Abstract
To assess the effect of a savings-led economic empowerment intervention on viral suppression among adolescents living with HIV. Using data from Suubi + Adherence, a longitudinal, cluster randomized trial in southern Uganda (2012–2017), we examine the effect of the intervention on HIV RNA viral load, dichotomized between undetectable (< 40 copies/ml) and detectable (≥ 40 copies/ml). Cluster-adjusted comparisons of means and proportions were used to descriptively analyze changes in viral load between study arms while multi-level modelling was used to estimate treatment efficacy after adjusting for fixed and random effects. At 24-months post intervention initiation, the proportion of virally suppressed participants in the intervention cohort increased tenfold (ΔT2–T0 = + 10.0, p = 0.001) relative to the control group (ΔT2–T0 = + 1.1, p = 0.733). In adjusted mixed models, simple main effects tests identified significantly lower odds of intervention adolescents having a detectable viral load at both 12- and 24-months. Interventions addressing economic insecurity have the potential to bolster health outcomes, such as HIV viral suppression, by improving ART adherence among vulnerable adolescents living in low-resource environments. Further research and policy dialogue on the intersections of financial security and HIV treatment are warranted.
Keywords: ART, HIV, SUUBI, Adolescents, Assets, Economic empowerment, Savings-led intervention
Introduction
An estimated 2.1 million adolescents, aged 10–19 years, are living with HIV/AIDS (ALWHA) worldwide, with greater than 80% in sub-Saharan Africa [1]. Research has shown that early initiation of antiretroviral therapy (ART) and continued adherence to ART in persons living with HIV can lead to viral load suppression, extended life expectancy, and reduced risk of HIV transmission to uninfected sexual partners [2, 3]. ART adherence also delays disease progression to AIDS and decreases the incidence of serious non-AIDS events [4, 5]. With expanded coverage of ART globally to children; there is a growing number of perinatally-infected adolescents who are increasingly responsible for managing their ART regimens [6, 7]. However, studies have shown that adolescents have worse adherence compared to adults and younger children [8, 9]. Adolescents are the only group for which AIDS-related mortality has not decreased [10, 11] with AIDS being the leading cause of adolescent death in sub-Saharan Africa [12].
Financial instability remains a threat to adherence to ART and its potential effectiveness in persons living with HIV [13, 14]. The current evidence has primarily centered on HIV-infected adults, and suggests several mechanisms for why this may be the case. HIV-infected individuals may have inadequate economic access to ART, limiting resources to pay for real or perceived costs related to medications and treatment, including travel costs to remain engaged in care [15–17]. Many individuals living with HIV, including families affected by AIDS, may have lost employment or other sources of income over the course of their illness. They may subsequently avoid treatment or experience disruptions in ART due to lack of food or other financial support [18]. Food insecurities may hinder adherence to ART as a result of stress, anxiety, or physical ramifications of hunger [18, 19]. Once initiating ART, individuals and families affected by AIDS may also struggle to resume income-earning work if household assets were sold to sustain food or other living expenses during periods of illness and unemployment [18, 20]. For adolescents in particular, studies have also shown that caregiver inability to pay a fee for ART, inadequate meals to support medication consumption, and resource prioritization towards school expenses are all associated with low ART adherence in youth populations [21–23].
A limited number of economic interventions have been evaluated to address low ART adherence in resource-poor settings. The published literature has largely focused on poor adults who are living with HIV and includes strategies relating to financial and economic incentives [18, 24], food assistance [18, 25], medication vouchers and subsidies [26, 27], as well as microfinance [26] and entrepreneurial education [27]. By and large, these studies have found significant improvements in treatment adherence among HIV-infected adults.
Many of these studies were limited by non-experimental designs. The few studies targeting adolescent ART adherence have rarely addressed economic factors as key drivers of disengagement in care. In a recent systematic review of ARV adherence enhancing interventions for adolescents and young adults, aged 13–24 [28], only one of the ten intervention studies included an economic assistance component [29]. Since then, to our knowledge, only one other published intervention [21] has evaluated use of cash assistance to improve adolescent adherence to ART. In addition, while the literature on economic interventions and adolescent adherence is limited, the evidence examining cash assistance on HIV incidence among adolescents has been somewhat mixed [30], indicating that structural interventions may not always achieve similar results in all contexts and highlighting the need for further research in this area. To address this knowledge gap, our study assesses viral load suppression, a clinical marker of ART adherence, among PHIV adolescents randomized to receive an economic intervention that included a Child Savings Account (CSA) compared to adolescents randomized to receive a bolstered standard of care.
Methods
Study Design and Setting
This paper used the first three waves of data (baseline, 12-, and 24-months post-intervention initiation) from a longitudinal NICHD-funded cluster randomized trial in southern Uganda, entitled Suubi + Adherence (2012–2017). The Suubi + Adherence project is a family-based savings-led economic empowerment intervention focused on addressing ARV adherence for HIV-infected adolescents in four districts of southern Uganda (Rakai, Masaka, Kalungu & Lwengo), a region heavily affected by HIV/AIDS, with an HIV prevalence rate of 10.6%, three percentage points higher than the national average of 7.4% [31]. Despite rapid declines in poverty in the past decade, approximately 35% of Ugandans continue to live on $1.90 USD or less per day [32]. Such economic insecurity suggests that costs associated with HIV treatment, including transportation to clinics and access to food (to meet ART dietary requirements and avoid medication related gastrointestinal side effects), may negatively affect adherence, even where ART is provided without cost.
Sample
Nine hundred ninety adolescents from 40 HIV clinics were screened for eligibility. To be eligible for the study, adolescents must have met the following conditions: (i) tested positive for HIV (confirmed by medical report and aware of status); (ii) living within a family; (iii) aged 10–16 years; (iv) prescribed ART; and (v) enrolled in care at a medical clinic participating in the Suubi + Adherence project. Two-hundred and eighty-eight adolescents were excluded, predominately for lack of awareness of HIV status and being under or over age. Participants were also excluded if they had a cognitive or severe psychiatric impairment that would prevent comprehension of study procedures as assessed during the Informed Consent process. Seven hundred and two adolescents were enrolled in the study and interviewed at baseline.
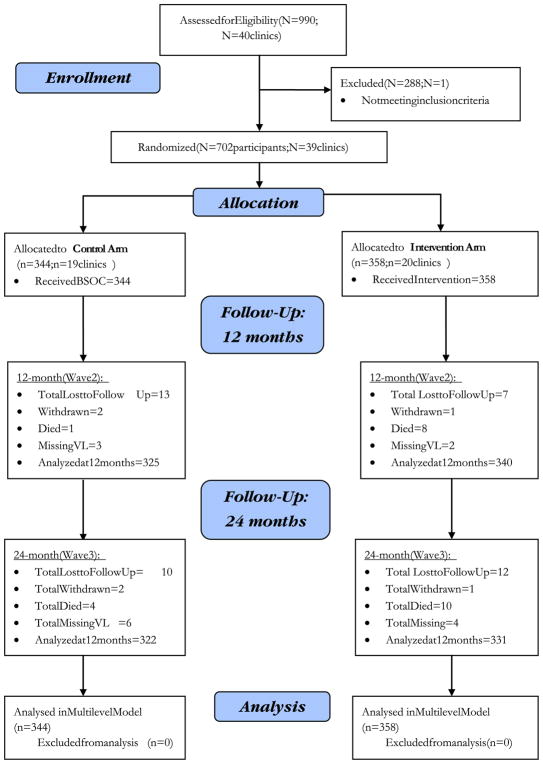
Clinics were eligible to participate if they (1) had existing procedures tailored to adolescent adherence (including adolescent clinic days and peer counseling); and (2) were accredited by the Ugandan Ministry of Health as a provider of ART within the four study districts. Based on that criterion one clinic was excluded leaving a total of 39 clinics within the study sample, representing all clinics that met the inclusion criteria within the study districts. See Consort Diagram (Fig. 1).
Fig. 1.
Consort diagram adherence study
Study Group Assignments
Following enrollment and baseline assessments, 39 participating clinics were randomly assigned to one of two study groups, resulting in 19 clinics (n = 344 participants) assigned to the control and 20 clinics (n = 358 participants) assigned to the intervention condition. A two-arm, cluster randomized trial design was employed where all participants attending the same clinic were assigned to the same study group. This was intended to reduce issues of contamination.
Randomization of clinics was carried out by an independent Columbia University Research Assistant. All participants received medical Standard of Care (SOC), as defined by the Uganda Ministry of Health Guidelines for pediatric and adolescent HIV care and treatment; and psychosocial SOC, consisting of information leaflets on adherence and support provided by lay counselors including persons living with HIV who have been trained in ART adherence counseling, known as “expert clients”. Due to inconsistency in which SOC is often provided in the region, the Suubi-Adherence study provided adolescents in both the control and intervention conditions with a bolstered standard of care (BSOC), which included eight information sessions on adherence to ART adapting evidence-based, print cartoons to portray adherence topics in a relatable manner [33]. Adolescents in the intervention group received the BSOC as well as an economic empowerment intervention consisting of a Child Savings Account (CSA), matched at a rate of 1:1 and from which financial savings could be used for medical related expenses, family small business development or education related expenses (including school lunches and fees). As part of the CSA package, the intervention group also received four workshops blending financial management and life skills with topics including asset building, small business development, goal setting, and risk mitigation, components previously tested among adolescents in the region [34, 35].
Outcomes
The primary outcome of this analysis was viral suppression as reflected in HIV RNA viral load (VL). VL testing was done by the Rakai Health Sciences Program at each of the study’s time points: baseline/pre-intervention initiation (time 0), 12-months post intervention initiation (time 1), and 24-months post intervention initiation (time 2). Plasma VL measurements were quantified in blood samples collected in EDTA tubes using Abbott RealTime HIV-1 RNA PCR, version 5.00. In accordance with the Abbott platform, VL was dichotomized between undetectable (< 40 copies/ml) and detectable (≥ 40 copies/ml) levels. Binary measures of VL have greater clinical relevance as health care practitioners aim to for all patients to achieve viral suppression. However, continuous measures of VL offer greater granularity for patient monitoring and data analysis. As such, in addition to our primary endpoint, we have included an exploratory analysis of the intervention’s effect on the continuous measure of VL, normalized using log10 transformation, reducing both skew and kurtosis (SK = 10.7 v. 1.1; KR = 159.4 v. 2.9).
Analysis
Cluster-adjusted comparisons of means and proportions using linear regressions with cluster-adjusted standard errors and Rao-Scott Chi square tests, respectively, were used to examine demographic and adherence characteristics at baseline and to descriptively analyze changes in adherence over time between study arms. Multi-level models were then used to estimate the effects of the intervention on adherence while accounting for clustering, specifically the nested nature of children within the same clinic and the repeated measures for each child. Mixed effects linear (Stata mixed command) and logistic (Stata melogit command) regression models were used for continuous and dichotomous outcomes, respectively. Linear mixed models were estimated using maximum likelihood estimation (ML); logistic mixed models were estimated via maximum likelihood using the adaptive Gaussian quadrature approach with 7 integration points. In initial unadjusted models, study group, time, and group-by-time interactions were incorporated to enable planned simple main effects comparisons of study arms within each time point and time points within each group. Subsequent covariate-adjusted mixed effects models were used to account for age, gender, and number of prescribed pills [36, 37], and to account for random effects as imposed by unmeasured characteristics at the clinic and individual level. An intention- to-treat (ITT) analysis inherent within the multi-level model allowed for the inclusion of every participant within the analysis, by the study group to which they were originally randomized, under the missing at random (MAR) assumption for cases with incomplete data via maximum likelihood estimation. All hypothesis tests were considered statistically significant at p < 0.05. Data were analyzed using STATA v14 (College Station, TX, USA).
Ethical Considerations
This study received ethics approval from Columbia University (Protocol #AAAK3852), the Makerere University School of Public Health (#IRB00011353) and the Uganda National Council for Science and Technology (Protocol #IRB00011353). Both adolescent assent and caregiver/guardian consent were obtained prior to the study. Adolescents were consented separately from their caregivers/guardians to avoid coercion to participate. The study’s protocol is registered in the clinicaltrial.gov database (ID#NCT01790373).
Results
Sample Demographic Characteristics
Among the 702 enrolled participants, the mean age was 12 years and 56% were girls. On average, two ARV pills were taken per day by study participants. The mean VL was between 4.96 and 5.23 log units with approximately 62% of adolescents in the control arm and 56% in the treatment arm recording suppressed VL (< 40 copies/ml) at baseline. No statistically significant differences were observed between study groups at baseline (Table 1). Between baseline and follow-up at 24 months, attrition rates between study arms were relatively consistent with a 5.5% rate for adolescents in the control group and 6.5% rate for the treatment arm (Fig. 1). Seventy-eight percent of participants attended all eight BSOC adherence sessions over the course of 8 months while 72% of intervention participants also attended the additional four workshops on financial management and life skills within a four-month time frame. Among intervention participants, mean self-reported savings increased from USD equivalent of $2.15 at baseline to $19.34 at 24-months while control group participant savings increased from $1.78 to $4.44 over the same time period. At 12- and 24-month follow-up, both control and treatment groups had approximately 1.0% of VL values missing for reasons other than loss to follow-up, withdrawal from the study, or death for which the attrition rate accounted.
Table 1.
Characteristics of enrolled adolescents at baseline by randomly assigned group
| Control N = 344 | Intervention N = 358 | p-valuea | |
|---|---|---|---|
| Demographic characteristics N(%) | |||
| Gender | |||
| Female | 193 (56.1) | 203 (56.7) | 0.88 |
| Male | 151 (43.9) | 155 (43.3) | |
| Age | |||
| Mean (SD) | 12.4 (2.0) | 12.5 (2.0) | 0.77 |
| Age 10–12 | 184 (53.5) | 181 (50.6) | 0.67 |
| Age 13–16 | 160 (46.5) | 177 (49.4) | |
| Number of pills in daily regimen | |||
| Mean (SD) | 1.95 (0.7) | 2.05 (0.7) | 0.33 |
| Adherence measures N(%) | |||
| Viral load | |||
| Mean (SD) | 12,344 (48,688) | 23,710 (111,706) | 0.08 |
| Mean (SD) Log10 VL | 4.96 (2.97) | 5.23 (3.11) | 0.25 |
| Viral load (copies/ml) | |||
| Below detectable (< 40 ml) | 214 (62.2) | 200 (55.9) | 0.18 |
| Detectable (≥ 40 ml) | 130 (37.8) | 158 (44.1) | |
Adjusted for clustering
Descriptive Analysis of Virologic Suppression by Study Arm
Analysis of pre-intervention VL data demonstrated no statistically significant difference between study arms in regard to the proportion of participants with suppressed VL. Likewise, exploratory analysis of the log of the VL showed no significant differences between the control and intervention arms (Table 1).
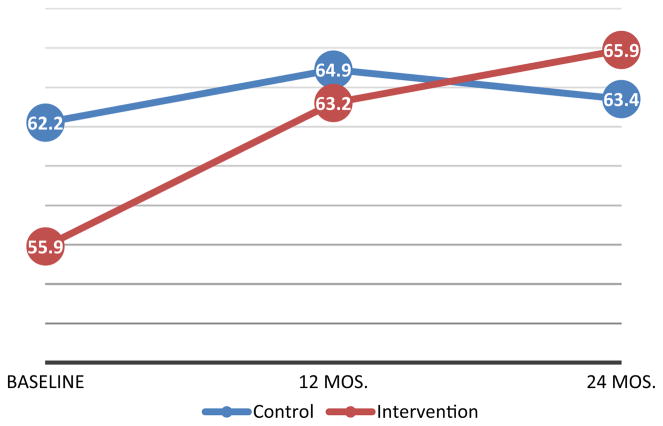
When measuring VL dichotomously to quantify viral suppression (< 40 vs. ≥ 40 copies/ml), adolescents in the control arm showed a small net increase in the proportion of participants with viral suppression between baseline and 24-months post-intervention initiation (ΔT2–T0 = + 1.1, p = 0.733), though the findings were not statistically significant. In contrast, during the same period, there was a statistically significant increase in the proportion of virally suppressed participants in the intervention cohort (ΔT2–T0 = + 10.0, p = 0.001) (Table 2, Fig. 2). Among intervention adolescents, a higher proportion of participants that achieved viral suppression did so within the 1st year of the intervention (ΔT1–T0 = + 7.4, p = 0.015) (Table 2, Fig. 2).
Table 2.
Descriptive analysis of viral suppression by study arm Adjusted for clustering of research participants within clinics
| Control | Intervention | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| T0 | T1 | T2 | ΔT1–T0 | ΔT2–T1 | ΔT2–T0 | T0 | T1 | T2 | ΔT1–T0 | ΔT2–T1 | ΔT2–T0 | |
| Sample size | 344 | 325 | 322 | – | – | – | 358 | 340 | 331 | – | – | – |
| Viral load (copies/ml) | ||||||||||||
| % Undetectable (< 40 copies/ml) | 62.2 | 64.9 | 63.4 | + 2.7 | − 1.6 | + 1.1 | 55.9 | 63.2 | 65.9 | + 7.4* | + 2.6 | + 10.0** |
| Viral load Log10 | ||||||||||||
| Mean (SD) | 4.96 (± 2.96) | 4.81 (± 2.82) | 5.19 (± 3.18) | − 0.15 | + 0.38 | + 0.23 | 5.23 (± 3.11) | 4.90 (± 2.91) | 4.95 (± 3.09) | − 0.33* | + 0.05 | − 0.28 |
p < 0.05,
p < 0.01
Fig. 2.
Percentage of virally suppressed adolescents by study arm
Comparisons of mean VL logs showed no statistically significant difference between the control and intervention arms from baseline to 12-months post intervention initiation or from 12- to 24-months. However, while the control group recorded a net increase in VL log units from baseline to 24-months (ΔT2–T0 = + 0.23, p = 0.124), the intervention group demonstrated a net decrease (ΔT2–T0 = − 0.28, p = 0.240); though these effects were not statistically significant (Table 2). For adolescents in the intervention group, a statistically significant reduction in VL log units did occur between baseline and 12-months post intervention initiation (ΔT1–T0 = − 0.33, p = 0.044) (Table 2).
Adjusted Effect of Suubi + Adherence Intervention
In mixed models, adjusting for pre-specified covariates of gender, age, and number of medications, no statistically significant group effect was identified for our primary outcome of analysis, the binary measure of VL. A significant time main effect was identified, indicating that the odds of viral suppression increased over time for both study groups (Table 3). However, the statistically significant group-by-time interaction qualified this time main effect such that the improvement in viral suppression was driven by adolescents in the intervention cohort (Table 3; Fig. 2). Simple main effects tests clarified this interaction by showing no significant differences for groups at each time point (all ps > 0.22), but a significant reduction in lower odds of having a detectable VL at 12 months (OR 0.424; 95% CI 0.248, 0.723; p = 0.002) and 24 months (OR 0.299; 95% CI 0.161, 0.554; p = <0.001) for intervention adolescents. In contrast, no significant differences were found across time for the control group (Fig. 2).
Table 3.
Multi-level regression analysis of viral suppression by study arm (N = 344)
| Adjusted estimate (± SE) and p valuea
|
||
|---|---|---|
| Detectable viral load (≥ 40 copies/ml) (adjusted odds ratio) | Viral load log10 (linear B) | |
| Constant | 0.099 (0.101) | 5.081 (0.652) |
| 0.023* | <30.001*** | |
| Intervention (N = 358) | 1.631 (0.662) | 0.191 (0.278) |
| 0.228 | 0.492 | |
| 12 month | 0.656 (0.187) | − 0.222 (0.158) |
| 0.139 | 0.161 | |
| 24 month | 0.742 (0.236) | 0.147 (0.184) |
| 0.348 | 0.425 | |
| Intervention × 12 month | 0.646 (0.232) | − 0.142 (0.198) |
| 0.224 | 0.475 | |
| Intervention × 24 month | 0.403 (0.147) | − 0.497 (0.199) |
| 0.013* | 0.013* | |
| Group × time χ2 (2) | 6.22 (p = 0.045)* | 6.57 (p = 0.037)* |
Results shown in italics are Chi square omnibus tests of the group-by-time interaction effect
Control N = 344; Intervention N = 358.
p < 0.05,
p < 0.01,
p < 0.001
Adjusted for clinic ID and child ID (random effects) and gender, age, and number of medications (fixed effects)
In exploratory analysis of the intervention effect on the log of the VL, results supported our findings on the primary outcome of analysis. A group-by-time interaction was identified with intervention group participants, driven by the difference in the log VL between baseline and 24-months in the intervention group (Table 3). Simple main effects tests showed no significant differences for groups at each time point (all ps > 0.28), but did reveal a significant reduction in log10 VL from baseline to 12-months (mean difference = − 0.364; 95% CI − 0.667, − 0.060; p = 0.019) in the intervention group. Though not statistically significant at the conventional alpha level of 0.05, the simple main effect difference from baseline to 24 months exhibited a similar reduction (mean difference = − 0.350; 95% CI − 0.702, 0.001; p = 0.051).
Of note, while VL differences did exist at baseline (Fig. 2), they were not statistically significant in contrast with the differences found at 24-months, which were significant. Further, the significant group-by-time interactions suggest differential change over time across the two groups with intervention group participants improving.
Discussion
In a large sample of Ugandan adolescents between the ages of 10 and 16 years and living with HIV, a multi-component economic empowerment intervention, Suubi + Adherence, was found to significantly improve viral suppression, the primary marker of adherence to ART, as measured through the reduced mean log of VL and reduced odds of having a detectable VL (≥ 40 ml).
Our findings contribute to the scientific literature examining the efficacy of economic interventions in improving HIV treatment adherence [18, 24–27], and expand this evidence base to an adolescent population. To our knowledge, this is the first study to utilize a randomized experimental design to assess the longitudinal effects of an economic intervention on HIV viral suppression among adolescents.
There are several potential mechanisms by which the Suubi + Adherence economic empowerment intervention may have reduced detectable VL in adolescents, mechanisms that will be explored in future papers incorporating the final time point of the study. For adolescents who live in family care and remain dependent on their caregivers, financial hardship in the household may prevent consistency in care utilization. The promotion of financial stability may have addressed transport costs and food security, which are factors that have previously been identified as barriers to ART adherence [15–19]. Further, given that adolescents affected by HIV/AIDS and living in low resource communities are more likely to work in order to help improve the financial situation of their household [38], it is plausible that opportunities for income generation may interfere with clinic appointments to obtain ART refills. By improving economic security for the household, adolescents may feel less pressure to seek employment and thus have greater availability to travel to clinics to obtain their medication.
Beyond economic considerations, the asset-based economic intervention may have also improved psychosocial outcomes, such as hope, self-efficacy, and future orientation, which in turn facilitated health-seeking behavior. This pathway aligns with Asset Theory, which posits that tangible assets, including monetary savings, can fuel optimism, risk aversion, and greater future orientation [39]. Given that adolescence is characterized by a greater need for autonomy and control, markers of the forthcoming transition to adulthood, the accumulation of assets can improve an adolescent’s self-concept and trajectory, with secondary education or a successful microenterprise endeavor a realizable goal. This enhanced sense of agency may motivate adolescents to take greater control over their HIV treatment. In other words, an asset-based economic intervention may provide a tangible foundation on which adolescents can construct a secure future, with hope and optimism mediating the positive relationship between savings and improved ART adherence. From the perspective of parents and caregivers of ALWHA, being able to imagine a positive future for adolescents under their care may also motivate stronger social and emotional support to ensure that their children adhere to therapy.
Relatedly, it is well documented that stigma is a key reason why adolescents do not want to disclose HIV status to their peers, as adolescence is a time when positive self-image and acceptance are some of the biggest considerations as adolescents build their network of peers and find their footing in the community [22]. As Suubi + Adherence is a combination intervention that integrates matched savings, financial education, and life skills for adolescents receiving the treatment, it is possible that participation in these program components provided ALWHA exposure to other adolescents with the same status and life circumstances, promoting confidence and alleviating perceived stigma on ART usage.
The ten percent increase in participants with viral suppression falls at the lower-middle range of outcome effects for adherence interventions in Sub-Saharan Africa [40]. However, the meta-analysis referred to above largely captures outcomes for adults who have been shown to be as much as 2–3 times more adherent than adolescents in the region [8], suggesting our findings to be clinically meaningful for an adolescent population. In addition, because viral suppression for intervention adolescents was most pronounced at 12 months as opposed to 24 months, it can be suggested that SUUBI + adherence can have rapid effects on treatment adherence. What is yet to be explored is whether the proposed pathways from economic and psychosocial benefit to ART adherence can be sustained over longer periods of time. Further, after the intervention has ended, is adherence maintained? Tracer studies would be useful to understand these effects longitudinally.
Taken together, our findings show that a combination intervention with an emphasis on economic strengthening may improve adherence to ART, but also highlight the need to further explore the intricacies linking economic vulnerability and ART adherence outcomes among adolescents in low-resource settings, such that future interventions can effectively target all causal pathways. This study reiterates the importance of a multidisciplinary, combination intervention approach to HIV treatment that includes an economic component. The findings also support efforts to promote a broader dialogue on the ability of social protection policies to improve public health outcomes.
Limitations
Several limitations are inherent within our treatment-effects analysis. First, given the nature of the Suubi + Adherence intervention as one with multiple components, it is difficult to disentangle the effects of savings alone; therefore, it is the entire package of services that has been evaluated. Nevertheless, public health scholars have widely called for greater research on combination interventions, recognizing that singular solutions to HIV prevention and treatment will likely be less effective given the interconnected vulnerabilities faced by persons at risk of or living with HIV [41–43].
Relatedly, the focus of our investigation was to test the efficacy of Suubi + Adherence to enhance viral suppression. While the study was efficacious in improving viral suppression for adolescents in the intervention arm, the relative comparability in viral load levels between study groups suggests that more research is needed to take into account and address ceiling effects when analyzing viral suppression among adolescents.
Future studies should explore mediators and moderators of the intervention effect in order to quantitatively examine various mechanisms of change. Scientific inquiry into the cost-effectiveness of this type of economic intervention for improving adherence is also warranted.
Further, our study was quantitative by design and thus we did not obtain qualitative data on adolescent perceptions of the program, which could have added nuance and context to better elucidate the causal pathways between program participation and ART adherence. Additional research is needed to qualitatively capture these themes.
Public Health Implications
Prior studies have indicated that elevated HIV RNA viral loads are associated with faster progression to AIDS and higher risk of HIV transmission, making adherence to ART to promote viral suppression a critical component in achieving an AIDS-free generation. Our findings suggest that interventions addressing economic insecurity have the potential to improve viral suppression, a clinical marker of ART adherence, among vulnerable adolescents living in low-resource environments. This supports previous research demonstrating the ability of economic initiatives to improve ART adherence and extends this evidence base to an adolescent population. Such results prompt important questions for both policy and practice when aiming to address the public health challenge of HIV/AIDS, necessitating further research and policy dialogue on the intersections of financial security and HIV prevention and treatment.
Acknowledgments
We are grateful to Godfrey Kigozi at Rakai Health Sciences Program in Uganda; Fred Makumbi at Makerere University School of Public Health; Abel Mwebembezi at Reach the Youth— Uganda; and Christopher Damulira and Apollo Kivumbi at the International Center for Child Health and Development (Ichad) for their respective contributions to the study design, implementation, and data management. We are also grateful to Jessica Pac for her statistical support on this paper. We wish to thank all of the participants and their families who have given their time for this trial.
Funding This work was supported by the National Institute of Child and Human Development (NICHD) under Grant number 1R01HD074949-01 (FS, PI). The fifth author’s work on this manuscript was supported by the NIMH (K01MH107310) and the Johns Hopkins University Center for AIDS Research (P30AI094189).
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.UNICEF. UNICEF Data: Adolescents and Young People Current Status and Progress. 2016 http://data.unicef.org/hiv-aids/adolescents-young-people.html.
- 2.Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335–43. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the strategic timing of AntiRetroviral treatment (start) study. Clin Trials. 2013;10:S5–36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. doi: 10.1093/pch/13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6:194–200. doi: 10.1007/s11904-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in Southern Africa. J Acquir Immune Defic Syndr. 2009;51:65. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams PL, Van Dyke R, Eagle M, Smith D, Vincent C, Ciupak G, et al. Association of site-specific and participant-specific factors with retention of children in a long-term pediatric HIV cohort study. Am J Epidemiol. 2008;167:1375–86. doi: 10.1093/aje/kwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS ONE. 2012;7:e52856. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–85. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS. 2015 HIV and AIDS estimates. Geneva: UNAIDS; 2015. [Google Scholar]
- 13.Reddi A, Powers MA, Thyssen A. HIV/AIDS and food insecurity: deadly syndemic or an opportunity for healthcare synergism in resource-limited settings of sub-Saharan Africa? AIDS. 2012;26:115–7. doi: 10.1097/QAD.0b013e32834e14ac. [DOI] [PubMed] [Google Scholar]
- 14.Weiser SD, Young SL, Cohen CR, Kushel MB, Tsai AC, Tien PC, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. 2011;94(6):1729S. doi: 10.3945/ajcn.111.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAllister J, Beardsworth G, Lavie E, MacRae K, Carr A. Financial stress is associated with reduced treatment adherence in HIV-infected adults in a resource-rich setting. HIV Med. 2013;14(2):120–4. doi: 10.1111/j.1468-1293.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SS, Juday T, Seekins D, Espindle D, Chu BC. Association between prescription cost sharing and adherence to initial combination antiretroviral therapy in commercially insured antiretroviral-naïve patients with HIV. J Manag Care Pharm. 2012;18(2):129–45. doi: 10.18553/jmcp.2012.18.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik E, Casanas B, Pazare A, Wabale G, Sinnott J, Salihu H. Cost of treatment: the single biggest obstacle to HIV/AIDS treatment adherence in lower-middle class patients in Mumbai, India. Indian J Sex Transm Dis. 2009;30(1):23–7. doi: 10.4103/2589-0557.55476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy SI, Njau PF, Czaicki NL, Kadiyala S, Jewell NP, Dow WH, Padian NS. Rationale and design of a randomized study of short-term food and cash assistance to improve adherence to antiretroviral therapy among food insecure HIV-infected adults in Tanzania. BMC Infect Dis. 2015;28(15):490. doi: 10.1186/s12879-015-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide, Edited by (FANTA) FaNTAP. Washington, D.C: United States Agency for International Development; 2007. [Google Scholar]
- 20.Bor J, Tanser F, Newell ML, Barnighausen T. In a study of a population cohort in South Africa, HIV patients on antiretrovirals had nearly full recovery of employment. Health Aff (Millwood) 2012;31(7):1459–69. doi: 10.1377/hlthaff.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cluver LD, Toska E, Orkin FM, Meinck F, Hodes R, Yakubovich AR, Sherr L. Achieving equity in HIV-treatment outcomes: can social protection improve adolescent ART-adherence in South Africa? AIDS Care. 2016;28(Suppl 2):73–82. doi: 10.1080/09540121.2016.1179008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabukeera-Barungi N, Elyanu P, Asire B, Katureebe C, Lukabwe I, Namusoke E, Musinguzi J, Atuyambe L, Tumwesigye N. Adherence to antiretroviral therapy and retention in care for adolescents living with HIV from 10 districts in Uganda. BMC Infect Dis. 2015;14(15):520. doi: 10.1186/s12879-015-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudelson C, Cluver L. Factors associated with adherence to antiretroviral therapy among adolescents living with HIV/AIDS in low- and middle-income countries: a systematic review. AIDS Care. 2015;27(7):805–16. doi: 10.1080/09540121.2015.1011073. [DOI] [PubMed] [Google Scholar]
- 24.Yotebieng M, Thirumurthy H, Moracco KE, Edmonds A, Tabala M, Kawende B, Wenzi LK, Okitolonda EW, Behets F. Conditional cash transfers to increase retention in PMTCT care, antiretroviral adherence, and postpartum virological suppression: a randomized controlled trial. J Acquir Immune Defic Syndr. 2016;1(72 Suppl 2):S124–9. doi: 10.1097/QAI.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantrell RA, Sinkala M, Megazinni K, Lawson-Marriott S, Washington S, Chi BH, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;49(2):190–5. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz M, Bayona J, Sanchez E, Arevalo J, Sebastian JL, Arteaga F, Guerra D, Zeladita J, Espiritu B, Wong M, Caldas A, Shin S. Matching social support to individual needs: a community-based intervention to improve HIV treatment adherence in a resource-poor setting. AIDS Behav. 2011;15(7):1454–64. doi: 10.1007/s10461-010-9697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrivillaga M, Salcedo JP, Pérez M. The IMEA project: an intervention based on microfinance, entrepreneurship, and adherence to treatment for women with HIV/AIDS living in poverty. AIDS Educ Prev. 2014;26(5):398–410. doi: 10.1521/aeap.2014.26.5.398. [DOI] [PubMed] [Google Scholar]
- 28.Shaw S, Amico KR. Antiretroviral therapy adherence enhancing interventions for adolescents and young adults 13–24 years of age: a review of the evidence base. J Acquir Immune Defic Syndr. 2016;72(4):387–99. doi: 10.1097/QAI.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster C, McDonald S, Frize G, Ayers S, Fidler S. “Payment by Results”–financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS. 2014;28(1):28–32. doi: 10.1089/apc.2013.0262. [DOI] [PubMed] [Google Scholar]
- 30.Pettifor A, MacPhail C, Hughes JP, Selin A, Wang J, Gómez- Olivé FX, Eshleman SH, Wagner RG, Mabuza W, Khoza N, Suchindran C. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health. 2016;4(12):e978–88. doi: 10.1016/S2214-109X(16)30253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Republic of Uganda. The HIV and AIDS Uganda Country Progress Report. 2014 http://www.unaids.org/sites/default/files/country/documents/UGA_narrative_report_2015.pdf.
- 32.The World Bank. Uganda Poverty Assessment: Fact Sheet. 2016 http://www.worldbank.org/en/country/uganda/brief/uganda-poverty-assessment-2016-fact-sheet.
- 33.Bhana A, Mellins CA, Petersen I, Alicea S, Myeza N, Holst H, Abrams E, John S, Chhagan M, Nestadt DF, Leu CS. The VUKA family program: piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care. 2014;26(1):1–10. doi: 10.1080/09540121.2013.806770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han CK, Ssewamala FM, Wang JS. Family economic empowerment and mental health among AIDS-affected children living in AIDS-impacted communities: evidence from a randomised evaluation in southwestern Uganda. J Epidemiol Commun Health. 2013;67(3):225–30. doi: 10.1136/jech-2012-201601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ssewamala FM, Wang JS, Neilands TB, Bermudez LG, Garfinkel I, Waldfogel J, Brooks-Gunn J, Kirkbride G. Cost-effectiveness of a savings-led economic empowerment intervention for AIDS-affected adolescents in Uganda: implications for scale-up in low-resource communities. J Adolesc Health. 2018;62(1):S29–36. doi: 10.1016/j.jadohealth.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Sklar GE, Oh VM, Li SC. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269. doi: 10.2147/tcrm.s1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PL, Storm D, Montepiedra G, Nichols S, Kammerer B, Sirois PA, Farley J, Malee K. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics. 2006;118(6):e1745–57. doi: 10.1542/peds.2006-0493. [DOI] [PubMed] [Google Scholar]
- 38.Franco LM, Burkhalter B, De Wagt A, Jennings L, Kelley AG, Hammink ME. Evidence base for children affected by HIV and AIDS in low prevalence and concentrated epidemic countries: applicability to programming guidance from high prevalence countries. AIDS care. 2009;21(S1):49–59. doi: 10.1080/09540120902923089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherraden M, Gilbert N. Assets and the poor: New American welfare policy. Abington: Routledge; 2016. [Google Scholar]
- 40.Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell ML. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis. 2011;11(12):942–51. doi: 10.1016/S1473-3099(11)70181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376(9737):285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 42.Hankins CA, de Zalduondo BO. Combination prevention: a deeper understanding of effective HIV prevention. AIDS. 2010;24:70–80. doi: 10.1097/01.aids.0000390709.04255.fd. [DOI] [PubMed] [Google Scholar]
- 43.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]