an intelligence quotient (IQ) of less than 80 as determined by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Participants were also excluded if they had any MRI contraindications such as braces, claustrophobia, or non-MRI safe implants. For the purposes of the present study, participants were selected if they planned to receive 8 weeks of SSRI treatment as recommended by own provider.
2.3. Clinical Assessments
Following a phone screen to determine initial eligibility requirements, all participants deemed eligible for participation were invited for an in-person clinical evaluation. Upon arrival to the study site, all participants or their legal guardians (< 18 years) provided written informed consent or assent when appropriate. During this visit, all adolescent and parents completed clinical interviews that were conducted separately, which included the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) and the clinician-administered Children’s Depression Rating Scale - Revised (CDRS-R; Poznanski et al., 1985). Handedness (Edinburgh Handedness Inventory; Oldfield, 1971) was assessed at baseline. Patients provided self-reports on depression severity in the past two weeks using the Beck Depression Inventory II (BDI-II; Beck et al., 1996; Osman et al., 2004). At the final visit following 8 weeks of SSRI treatment, participants completed another BDI-II, which was the primary outcome measure of the study.
2.4. Structural Neuroimaging
Neuroimaging data were acquired at baseline and after treatment at the Center for Magnetic Resonance Research at UMN using a Siemens 3T TIM Trio scanner and an identical neuroimaging protocol. A five-minute structural scan was acquired using a T1-weighted high-resolution magnetization prepared gradient echo (MPRAGE) sequence: TR = 2530ms; TE = 3.65ms; TI = 1100ms; flip angle = 7 degrees; 1mm slices, FOV = 256, voxel size 1×1×1mm; GRAPPA = 2. Free Surfer Version 5.3 (https://surfer.nmr.mgh.harvard.edu) was used to process T1 data including brain extraction and parcellation of data into a standard set of anatomically-based regions of white and grey matter. Cortical thickness was calculated using automated cortical parcellation from Free Surfer version 5.3 using the Desikan-Killiany atlas (Desikan et al., 2006). This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white matter boundary to the gray matter/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). Analyses of structural thickness included 9 ROIs each for the left and the right hemisphere: Caudal ACC, Caudal MFG, Lateral OFC, Medial OFC, Rostral ACC, Rostral MFG, Superior Frontal, Frontal Pole and the Insula. The selection of ROIs was based on previous studies addressing the association of structural thickness and vagally-mediated HRV (Koenig et al., 2017a; Winkelmann et al., 2016; Woodward et al., 2008; Yoo et al., 2017)
2.5. Analysis of Heart Rate and its Variability
Reporting of HRV processing and analyses adheres to the Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH; Quintana et al., 2016). Pulse-to-pulse intervals were derived using the built-in Siemens MRI-compatible photo plethysmograph (PPG) placed on the right index finger, recorded at a sample rate of 50Hz for 6 minutes during resting state scans at baseline and after treatment. During the resting state scans, subjects were instructed to rest awake with their eyes closed, to try not to fall asleep, and to not think about anything in particular. A segment of 5 minutes each (baseline and post-line) was selected for analysis of HR and HRV, discarding 30 seconds each at the beginning and end of the recoding. Pulse-to-pulse intervals are a sufficient proxy for cardiac inter-beat-intervals (IBIs), and suitable for analysis of HR and HRV (Gil et al., 2010). Readout of pulse oximetry data was automated in MATLAB to determine IBIs based on the built-in Siemens peak detection algorithm. IBIs were written in a text file and later analyzed using Kubios HRV analysis package 2.2 (Tarvainen et al., 2014), allowing for the calculation of time- and frequency-domain indices of resting vagal activity. Data processing was done by the first author, who was blinded to the time point of assessment when processing the data. The level of artifact removal was selected based on visual inspection of the IBI signal in Kubios. Smoothing priors were selected as detrending method (λ for IBI data. The frequency band for the assessment of power in the high-frequency (HF) band was adjusted to the adolescent population as recently suggested (0.2 – 1 Hz; Shader et al., 2018). RMSSD and HF-HRV were derived as time- and frequency-domain measures of vagally-mediated HRV (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). RMSSD was measured in milliseconds (ms) and HF-HRV was determined as absolute power (ms²) from autoregressive models.
2.6. Statistical Analysis
A series of analyses were conducted to measure changes from baseline to follow-up in BDI-II scores, indices of resting state autonomic function (HR, RMSSD, HF-HRV) and cortical thickness in the selected ROIs listed above. Specifically, we used mixed models with robust variance estimation to account for the small sample size, included TIME (baseline versus follow-up) as a fixed factor and subject ID as a random factor, and included each clinical and neurobiological variable as the outcome variable in separate analyses. Effect sizes (Cohen’s d with 95% confidence intervals (CI)) were derived from mixed models corrected for dependence among means to describe the size of observed effects. Observed difference scores (i.e., ∆ follow-up minus baseline) were calculated to investigate the association of change across measures using pairwise correlations. All analyses were performed using Stata (Version 15; StataCorp LP, College Station, TX, USA), at an alpha level of 0.05. Given the preliminary nature of the study and the small sample size, we did not correct for multiple testing. All graphs were prepared using GraphPad Prism version 7.0 (GraphPad Software Inc.).
3. Results
Overall changes after treatment.
At follow-up, patients reported decreased depressive symptoms (BDI: χ2(1) = 11.40, p = .001; MD: −16.80, coef. 95%CI [−26.55; −7.05]) and showed significantly increased HF-HRV (χ2(1) = 4.94, p .026; MD: 456.44 ms2, coef. 950025CI [54.10; 858.79]; Table 1). Mean HR (χ2(1) = 0.82, p = .366; MD: −3.64 bpm, coef. 95%CI [−11.54;4.26]) and RMSSD (χ2(1) = 2.79, p = .095; MD: 9.89 ms, coef. 95%CI [−1.71; 21.49]) did not change significantly from baseline to follow-up. Cortical thickness of left medial OFC (χ2(1) = 4.38, p = .036; MD: 0.07 mm, coef. 95%CI [.01; .14]) was significantly increased at follow-up. However, no other ROI showed significant changes in cortical thickness following treatment (Table 2). Significant findings from these pre-post comparison analyses are illustrated in Figure 1. At baseline, mean HR was inversely correlated with RMSSD (r(12) = −.665, p = .018, 95%CI [−.897; −.147]) but not HF-HRV (r(12) = −.417, p = .177, 95%CI [−.800; .206]). Similarly, at follow-up, mean HR was inversely correlated with RMSSD (r(12) = −.633, p = .027, 95%CI [−.885; −.092]) but not HF-HRV (r(12) = −.336, p = .285, 95%CI [−.763; −.295]). RMSSD and HF-HRV were positively correlated at baseline (r(12) = .911, p < .0001, 95%CI [.705; .975]) and follow-up (r(12) =.862, p < .001, 95%CI [.570; .961]).
Table 1.
Sample Characteristics by Time of Measurement
| Baseline | Follow-Up | r | d [95% CI] | |
|---|---|---|---|---|
| Age, mean years (SD) | 15.52 (2.11) | 15.72 (2.12) | .999 | 2.12 [1.12; 3.12] |
| Caucasian, n (%) | 8 (66.67) | |||
| Female, n (%) | 8 (66.67) | |||
| Right handed, n (%) | 12 (100.00) | |||
| Height, mean inch (SD) | 65.77 (3.20) | |||
| Weight, mean pounds (SD) | 162.36 (51.09) | |||
| CDRS (T score), mean (SD) | 74.39 (8.35) | |||
| HR, mean bpm (SD) | 70.91 (8.10) | 67.27 (11.48) | .013 | −.32 [−1.13; .49] |
| RMSSD, mean ms (SD) | 58.40 (12.26) | 68.29 (17.51) | .086 | .60 [−.22; 1.41] |
| HF-HRV, mean ms² (SD) | 1049.88 (374.15) | 1506.32 (697.54) | .232 | .98 [.14; 1.83] |
| BDI, mean (SD) | 31.08(10.69) | 14.09 (15.86) | .261 | −1.31 [−2.23; −.39] |
Notes: missing BDI data at Follow-Up for n = 1 patient; missing CDRS data at Baseline for n = 3 patients; case-wise data presented; d [95%CI]: Cohen’s d corrected for dependence among means
Table 2.
Cortical Thickness in Fronto-Limbic Regions at Baseline and After Treatment
| Baseline | Follow-Up | r | d [95%CI] | |||||
|---|---|---|---|---|---|---|---|---|
| LH | RH | LH | RH | LH | RH | LH | RH | |
| Caudal ACC | 2.81 (.14) | 2.69 (.17) | 2.74 (.23) | 2.67 (.18) | .710 | .783 | −.66 [−1.48; .17] | −.18 [−.98; .62] |
| Caudal MFG | 2.76 (.11) | 2.67 (.09) | 2.73 (.16) | 2.65 (.12) | .884 | .668 | −.57 [−1.38; .25] | −.27 [−1.08; .53] |
| Lateral OFC | 2.64 (.12) | 2.60 (.13) | 2.67 (.11) | 2.60 (.11) | .451 | .559 | .24 [−.56; 1.04] | .00[−.80; .80] |
| Medial OFC | 2.43 (.13) | 2.51 (.13) | 2.51 (.10) | 2.53 (.13) | .482 | .560 | .61 [−.21; 1.42] | .16[−.64; .97] |
| Rostral ACC | 3.08 (.16) | 3.05 (.22) | 3.04 (.19) | 3.04 (.20) | .782 | .346 | −.38 [−1.19; .43] | −.04 [−.84; .76] |
| Rostral MFG | 2.56 (.09) | 2.45 (.09) | 2.56 (.08) | 2.45 (.07) | .860 | .700 | .00 [−.80; .80] | .00 [−.80; .80] |
| Superior Frontal | 2.99 (.12) | 2.92 (.11) | 2.95 (.15) | 2.90 (.11) | .621 | .721 | −.38 [−1.19; .43] | −.24 [−1.05; .56] |
| Frontal Pole | 2.84 (.40) | 2.87 (.22) | 2.91 (.33) | 2.86 (.27) | .748 | .655 | .25 [−.56; 1.05] | −.06 [−.86; .75] |
| Insula | 3.15 (.11) | 3.05 (.12) | 3.15 (.11) | 3.06 (.19) | .599 | .595 | .00 [−.80; .80] | .09 [−.71; .89] |
Notes: LH: left hemisphere; RH: right hemisphere; ACC: anterior cingulate cortex; MFG: middle frontal gyrus; OFC: orbitofrontal cortex; all data given as mean and standard deviation; d [95%CI]: Cohen’s d from mean comparisons corrected for dependence among means
Figure 1. Significant Differences in Patients from Baseline to Follow-Up.
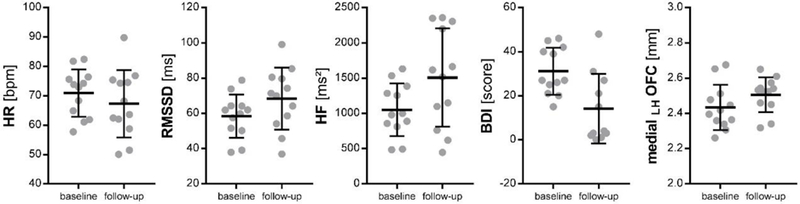
HR: heart rate; bpm: beats per minute; RMSSD: root of the mean squared difference of successive inter-beat-intervals; ms: milliseconds; HF: high-frequency heart rate variability; BDI: depressive symptoms assessed on the Beck Depression Inventory; LH: left hemisphere; OFC: orbitofrontal cortex; mm: millimeter; illustrated are means and standard deviations (SD) for each time point and patient.
Relationship between clinical improvement and biological changes.
Clinical improvement in depression symptoms as measured by BDI-II was associated with decreased HR (r(11) = .799, p = .003, 95%CI [.382; .946]) and increased RMSSD (r(11) = −.639, p = .034, 95%CI [−.896; −.064]). There was no significant association between clinical improvement and change in HF-HRV (r(11) = −.500, p = .117, 95%CI [−.846; .143]). No correlations were found between clinical improvement and change in cortical thickness.
Relationship between changes in each biological variable with change in the others.
Increase in left lateral OFC cortical thickness was associated with increase in RMSSD (r(12) = .582, p = .047, 95%CI [.012; .866]) and HF-HRV (r(12) = .641, p = .025, 95%CI [.106; .888]), but there was not a significant correlation with HR (r(12) = −.410, p = .186, 95%CI [−.796; .215]). Further, relative change in cortical thickness of the left frontal pole was associated with changes in HF-HRV (r(12) = .582, p = .047, 95%CI [.012; .866]), but not RMSSD (r(12) = .545, p = .067, 95%CI [−.043; .852]), or HR (r(12) = −.345, p = .272, 95%CI [−.767; .285]).
4. Discussion
This preliminary study examined treatment-related changes in depression severity, cortical thickness and autonomic function in 12 adolescents with MDD. Following 8 weeks of SSRI treatment, adolescents reported decreased depressive symptoms, showed increased cortical thickness of the left medial OFC, and showed increased vagally-mediated HRV as indexed by HF-HRV. Clinical improvement was associated with changes in autonomic function indexed by greater RMSSD and lower HR. Increased vagally-mediated HRV following treatment was positively associated with changes in cortical thickness of the left lateral OFC (RMSSD and HF-HRV) and left frontal pole (HF-HRV). Changes in cortical thickness were unrelated to changes in HR and depression severity. Thus, although we illustrated a link between symptom improvement and changes in autonomic function, as well as between changes in autonomic function and cortical thickness, in this small sample we found no association between symptom improvement and cortical thickness.
The associations found between improvement in depression severity and changes in autonomic function are in line with previous studies in adults. Although only HF-HRV showed significant differences comparing recordings taken before and after treatment, on a dimensional level, increased RMSSD and decreased HR were associated with improvements in depression severity. Changes in RMSSD and HF-HRV were medium to large in size (Cohen’s d = .60 to .98), while changes in HR were of small to medium magnitude (Cohen’s d = −.32; Quintana, 2017). Chambers & Allen (2002), reported that reduction in depression symptoms, in a sample of 16 women with MDD that received 8 weeks of acupuncture, was associated with greater increase in RSA (r = − .61) and with greater decrease in HR (r = .54). Correlation coefficients in the present study were stronger for RMSSD (r = .64) and HR (r = .80), but missed the set level of significance for HF-HRV (r = −.50). The present study suggests that indices of autonomic function might be well suitable to track symptom severity over time in adolescents with depression (Koenig et al., 2016). Importantly, although acute SSRI intake may be associated with reduced HRV (Kemp et al., 2016), findings show that improvements in depression severity following pharmacological treatment are associated with increased HRV. Unlike an earlier review (van Zyl et al., 2008) the largest meta-analysis to date (Kemp et al., 2010) found that acute pharmacological antidepressant treatment has minimal effects on HRV, and that unmedicated patients with MDD also show reduced HRV. The implication from this study was that reduced HRV in adults with MDD was more attributable to their diagnosis than to the pharmacological properties of their treatment. The mechanisms of SSRI treatment are still not fully understood, but it is generally accepted that although they lead to an acute effect of increased serotonin levels in the synapse, the delayed antidepressant effect is achieved through neuro-adaptive processes that are weeks in the making. Since treatment of depression requires long-term treatment, improving our understanding of the long-term effects of SSRI treatment on autonomic functioning and their association with symptom improvement will be critical to the field.
Changes in cortical thickness from baseline to post-treatment scans were small in size with the exception of the left medial OFC (Cohen’s d = .62), which showed a significant increase in cortical thickness (0.07mm on average). The OFC is known to play a critically important role in the pathophysiology of depression, based on prior studies that identified abnormal structure and function (i.e., glucose metabolism and cerebral blood flow) of the medial OFC in adults with MDD (Drevets, 2007). Previous pharmacological studies have reported similar treatment effects on gray matter volume (Dusi et al., 2015). Further, studies highlighted the importance of the OFC in predicting remission from depression in adults (Phillips et al., 2015), and depression onset in adolescents girls (Foland-Ross et al., 2015). In the present analyses, increases in left lateral OFC thickness were associated with increases in cardiac vagal activity. Similarly, although change in left frontal pole thickness was in itself not significant, we found that increased thickness in this region was associated with increases in vagal activity (HF-HRV only). Interestingly, we have previously reported a general pattern of inverse associations between HRV and cortical thickness (not including the OFC) in healthy non-depressed adolescents (Koenig et al., 2017a). This pattern was inverted (i.e., positive association between HRV and cortical thickness) in depressed adolescents (Koenig et al. 2018), similar to findings in adults (Winkelmann et al., 2016; Wood et al., 2017; Yoo et al., 2017). We previously suggested a compensatory mechanism where increased cortical thickness in adolescent MDD may facilitate maintenance of autonomic function (Koenig et al. 2018). The present findings add that effective SSRI treatment does not normalize the pattern of association between HRV and thickness, but seems to support the hypothesis that a compensatory mechanism is at play via increases in cortical thickness in the OFC.
Our previous cross-sectional study (Koenig et al. 2018) found that left lateral OFC and left frontal pole thickness interacted with depression severity (assessed at baseline via clinician interviews) in predicting only HR – not vagally-mediated HRV –in adolescents with MDD. The present results suggest that pharmacological treatment may improve top-down control of cardiac function via the lateral OFC. Brain structural effects of SSRI treatment are considered neuroprotective, in that they reduce structural shrinkage processes (Dusi et al., 2015). It is suggested that SSRIs enhance neuroplasticity as evidenced by an increase in functional connectivity (Kraus et al., 2014). Consequences of MDD and its treatment on autonomic function are likely attributable to changes in norepinephrine and serotonin metabolism (Ressler and Nemeroff, 1999). Experimental lowering of serotonin via tryptophan depletion has been shown to reduce HRV and increase depressive symptoms in remitted MDD patients (Booij et al., 2006). However, functional neuroimaging studies including assessments of neurotransmitter concentration in adolescent MDD are necessary to confirm these assumptions. Interestingly, a recent PET study in 10 women with MDD and 7 healthy controls found regional cerebral blood flow in the medial OFC to be positively associated with the low-frequency (LF)/HF ratio during a handgrip task (Nugent et al., 2011). Although LF/HF may not be an optimal measure of sympathetic-parasympathetic balance (Billman, 2013; Heathers and Goodwin, 2017; Heathers, 2014) the findings from this small sample point to a potential role of the medial OFC in autonomic dysregulation associated with depression.
Vagal activity, indexed by HRV, has been suggested to serve as a proxy for mapping the function of these pre-frontal inhibitory mechanisms within the Model of Neurovisceral Integration (Thayer and Lane, 2000). Executive brain areas, such as the PFC, exert an inhibitory influence on sub-cortical structures, such as the amygdala, allowing the organism to adaptively respond to demands from the environment, and organize their emotional and behavioral responses effectively (Thayer et al., 2012). The same set of neural structures is implicated in regulating ANS function. It is suggested that this reciprocal inhibitory cortico-subcortical neural circuit serves as a structural link between psychological processes such as emotion regulation, and health-related physiological outcomes. Adaptive functioning of this circuit can be indexed by vagally-mediated HRV (Thayer et al., 2012) and is suggested to be compromised in MDD. Pharmacological treatment of MDD is associated with changes in brain function, suggesting a normalization of affective processing (i.e., a reduction of limbic activation in response to negative stimuli; Delaveau et al., 2011; Wessa and Lois, 2015). The present findings add to current knowledge that SSRI-enhanced neuroplasticity of the OFC may aid to restore function of the inhibitory cortico-subcortical neural circuit also involved in regulating autonomic function. A previous study was able to show that sertraline treatment over 12 weeks led to a significant increase in brain-RSA covariation for adult patients with MDD after treatment (Schafer et al., 2015). Treatment studies with multiple repeated assessments including functional MRI may help to elucidate similar processes in adolescents with MDD.
Future studies in larger samples will further allow researchers to address the lack of association reported between symptom improvement and cortical thickness in the present study. Currently we can only speculate that the correlation was too weak to meet the set level of significance in such a small sample. It is possible that changes in ANS precede observable changes in cortical thickness; testing this possibility will require long-term follow-up scans to reveal meaningful correlations with symptom improvement. It is also possible that the association between symptom improvement and cortical thickness is only mediated via ANS function, and not directly related. Following this line of thought, and pioneering work by Mather and Thayer (Mather & Thayer 2018) increases in HRV via pharmacological treatment may entrain brain rhythms and architecture, thereby improving emotion related outcome (i.e., depression).
4.1. Limitations
The study has several limitations that need to be addressed. First, due to the small sample size, results should be considered as preliminary findings that need further replication. Given the small sample size, we were not able to address potential confounding factors such as sex in the present analyses. Further, we did not adjust for multiple comparisons. Thus, caution is required when interpreting these findings. Second, in the absence of a placebo group, we cannot confirm that the neurobiological changes are due solely to medication effect. Future studies would do well to integrate a placebo arm. Third, we drew on a sample from a naturalistic study design (Cullen et al., 2016), in that we did not control the type of antidepressant medication or the dose that the adolescents received between scans. Patients either received fluoxetine or sertraline and a range of different doses. It is possible that the different SSRI medications and doses have different effects on the reported outcomes. Fourth, we relied on self-report assessment to measure clinical change in depression severity. Inclusion of a clinician-administered interview such as the CDRS-R at the post-treatment assessment would have provided additional and important information about clinical change. Finally, HRV analyses were based on pulse oximetry data. Although pulse oximetry is acceptable for the analyses of HRV, future studies would do well to implement recordings of vagally-mediated HRV and HR using ECG at a higher sample-rate.
4.2. Conclusion
This preliminary study suggests that symptom improvements in adolescents with MDD following 8 weeks of SSRI treatment are associated with improvement of autonomic function, characterized by increases in vagally-mediated HRV (RMSSD only) and decreases in HR, and increases in OFC cortical thickness. Post treatment increases in cortical thickness of the left lateral OFC (RMSSD and HF-HRV) and the left frontal pole (HF-HRV only) were associated with increases in vagally-mediated HRV. These findings suggest biological mediators of treatment response. Although SSRI treatments are known to be effective for depression in adolescents, many adolescents do not respond to these treatments and mechanisms underlying response and non-response have not been fully understood. Research documenting the biological mediators of response to existing treatments can provide a foothold for monitoring and optimizing treatment based on neurobiological changes. ANS function seems suitable to monitor treatment outcome in adolescents with MDD, providing a window to underlying changes in brain morphology. Findings are preliminary and require future replication and extension addressing the causality and temporal dependency underlying the associations between ANS function, cortical thickness and depression.
Figure 2. Correlations of Change in Depression Severity and Change in Resting State Autonomic Function.
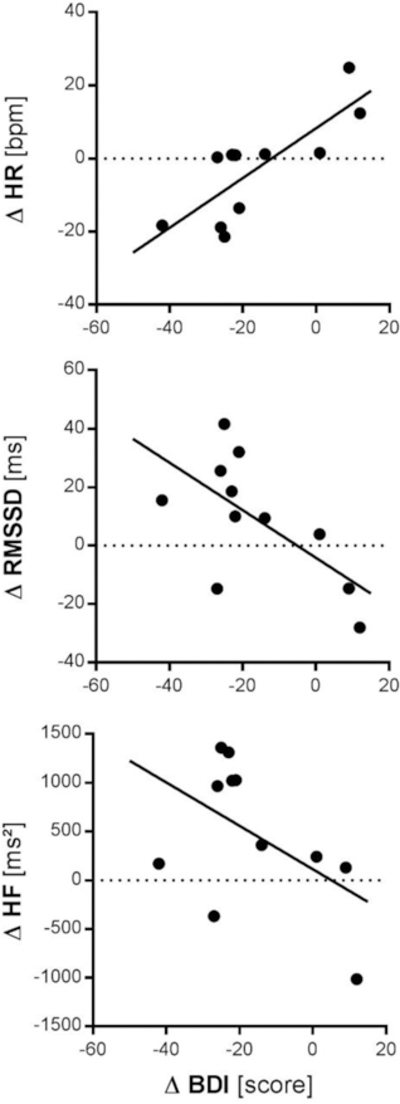
HR: heart rate; bpm: beats per minute; RMSSD: root of the mean squared difference of successive inter-beat-intervals; ms: milliseconds; HF: high-frequency heart rate variability; BDI: depressive symptoms assessed on the Beck Depression Inventory; ∆: observed differences score (Follow-Up minus Baseline)
Figure 3. Correlations of Change in Resting State Autonomic Function and Cortical Thickness.
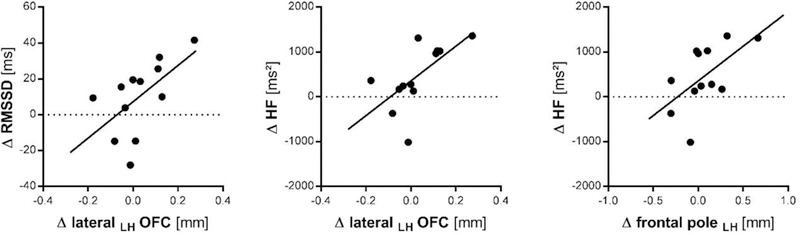
RMSSD: root of the mean squared difference of successive inter-beat-intervals; ms: milliseconds; HF: high-frequency heart rate variability; LH: left hemisphere; OFC: orbitofrontal cortex; mm: millimeter ∆: observed differences score (Follow-Up minus Baseline)
Highlights.
Treatment of MDD is associated with increases in HRV and cortical thickness in adults
Preliminary data in n=12 adolescents with MDD following 8 weeks of SSRI treatment
Decreased symptoms, increased HRV and thickness of the left medial OFC at follow-up
Improvement of MDD severity was associated with increased HRV and decreased HR
Increased HRV, left lateral OFC and superior frontal cortex thickness correlated
5. Acknowledgement
The authors would like to first and foremost thank the adolescents and families that contributed to this study. The study was funded by grants to Dr. Cullen including the National Institute of Mental Health (K23MH090421), the National Alliance for Research on Schizophrenia and Depression, the University of Minnesota Graduate School, and the Minnesota Medical Foundation. Dr. Koenig is supported by a Physician-Scientist-Fellowship provided by the Medical School, University of Heidelberg, Germany, and acknowledges the financial support through a Post-Doctoral Scholarship provided by the Daimler and Benz Foundation (Ladenburg, Germany) and the Thrasher Research Fund Early Career Award provided by the Thrasher Research Fund (Salt Lake City, UT, USA). These resources supported the roles of design and conduct of the study; collection, management, and analysis of the data; and interpretation of results and preparation of the publication. We thank Michael Gaebler for his helpful support regarding the Siemens PPG data format and readout.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
8. References
- Balogh S, Fitzpatrick DF, Hendricks SE, Paige SR, 1993. Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacol. Bull 29, 201–206. [PubMed] [Google Scholar]
- Beck A, Steer R, Brown K, 1996. Beck Depression Inventory - Revised. Harcourt Brace, Texas
- Billman GE, 2013. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol 4 10.3389/fphys.2013.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Swenne CA, Brosschot JF, Haffmans PMJ, Thayer JF, Van der Does AJW, 2006. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol. Psychiatry 60, 507–514. 10.1016/j.biopsych.2006.02.010 [DOI] [PubMed] [Google Scholar]
- Carnevali L, Koenig J, Sgoifo A, Ottaviani C, 2018. Autonomic and Brain Morphological Predictors of Stress Resilience. Front Neurosci 12:228 10.3389/fnins.2018.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AS, Allen JJB, 2002. Vagal tone as an indicator of treatment response in major depression. Psychophysiology 39, 861–864. 10.1017/S0048577202010442 [DOI] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Vu DP, Westlund Schreiner M, Mueller BA, Eberly LE, Camchong J, Westervelt A, Lim KO, 2016. Neural Correlates of Antidepressant Treatment Response in Adolescents with Major Depressive Disorder. J. Child Adolesc. Psychopharmacol 26, 705–712. 10.1089/cap.2015.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO, 2014. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry 71, 1138–1147. 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P, 2011. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J. Affect. Disord 130, 66–74. 10.1016/j.jad.2010.09.032 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Drevets WC, 2007. Orbitofrontal cortex function and structure in depression. Ann. N. Y. Acad. Sci 1121, 499–527. 10.1196/annals.1401.029 [DOI] [PubMed] [Google Scholar]
- Dusi N, Barlati S, Vita A, Brambilla P, 2015. Brain Structural Effects of Antidepressant Treatment in Major Depression. Curr. Neuropharmacol 13, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic Resonance images. Proc. Natl. Acad. Sci. U. S. A 97, 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Sacchet MD, Prasad G, Gilbert B, Thompson PM, Gotlib IH, 2015. Cortical Thickness Predicts the First Onset of Major Depression in Adolescence. Int. J. Dev. Neurosci.Off.J.Int.Soc.Dev.Neurosci 46, 125–131. 10.1016/j.ijdevneu.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil E, Orini M, Bailón R, Vergara JM, Mainardi L, Laguna P, 2010. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas 31, 1271–1290. 10.1088/0967-3334/31/9/015 [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Gaffney M, Zyl LTV, 2007. Heart Rate Variability in Acute Coronary Syndrome Patients With Major Depression: Influence of Sertraline and Mood Improvement. Arch. Gen. Psychiatry 64, 1025–1031. 10.1001/archpsyc.64.9.1025 [DOI] [PubMed] [Google Scholar]
- Hall LMJ, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, Mueller BA, Lim KO, Cullen KR, 2014. An fMRI study of emotional face processing in adolescent major depression. J. Affect. Disord 168, 44–50. 10.1016/j.jad.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathers J, Goodwin M, 2017. LF/HF HRV: The „Life After Death‟ Of A Refuted Theory. PsyArXiv https://doi.org/10.17605/OSF.IO/637YM
- Heathers JAJ, 2014. Everything Hertz: methodological issues in short-term frequency-domain HRV. Front. Physiol 5 10.3389/fphys.2014.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A, Hwang S-K, Padhye NS, Meininger JC, 2017. Effects of Cognitive Behavior Therapy on Heart Rate Variability in Young Females with Constipation-predominant Irritable Bowel Syndrome: A Parallel-group Trial. J. Neurogastroenterol. Motil 23, 435–445. 10.5056/jnm17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–8. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kemp AH, Fráguas R, Brunoni AR, Bittencourt MS, Nunes MA, Dantas EM, Andreão RV, Mill JG, Ribeiro ALP, Koenig J, Thayer JF, Benseñor IM, Lotufo PA, 2016. Differential Associations of Specific Selective Serotonin Reuptake Inhibitors With Resting-State Heart Rate and Heart Rate Variability: Implications for Health and Well-Being. Psychosom. Med 78, 810–818. 10.1097/PSY.0000000000000336 [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM, 2010. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67, 1067–1074. 10.1016/j.biopsych.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar‐ Gaxiola S, Alonso J, Lee S, Ustun TB, 2007. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatry 20, 359–364. 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaykin Y, Dorian P, Baker B, Shapiro C, Sandor P, Mironov D, Irvine J, Newman D, 1998. Autonomic correlates of antidepressant treatment using heart-rate variability analysis. Can. J. Psychiatry Rev. Can. Psychiatr 43, 183–186. 10.1177/070674379804300209 [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Eberly LE, Schreiner MW, Kurkiewicy P, Houri A, Schlesinger A, Thomas KM, Mueller BA, Lim KO, Cullen KR, 2014. Multilevel assessment of the neurobiological threat system in depressed adolescents: Interplay between the limbic system and hypothalamic–pituitary–adrenal axis. Dev. Psychopathol 26, 1321–1335. 10.1017/S0954579414001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Westlund Schreiner M, Thai M, Gunlicks-Stoessel M, Reigstad K, Cullen KR, 2018. Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: A preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 194–202. 10.1016/j.pnpbp.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Beauchaine TP, Thayer JF, Kaess M, 2016. Depression and resting state heart rate variability in children and adolescents - A systematic review and meta-analysis. Clin. Psychol. Rev 46, 136–150. 10.1016/j.cpr.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Koenig J, Parzer P, Reichl C, Ando A, Thayer J, Brunner R, Kaess M, 2017a. Cortical Thickness, Resting State Heart Rate and Heart Rate Variability in Female Adolescents [DOI] [PubMed]
- Koenig J, Weise S, Rinnewitz L, Parzer P, Resch F, Kaess M, 2017b. Longitudinal covariance of resting-state cardiac function and borderline personality disorder symptoms in adolescent non-suicidal self-injury. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 1–6. 10.1080/15622975.2017.1342046 [DOI] [PubMed]
- Koenig J, Westlund Schreiner M, Klimes-Dougan B, Ubani B, Mueller B, Kaess M, Cullen KR, 2018. Brain Structural Thickness and Resting State Autonomic Function in Adolescents with Major Depression. Soc Cogn Affect Neurosci 10.1093/scan/nsy046. [DOI] [PMC free article] [PubMed]
- Kraus C, Ganger S, Losak J, Hahn A, Savli M, Kranz GS, Baldinger P, Windischberger C, Kasper S, Lanzenberger R, 2014. Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. NeuroImage 84, 236–244. 10.1016/j.neuroimage.2013.08.036 [DOI] [PubMed] [Google Scholar]
- Mackin RS, Tosun D, Mueller SG, Lee J-Y, Insel P, Schuff N, Truran-Sacrey D, Arean P, Nelson JC, Weiner MW, 2013. Patterns of Reduced Cortical Thickness in Late-Life Depression and Relationship to Psychotherapeutic Response. Am. J. Geriatr. Psychiatry 21, 794–802. 10.1016/j.jagp.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Nakamura EF, Kessler RC, 2009. Epidemiology of mental disorders in children and adolescents. Dialogues Clin. Neurosci 11, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Thayer J, 2018. How heart rate variability affects emotion regulation brain networks. Curr Opin Behav Sci 19:98–104. 10.1016/j.cobeha.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC, 2011. Heart rate variability during motor and cognitive tasks in females with major depressive disorder. Psychiatry Res 191, 1–8. 10.1016/j.pscychresns.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL, 2004. Reliability and validity of the Beck depression inventory--II with adolescent psychiatric inpatients. Psychol. Assess 16, 120–132. 10.1037/1040-3590.16.2.120 [DOI] [PubMed] [Google Scholar]
- Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P, 2015. A Prospective, Longitudinal Study of the Effect of Remission on Cortical Thickness and Hippocampal Volume in Patients with Treatment-Resistant Depression. Int. J. Neuropsychopharmacol 18 10.1093/ijnp/pyv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Freman L, Mokros H, 1985. Childre’s depression rating scale-revised. Psychopharmacol. Bull 979–989.
- Quintana DS, 2017. Statistical considerations for reporting and planning heart rate variability case-control studies. Psychophysiology 54, 344–349. 10.1111/psyp.12798 [DOI] [PubMed] [Google Scholar]
- Quintana DS, Alvares GA, Heathers J. a. J., 2016. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl. Psychiatry 6, e803 10.1038/tp.2016.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB, 1999. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol. Psychiatry 46, 1219–1233. 10.1016/S0006-3223(99)00127-4 [DOI] [PubMed] [Google Scholar]
- Schafer SM, Wager TD, Mercado RAJ, Thayer JF, Allen JJB, Lane RD, 2015. Partial Amelioration of Medial Visceromotor Network Dysfunction in Major Depression by Sertraline. Psychosom. Med 77, 752–761. 10.1097/PSY.0000000000000200 [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Carnevali L, Alfonso Mde L, Amore M, 2015. Autonomic dysfunction and heart rate variability in depression. Stress 18, 343–352. 10.3109/10253890.2015.1045868 [DOI] [PubMed] [Google Scholar]
- Shader TM, Gatzke-Kopp LM, Crowell SE, Jamila Reid M, Thayer JF, Vasey MW, Webster-Stratton C, Bell Z, Beauchaine TP, 2018. Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Dev. Psychopathol 30, 351–366. 10.1017/S0954579417000669 [DOI] [PubMed] [Google Scholar]
- Singh MK, Gotlib IH, 2014. The Neuroscience of Depression: Implications for Assessment and Intervention. Behav. Res. Ther 62, 60–73. 10.1016/j.brat.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA, 2014. Kubios HRV--heart rate variability analysis software. Comput. Methods Programs Biomed 113, 210–220. 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers III JJ, Wager TD, 2012. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health Neurosci. Biobehav. Rev 36, 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord., Arousal in Anxiety 61, 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- van Zyl LT, Hasegawa T, Nagata K, 2008. Effects of antidepressant treatment on heart rate variability in major depression: A quantitative review. Biopsychosoc. Med 2, 12 10.1186/1751-0759-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp LM, 2009. Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. J. Child Psychol. Psychiatry 50, 1357–1364. 10.1111/j.1469-7610.2009.02172.x [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence The Psychological Corporation: Harcourt Brace & Company, New York, NY. [Google Scholar]
- Wessa M, Lois G, 2015. Brain Functional Effects of Psychopharmacological Treatment in Major Depression: a Focus on Neural Circuitry of Affective Processing. Curr. Neuropharmacol 13, 466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann T, Thayer JF, Pohlack S, Nees F, Grimm O, Flor H, 2016. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Struct. Funct 10.1007/s00429-016-1185-1 [DOI] [PubMed]
- Wood KN, Badrov MB, Speechley MR, Shoemaker JK, 2017. Regional cerebral cortical thickness correlates with autonomic outflow. Auton. Neurosci. Basic Clin 10.1016/j.autneu.2017.05.012 [DOI] [PubMed]
- Woodward SH, Kaloupek DG, Schaer M, Martinez C, Eliez S, 2008. Right anterior cingulate cortical volume covaries with respiratory sinus arrhythmia magnitude in combat veterans. J. Rehabil. Res. Dev 45, 451–463. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Thayer JF, Greening S, Lee T-H, Ponzio A, Min J, Sakaki M, Nga L, Mather M, Koenig J, 2017. Brain structural concomitants of resting state heart rate variability in the young and old: evidence from two independent samples. Brain Struct. Funct 10.1007/s00429-017-1519-7 [DOI] [PMC free article] [PubMed]


