Cholangiocarcinoma (CCA), the most common biliary tract malignancy, represents a heterogeneous group of epithelial cancers arising from varying locations within the biliary tree. The anatomic subtypes of CCA are intrahepatic CCA (iCCA) which arises in the liver parenchyma above the second order bile ducts, perihilar CCA (pCCA) which arises from the large bile ducts in the hepatic hilum and above the insertion of the cystic duct, and distal CCA (dCCA) which arises below the insertion of the cystic duct.1 The different anatomic subtypes are unified by delayed diagnosis and consequently poor outcomes as potentially curative surgical options are limited to early-stage disease. The practice standard for patients with advanced/unresectable disease is systemic chemotherapy with gemcitabine and cisplatin which has limited efficacy (median overall survival 11.7 months).1 Currently, there are no approved medical therapies for CCA patients who have progressive disease despite gemcitabine/cisplatin therapy. Moreover, non-targeted therapies have poor efficacy in advanced CCA.
The CCA subtypes have divergent biological behaviors with each having a distinct mutational landscape. For instance, fibroblast growth factor receptor (FGFR) gene fusions have been reported almost exclusively in iCCAs.2, 3 FGFR2 gene fusions have been detected in approximately 15% of iCCAs.2, 3 FGFR fusions result from various translocations which permit activating dimerization of the receptor in the absence of the ligand.3 Gene fusions are frequently driver mutations which enable malignant transformation and targeting these may have high therapeutic impact. For instance, the detection of anaplastic lymphoma kinase (ALK) gene fusions in lung cancer has led to accelerated approval of therapies for ALK-positive lung cancer. Hence, discovery of the FGFR2 gene fusions in iCCA has garnered much excitement, and there are several FGFR inhibitors being evaluated in clinical trials of CCA patients with tumors containing FGFR2 gene fusions. These include BGJ398, TAS-120, E7090, and ARQ-087. E7090 is a selective FGFR inhibitor being evaluated in phase I clinical trial in patients with advanced solid tumors (NCT02275910). In a phase 1/2 clinical trial (NCT01752920) of patients with advanced solid tumors, ARQ 087 had encouraging anti-tumor activity in the subset of patients with iCCA (n=30) with a partial response in 6 patients (20%) and stable disease in 17 patients (57%).4 BGJ398 has been the most extensively studied FGFR inhibitor in human clinical trials.
BGJ398 is an orally bioavailable, small molecule, selective pan-FGFR inhibitor which has demonstrated antitumor activity in preclinical models of CCA.1 In a phase I, dose-escalation and dose-expansion study, BGJ398 was evaluated in 132 patients with advanced solid organ malignancies containing FGFR genetic alterations including FGFR gene fusions, amplifications, and mutations.5 In this study, BGJ398 exhibited antitumor activity (seven partial responses) in different tumor types and had a tolerable safety profile with the most common adverse events (AE) being hyperphosphatemia, constipation, decreased appetite, and stomatitis.5 Of note, hyperphosphatemia is an on-target side effect due to the prominent role of FGF signaling in phosphate homeostasis.6
Javle et al. have now reported the results of a multi-center, open-label, phase II study of BGJ398 in patients with advanced or metastatic CCA harboring FGFR genetic alterations who either had prior treatment discontinuation due to toxicity or progressive disease despite gemcitabine-based therapy.6 Patients with cancer of the gallbladder or ampulla of Vater were excluded, as were patients with prior/current treatment with a selective FGFR inhibitor or a MEK inhibitor. BJG398 therapy was continued until patients experienced one of the following: disease progression, unacceptable toxicity, or consent withdrawal. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Of the 61 patients who participated in the study, the majority (n=48) had tumors with FGFR2 gene fusions.6 The overall response rate (ORR; complete response plus partial response), the primary study endpoint, was 14.8% (18.8% in patients with FGFR2 fusion tumors). BGJ398 had a disease control rate (DCR; complete response plus partial response plus stable disease) of 75.4% and the median duration of disease control amongst the patients (n=46) with either complete or partial response or stable disease was 7.5 months.6 The DCR in patients with FGFR2 fusion tumors was 83.3%. No response to BGJ398 was observed in the four patients with CCAs harboring FGFR3 amplification. A correlation between serum carbohydrate antigen 19-9 concentration and tumor response to BGJ398 was noted, suggesting that CA 19-9 may be a biomarker for disease response in this subset of patients. The median overall progress-free survival was estimated at 5.8 months (95% confidence interval [CI], 4.3 to 7.6 months). At the time of the publication, 50 patients had discontinued BGJ398 treatment mainly due to progressive disease (60.7%). Almost two-thirds of patients required a dose reduction of BGJ398, primarily due to AEs. The most commonly observed AEs were hyperphosphatemia, stomatitis, and alopecia.
Overall, this study suggests efficacy of FGFR inhibition in a subset of CCA tumors with FGFR dependence. Although the DCR of 83.3% in patients with FGFR2 fusion tumors may appear encouraging, the durability of response is limited as demonstrated by a median duration of disease control of 7.5 months. This indicates that almost all patients will ultimately have disease progression on BGJ398 therapy, perhaps due to the emergence of secondary FGFR2 active site kinase mutations. An integrative molecular analysis of cell-free circulating tumor DNA, primary tumors, and metastases in three patients with advanced FGFR2 fusion-positive iCCA revealed the emergence of secondary FGFR2 active site kinase mutations, which lead to BGJ398 resistance.7 Interestingly, distinct mutations were detected in different metastatic lesions from the same patient, signifying the presence of intra-lesional heterogeneity. All three patients had the p.V564F “gatekeeper” mutation. The gatekeeper residue is present in the ATP-binding pocket of FGFR and mutation of this residue inhibits binding of BGJ398, an ATP-competitive FGFR inhibitor. Serial analysis of cell-free circulating DNA from a subset of patients with progressive disease in the BGJ398 trial demonstrated the presence of secondary resistance mutations in the FGFR2 kinase domain. Next-generation covalent irreversible FGFR inhibitors can overcome the gatekeeper mutations and inhibit cells dependent on gatekeeper mutants.8 TAS-120 is a covalent, irreversible FGFR inhibitor currently under investigation in early phase clinical trials of patients with advanced solid organ malignancy harboring FGF/FGFR genetic alterations (NCT02052778). Hence, TAS-120 has the potential to have improved efficacy and more durable disease control in FGFR2 fusion driven iCCA, analogous to next-generation ALK inhibitors in ALK fusion positive non-small cell lung cancer (NSCLC). For instance, brigatinib, a next-generation ALK inhibitor has recently received Food and Drug Administration approval for the treatment of ALK fusion positive NSCLC. The biology of FGFR2 genetic aberrations in iCCA still needs greater definition. Nonetheless, it is likely a small subset of iCCA patients may have durable responses to FGFR inhibitors, opening the door slightly for personalized medicine in iCCA. We also note the ongoing studies exploring isocitrate dehydrogenase inhibitors 1 (IDH1) in patients with IDH1 genetic aberrations in iCCA. In this respect, the door for effective precision therapy of iCCA hopefully will continue to be opened.
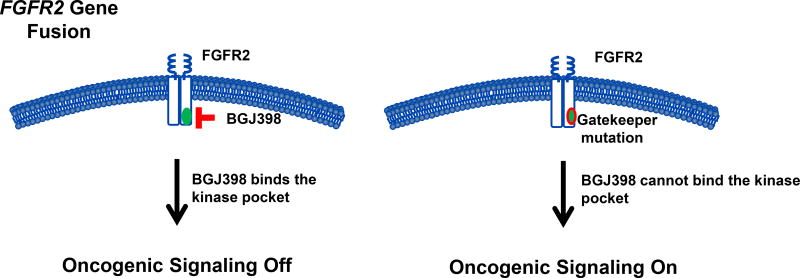
Figure 1.
FGFR2 gatekeeper mutations prevent binding of BGJ398. The gatekeeper residue is present in the ATP-binding pocket of FGFR. BGJ398 binds to the gatekeeper residue with resultant inhibition of oncogenic signaling (left panel). Mutation of the gatekeeper residue inhibits binding of BGJ398 and oncogenic signaling remains on (right panel).
Abbreviations
- AE
adverse event
- ALK
anaplastic lymphoma kinase
- CA 19-9
carbohydrate antigen 19-9
- CCA
cholangiocarcinoma
- dCCA
distal cholangiocarcinoma
- DCR
disease control rate
- FGFR
fibroblast growth factor receptor
- iCCA
intrahepatic cholangiocarcinoma
- IDH
isocitrate dehydrogenase
- NSCLC
non-small cell lung cancer
- ORR
objective response rate
- pCCA
perihilar cholangiocarcinoma
- RECIST
Response Evaluation Criteria in Solid Tumors
Footnotes
The authors have nothing to disclose.
References
- 1.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1630–8. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–47. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, El-Rayes BF, Cotsoglou C, et al. ARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrations. J Clin Oncol. 2017;35:4017. [Google Scholar]
- 5.Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1–3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I. Dose-Escalation and Dose-Expansion Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.2048. JCO2016672048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javle M, Lowery M, Shroff RT, et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal L, Saha SK, Liu LY, et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan L, Wang J, Tanizaki J, et al. Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors. Proc Natl Acad Sci U S A. 2014;111:E4869–77. doi: 10.1073/pnas.1403438111. [DOI] [PMC free article] [PubMed] [Google Scholar]