Abstract
Selective attention is central to cognition. Dramatic advances have been made in understanding the neural circuits that mediate selective attention. Forebrain networks, most elaborated in primates, control all forms of attention based on task demands and the physical salience of stimuli. These networks contain circuits that distribute top-down signals to sensory processing areas and enhance information processing in those areas. A midbrain network, most elaborated in birds, controls spatial attention. It contains circuits that continuously compute the highest priority stimulus location and route sensory information from the selected location to forebrain networks that make cognitive decisions. The identification of these circuits, their functions and mechanisms represent a major advance in our understanding of how the vertebrate brain mediates selective attention.
Keywords: Mechanisms of attention, Visual attention, Cognition
Networks that mediate information selection
Since the beginning of vertebrate evolution, neural mechanisms of attention have selected the information that gains access to networks that make cognitive decisions. When an animal, be it fish or primate, is engaged in complex behavior, such as social interactions, navigation or foraging, information selection is based on the task and goals of the animal [1–3]. During such periods, the mechanisms of attention are controlled by forebrain networks [3–7]. However, when an unexpected or highly salient stimulus occurs, information selection is dominated by the physical properties of the stimulus. When the stimulus has a location, a midbrain network acts with speed to direct spatial attention to that location and, when appropriate, also the gaze of the animal [1, 8–10]. Following capture of spatial attention by a physically salient stimulus, forebrain networks identify and evaluate the risks and benefits of the stimulus.
The forebrain and midbrain networks that mediate these complementary aspects of attention-control each contain specialized circuits that compute the highest priority information at each moment for decision-making (Fig. 1, Key Figure). Forebrain networks select information, based on task demands or the physical salience of stimuli, from all available sources, including sensory input, plans for action, and memory stores. They direct attention either to locations, sensory modalities, stimulus features, objects or memory stores [4]. In contrast, the midbrain network is concerned only with the relative priorities of locations (Fig. 1A, purple box), based on the physical salience of stimuli and their behavioral relevance, and directs spatial attention to the highest priority location [1, 8, 11, 12].
Figure 1.
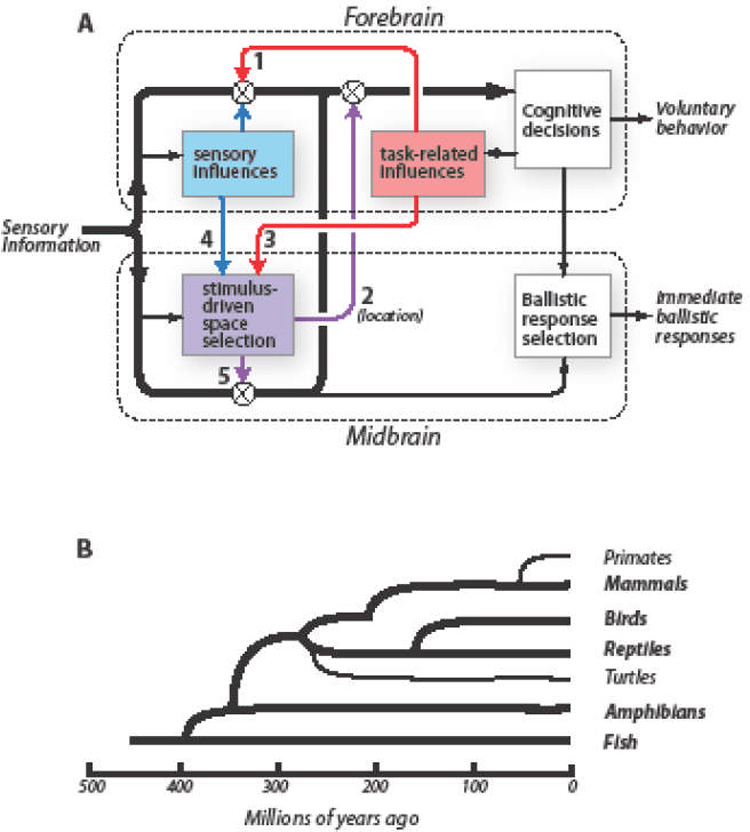
Key Figure. Schemata for attention-dependent modulation of sensory information and for the evolutionary relationships of living vertebrate taxonomic groups.
A: Sensory information is processed in parallel by the forebrain (upper dashed rectangle) and the midbrain (lower dashed rectangle). Thick black arrows: information used for stimulus identification. Red rectangle: information selection based on task relevance. Blue rectangle: stimulus selection based on the physical properties of stimuli. Purple rectangle: stimulus selection based on the priority of stimulus location (stimulus-driven space selection). Symbols: circled X, regulation of sensory information by a selection signal; arrow 1, sites where task-related signals modulate sensory representations in the forebrain; arrow 2, sites where midbrain selection signals modulate forebrain sensory information processing based on stimulus location; arrow 3, sites where task-related signals bias space selection by the midbrain network; arrow 4, sites where sensory information from the forebrain biases space selection by the midbrain network; arrow 5, sites where midbrain selection signals modulate visual information in the OT.
B: Diagram showing the evolutionary relationships of extant Classes and two key Orders of vertebrate animals. Based on [140].
This review summarizes the contributions made by specific midbrain and forebrain circuits to the selection or enhancement of information used for cognitive decisions. The data come from a variety of vertebrate species. The modular architecture of these selection networks provides a substrate for evolution to improve their performance. Hence, differences across species are to be expected. Nevertheless, some basic circuits have been conserved through evolution, demonstrating their adaptive value. Detailed exploration of both conserved and specialized circuits in diverse species will lead to a deeper understanding of how the vertebrate brain enables the amazing capacities of selective attention.
Selection circuits in the midbrain
The midbrain selection network
The midbrain stimulus selection network, which monitors the environment continuously for behaviorally relevant stimuli, appeared already well differentiated at the beginning of vertebrate evolution (Fig. 1B) [1, 8, 13]. The hub of the network is the optic tectum (OT), also referred to as the superior colliculus in mammals, a conspicuously laminated structure even in the earliest vertebrate species [13, 14]. The OT interconnects with the isthmic nuclei in the midbrain tegmentum (see Glossary); in mammals, these nuclei are named the parabigeminal nucleus and the peri-parabigeminal lateral tegmental nucleus [15]. The isthmic nuclei mediate the specialized computations carried out by the midbrain network that are essential to stimulus-driven selection across space [16]. They receive direct, topographic input from the OT, and they project back to the OT, as well as to the thalamus and basal ganglia [17]. Together, these structures constitute the midbrain selection network.
Information in each component structure of the network is organized as a topographic map of space in a retinocentric frame of reference. The OT itself is divided into two functional subdivisions. The superficial subdivision, referred to as the visual OT (OTv), is concerned primarily with visual information: it receives input directly from the retina (retinotectal tract) and indirectly from the visual forebrain, and it sends output to brainstem and thalamic nuclei involved in visual processing (including the dorsal lateral geniculate nucleus; dLGN [18]) or reflexive responses of the eye [13, 16, 19, 20]. The deep subdivision, referred to as the multimodal OT (OTm), receives visual input from the retina, but also input from all other sensory modalities that provide an animal with spatial information, as well as input from the forebrain indicating the spatial goal of impending orienting movements and the behavioral relevance of stimuli [19, 21]. The OTm sends descending output to premotor nuclei in the brainstem and spinal cord that orchestrate rapid, ballistic movements and, more importantly for the purposes of this review, it sends ascending output to thalamic nuclei involved in sensory information processing and voluntary control of behavior (Fig. 1A) [8, 9, 22].
In monitoring the environment for behaviorally relevant stimuli, the midbrain network combines information about the physical salience of stimuli with information about the relevance of particular stimuli and locations to an animal’s current task. By integrating and processing this information, the midbrain network generates a representation of the “priorities” of stimuli (Box 1) [8, 11, 23]. The network selects the highest priority location, enhances the processing of sensory information from that location (Fig. 1A, arrows 2 and 5), and if the stimulus is of sufficient priority, directs immediate ballistic orientation, attack, or defensive responses to the stimulus [8, 24].
Box 1.
Computation of the highest priority location by the OT
To compute the highest priority stimulus, the OT is provided with sensory information that represents the physical salience of stimuli [8, 9, 20, 23]. Topographic visual input originates from the entire contralateral retina (except in primates, in which the representation extends to just past the vertical meridian) as well as from those portions of the ipsilateral retina that represent corresponding locations [130]. OT neurons exhibit a strong preference for small stimuli, due in part to lateral inhibition in the OTv [131]. The activity of OT neurons increases with increasing luminance or motion speed, and decreases when the same stimulus is presented repeatedly, largely due to adaptation mechanisms that operate within the OT [8, 19]. The OT also receives information about stimulus properties from the forebrain. The forebrain indicates, for instance, when a particular stimulus feature value (for example, a color) occurs rarely across space, referred to as “popout,” (Fig. 1A, arrow 4) [23, 34, 132, 133]. Afferent inputs from other sensory modalities, including auditory and somatosensory, encode the strength, motion, and novelty of a stimulus, but not its identity [21]. By combining these inputs, a population of neurons in the OT space map encodes the physical salience and novelty of a stimulus.
The OT generates a representation of stimulus priority by combining information about physical salience with activity that represents the immediate behavioral relevance of particular locations or stimuli [11]. Information about the relevance of a location or stimulus to an animal’s current task originates in the forebrain, and is conveyed to the OT via descending pathways (Fig. 1A, arrow 3) [6, 11]. Neural activity from voluntary gaze control areas in the forebrain, indicating a plan to orient toward a location and the learned benefits of a stimulus, facilitate sensory responses at corresponding locations in the OT space map [61]. The resulting relevance-modulated sensory responses compete for supremacy (highest priority) in the OT space map.
A circuit for competitive selection. The midbrain network selects the highest priority stimulus by implementing powerful competitive inhibition that acts globally across the entire space map. The effects of global competition are demonstrated experimentally by presenting an animal with two or more stimuli at different locations. Under these conditions, the responses of a neuron to the weaker stimulus are suppressed by responses to a stronger stimulus located anywhere else in the visual field. Such “global competitive surrounds” are a distinctive property of OTm neurons that have been documented in a wide range of vertebrate species [25–28].
A neural circuit that mediates global competitive surrounds is formed by specialized inhibitory neurons in the midbrain tegmentum. Although this circuit was first recognized anatomically in reptiles [29], its structural and functional properties have been fully explored only in birds (Box 2), in which the specialized inhibitory neurons cluster in the nucleus isthmi pars magnocellularis (Imc; Fig. 2A).
Box 2.
The midbrain circuit in birds that mediates global competitive inhibition
The circuit that mediates stimulus competition across space in the midbrain network of birds, begins with anatomically and biophysically specialized neurons located in the OT (Fig. 2A, orange) [134]. These neurons have radial, bipolar dendrites that extend into the OTv and OTm [36]. They receive direct retinal input as well as direct multimodal and behavioral relevance input, and they project topographically to specialized inhibitory neurons located in the midbrain tegmentum in the nucleus isthmi pars magnocellularis (Imc). Imc neurons, in turn, project broadly back to the OT, to the cholinergic isthmic nuclei, and to each other (Fig. 2A, black) [32, 135]. Because the inhibitory influence of Imc neurons acts globally across the maps in each of these structures, the activity evoked by any stimulus in each of these structures competes with the activities evoked by stimuli at all other locations (Fig. 2B). The stimulus that evokes the highest level of Imc activity wins this competition and suppresses sensory responses at all other locations [136].
The OT neurons that project to the Imc exhibit an unusual biophysical property that enables their function [134]. They transform transient afferent drive into persistent, stochastic, exceptionally high firing rates (>100 spikes/s). This activity drives Imc neurons at similarly high rates, allowing them to powerfully suppress network responses to competing stimuli (Fig. 2B).
Figure 2.
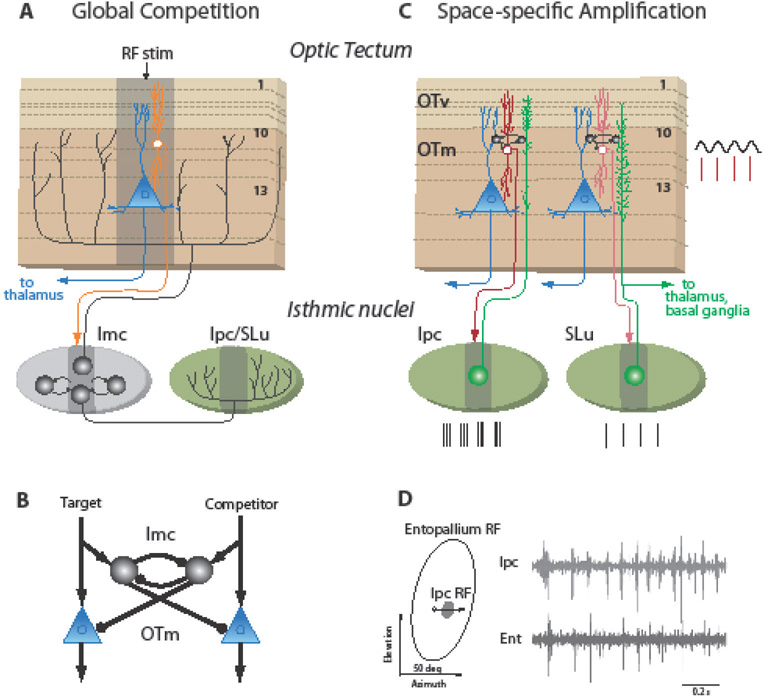
Circuits that generate selection signals in the bird midbrain selection network.
A: The circuit that mediates global competitive selection across the space map. Orange neuron: OT neurons that project to the nucleus isthmi pars magnocellularis (Imc). Blue neuron: OT neurons that project to the high-order thalamic nucleus Rotundus (Pulvinar, in mammals). Black neurons: GABAergic inhibitory neurons that project broadly to the space maps in the OT, the cholinergic isthmic nuclei (green), or to each other. Ipc: nucleus isthmi pars parvocellularis; SLu: nucleus isthmi pars semilunaris; numbers: prominent layers in the OT; RF stim: center of the region activated by a small, salient stimulus; dark gray areas: regions activated by a small stimulus. Data from [32, 36].
B: Circuit model of reciprocal lateral inhibition of feedforward inhibition that implements global competitive selection in the midbrain network. Black circles: Imc neurons; blue triangles: OTm output neurons that project to the thalamus; arrows: excitatory connections; dots: inhibitory connections. Based on [32].
C: The circuit that amplifies and rhythmically synchronizes activity in the network. Abbreviations as in A. GABAergic neurons (black) in layer 10a generate periodic depolarization (black oscillations) of neurons in layer 10b that project to the cholinergic Ipc or SLu [41]. Periodic spikes from layer 10b neurons (red lines) evoke periodic spike bursts in Ipc neurons (black lines) and periodic single spikes or doublets in SLu neurons (single black lines) [44]. Ipc and SLu neurons project back topographically across layers in the visual (OTv) and multimodal OT (OTm) [36]. SLu neurons also project to the thalamus and to the basal ganglia [17].
D: Electrophysiological recordings of synchronized, periodic visual responses measured simultaneously in the pigeon Ipc (top trace) and high-order forebrain pallial area, (Entopallium; bottom trace), at sites with overlapping visual RFs, as shown on the left. The open circle and vector represent the position and trajectory, respectively, of a small bright visual stimulus that was swept across the RFs. Figure 2D is from Marin et. al. [44].
When the relative priorities of competing stimuli change and neural activity representing one location exceeds the activity at all other locations in the midbrain space map (Box 1), this competitive inhibitory circuit powerfully suppresses responses at all other locations (Box 2) [30, 31]. This property causes the population activity in the OT to represent categorically the winning stimulus as the location in the space map with the highest activity, a code easily read-out by downstream neurons. Response adaptation allows the network to select, across time, different stimuli as highest priority [8, 19].
Long distance, reciprocal inhibition acting within the Imc [32] enables the competition circuit to compare relative activity levels with high precision. Computational studies show that this microcircuit motif of ‘lateral inhibition of feedforward inhibition’ (Fig. 2 B) is an extremely efficient mechanism for rapid adjustment of the categorization boundary for “highest priority” in response to changing stimulus strengths [33]. In addition, this mechanism sharpens the categorization boundary, enabling improved discrimination of the highest priority location by a decoding network.
The beneficial effects of the global competitive inhibitory circuit are apparent only when an animal must select among multiple competing stimuli. When an animal confronts only a single stimulus (which occurs rarely in nature, but often in the laboratory), the advantage gained from suppressing responses at all other locations is minimized. This can account for the observation that, in monkeys trained to perform attention-demanding tasks, OT inactivation has devastating effects on target selection only when competing stimuli are present [9, 34].
A circuit for space-specific amplification. In addition to suppressing neural responses to lower priority stimuli, the midbrain selection network amplifies and, at least in birds, causes periodic (25–60 Hz; “ low gamma-band”) synchronization of neural responses to the selected stimulus [16]. Response amplification is mediated by a different specialized circuit, which is established in parallel with the global competition circuit and includes cholinergic neurons in the isthmic nuclei (Fig. 2C, green). The circuit involves precise, reciprocal, topographic connections between the OT and isthmic cholinergic neurons. This circuit was first described in mammals, in which the cholinergic isthmic neurons are referred to as the parabigeminal nucleus [35]. However, the structural and functional properties of these neurons have been explored primarily in birds (Box 3), in which they are called the nucleus isthmi pars parvocellularis (Ipc) and pars semilunaris (SLu) (Fig. 2C) [36].
Box 3.
The midbrain circuit in birds for space-specific amplification and synchronization
The circuit that amplifies and synchronizes sensory responses in the midbrain network of birds (Fig. 2C), begins with specialized OT neurons with bipolar dendrites that sample inputs to both the OTv and OTm, in parallel with the OT neurons that project to the Imc [36, 134]. The specialized OT neurons send axons topographically to the Ipc and SLu, creating space maps in both nuclei [36]. The Ipc and SLu send topographic projections back to the OTv and OTm, precisely to the location that provides their input. SLu neurons also send cholinergic projections to the thalamus and to the source of basal ganglia input to the OT in the brainstem [17].
In addition to amplifying OT responses, these cholinergic circuits also cause network responses to synchronize at a periodicity of 25–60 Hz (low gamma frequencies). The oscillatory activity originates in the OT: the Ipc-projecting neurons receive periodic inhibition from local inhibitory neurons (Fig. 2C, black) [41]. The periodicity of this inhibitory microcircuit is regulated by the strength of γ-aminobutyric acid (GABA)A –receptor currents, and the amplitude of the resulting periodic inhibition is regulated by the activation of non-alpha 7 nicotinic acetylcholine (ACh) receptors on a subset of these interneurons [40]. As a result, Ipc-projecting neurons in the OT respond to sensory input with periodic spikes at low gamma frequencies (Fig. 2C, red lines). Neurons in the Ipc transform these periodic spikes into spike bursts (Fig. 2C, black lines), which are transmitted to neurons in most layers of the OT [137]. These bursts also entrain the discharges of SLu neurons [44]. The resulting synchronization of activity across OT layers can enhance the transmission of visual responses within the OT, specifically for this location in the space map (Fig. 2D) [47]. The persistence of responses that is caused by this circuit retains information for a brief period and momentarily stabilizes the activity of the network in an attractor state [41]. Response adaptation of the inputs to this circuit would enable the network to select different attractor states across time.
Activity in this circuit controls the sensitivity and gain of visual responses in the OT [37]. Cholinergic isthmic neurons project heavily to the retino-recipient layers of the OT. In these layers, the release of ACh facilitates the release of glutamate from the afferent terminals of retinal ganglion cells, thereby enhancing their effects [38, 39]. ACh release also drives local OT inhibitory neurons, which, in turn, inhibit other inhibitory neurons [40], an “inhibition-of-inhibition” motif that could increase the response gain of OT output neurons. In addition, ACh release increases the amplitude of the synchronizing, gamma-band oscillations (Box 3) [40, 41].
The space-specific amplification circuit provides a critical node where top-down information from the forebrain can bias the competition that takes place within the midbrain network. In birds, space-specific top-down signals, by facilitating sensory responses to a particular location, shift the balance of competition to favor the location signaled by the forebrain [42]. Conversely, focal inactivation of the Ipc or OT prevents any stimulus at the represented location from being selected as “highest priority,” even when there are no competing stimuli [43].
In birds, the periodic synchronization of activity in the midbrain network provides a physiological tag of neural responses that correspond to the stimulus at the “highest priority” location [44]. Ascending projections from the SLu and OT transmit this distinctively periodic activity to the thalamus (specifically, the nucleus Rotundus, ROT; pulvinar in mammals) which, in turn, transmits it to high-order areas in the forebrain pallium (Fig. 2D, Entopallium) [45]. Although visual and multimodal neurons in the ROT have large receptive fields and exhibit little evidence of topographic RF organization [46], their responses, when periodic at low gamma frequencies, represent stimuli at the highest priority location, and the location itself is signaled by the site of synchronized activity in the midbrain space maps. Periodic ascending activity from the midbrain could influence how the forebrain selects both the visual information it receives from the OT as well as simultaneous information that converges in the forebrain via OT-independent pathways [47].
Two major roles of the activity that ascends from the OT to the forebrain are 1) to provide the forebrain with visual information to analyze the identity of a stimulus (Fig. 1A, arrow 5), critically important for non-mammalian vertebrate species (Box 4), and 2) to alert the forebrain to the location of the highest priority stimulus and filter forebrain sensory information for that location (Fig. 1A, arrow 2). The importance of OT activity in both of these roles has changed dramatically across vertebrate evolution (Box 4).
Box 4.
Evolution of midbrain network functions
The role of the midbrain as a site where information is selected for processing in the forebrain has decreased dramatically across vertebrate evolution. Early in vertebrate evolution, as represented by fish, amphibians and reptiles (Fig. 1B), the vast majority of visual information passed through the OT on its way to the forebrain, where complex behaviors were planned [1, 8, 45]. In these species, visual information used by the forebrain for stimulus identification is filtered in the OT for physical salience and, presumably, for priority through top-down modulation. Such spatial filtering (Fig. 1A, arrow 5) would cause visual information from one location at a time to dominate the information that the OT transmits to the forebrain (Fig. 1A, black ascending arrow). This dimension-reduction strategy enables the anatomically limited resources of the early vertebrate forebrain [45] to analyze one, selected stimulus in detail. Thus, in these species, the OT serves as the primary site where visual information for cognitive decisions is filtered, based on both exogenous and endogenous information.
The retino-geniculate pathway and the architecture of the forebrain are more elaborated in birds and are far more elaborated in mammals [138–140]. Correspondingly, the importance of the midbrain selection signal in filtering visual information that passes through the OT is reduced (Fig. 1A, arrow 5), while its importance in filtering information that passes through the dLGN is increased (Fig. 1A, arrow 2).
In primates, the role of the OT as a site of visual information filtering decreases further. Primates have foveae, and the retinogeniculate pathway and the forebrain (frontoparietal) selection networks are uniquely well differentiated [4, 141]. Conversely, only ~10% of retinal afferents project to the OT, in contrast to the massive projection of retinal afferents to the OT that exists in all non-primate species [139]. Nevertheless, focal electrical microstimulation of the OT in monkeys mimics the behavioral effects of spatial attention: it improves the detection and discrimination of visual stimuli specifically for target stimuli at the microstimulated location [142, 143]. As in other vertebrate species, the primate OT monitors the environment for physically salient stimuli, its ascending signal can override task-related spatial selection and direct attention to a particular location, and it contributes to space selection for cognitive decisions, especially when distracting stimuli are present [9, 12, 24, 144].
The midbrain network achieves its highest degree of anatomical differentiation in birds, the Class of vertebrates that, due to flight, must make extremely rapid, reliable decisions about approaching objects [8]. In birds, specialized circuits in the midbrain generate a conspicuously rhythmic, categorical selection signal indicating the location of the highest priority stimulus [41, 136]. This selection signal spatially filters visual information within the OT as well as in the forebrain (Fig. 2D) [43].
It is important to underscore the specific effects that the amplification circuit have on activity in the midbrain network. The mechanisms engaged by this circuit, including presynaptic afferent facilitation, amplification of inhibition, disinhibition, and oscillation gain-control, do not alone drive the activity of OT output neurons. Instead, they modulate their responses and regulate the balance of competition across the space map. Specifically, these mechanisms (1) increase the sensitivity, gain and resolution of the system (and, thereby, provide a means for task-related signals to influence the competitive selection of a particular location), (2) increase the persistence of responses, thereby retaining information for short periods and stabilizing the dynamics of the system, and (3) impose a temporal signature and windowing of the visual activity that passes through the OT. It will be important to determine which of the mechanisms that operate in birds (Boxes 2 and 3) are also found in species from other vertebrate Classes, and which may be specializations to meet the particularly high demands that flight places on decision-making.
Selection circuits in the forebrain
Forebrain selection networks
Forebrain selection networks have been studied almost exclusively in mammals, particularly in primates and rodents. The mammalian networks comprise mutually connected areas of the prefrontal and posterior parietal cortex (frontoprietal network), sensory cortices, and subcortical structures in the thalamus, basal ganglia and brainstem [4, 7, 48].
Unlike the midbrain network described above, the forebrain networks are thought to encode information less topographically, with more mixed and distributed representations, and with heterogeneous dynamics [49–52]. Computations for the identification of stimuli or the accumulation of information toward cognitive decisions are readout from the trajectory of activity (population vector) across the population of neurons [53]. The information that is selected for decision-making may influence the recurrent dynamics of the network as well as drive the trajectory of the population vector toward a decision [51].
Forebrain networks in the earliest vertebrate species probably operated in a fashion similar to the one discussed above. In turtles (Fig. 1B) for example, even the primary visual cortex (V1) acts as a distributed, dynamical system, with neurons exhibiting mixed selectivities, extremely large visual receptive fields, and sustained oscillatory activity that develops over time [54]. In fish, amphibians and reptiles, various forebrain areas process sensory, motor, or high-order information [2, 3, 45, 55, 56]. These areas are likely to be organized hierarchically, in a layered control architecture [57], as they are in birds and mammals, but the number of hierarchical levels is extremely limited, and there is as yet no evidence that the features of stimuli, such as visual contour orientation or motion, are analyzed in separate specialized areas.
Distributed dynamical networks, although slower to categorize information than feedforward hierarchies, are far more versatile and efficient for small populations of neurons to convert high dimensional information into low dimensional adaptive decisions [58]. This versatile neural architecture, which presumably evolved first in the vertebrate forebrain to analyze olfactory information, eventually led to the distributed, dynamical processing networks of the prefrontal, parietal and inferotemporal cortices [55, 59].
Most research on forebrain attention networks focuses not on the mechanisms that generate selection signals, but rather on pathways that distribute selection signals and the mechanisms by which these signals regulate the quality of the selected information [4, 7, 60, 61]. Effects on neural activity that correlate with attention include increases in neuronal sensitivity, gain, decorrelation of activity with neighboring neurons, periodic synchronization of activity both within a cortical area and across areas and, at high levels of processing, dynamic shifts or sharpening of receptive field tuning (e.g., for location or feature values), resulting in the preferential representation of the attended stimulus [62–65]. At the same time, neuronal responses to distracting stimuli tend to be suppressed [66, 67]. In addition to enhancing the representation of information, forebrain networks may also improve decisions by adjusting the criteria used in making cognitive decisions [68]. The influence of these effects on neuronal responses increases at progressively higher levels in hierarchies of forebrain information processing.
Mechanisms have been identified in rodents and primates that can account for many of these neural correlates of attention [60, 69, 70]. In a few cases, specific circuits have been shown to be causally involved in attending animals. These include (1) feedback circuits from higher order to lower order cortical areas [71, 72], (2) thalamic circuits that include inhibitory neurons in the thalamic reticular nucleus (TRN) [73]; and (3) circuits formed by broadly projecting cholinergic neurons in the basal forebrain (BF) [74, 75]. These circuits act cooperatively, with different degrees of anatomical and functional precision, to enhance the representation of selected information. However, all of these circuits act with high temporal precision, enabling the rapid modulation of information processing that is required for selective attention in a rapidly changing world.
Corticocortical feedback circuits
In birds and mammals, selection signals computed in high-order forebrain areas propagate to progressively lower order areas, modulating the processing and representation of selected information [76–78]. These circuits have the anatomical precision to differentially modulate the responses of specific functional subgroups of neurons within an area (Fig. 3A), as occurs in the context of space-, feature-, object-specific attention. Although these circuits may contribute to all forms of selective attention, they have been studied primarily in the context of visual spatial attention.
Figure 3.
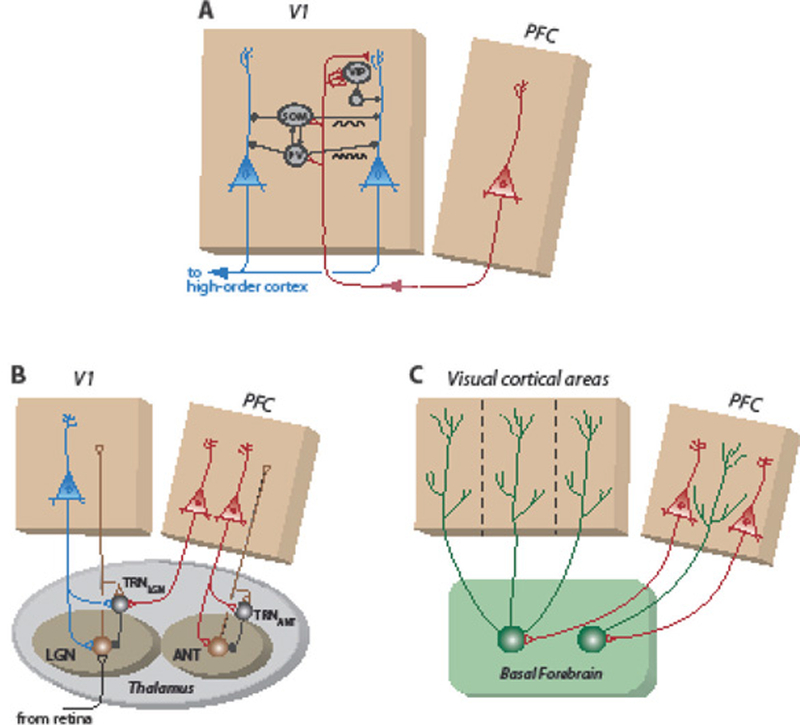
Circuits that enhance or gate sensory responses in the mammalian neocortex.
A: Corticocortical feedback circuits. Red neuron: pyramidal neurons in the cingulate region of the mouse prefrontal cortex (PFC) that project to the primary visual cortex (V1). Blue neurons: layer 2/3 V1 pyramidal neurons. Black neurons: local inhibitory interneurons. PV: parvalbumin-positive; SOM: somatostatin-positive; VIP: vasoactive intestinal peptide-positive. Based on [71]. Rhythmic activation of SOM interneurons entrains resonant activity in the 5–30 Hz band, and of PV interneurons in the 20–80 Hz band (black oscillations) [98]. In monkeys, the anatomically dominant path is the direct connection from the PFC to V4 pyramidal neurons [94].
B: Thalamic circuits. Brown neurons: excitatory output neurons to the cortex. Black neurons: GABAergic inhibitory neurons in the thalamic reticular nucleus (TRN) that connect with output neurons either in the lateral geniculate nucleus (LGN) or in the anterior nuclei (ANT) [73, 111].
C: Circuits formed by cholinergic neurons in the basal forebrain (BF). Red neurons: pyramidal neurons in the PFC that project to the BF. Green neurons: cholinergic BF neurons that project to functionally related regions of the cortex or PFC.
In primates, selection signals for visual spatial attention often originate in high-order areas of the PFC [52, 79–81], where task demands and multiple sources of information are integrated in non-retinocentric frames of reference (space representations that do not move with the eyes [82, 83]). These selection signals are translated into retinocentric signals, perhaps in the lateral intraparietal area (LIP) [4, 84, 85], and are conveyed to the frontal eye field (FEF), a gaze control area in the PFC. The FEF encodes spatial information in retinocentric coordinates [61, 86] and communicates topographically with retinocentric visual areas in the posterior cortex, as well as with the OTm. Under most conditions, the FEF is not likely to be the site where selection signals are initially generated. However, when a behavioral task does not require the translation of information across spatial frames of reference (for example, during explicit spatial cueing or selection based on the physical salience of a stimulus), the FEF, like the midbrain OTm, may itself generate the selection signal for the highest priority location [61, 87].
The FEF broadcasts retinocentric selection signals to the visual cortex [88, 89]. In monkeys, these signals produce the neurophysiological correlates and behavioral effects of voluntary spatial attention [61]. Focal electrical microstimulation of the FEF improves behavioral discrimination of target stimuli and increases the responses of high-order visual neurons in area V4, specifically for targets at the microstimulated location [61, 72]. During attention-demanding tasks, visual responses of FEF neurons are strongly enhanced when a receptive field stimulus is of highest priority, either because of its physical salience (sensory-driven) or its relevance to a current task (task-driven). Activity from the FEF, in turn, increases the strength and synchronization of neuronal responses in V4 in a space-specific, attention-dependent manner [90]. Attention also increases the periodic synchronization of visual responses both within the FEF and between the FEF and V4 [91]. These effects can cascade to progressively lower order visual areas via corticocortical projections [78].
In mice, the cingulate region (Cg) of the PFC, like the FEF in monkeys, has been shown to transmit selection signals to the visual cortex (mouse V1; Fig. 3A) [71]. Optogenetic activation of Cg axons in V1 improves the ability of behaving mice to discriminate visual contour orientations at the corresponding location. Neurophysiologically, Cg axon activation increases the gain and orientation discriminability of nearby V1 neurons and decreases the responses of more distant neurons.
The visual cortex provides specific examples of circuits that enhance the representation of selected information. For instance in the mouse V1, Cg axons connect directly with several classes of inhibitory interneurons as well as directly with pyramidal output neurons (Fig. 3A) [71]. One class of interneuron (VIP+), which in mice receives the strongest input from Cg axons, inhibits locally acting inhibitory interneurons, thereby releasing pyramidal neurons from inhibition (disinhibition). This circuit increases the responses of cortical output neurons to the selected stimulus. In addition, the direct Cg input to pyramidal neurons could act cooperatively to increase the responsiveness of pyramidal neurons, possibly via activation of NMDA receptors. As a comparative side note, in contrast to the circuitry in the mouse, in the monkey the dominant connection of FEF axons with V4 pyramidal neurons is direct [94], even though the monkey neocortex contains similar chemically and anatomically distinct classes of neurons [92, 93]. Returning to the mouse V1, Cg input to two other classes of interneurons (PV+ and SOM+) causes inhibition of more distant pyramidal neurons. Activation of PV+ interneurons sharpens pyramidal neuron tuning for stimulus orientation [95]. These same classes of interneurons are also driven by sensory input, and their activity normalizes responses across the cortex [96, 97]. In addition, these classes of interneurons generate and regulate the periodic synchronization of visual responses at beta (5–30 Hz; SOM) and low gamma (30–80 Hz; PV) frequencies [98–100]. Thus, these circuits in mice can account for many of the neurophysiological effects of selection signals in the visual cortex.
In birds, the forebrain area analogous to the primate FEF is called the arcopallial gaze field (AGF) [101]. Like the FEF and Cg, the AGF projects to the OTm, which in birds is a major pathway for transmitting visual information to the forebrain (Box 4) [46]. Focal microstimulation of the AGF in owls increases the gain and sensitivity of OTm visual neurons, and it sharpens and shifts their spatial tuning toward the location encoded at the AGF stimulation site [102]. These effects (Fig. 1A, arrow 3) reflect, at least in part, the action of the midbrain cholinergic amplification circuit (Fig. 2C), which receives direct input from the AGF [37]. At the same time, AGF microstimulation increases the inhibition of visual responses at surrounding locations in the space map [30], an effect that is mediated by the GABAergic Imc circuit (Fig. 2A,B). Thus, midbrain circuits that participate in the generation of selection signals are also engaged by top-down feedback circuits to regulate the processing of ascending visual information (Fig. 1A, arrow 5), at least in birds.
Thalamic circuits
The thalamus controls the access of information to the forebrain pallium (cortex, in mammals [45]) as well as regulates the transmission of information between pallial areas [103]. The thalamus is an ancestral structure that receives ascending information from the brainstem and spinal cord and descending information from pallial areas [104, 105]. Discrete thalamic nuclei interconnect with functional sectors of the pallium such that the functional topography of the thalamus reflects the topography of the pallium (Fig. 3B).
The thalamus is a key node in the forebrain selection network [103]. In primates, deactivation of the thalamic nuclei that connect with the posterior parietal cortex or high-order visual cortex results in severe attention deficits [106]. Thalamic circuits modulate the amplitude of periodic synchronization of cortical responses to attended targets [107]. Attentive viewing synchronizes oscillations of local field potentials (LFPs) in connected sectors of the thalamic LGN and V1 in cats [108]. It also increases the mutual coherence of LFPs in high-order visual areas, such as V4 and TEO in monkeys [107]. This coupling of oscillations across brain structures, mediated by the thalamus, could provide a mechanism for selectively routing task-relevant information for decision-making.
Distributed among the thalamic nuclei are GABAergic inhibitory neurons that receive ascending input from thalamo-pallial neurons as well as feedback input from the pallium (Fig. 3B). In reptiles, birds and mammals, these inhibitory neurons are referred to as the thalamic reticular nucleus (TRN) [104]. Based on its unique anatomical position and functional properties, Crick proposed that the TRN controls the “searchlight of attention” [109]. In mammals, the TRN exerts powerful inhibitory control over thalamo-cortical transmission. The TRN surrounds the other thalamic nuclei, and its functional topography reflects their functional topography [110]. Although the PFC in monkeys connects most strongly with the anterior TRN, it projects widely across the TRN [111].
TRN neurons inhibit thalamocortical neurons and also each other [110, 112]. They discharge at high rates, and their discharge rates are modulated selectively by the task relevance of a stimulus during attention-demanding behaviors [113]. TRN activity suppresses thalamic responses to non-selected information. One example comes from experiments in mice in which animals were cued to select either a visual target or a simultaneously presented auditory target for making a perceptual decision. In this study, optogenetic inhibition of the TRN neurons that connect with the LGN (TRNLGN; Fig. 3B) eliminated the cue-dependent modulation of both LGN neuronal responses and behavioral discrimination in the task [73]. In addition, lesions of the TRN eliminate faster behavioral responses to cued stimuli, a hallmark of attention [114].
In mammals, thalamocortical inputs to the PFC are regulated by the anterior portion of the TRN (Fig. 3B). Hence, the TRNANT is likely to play a key role in the generation of task-related selection signals. The functional precision of the signals from the PFC to the thalamus is sufficient to differentially select a location or a sensory modality for enhanced processing and decision-making [73, 113, 115]. The limit of the functional precision of this circuit and the degree to which analogous circuits and mechanisms operate in other Classes of vertebrates remain to be determined.
Cholinergic circuits in the basal forebrain
The ancestral vertebrate brain also contained diffusely projecting systems capable of modulating information processing in the forebrain [116]. The systems are identified by the distinguishing neurotransmitter that mediates their respective effects: acetylcholine (ACh), dopamine (DA), noradrenaline and serotonin. In mammals, each of these neuromodulatory systems can influence behavioral performance in attention-demanding tasks. Many of these influences can be attributed to general effects on brain state, working memory, reinforcement learning, action selection or inhibitory control [60]. The cholinergic system, however, has recently been shown to have an anatomical precision that enables selective modulation of functional systems and the capacity to modulate neural activity with a temporal precision that enables trial-by-trial modulation of sensory processing [75, 117]. Although others of the neuromodulatory systems (particularly the DA system [60, 118]) may be shown, by future experiments, to contribute specifically to selective attention, the evidence that the cholinergic system does so is already compelling.
The cholinergic neurons that establish this circuit are located in the basal forebrain (BF) of all vertebrate species [74, 116, 117, 119]. The cholinergic BF is the major source of ACh in the forebrain (Fig. 3C). In mammals, these neurons receive strong inputs from the PFC, amygdala, and nucleus accumbens, and they project broadly to the thalamus and to the cortex, particularly to the PFC [120].
Definitive evidence from multiple lines of inquiry, links the cholinergic BF in mammals to the control of selective attention. Lesions of the cholinergic BF in mice impair the behavioral identification of target stimuli [74, 121]. The activity of cholinergic BF neurons correlates with attentional demand in these behavioral tasks. Neurons in the BF are activated by high priority sensory stimuli and are spatially selective for stimuli that predict reward [122, 123]. They are activated top-down, when distractors must be identified or when task conditions change. Electrical or optogenetic stimulation of cholinergic BF neurons increases the gain and reliability of neuronal responses in mouse V1 and decreases the mutual correlation of those responses [75]. And in the monkey V1, local application ACh receptor antagonists reduces attentional modulations of neuronal responses [124].
Cholinergic mechanisms in the forebrain that enhance information processing are similar to those that operate in the midbrain network, discussed previously. In the cortex, ACh release increases sensory drive by presynaptic facilitation of glutamate release from thalamic afferent terminals [125, 126]; it drives inhibitory neurons that increase the gain of output neurons by inhibiting local inhibitory neurons (disinhibition) [127]; it drives other inhibitory neurons that provide surround inhibition at distant locations [128]; and it increases the periodic synchrony of sensory responses, by acting on local oscillatory circuits [98, 129].
Although cholinergic BF neurons project broadly, they project to functionally related regions of the cortex [117, 120]. The coarseness of their projections implies that they are unlikely to contribute to the precision of space-or feature-specific attention (but see [60]). On the other hand, the modulation of responses in the cortex by cholinergic BF neurons is fast: their temporal resolution in mice is <1 s [75]. The functional selectivity and temporal resolution of cholinergic BF neurons, together with the effects of BF lesions, suggest that this circuit contributes to selective attention.
Coordination of forebrain and midbrain selection signals
The forebrain and midbrain networks carry out computations in parallel to determine the information that has the highest priority for access to forebrain networks that make cognitive decisions (Fig. 1A). Our understanding of how the networks coordinate their computations is at an early stage. Coordination depends on communication between the networks and the relative dominance of their effects (Box 5). Descending inputs from the forebrain bias the competition that takes place in the midbrain [11, 24]. However, they do not dictate the winner: when a stimulus at a location different from the location signaled by the forebrain network is sufficiently more salient, it wins the midbrain competition for “highest priority,” overriding the top-down signals [42].
Box 5.
Resolution of forebrain-midbrain selection conflicts
Conflicting forebrain and midbrain signals have been induced experimentally by focal inactivation of the OT in trained monkeys [145]. When a cued target and a task-relevant distractor are presented simultaneously, and the cued target is positioned at an OT-inactivated location, monkeys fail to report the feature properties of a cued stimulus and, instead, report the properties of the distractor. Enhanced processing of information about the cued stimulus persists in the visual cortex. Nevertheless, the midbrain network selects information from the distractor stimulus location (not inactivated) for decision-making. This implies that a midbrain influence on information selection in the forebrain (Fig. 1A, arrow 2) acts downstream from task-related influences on cortical sensory processing (Fig. 1A, arrow 1).
Computational studies that employed a multidimensional, signal detection framework to analyze behavioral results from OT inactivation experiments in monkeys concluded that the OT exerts its effects on perceptual decisions primarily by altering an animal’s spatial choice bias for selecting information, rather than by altering the quality of the sensory information itself [146]. Spatial choice bias could be implemented by gating forebrain sensory information based on spatial location (Fig. 1A, arrow 2). Results from electrophysiological and microstimulation experiments are consistent with this conclusion [12, 24, 144]. The mechanisms by which the midbrain network exerts its effects on information processing in the forebrain remain unknown. A likely possibility, however, is that they involve the engagement of gating circuits in the thalamus (Fig. 3B).
The influence of forebrain networks in controlling attention has increased across vertebrate evolution, reflecting the increasing computational capacities of the forebrain. Conversely, the role of the OT as a site of spatial selection of sensory information (Fig. 1A, arrow 5) has decreased (Box 4). In addition, the relative dominance of the forebrain and midbrain networks is likely to vary across prey versus predatory species within a taxonomic group: to avoid predation, there is an intuitive advantage for the midbrain network (Fig. 1A, purple box), which monitors the environment for unexpected stimuli, to dominate information selection; conversely, in the context of hunting, there is an intuitive advantage for forebrain networks to dominate (Fig. 1A, red box). Moreover, the relative influence of the networks probably varies dynamically within individuals, with the midbrain network becoming more dominant in times of stress or increased likelihood of unexpected stimuli, and forebrain networks becoming more dominant in times of focused, voluntary behavior.
Concluding remarks
We have begun to identify some of the circuits and mechanisms in the vertebrate brain that mediate selective attention. This progress notwithstanding, much remains unknown (see Outstanding Questions). In particular, further research is needed to understand how forebrain networks generate and transform task-related selection signals, and the mechanisms by which selection signals generated in the midbrain network control the access of information to forebrain networks involved in making cognitive decisions.
Outstanding Questions.
How do forebrain networks generate selection signals based on task demands or on the relative priorities of stimuli? How do these networks translate selection signals into representations that can modulate the processing of information that is encoded in different formats and coordinate spaces in various parts of the brain?
Do the circuits that have been shown to regulate information processing in the neocortex of mice or monkeys exist also in other mammalian species? Do similar circuits exist in other Classes of vertebrates that do not have a neocortex?
Do the forebrains of fish, amphibians and reptiles contain feedback circuits from high-order to low-order pallial areas? Given the limited differentiation of their pallial areas and the likelihood that these areas act as distributed, dynamical systems, it is possible that interpallial feedback circuits (and, indeed, the capacity to attend to specific feature values) do not exist in these species.
Do the circuits of the midbrain selection network in other Classes of vertebrate species perform the same computations and employ the same mechanisms as in birds, in which the network is so highly differentiated?
What is the functional role of low gamma-band activity synchronization in attention? The mechanisms that generate and shape gamma-band oscillations are strikingly similar in the mammalian neocortex and the bird midbrain. The presence of these oscillations in widely different structures and species suggests that the periodic synchronization of activity at gamma frequencies plays a critical role in the processing of attended information.
How do selection signals from the midbrain network control information processing in the forebrain? The midbrain network projects heavily to the thalamus in all Classes of vertebrates, and the thalamus contains circuits capable of gating information in the cortex. The thalamus is, therefore, an excellent site to begin such studies.
How do the forebrain and midbrain networks coordinate so that the information selected at each moment is of the highest priority to the animal? Particularly vital is the ability to shift rapidly between current task demands and attention to unexpected stimuli. How is this flexibility and coordination of information selection achieved?
It is important to remember that each of the circuits discussed in this review has been explored thoroughly only in one or a few species. Studies in a wide range of species, from all Classes of vertebrates, are required in order to determine which of these circuits and mechanisms are shared universally and which are specializations that reflect specific demands or novel brain architectures of species. The answer to this comparative question will lead to a deeper understanding of how selective attention is achieved in vertebrate brains and how the capacities and mechanisms of attention have evolved over time.
Highlights.
Cognitive decisions rely on neuronal circuits that select or differentially process information. Such circuits have been identified in mammalian and bird species. For many of these circuits, anatomical precursors exist also in earlier Classes of vertebrate species.
Circuits in the mammalian forebrain include: feedback circuits from the prefrontal to sensory cortex that distribute top-down selection signals; circuits within sensory cortex; thalamic circuits; and cholinergic circuits in the basal forebrain.
A midbrain network continuously monitors the world for behaviorally relevant stimuli and controls spatial attention. Specific circuits in the bird midbrain network signal the highest priority stimulus location by implementing global competitive inhibition across all of space, space-specific amplification, and periodic synchronization of activity at low gamma-band frequencies.
Acknowledgements
I thank T. Buschman, S. Druckman, S. Evans, S. Kastner, R. Krauzlis, J. Maunsell, T. Moore, S. Mysore and N. Steinmetz for helpful comments on the manuscript and P. Knudsen for preparing figures. This work was supported by funding from the NIH (1R01 EY024243).
Glossary
- Dynamical system
In the context of the brain, a population of neurons that encodes information as time-dependent patterns of activity across the population.
- Gamma-band oscillations
Rhythmic fluctuations in the local field potential that contain spectral power in the frequency range of 25–140 Hz.
- Layered control architecture
An architecture consisting of multiple brain areas, organized in a functional hierarchy of layers. Within a layer are modules mediating sensory, planning and motor functions. Higher layers support progressively more sophisticated tasks. Higher layers often operate by modulating the activity of lower layers to generate more complex behaviors. Higher levels can subsume the roles of lower levels by suppressing the outputs of lower layers and substituting their own.
- Local field potential (LFP)
The electrical potential that is generated by the summed electrical currents of nearby neurons and glia, as measured extracellularly using a low-impedance electrode and processed to remove action potentials.
- Midbrain tegmentum
The ventral portion of the midbrain, located beneath the cerebral aqueduct or tectal ventricle.
- Mixed selectivity
Neuronal activity that correlates with multiple stimulus modalities, conditions, or components of a task.
- Oscillations
In the context of the brain, fluctuations in neural activity that are periodic. The frequency of the oscillations indicates the periodicity of the neural activity.
- Pallium
The portion of the telencephalon in all Classes of vertebrate species that, in mammals, gives rise to cortical structures.
- Population vector
A decoding approach to identify the information represented in the pattern of activity in a population of neurons, derived as the sum of the preferred tuning properties of each neuron weighted by its spike rate, and summed across all neurons in the population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Krauzlis RJ et al. (2017) Selective attention without a neocortex. Cortex [DOI] [PMC free article] [PubMed]
- 2.Loveland JL and Fernald RD (2017) Differential activation of vasotocin neurons in contexts that elicit aggression and courtship. Behav Brain Res 317, 188–203. [DOI] [PubMed] [Google Scholar]
- 3.Ewert JP et al. (2001) Neural modulation of visuomotor functions underlying prey-catching behaviour in anurans: perception, attention, motor performance, learning. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology 128 (3), 417–461. [DOI] [PubMed] [Google Scholar]
- 4.Buschman TJ and Kastner S (2015) From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron 88 (1), 127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EK and Buschman TJ (2013) Cortical circuits for the control of attention. Curr Opin Neurobiol 23 (2), 216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipp S (2004) The brain circuitry of attention. Trends Cogn Sci 8 (5), 223–30. [DOI] [PubMed] [Google Scholar]
- 7.Maunsell JHR (2015) Neuronal Mechanisms of Visual Attention. Annual Review of Vision Science, Vol 1 1, 373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen EI and Schwarz JS (2017) The optic tectum: a structure evolved for stimulus selection. In Evolution of the Nervous System 2nd edition (Kaas JH ed), Elsevier. [Google Scholar]
- 9.Krauzlis RJ et al. (2013) Superior colliculus and visual spatial attention. Annu Rev Neurosci 36, 165–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen EI (2007) Fundamental components of attention. Annu Rev Neurosci 30, 57–78. [DOI] [PubMed] [Google Scholar]
- 11.Fecteau JH and Munoz DP (2006) Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 10 (8), 382–90. [DOI] [PubMed] [Google Scholar]
- 12.Crapse TB et al. (2018) A Role for the Superior Colliculus in Decision Criteria. Neuron 97 (1), 181–194 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northmore DPM, The optic tectum, in: Farrell AP (Ed.) Encyclopedia of fish physiology: from genome to environment, Elsevier, 2011, pp. 131–142. [Google Scholar]
- 14.May PJ (2006) The mammalian superior colliculus: laminar structure and connections. Neuroanatomy of the Oculomotor System 151, 321–378. [DOI] [PubMed] [Google Scholar]
- 15.Gruberg E et al. (2006) Influencing and interpreting visual input: the role of a visual feedback system. J Neurosci 26 (41), 10368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudsen EI (2011) Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci 33, 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann B et al. (2001) Nucleus isthmi, pars semilunaris as a key component of the tectofugal visual system in pigeons. J Comp Neurol 436 (2), 153–66. [PubMed] [Google Scholar]
- 18.Harting JK et al. (1991) Projection of the Mammalian Superior Colliculus Upon the Dorsal Lateral Geniculate-Nucleus -Organization of Tectogeniculate Pathways in 19 Species. Journal of Comparative Neurology 304 (2), 275–306. [DOI] [PubMed] [Google Scholar]
- 19.Wurtz RH and Albano JE (1980) Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3, 189–226. [DOI] [PubMed] [Google Scholar]
- 20.White BJ et al. (2017) Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. Nat Commun 8, 14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein BE and Meredith MA (1993) The Merging of the Senses, MIT Press. [Google Scholar]
- 22.Wurtz RH et al. (2005) Drivers from the deep: the contribution of collicular input to thalamocortical processing. Prog Brain Res 149, 207–25. [DOI] [PubMed] [Google Scholar]
- 23.White BJ et al. (2017) Superior colliculus encodes visual saliency before the primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America 114 (35), 9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basso MA and May PJ (2017) Circuits for Action and Cognition: A View from the Superior Colliculus. Annual Review of Vision Science, Vol 3 3, 197–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzolatti G et al. (1974) Inhibitory effect of remote visual stimuli on visual responses of cat superior colliculus: spatial and temporal factors. J Neurophysiol 37 (6), 1262–75. [DOI] [PubMed] [Google Scholar]
- 26.Schellart NA et al. (1979) Center-surround organisation and interactions in receptive fields of goldfish tectal units. Vision Res 19 (4), 459–67. [DOI] [PubMed] [Google Scholar]
- 27.Munoz DP and Istvan PJ (1998) Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79 (3), 1193–209. [DOI] [PubMed] [Google Scholar]
- 28.Mysore SP et al. (2010) Global inhibition and stimulus competition in the owl optic tectum. J Neurosci 30 (5), 1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sereno MI and Ulinski PS (1987) Caudal topographic nucleus isthmi and the rostral nontopographic nucleus isthmi in the turtle, Pseudemys scripta. J Comp Neurol 261 (3), 319–46. [DOI] [PubMed] [Google Scholar]
- 30.Mysore SP and Knudsen EI (2013) A shared inhibitory circuit for both exogenous and endogenous control of stimulus selection. Nat Neurosci 16 (4), 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mysore SP et al. (2011) Signaling of the strongest stimulus in the owl optic tectum. J Neurosci 31 (14), 5186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goddard CA et al. (2014) Spatially reciprocal inhibition of inhibition within a stimulus selection network in the avian midbrain. PLoS One 9 (1), e85865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mysore SP and Knudsen EI (2012) Reciprocal inhibition of inhibition: a circuit motif for flexible categorization in stimulus selection. Neuron 73 (1), 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPeek RM and Keller EL (2004) Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7 (7), 757–63. [DOI] [PubMed] [Google Scholar]
- 35.Graybiel AM (1978) A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res 145 (2), 365–74. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y et al. (2006) Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol 494 (1), 7–35. [DOI] [PubMed] [Google Scholar]
- 37.Asadollahi A and Knudsen EI (2016) Spatially precise visual gain control mediated by a cholinergic circuit in the midbrain attention network. Nat Commun 7, 13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binns KE and Salt TE (2000) The functional influence of nicotinic cholinergic receptors on the visual responses of neurones in the superficial superior colliculus. Vis Neurosci 17 (2), 283–9. [DOI] [PubMed] [Google Scholar]
- 39.Edwards JA and Cline HT (1999) Light-induced calcium influx into retinal axons is regulated by presynaptic nicotinic acetylcholine receptor activity in vivo. J Neurophysiol 81 (2), 895–907. [DOI] [PubMed] [Google Scholar]
- 40.Bryant AS et al. (2015) Cholinergic control of gamma power in the midbrain spatial attention network. J Neurosci 35 (2), 761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goddard CA et al. (2012) Gamma oscillations are generated locally in an attention-related midbrain network. Neuron 73 (3), 567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mysore SP and Knudsen EI (2014) Descending control of neural bias and selectivity in a spatial attention network: rules and mechanisms. Neuron 84 (1), 214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knudsen EI et al. (2017) Space-Specific Deficits in Visual Orientation Discrimination Caused by Lesions in the Midbrain Stimulus Selection Network. Curr Biol 27 (14), 2053–2064 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marin GJ et al. (2012) Attentional capture? Synchronized feedback signals from the isthmi boost retinal signals to higher visual areas. J Neurosci 32 (3), 1110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karten HJ (2015) Vertebrate brains and evolutionary connectomics: on the origins of the mammalian ‘neocortex’. Philosophical Transactions of the Royal Society B-Biological Sciences 370 (1684). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wylie DR et al. (2009) The optic tectum of birds: mapping our way to understanding visual processing. Can J Exp Psychol 63 (4), 328–38. [DOI] [PubMed] [Google Scholar]
- 47.Sridharan D and Knudsen EI (2015) Gamma oscillations in the midbrain spatial attention network: linking circuits to function. Current Opinion in Neurobiology 31, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desimone R and Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18, 193–222. [DOI] [PubMed] [Google Scholar]
- 49.Murray JD et al. (2017) Working Memory and Decision-Making in a Frontoparietal Circuit Model. J Neurosci 37 (50), 12167–12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rigotti M et al. (2013) The importance of mixed selectivity in complex cognitive tasks. Nature 497 (7451), 585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mante V et al. (2013) Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503 (7474), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay S et al. (2015) Attentional Filtering of Visual Information by Neuronal Ensembles in the Primate Lateral Prefrontal Cortex. Neuron 85 (1), 202–215. [DOI] [PubMed] [Google Scholar]
- 53.Chaisangmongkon W et al. (2017) Computing by Robust Transience: How the Fronto-Parietal Network Performs Sequential, Category-Based Decisions. Neuron 93 (6), 1504–1517 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fournier J et al. (2018) Spatial Information in a Non-retinotopic Visual Cortex. Neuron 97 (1), 164–180 e7. [DOI] [PubMed] [Google Scholar]
- 55.Wicht H and Northcutt RG (1992) The Forebrain of the Pacific Hagfish -a Cladistic Reconstruction of the Ancestral Craniate Forebrain. Brain Behavior and Evolution 40 (1), 25–64. [DOI] [PubMed] [Google Scholar]
- 56.Butler AB et al. (2011) Evolution of the amniote pallium and the origins of mammalian neocortex. New Perspectives on Neurobehavioral Evolution 1225, 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prescott TJ et al. (1999) Layered control architectures in robots and vertebrates. Adaptive Behavior 7 (1), 99–127. [Google Scholar]
- 58.Wang XJ (2008) Decision making in recurrent neuronal circuits. Neuron 60 (2), 215–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aboitiz F and Montiel JF (2015) Olfaction, navigation, and the origin of isocortex. Frontiers in Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thiele A and Bellgrove MA (2018) Neuromodulation of Attention. Neuron 97 (4), 769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore T and Zirnsak M (2017) Neural Mechanisms of Selective Visual Attention. Annu Rev Psychol 68, 47–72. [DOI] [PubMed] [Google Scholar]
- 62.Nandy AS et al. (2017) Laminar Organization of Attentional Modulation in Macaque Visual Area V4. Neuron 93 (1), 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zirnsak M and Moore T (2014) Saccades and shifting receptive fields: anticipating consequences or selecting targets? Trends in Cognitive Sciences 18 (12), 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conner CE et al. (1997) Spatial attention effects in macaque area V4. Journal of Neuroscience 17 (9), 3201–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartsch MV et al. (2017) Attention to Color Sharpens Neural Population Tuning via Feedback Processing in the Human Visual Cortex Hierarchy. Journal of Neuroscience 37 (43), 10346–10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Trujillo JC and Treue S (2004) Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol 14 (9), 744–51. [DOI] [PubMed] [Google Scholar]
- 67.Malek N et al. (2017) Distracter suppression dominates attentional modulation of responses to multiple stimuli inside the receptive fields of middle temporal neurons. Eur J Neurosci 46 (12), 2844–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo TZ and Maunsell JHR (2018) Attentional Changes in Either Criterion or Sensitivity Are Associated with Robust Modulations in Lateral Prefrontal Cortex. Neuron [DOI] [PMC free article] [PubMed]
- 69.Isaacson JS and Scanziani M How inhibition shapes cortical activity. Neuron 72 (2), 231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang GR et al. (2016) A dendritic disinhibitory circuit mechanism for pathway-specific gating. Nature Communications 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S et al. (2014) Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345 (6197), 660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore T and Fallah M (2004) Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol 91 (1), 152–62. [DOI] [PubMed] [Google Scholar]
- 73.Wimmer RD et al. (2015) Thalamic control of sensory selection in divided attention. Nature 526 (7575), 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarter M et al. (2005) Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev 48, 98–111. [DOI] [PubMed] [Google Scholar]
- 75.Pinto L et al. (2013) Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature Neuroscience 16 (12), 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore T et al. (2003) Visuomotor origins of covert spatial attention. Neuron 40 (4), 671–83. [DOI] [PubMed] [Google Scholar]
- 77.Winkowski DE and Knudsen EI (2007) Top-down control of multimodal sensitivity in the barn owl optic tectum. J Neurosci 27 (48), 13279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hochstein S and Ahissar M (2002) View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron 36 (5), 791–804. [DOI] [PubMed] [Google Scholar]
- 79.Ma YY et al. (2012) The egocentric spatial reference frame used in dorsal-lateral prefrontal working memory in primates. Neuroscience and Biobehavioral Reviews 36 (1), 26–33. [DOI] [PubMed] [Google Scholar]
- 80.Bichot NP et al. (2015) A Source for Feature-Based Attention in the Prefrontal Cortex. Neuron 88 (4), 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossi AF et al. (2007) Top-down attentional deficits in macaques with lesions of lateral prefrontal cortex. Journal of Neuroscience 27 (42), 11306–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Committeri G et al. (2004) Reference frames for spatial cognition: Different brain areas are involved in viewer-, object-, and landmark-centered judgments about object location. Journal of Cognitive Neuroscience 16 (9), 1517–1535. [DOI] [PubMed] [Google Scholar]
- 83.Genovesio A et al. (2006) Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. Journal of Neuroscience 26 (27), 7305–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andersen R et al. (2004) Sensorimotor transformations in the posterior parietal portex. In The Cognitive Neurosciences III (Gazzaniga MS ed), pp. 463–474, MIT. [Google Scholar]
- 85.Bisley JW (2011) The neural basis of visual attention. J Physiol 589 (Pt 1), 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joiner WM et al. (2017) Visual Responses in FEF, Unlike V1, Primarily Reflect When the Visual Context Renders a Receptive Field Salient. Journal of Neuroscience 37 (41), 9871–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knudsen EI (2012) Midbrain and forebrain systems for bottom-up control of spatial attention. In The neuroscience of attention: attentional control and selection (Mangun GR ed), Oxford University Press. [Google Scholar]
- 88.Noudoost B et al. (2014) A Distinct Contribution of the Frontal Eye Field to the Visual Representation of Saccadic Targets. Journal of Neuroscience 34 (10), 3687–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merrikhi Y et al. (2017) Spatial working memory alters the efficacy of input to visual cortex. Nature Communications 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ekstrom LB et al. (2008) Bottom-up dependent gating of frontal signals in early visual cortex. Science 321 (5887), 414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gregoriou GG et al. (2009) High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324 (5931), 1207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang XJ and Yang GR (2018) A disinhibitory circuit motif and flexible information routing in the brain. Curr Opin Neurobiol 49, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilman JP et al. (2017) Area-Specific Features of Pyramidal Neurons-a Comparative Study in Mouse and Rhesus Monkey. Cereb Cortex 27 (3), 2078–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson JC et al. (2011) Pathways of Attention: Synaptic Relationships of Frontal Eye Field to V4, Lateral Intraparietal Cortex, and Area 46 in Macaque Monkey. Journal of Neuroscience 31 (30), 10872–10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SH et al. (2012) Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488 (7411), 379-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J and Maunsell JH (2009) A normalization model of attentional modulation of single unit responses. PLoS One 4 (2), e4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reynolds JH and Heeger DJ (2009) The normalization model of attention. Neuron 61 (2), 168–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen G et al. (2017) Distinct Inhibitory Circuits Orchestrate Cortical beta and gamma Band Oscillations. Neuron 96 (6), 1403–1418 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veit J et al. (2017) Cortical gamma band synchronization through somatostatin interneurons. Nature Neuroscience 20 (7), 951-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cardin JA et al. (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459 (7247), 663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knudsen EI et al. (1995) Characterization of a forebrain gaze field in the archistriatum of the barn owl: microstimulation and anatomical connections. J Neurosci 15 (7 Pt 2), 5139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winkowski DE and Knudsen EI (2008) Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron 60 (4), 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saalmann YB and Kastner S (2011) Cognitive and perceptual functions of the visual thalamus. Neuron 71 (2), 209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Butler AB (2008) Evolution of the thalamus: a morphological and functional review. In Thalamus and related systems, pp. 1–24, Cambridge University Press. [Google Scholar]
- 105.Reiner A et al. (2005) Organization and evolution of the avian forebrain. Anatomical Record Part a-Discoveries in Molecular Cellular and Evolutionary Biology 287a (1), 1080–1102. [DOI] [PubMed] [Google Scholar]
- 106.Zhou HH et al. (2016) Pulvinar-Cortex Interactions in Vision and Attention. Neuron 89 (1), 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saalmann YB et al. (2012) The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science 337 (6095), 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wrobel A et al. (2007) Two streams of attention-dependent beta activity in the striate recipient zone of cat’s lateral posterior-pulvinar complex. Journal of Neuroscience 27 (9), 2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crick F (1984) Function of the Thalamic Reticular Complex -the Searchlight Hypothesis. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences 81 (14), 4586–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pinault D (2004) The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 46 (1), 1–31. [DOI] [PubMed] [Google Scholar]
- 111.Zikopoulos B and Barbas H (2006) Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. Journal of Neuroscience 26 (28), 7348–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crabtree JW and Isaac JT (2002) New intrathalamic pathways allowing modality-related and cross-modality switching in the dorsal thalamus. J Neurosci 22 (19), 8754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McAlonan K et al. (2006) Attentional modulation of thalamic reticular neurons. J Neurosci 26 (16), 4444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weese GD et al. (1999) Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. Journal of Neuroscience 19 (22), 10135–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marton T et al. (2018) Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. J Neurosci [DOI] [PMC free article] [PubMed]
- 116.Wullimann MF and Rink E (2002) The teleostean forebrain: A comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Research Bulletin 57 (3–4), 363–370. [DOI] [PubMed] [Google Scholar]
- 117.Zaborszky L (2002) The modular organization of brain systems. Basal forebrain: the last frontier. Changing Views of Cajal’s Neuron 136, 359–372. [DOI] [PubMed] [Google Scholar]
- 118.Schultz W et al. (2017) The phasic dopamine signal maturing: from reward via behavioural activation to formal economic utility. Current Opinion in Neurobiology 43, 139–148. [DOI] [PubMed] [Google Scholar]
- 119.Medina L and Reiner A (1994) Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J Comp Neurol 342 (4), 497–537. [DOI] [PubMed] [Google Scholar]
- 120.Li X et al. (2018) Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A 115 (2), 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burk JA and Sarter M (2001) Dissociation between the attentional functions mediated via basal forebrain cholinergic and gabaergic neurons. Neuroscience 105 (4), 899–909. [DOI] [PubMed] [Google Scholar]
- 122.Peck CJ and Salzman CD (2014) The Amygdala and Basal Forebrain as a Pathway for Motivationally Guided Attention. Journal of Neuroscience 34 (41), 13757–13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gielow MR and Zaborszky L (2017) The Input-Output Relationship of the Cholinergic Basal Forebrain. Cell Reports 18 (7), 1817–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herrero JL et al. (2008) Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454 (7208), 1110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cox BC et al. (2008) Transport of multiple nicotinic acetylcholine receptors in the rat optic nerve: high densities of receptors containing alpha 6 and beta 3 subunits. Journal of Neurochemistry 105 (5), 1924–1938. [DOI] [PubMed] [Google Scholar]
- 126.Disney AA et al. (2007) Gain modulation by nicotine in macaque v1. Neuron 56 (4), 701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Letzkus JJ et al. (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480 (7377), 331–5. [DOI] [PubMed] [Google Scholar]
- 128.Rudy B et al. (2011) Three Groups of Interneurons Account for Nearly 100% of Neocortical GABAergic Neurons. Developmental Neurobiology 71 (1), 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Howe WM et al. (2017) Acetylcholine Release in Prefrontal Cortex Promotes Gamma Oscillations and Theta-Gamma Coupling during Cue Detection. Journal of Neuroscience 37 (12), 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kruger L (1970) The topography of the visual projection to the mesencephalon: a comparative survey. Brain Behav Evol 3 (1), 169–77. [DOI] [PubMed] [Google Scholar]
- 131.Del Bene F et al. (2010) Filtering of visual information in the tectum by an identified neural circuit. Science 330 (6004), 669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burrows BE and Moore T (2009) Influence and limitations of popout in the selection of salient visual stimuli by area V4 neurons. J Neurosci 29 (48), 15169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.White BJ et al. (2009) Color-related signals in the primate superior colliculus. J Neurosci 29 (39), 12159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goddard CA et al. (2014) Parallel midbrain microcircuits perform independent temporal transformations. J Neurosci 34 (24), 8130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y et al. (2004) Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus). J Comp Neurol 469 (2), 275–97. [DOI] [PubMed] [Google Scholar]
- 136.Mysore SP and Knudsen EI (2011) Flexible categorization of relative stimulus strength by the optic tectum. J Neurosci 31 (21), 7745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marin G et al. (2005) Oscillatory bursts in the optic tectum of birds represent re-entrant signals from the nucleus isthmi pars parvocellularis. J Neurosci 25 (30), 7081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karten HJ et al. (1973) Neural connections of the “visual wulst” of the avian telencephalon. Experimental studies in the piegon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol 150 (3), 253–78. [DOI] [PubMed] [Google Scholar]
- 139.Zhaoping L (2016) From the optic tectum to the primary visual cortex: migration through evolution of the saliency map for exogenous attentional guidance. Curr Opin Neurobiol 40, 94–102. [DOI] [PubMed] [Google Scholar]
- 140.Jarvis ED et al. (2005) Avian brains and a new understanding of vertebrate brain evolution. Nature Reviews Neuroscience 6 (2), 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Livingstone M and Hubel D (1988) Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240 (4853), 740–9. [DOI] [PubMed] [Google Scholar]
- 142.Cavanaugh J and Wurtz RH (2004) Subcortical modulation of attention counters change blindness. J Neurosci 24 (50), 11236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muller JR et al. (2005) Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A 102 (3), 524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lovejoy LP and Krauzlis RJ (2017) Changes in perceptual sensitivity related to spatial cues depends on subcortical activity. Proceedings of the National Academy of Sciences of the United States of America 114 (23), 6122–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zenon A and Krauzlis RJ (2012) Attention deficits without cortical neuronal deficits. Nature 489, 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sridharan D et al. (2017) Does the superior colliculus control perceptual sensitivity or choice bias during attention? Evidence from a multialternative decision framework. J. Neurosci 37 (3), 480–511. [DOI] [PMC free article] [PubMed] [Google Scholar]


