Abstract
Purpose:
History of cancer is significantly associated with increases in healthcare costs, worse work performance, and higher absenteeism in the workplace. This is particularly important as most cancer survivors return to employment. Sleep disturbance is a largely overlooked potential contributor to these changes.
Methods:
Data from 9,488 state employees participating in the Kansas State employee wellness program were used to assess cancer history, sleep disturbance, healthcare expenditures, work performance ratings, and absenteeism. Participants were categorized as having had no history of breast or prostate cancer, a past history only with no current cancer treatment, or current treatment for breast or prostate cancer. Indirect mediation analyses determined whether sleep disturbance mediated the influence of cancer status on outcomes.
Results:
Employees receiving treatment for breast or prostate cancer had significantly greater healthcare expenditures and absenteeism than those with a past history or no history of cancer (ps<.0001). Sleep disturbance significantly mediated the impact of cancer on healthcare expenditures and absenteeism (ps<.05), accounting for 2% and 8% of the impact of cancer on healthcare expenditure and missed full days of work, respectively.
Conclusions:
The worse outcomes observed among employees receiving treatment for breast and prostate cancer, the most common forms of cancer among women and men, were partially explained by the impacts of cancer and treatment for cancer on sleep disturbance. These findings suggest that preventing or addressing sleep disturbance may result in economic benefits in addition to improvements in health and quality of life.
Keywords: Cancer, Sleep, Healthcare Costs, Absenteeism, Quality of Life
Introduction
Cancer survivors comprise a large and growing population in the U.S. As of January 2016, there were an estimated 15.5 million survivors; this population is projected to grow to 20.3 million by 2026 [1]. Annual costs of cancer care in the United States is expected to rise to over $170 billion in 2020 [2]. Care for breast and prostate cancer, the most common cancers among women and men[3], account for nearly a quarter of cancer care expenditures [2]. Although the time periods soon after diagnosis and at the end of life contribute to a significant portion of cancer care costs, the survivorship phase for breast and prostate cancers account for over 40% and 50% of cancer care expenditures, respectively [2]. Given that almost 90% of cancer survivors who were employed before diagnosis return to work within two years after diagnosis [4], employers are interested in reducing the economic burden of cancer care among their cancer survivor employees [5,6]. Moreover, emerging evidence suggests sleep disturbance, through multiple biological pathways, may be associated with greater risk of cancer progression [7–11].
One method for employers to reduce healthcare costs is through employee wellness programs (EWPs), employer-sponsored programs aimed at promoting healthy behaviors and reducing risk of chronic diseases [12]. Promoting healthy behaviors can not only improve cancer survivorship outcomes, but also help manage multiple chronic diseases (e.g. diabetes, hypertension, obesity) that are more prevalent among cancer survivors than the cancer-free counterparts [13]. Most EWPs have focused on increasing employees’ physical activity, maintaining healthy weight, and smoking cessation [14]. Far fewer EWP’s have addressed the role of sleep in maintaining a healthy lifestyle, despite the fact that sleep disturbance is associated with many adverse health outcomes (e.g. diabetes, cardiovascular diseases) [15]. Sleep disturbance is associated with increased healthcare costs, absenteeism, and impaired work performance [16–19]. Absenteeism due to sleep disturbance has been estimated to cost $150 billion each year [20]. For instance, one study found that employees with sleep disturbance exhibit increased healthcare utilization and increased healthcare costs compared to employees without sleep disturbance [21]. Moreover, sleep disturbance is a very common symptom experienced by cancer survivors [22,23]. Thus, the contribution of sleep disturbance associated with cancer or cancer treatment to healthcare expenditures, absenteeism, and work performance among cancer survivors in the workplace merit examination.
Previous studies have yet to examine the additional contribution of sleep disturbance associated with cancer or cancer treatment on these outcomes. This study examines these relationships in a large panel of state employees in the context of diagnoses of breast or prostate cancer. It was hypothesized that sleep disturbance would mediate the impact of history of or treatment for breast or prostate cancer on healthcare expenditures, absenteeism, and work performance.
Participants and Methods
Data Source
The data analyzed in the current study were collected by from an online health risk assessment (HRA) survey distributed to Kansas state employees as part of the EWP in 2008. The data were obtained through a data use agreement in 2010 between the Kansas Health Policy Authority and Kansas Medical Center. The data obtained included deidentified responses to HRA questions as well as basic demographic data for each participant. Eligible participants in the Kansas EWP were those enrolled in a Kansas health plan. Because the dataset did not contain any identifiable information, this study was deemed exempt by the Human Subjects Committee at the University of Kansas Medical Center.
Measures
Measures of cancer history, sleep disturbance, absenteeism, and work performance were self-reported on the HRA questionnaire distributed in 2008. The HRA inquires about personal, familial, lifestyle, and emotional risk factors of common chronic diseases. Participants were provided a $50 gift certificate for completion of their online HRA and biometric screening.
To identify cancer status, participants were asked whether they had ever been diagnosed with breast or prostate cancer, depending on their sex. Those who reported a history of breast or prostate cancer were asked if they were currently undergoing treatment for their cancer diagnosis. To assess sleep disturbance, participants were asked, “During the past 4 weeks, how often have you been bothered by any of the following problems?” with “trouble sleeping” as one item in the series. Response options included “never,” “seldom,” “sometimes,” “often,” and “always.”
Health care costs data were collected from health services claims processed by the state employee health plans offered by the former Kansas Health Policy Authority. Absenteeism was measured by asking participants how many full days and partial days they had missed in the past month due to their own physical or mental health issues. Self-rated work performance was measured by asking, “On a scale of 0 (worst) to 10 (best), how would you rate your overall job performance on the days you worked during the past 4 weeks (28 days)?”
Statistical Analyses
Cancer status was treated as an ordinal variable coded as 0=no history of breast or prostate cancer, 1=past history of breast or prostate cancer and not currently on treatment for cancer, and 2=currently receiving treatment for breast or prostate cancer. Sleep disturbance was also treated as an ordinal variable with frequency of sleep disturbance coded as 0=never, 1=seldom or sometimes, and 2=often or always.
Analysis of variance tests and chi-square tests were first conducted to compare cancer status groups on demographic factors. Next, analysis of covariance tests were conducted to compare cancer status groups on outcomes while controlling for group differences on demographic factors. Post-hoc Tukey’s tests were used to compare groups for those outcomes in which significant variability was observed between groups.
Indirect mediation analyses were then conducted to determine whether the influence of cancer status on outcomes was mediated by sleep disturbance using version 3.0 of the PROCESS macro.[24] These mediation analyses produced regression coefficients for the association between cancer status on sleep disturbance (the a path), the association between sleep disturbance on the outcomes of interest (the b path), and the associations between cancer status on the outcomes of interest with and without accounting for the influence of sleep disturbance (c’ and c paths, respectively). The indirect effect of cancer status on outcomes through sleep disturbance is operationalized as the product of a and b. Indirect mediation analyses examine whether ab is different from zero by computing a 95% confidence interval for ab. However, a normal distribution theory approach to computing this confidence interval is generally not recommended[25] because the sampling distribution of the indirect effect (ab) is not normal and because a normal distribution theory approach results in unstable confidence intervals for the indirect effect. Thus, the PROCESS macro was used to compute the indirect effect and its confidence interval after resampling the sample 5,000 times, a process that accounts for both limitations of the normal distribution theory approach. Those indirect effects for which this bootstrap 95% confidence interval (CI) does not include zero are deemed statistically significant. In order to reduce the Type I error rate and the number of statistical tests conducted, mediation analyses were limited to only those outcomes for which a significant effect of cancer status was found. Consistent with recommendations, indirect mediation effect sizes were examined using PROCESS macro version 2.16.3 to calculate the ratio of the indirect effect to the total effect due to the large sample size in this study and because the c path is larger than and of the same sign as the indirect effect.[26] All analyses used an alpha level of .05 and were conducted using SAS 9.4 (Cary, NC).
Results
Demographic characteristics are presented in Table 1. On average, those with no history of cancer were more likely to be male than those with a history of cancer or on treatment for cancer. Those on treatment for cancer were less well educated than those with no history of cancer or a past history of cancer. As expected, age was also associated with cancer status, such that those with a past history of cancer or on treatment for cancer were older than those with no history of cancer. Because age is strongly associated with incidence of cancer (i.e., the greatest risk factor for breast and prostate cancer) only sex and education were controlled for in multivariate analyses in order to avoid variable suppression [27,28].
Table 1.
Demographic Characteristics of the Sample
| Total Sample N = 9,488 |
No History of Breast or Prostate Cancer n = 9,330 |
Past History of Breast or Prostate Cancer n = 105 |
Receiving Treatment for Breast or Prostate Cancer n = 53 |
F | p | |
|---|---|---|---|---|---|---|
| Age, years | 65.90 | < .001 | ||||
| Mean (SD) | 45 (12) | 44 (12) | 56 (8) | 54 (8) | ||
| Range | 18 – 82 | 18 – 81 | 37 – 82 | 33 – 66 | ||
| Χ2 | p | |||||
| Sex | 26.54 | < .001 | ||||
| Female | 5,415 (57%) | 5,293 (57%) | 80 (76%) | 42 (79%) | ||
| Male | 4,059 (43%) | 4,023 (43%) | 25 (42%) | 11 (21%) | ||
| Race/Ethnicity | 10.76 | .38 | ||||
| Black/African American | 374 (4%) | 367 (4%) | 5 (5%) | 2 (4%) | ||
| Native American | 44 (<1%) | 42 (<1%) | 2 (2%) | 0 (0%) | ||
| Asian | 254 (3%) | 252 (3%) | 2 (2%) | 0 (0%) | ||
| White, Non-Hispanic | 8,486 (89%) | 8,341 (89%) | 96 (91%) | 49 (92%) | ||
| White, Hispanic | 201 (2%) | 200 (2%) | 0 (0%) | 1 (2%) | ||
| Other | 129 (1%) | 128 (1%) | 0 (0%) | 1 (2%) | ||
| Education | 16.46 | .04 | ||||
| < H.S. graduate | 66 (1%) | 66 (1%) | 0 (0%) | 0 (0%) | ||
| High school graduate | 1,260 (13%) | 1,234 (13%) | 13 (12%) | 13 (25%) | ||
| Some college | 2,557 (27%) | 2,515 (27%) | 29 (28%) | 13 (25%) | ||
| College graduate | 3,116 (33%) | 3,078 (33%) | 31 (30%) | 7 (13%) | ||
| Postgraduate | 2,489 (26%) | 2,437 (26%) | 32 (30%) | 20 (38%) | ||
| Annual Income, $ | 3.06 | .98 | ||||
| 0 – 20,000 | 654 (7%) | 645 (7%) | 6 (6%) | 3 (6%) | ||
| 20,000 – 35,000 | 3,043 (34%) | 2,986 (34%) | 37 (36%) | 20 (39%) | ||
| 35,001 – 55,000 | 3,493 (39%) | 3,434 (39%) | 42 (41%) | 17 (33%) | ||
| 55,001 – 85,000 | 1,290 (15%) | 1,270 (15%) | 12 (12%) | 8 (16%) | ||
| 85,001 – 100,000 | 191 (2%) | 187 (2%) | 3 (3%) | 1 (2%) | ||
| ≥ 100,001 | 210 (2%) | 205 (2%) | 3 (3%) | 2 (4%) |
Note. SD = standard deviation. F and Χ2 statistics calculated using analyses of variance and Fisher’s exact tests, respectively. Some categories do not add up to 9,488 due to missing data.
Estimated means for outcomes and p−values for group comparisons are presented in Table 2. Groups differed significantly on healthcare expenditures, number of missed full days of work, and missed part days of work. Post-hoc analyses showed that those receiving treatment for cancer incurred greater healthcare expenditures than those with no history of cancer (t=16.47, p < .0001) and those with a past history of cancer (t=12.46, p < .0001). Those on treatment for cancer missed more full days of work (t=5.09, p < .0001) and more part days of work (t=6.36, p < .0001) than those with no history of cancer. Similarly, those on treatment for cancer missed more full days of work (t=4.77, p < .0001) and more part days of work (t=5.18, p < .0001) than those with a past history of cancer. Significant between-group variability was not observed for self performance. Lastly, significantly variability between groups was observed for sleep disturbance. Of those on treatment for cancer, 15% reported sleep disturbance often/always, 60% reported sleep disturbance seldom/sometimes, and only 25% reported never experiencing sleep disturbance. Sleep disturbance among those on treatment for cancer was significantly worse than for those with no history of breast or prostate cancer (11%, 43%, and 45%; OR = 1.78, 95% CI = 1.07, 2.97). Although those with a past history of cancer (15%, 47%, and 38%) reported higher rates of sleep disturbance than those with no history of cancer, this difference was not statistically significant (OR = 1.24, 95% CI = 0.86, 1.79). Similarly, the differences in rates of sleep disturbance between those on treatment for cancer compared to those with a past history of cancer were not statistically significant (OR = 1.43, 95% CI = 0.76, 2.68). Post-hoc chi-square analyses found significant variability in cancer status when comparing only those reporting sleep disturbance never vs. those reporting sleep disturbing seldom/sometimes (χ2 = 9.86, p = .01) and when comparing those reporting sleep disturbance never vs. those reporting sleep disturbance often/always (χ2 = 6.44, p = .04). Significant variability in cancer status was not observed when comparing those who reported sleep disturbance seldom/sometimes vs. often/always (χ2 = 0.58, p = .75).
Table 2.
Estimated Means and Group Comparisons for Outcomes
| No History of Breast or Prostate Cancer M (SE) |
Past History of Breast or Prostate Cancer M (SE) |
Receiving Treatment for Breast or Prostate Cancer M (SE) |
F | p | |
|---|---|---|---|---|---|
| Healthcare Dollars Spent | $5,009.91 ($340.19) | $7,186.58 ($1,277.85) | $33,747.57 ($1,769.13) | 139.42 | <.0001 |
| Self Performance Rating | 9.45 (0.03) | 9.50 (0.12) | 9.66 (0.17) | 0.83 | .44 |
| Missed Full Days | 0.57 (0.04) | 0.42 (0.15) | 1.63 (0.21) | 14.00 | <.0001 |
| Missed Part Days | 0.40 (0.04) | 0.40 (0.14) | 1.59 (0.19) | 21.05 | <.0001 |
Note: N = 9,488. M = mean. SE = standard error. Estimated means calculated using analysis of covariance analyses while controlling for gender and education. F and p−values reference results of analyses examining whether groups differed on outcomes while controlling for gender and education.
Separate indirect mediation analyses demonstrated that cancer status influenced healthcare expenditures, missed full days of work, and missed part days of work in part through its effect on sleep disturbance (ps < .05, see Figure 1). After controlling for gender and education, each one-point increase in the cancer status variable was associated with increases in healthcare expenditures, full days of work missed, and part days of work missed of $10,379 (95% CI = $8,969 – 11,789), 0.30 (95% CI = 0.13 – 0.46), and 0.40 (95% CI = 0.25 – 0.55), respectively. The indirect effect on healthcare expenditures was independent of the direct effect of cancer status on healthcare expenditures. Sleep disturbance accounted for 2% of the impact of cancer status on healthcare dollars spent. Sleep disturbance accounted for 8% and 5% of the impact of cancer status on missed full days of work and missed part days of work, respectively.
Figure 1.
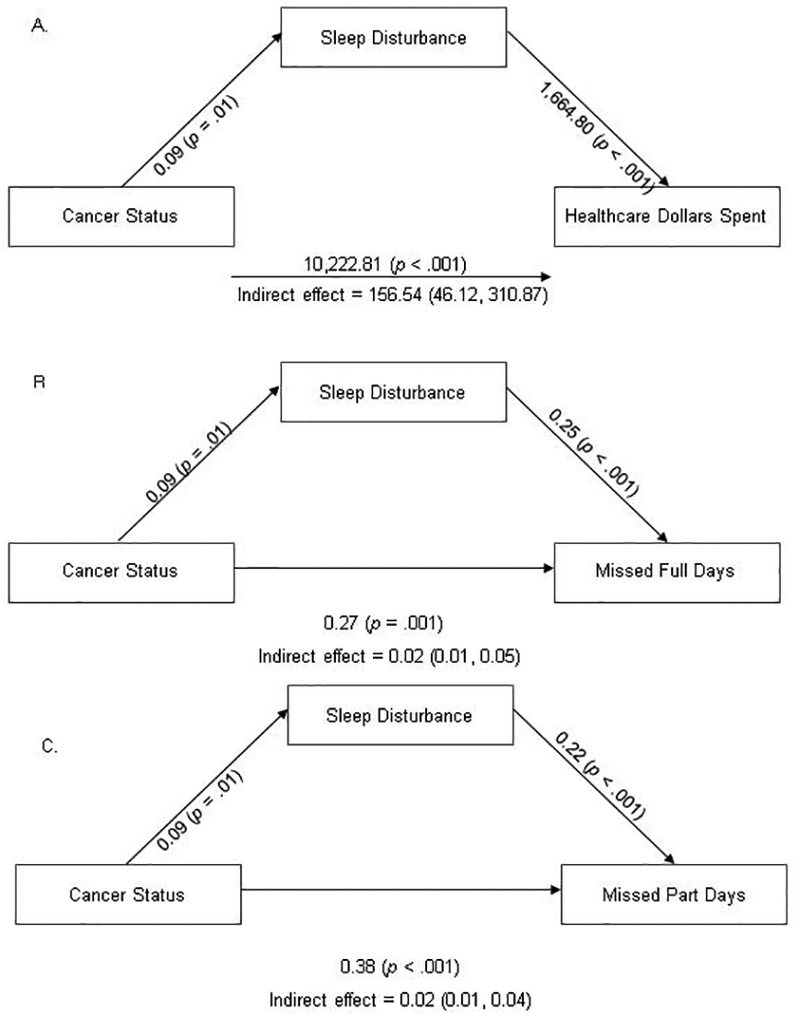
Indirect mediation analysis results
Note: Path estimates and 95% bias-corrected bootstrap confidence intervals obtained with the PROCESS macro version 3.0. The path estimates from cancer status to sleep disturbance, from sleep disturbance to the outcome, and from cancer status to the outcome are the a, b, and c’ (direct effect) paths, respectively.
Discussion
The current study investigated employees’ healthcare expenditures, work absenteeism, and work performance as a function of breast or prostate cancer diagnosis and treatment using a large state employee wellness program (EWP) panel. Whether the effect of cancer status on these outcomes was mediated by sleep disturbance was also measured. Undergoing treatment for cancer was associated with increased healthcare expenditures and work absenteeism. Healthcare expenditures for employees with a past breast or prostate cancer diagnosis were about $2,200 greater per person than for those without a cancer diagnosis. Expenditures for employees currently undergoing breast or prostate cancer treatment were greater by about $27,000 than for those with a past history of cancer. While it is to be expected that employees undergoing cancer treatment incur higher healthcare expenditures, the additional costs incurred by employees with a past cancer history but not currently being treated is still noteworthy, as regular follow-up and surveillance are needed after treatment completion. In addition, on average, individuals undergoing treatment for breast or prostate cancer also missed 1.08 more full days of work and 1.21 more half days of work than individuals with no cancer diagnosis. Furthermore, these effects were partly attributable to the impact of sleep disturbance that was possibly due to cancer and its treatment. That is, $314 of the increased healthcare expenditure associated with receiving treatment for cancer, relative to having no cancer diagnosis, was attributable to sleep disturbance. The prevalence of these diseases (over 400,000 new cases estimated for 2017 alone) suggests the increased expense and absenteeism place a large burden on employers, employees/cancer survivors, payers, and the US healthcare system [29].
Our results are consistent with previous findings that have demonstrated that sleep disturbance is associated with greater healthcare use [30] and that chronic diseases like breast and prostate cancer are related to work absenteeism and increased healthcare costs [6]. Other studies in the general population have found that sleep disturbance independently impacts work absenteeism and healthcare expenditures [16,18]. The existing literature suggests that employees with sleep disturbance exhibit increased healthcare utilization and increased medical costs compared to those employees without sleep disturbance [21]. Nonetheless, this is the first study of which the authors are aware of to find that sleep disturbance mediates the effects of cancer and treatment for cancer on health expenditures or absenteeism. This finding is in line with recent research in non-cancer populations showing that sleep disturbance is associated with healthcare expenditures and absenteeism [31]. Because cancer patients and survivors are vulnerable to sleep disturbance due to psychological distress, treatment side-effects, and tumor burden [9,32,7,33], understanding the role of sleep on increases in healthcare costs and work absenteeism is central to providing effective care for these patients in the workplace. This may be especially important for employers who generally pay the largest portion of healthcare expenses for working adults in the US and incur indirect costs of absenteeism. These findings may alert employers to take implement evidence-based cancer control interventions at their workplaces [34,35]. For example, cognitive behavioral therapy for insomnia has been shown to be efficacious among cancer populations and more efficacious than medication in non-cancer populations [36,37]. Employers are important stakeholders in improving health and well-being of employees, including cancer survivors, and reducing healthcare costs. Therefore, important implications of our findings may prompt employers to implement strategies to manage cancer survivorship in the workplace [38], such as including behavioral treatment for sleep disturbance in EWPs.
Strengths of this study include a large, diverse state employee sample, use of work productivity data, and objective healthcare expenditure data. Also, the three phases of breast or prostate cancer status (i.e., no history, past history, currently on treatment) among employees were available in the data for us to study the relationship between survivorship phase and our target outcome variables, as well as the mediating effect of sleep disturbance. However, there are also some limitations to this study. First, the cross-sectional nature of these analyses precludes any inferences about causality. Each construct was assessed using single-item measures; although the items are face-valid, they have not been tested for validity and reliability. In future studies, validated survey instruments should be used or HRA data should be linked with insurance panel data on medical diagnoses and/or medications prescribed to gather more valid data on participants’ sleep disturbance. Work absenteeism and work performance were also measured using subjective, unvalidated measures. Also, the sample was restricted to state employees in Kansas and only those who participated in the HRA, limiting the generalizability of this study’s findings. Although this study included two most common forms of cancer, breast and prostate cancer, data on whether or not employees were on treatment or had a history cancers were not available for other forms of cancer. The small percentage of participants who were on treatment or had a history of cancer reflects some of the difficulty cancer survivors face when attempting to return to work; however, this limits the generalizability of our findings. While numerous studies have demonstrated that cancer is associated with significant sleep disturbance [39,23,40,41], the phrasing of the sleep measure in this study allows for the possibility that sleep disturbance may be independent of cancer or treatment for cancer. In addition, this study did not include data on whether participants worked night shifts or had shift work, which have been linked to excess risk of cancer [42]. Lastly, time since cancer diagnosis was not assessed and therefore unavailable as a covariate in these analyses.
Future studies should employ longitudinal methods to examine the prospective impact of sleep disturbance after a cancer diagnosis, especially how it relates to work performance after returning to work. Future studies should also examine the potential influence of sex on the relationship between cancer status and healthcare expenditures and absenteeism. In addition, studies could employ propensity matching analyses to create more adequately compare employees with and without a history of cancer. Studies should also seek to elucidate the mechanisms by which sleep disturbance can influence healthcare expenditures and absenteeism in cancer patients and survivors in employee populations. Future studies should also seek to account for the impact of working night shifts or shift work on both sleep disturbance and risk of cancer. Lastly, future studies should examine the efficacy and cost-effectiveness of behavioral interventions to reduce sleep disturbance that are amenable to wide distribution in the context of large employers and third-party payers, such as internet-delivered or mobile device-delivered interventions through the EWP vendors.
Conclusions
Sleep disturbance partially mediated the higher healthcare expenditures and greater absenteeism observed among employees receiving treatment for breast and prostate cancer, the most common forms of cancer among women and men. In addition to improving health and quality of life, preventing or reducing sleep disturbance in cancer patients and survivors may result in economic and productivity benefits.
Acknowledgments
Funding: This work was supported by grants K01 CA211789 (PI: Gonzalez) from the National Cancer Institute and R25 HL105444 (PIs: Jean-Louis, Ogedegbe) from the National Heart, Lung, and Blood Institute.
The authors thank Drs. Ellerbeck and Shireman at University of Kansas Medical Center, and Ms. Cheryl Miller (the former Program Administrator of the Kansas Employee Wellness Program) for facilitating the data acquisition for this study.
Footnotes
The authors have no conflicts of interest to declare.
References Cited
- 1.American Cancer Society (2016) Cancer Treatment and Survivorship Facts & Figures 2016–2017. American Cancer Society, Atlanta [Google Scholar]
- 2.Yabroff KR, Lund J, Kepka D, Mariotto A (2011) Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiology Biomarkers & Prevention 20 (10):2006–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 66 (1):7–30 [DOI] [PubMed] [Google Scholar]
- 4.Mehnert A (2011) Employment and work-related issues in cancer survivors. Crit Rev Oncol Hematol 77 (2):109–130. doi: 10.1016/j.critrevonc.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine (2006) Employment, Insurance, and Economic Issues In: Hewitt ME, Greenfield S, Stovall E (eds) From Cancer Patient to Cancer Survivor: Lost in Transition. National Academies Press, Washington, DC, pp 363–342 [Google Scholar]
- 6.Sorensen G, Landsbergis P, Hammer L, Amick BC 3rd, Linnan L, Yancey A, Welch LS, Goetzel RZ, Flannery KM, Pratt C, Workshop Working Group on Worksite Chronic Disease P (2011) Preventing chronic disease in the workplace: a workshop report and recommendations. Am J Public Health 101 Suppl 1 (S1):S196–207. doi: 10.2105/AJPH.2010.300075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF (2013) Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw 11 (12):1523–1530 [DOI] [PubMed] [Google Scholar]
- 8.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D (2000) Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute 92 (12):994–1000 [DOI] [PubMed] [Google Scholar]
- 9.Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, Savard J, Rotatori M, Kesler S, Telli M, Mustian K (2012) Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep 4:151–162. doi: 10.2147/NSS.S18895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R (2012) Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. American journal of respiratory and critical care medicine 186 (2):190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P (2013) Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, behavior, and immunity 30:S163–S170 [DOI] [PubMed] [Google Scholar]
- 12.Schoenman J, Chockley N Building a stronger evidence base for employee wellness programs. In: National Institute for Health Care Management, Washington, DC, 2011. [Google Scholar]
- 13.Underwood JM, Townsend JS, Stewart SL, Buchannan N, Ekwueme DU, Hawkins NA, Li J, Peaker B, Pollack LA, Richards TB (2012) Surveillance of demographic characteristics and health behaviors among adult cancer survivors—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveillance Summaries 61 (1):1–23 [PubMed] [Google Scholar]
- 14.Mattke S, Liu H, Caloyeras JP, Huang CY, Van Busum KR, Khodyakov D, Shier V (2013) Workplace Wellness Programs Study. Rand Corporation, Santa Monica, CA: [PMC free article] [PubMed] [Google Scholar]
- 15.Grandner MA, Jackson NJ, Pak VM, Gehrman PR (2012) Sleep disturbance is associated with cardiovascular and metabolic disorders. Journal of Sleep Research 21 (4):427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinman NL, Brook RA, Doan JF, Melkonian AK, Baran RW (2009) Health Benefit Costs and Absenteeism Due to Insomnia From the Employer’s Perspective: A Retrospective, Case-Control, Database Study. J Clin Psychiat 70 (8):1098–1104. doi: 10.4088/JCP.08m04264 [DOI] [PubMed] [Google Scholar]
- 17.Kessler RC, Berglund PA, Coulouvrat C, Hajak G, Roth T, Shahly V, Shillington AC, Stephenson JJ, Walsh JK (2011) Insomnia and the performance of US workers: results from the America insomnia survey. Sleep 34 (9):1161–1171. doi: 10.5665/SLEEP.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson LM, Arnedt JT, Rosekind MR, Belenky G, Balkin TJ, Drake C (2011) Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. J Sleep Res 20 (3):487–494. doi: 10.1111/j.1365-2869.2010.00890.x [DOI] [PubMed] [Google Scholar]
- 19.Rahkonen O, Lallukka T, Kronholm E, Vahtera J, Lahelma E, Laaksonen M (2012) Sleep problems and sickness absence among middle-aged employees. Scand J Work Env Hea 38 (1):47–55. doi: 10.5271/sjweh.3186 [DOI] [PubMed] [Google Scholar]
- 20.World Economic Forum (2010) The New Discipline of Workforce Wellness: Enhancing Corporate Performance by Tackling Chronic Disease. World Economic Forum, Geneva [Google Scholar]
- 21.Rosekind MR, Gregory KB (2010) Insomnia risks and costs: health, safety, and quality of life. Am J Manag Care 16 (8):617–626 [PubMed] [Google Scholar]
- 22.Irwin MR, Olmstead RE, Ganz PA, Haque R (2013) Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun 30 Suppl:S58–67. doi: 10.1016/j.bbi.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM (2011) Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol 29 (26):3580–3586. doi: 10.1200/JCO.2010.33.2247 [DOI] [PubMed] [Google Scholar]
- 24.Hayes AF (2018) Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press, New York [Google Scholar]
- 25.Hayes AF (2018) The Simple Mediation Model In: Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 2nd edn Guilford Press, New York, pp 77–112 [Google Scholar]
- 26.Hayes AF (2018) Causal Steps, Confounding, and Causal Order In: Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press, New York, pp 113–146 [Google Scholar]
- 27.McPherson K, Steel C, Dixon J (2000) ABC of breast diseases: breast cancer—epidemiology, risk factors, and genetics. BMJ: British Medical Journal 321 (7261):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho Sm, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters DJ (2004) Human prostate cancer risk factors. Cancer 101 (S10):2371–2490 [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67 (1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 30.Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA (2002) The relationship between chronically disrupted sleep and healthcare use. Sleep 25 (3):289–296 [PubMed] [Google Scholar]
- 31.Burton WN, Chen C-Y, Schultz AB, Li X (2017) Association between employee sleep with workplace health and economic outcomes. Journal of Occupational and Environmental Medicine 59 (2):177–183 [DOI] [PubMed] [Google Scholar]
- 32.Phillips KM, Jim HS, Donovan KA, Pinder-Schenck MC, Jacobsen PB (2012) Characteristics and correlates of sleep disturbances in cancer patients. Support Care Cancer 20 (2):357–365. doi: 10.1007/s00520-011-1106-z [DOI] [PubMed] [Google Scholar]
- 33.Jim HS, Evans B, Jeong JM, Gonzalez BD, Johnston L, Nelson AM, Kesler S, Phillips KM, Barata A, Pidala J, Palesh O (2014) Sleep disruption in hematopoietic cell transplantation recipients: prevalence, severity, and clinical management. Biol Blood Marrow Transplant 20 (10):1465–1484. doi: 10.1016/j.bbmt.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atlantis E, Chow C-M, Kirby A, Singh MAF (2006) Worksite intervention effects on sleep quality: A randomized controlled trial. Journal of occupational health psychology 11 (4):291. [DOI] [PubMed] [Google Scholar]
- 35.Steffen MW, Hazelton AC, Moore WR, Jenkins SM, Clark MM, Hagen PT (2015) Improving sleep: outcomes from a worksite healthy sleep program. Journal of occupational and environmental medicine 57 (1):1–5 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell MD, Gehrman P, Perlis M, Umscheid CA (2012) Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Family Practice 13 (1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L, Campbell T (2014) Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat 10:1113–1124. doi: 10.2147/NDT.S47790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Business Group on Health and National Comprehensive Cancer Network (2011) An Employer’s Guide to Cancer Treatment and Prevention. National Business Group on Health, Washington, DC [Google Scholar]
- 39.Jim HS, Evans B, Jeong JM, Gonzalez BD, Johnston L, Nelson AM, Kesler S, Phillips KM, Barata A, Pidala J, Palesh O (2014) Sleep disruption in hematopoietic cell transplantation recipients: Prevalence, severity, and clinical management. Biology of Blood and Marrow Transplantation 20 (10):1465–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savard J, Simard S, Hervouet S, Ivers H, Lacombe L, Fradet Y (2005) Insomnia in men treated with radical prostatectomy for prostate cancer. Psychooncology 14 (2):147–156. doi: 10.1002/pon.830 [DOI] [PubMed] [Google Scholar]
- 41.Savard J, Villa J, Ivers H, Simard S, Morin CM (2009) Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol 27 (31):5233–5239. doi: 10.1200/JCO.2008.21.6333 [DOI] [PubMed] [Google Scholar]
- 42.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V, Group WIAfRoCMW (2007) Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology 8:1065–1066 [DOI] [PubMed] [Google Scholar]


