Abstract
Background
Studies examining the association of dialysate potassium concentration and mortality in hemodialysis patients show conflicting findings. We hypothesized that low dialysate potassium concentrations are associated with higher mortality, particularly in patients with high pre-dialysis serum potassium concentrations.
Methods
We evaluated 624 hemodialysis patients from the prospective Malnutrition, Diet, and Racial Disparities in Kidney Disease study recruited from 16 outpatient dialysis facilities over 2011-15 who underwent protocolized collection of dialysis treatment characteristics every six months. We examined the association of dialysate potassium concentration, categorized as 1, 2, and 3mEq/L, with all-cause mortality risk in the overall cohort, and stratified by pre-dialysis serum potassium (<5mEq/L vs. ≥5mEq/L) using case-mix adjusted Cox models.
Results
In baseline analyses, dialysate potassium concentrations of 1mEq/L were associated with higher mortality, whereas concentrations of 3mEq/L were associated with similar mortality in the overall cohort (reference: 2mEq/L): adjusted HRs (aHRs) (95% CI) 1.70 (1.01-2.88) and 0.95 (0.64-1.39), respectively. In analyses stratified by serum potassium, baseline dialysate potassium concentrations of 1mEq/L were associated with higher mortality in patients with serum potassium ≥5mEq/L but not in those with serum potassium <5mEq/L: aHRs (95% CI) 2.87 (1.51-5.46) and 0.74 (0.27-2.07), respectively (p-interaction=0.04). These findings were robust with incremental adjustment for serum potassium, potassium-binding resins, and potassium-modifying medications.
Conclusion
Low (1mEq/L) dialysate potassium concentrations were associated with higher mortality, particularly in hemodialysis patients with high pre-dialysis serum potassium. Further studies are needed to identify therapeutic strategies that mitigate inter-dialytic serum potassium accumulation and subsequent high dialysate-serum potassium gradients in this population.
Keywords: Dialysate potassium, serum potassium, gradient, hemodialysis, mortality
Introduction
Regulation of serum potassium is essential to health and survival, given the potent association between hyperkalemia and hypokalemia with cardiovascular complications, particularly ventricular arrhythmias.[1–6] Daily potassium intake typically ranges between 60 to 150mmol in the typical Western diet, with 92% and 8% excreted in urine and stool, respectively, in order to maintain total body potassium homeostasis.[7] In the context of normal kidney function, serum potassium levels can be maintained within normal ranges with up to a ten-fold higher intake of potassium, albeit with gradual increases in consumption. However, as kidney function declines, there is a compensatory increase in the fecal excretion of potassium up to a certain threshold below which hyperkalemia eventually ensues. [8, 9]
While it is well established that hemodialysis is a critical intervention for maintaining potassium homeostasis among end-stage renal disease patients, there remains wide debate regarding the appropriate prescription of dialysate potassium concentrations in this population. Although clinical practice guidelines do not currently provide recommendations on the prescription of dialysate potassium, many clinicians apply a “Rule of 7’s” in which the sum of a patient’s serum potassium and dialysate potassium concentrations should approximate 7mEq/L.[10] However, there is limited data supporting these methods of dialysate potassium concentration selection.
There is particular controversy regarding the safety of low dialysate potassium concentrations utilized in the maintenance hemodialysis population, particularly among patients with high pre-dialysis serum potassium levels. To date, several studies have observed that patients prescribed low dialysate potassium concentrations of 0mEq/L and 1mEq/L experience higher all-cause and cardiovascular mortality risk.[10–13] Adding to these aforementioned uncertainties, there has also been concern that abrupt changes in potassium kinetics during hemodialysis ensuing from a high serum-to-dialysate potassium gradient may increase risk of cardiovascular morbidity and mortality. For example, serum potassium levels decline most precipitously during the first two to three hours of hemodialysis, and following completion of dialysis treatment. In addition, while the vast majority of the body’s potassium is intracellular (98% vs. 2% stored in the intracellular vs. extracellular space, respectively)[4–6], dialysis has greater influence upon extracellular vs. intracellular potassium concentrations, post-dialysis rebound is frequently observed (i.e., 35% and 65% attenuation in intra-dialytic serum potassium reductions within one and six hours after hemodialysis treatment, respectively).[8] Thus, it has been hypothesized that a large differential between serum and dialysate potassium concentrations may exacerbate rapid changes in potassium kinetics, leading to instability in cardiac conduction and heightened risk of malignant arrhythmias in hemodialysis patients.[14, 15]
However, studies of the serum-to-dialysate potassium gradient and mortality in hemodialysis patients have shown mixed findings to date.[2, 10, 11, 16, 17] Thus, to better inform the field, we examined repeated measures of dialysate and serum potassium concentrations among a prospective multi-center cohort of hemodialysis patients in Southern California. We aimed to test the hypothesis that lower dialysate potassium concentrations prescribed to hemodialysis patients with higher serum potassium levels (i.e., high serum-to-dialysate potassium gradient) were associated with higher mortality risk.
Methods
Study Population
The study population was comprised of adult hemodialysis patients in the ongoing Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study (ClinicalTrials.gov study: NCT01415570), a prospective cohort study examining the differential association between dietary factors and nutritional status across racial and ethnic subgroups.[18–20] In the ongoing MADRAD study, patients undergo protocolized assessment of socio-demographics, comorbidities, medications, dialysis treatment characteristics, laboratory tests, nutritional status, and body anthropometry every six months (designated as study rounds).[18–20] The study population was a subcohort of MADRAD study patients recruited from 16 outpatient dialysis clinics in the Southern California region over October 2011 to February 2015, with follow up through November 2016.
Patients were included in the study provided that they had available dialysate and serum potassium data collected during the same round, were age 18 years or older at the time of study entry (i.e., date of first dialysate potassium concentration assessment), were receiving thrice-weekly in-center hemodialysis for at least four weeks, and signed a local institutional review board approved consent form. Patients were excluded if they were actively receiving treatment with peritoneal dialysis, had life expectancy less than six months, or were unable to provide consent. The study was approved by the institutional review committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA and the University of California Irvine School of Medicine.
Exposure Ascertainment
The exposure of interest was the dialysate potassium concentration ascertained every six months by MADRAD research coordinators while patients were undergoing routine dialysis treatments, categorized as 1mEq/L, 2mEq/L, and 3mEq/L. In co-primary analyses, we evaluated the association between (1) dialysate potassium concentration and all-cause mortality in the overall cohort, as well as (2) dialysate potassium concentration and all-cause mortality stratified by serum potassium level dichotomized as less <5mEq/L versus ≥5mEq/L. A cutoff of 5mEq/L was selected as the approximate median and mean serum potassium level in our study population, as well as an established threshold for higher mortality risk in previous studies.[13] Serum potassium levels were performed by the outpatient dialysis clinics on a minimum monthly basis, and other routine dialysis laboratory measurements were conducted on a monthly or quarterly basis.
In primary analyses, we sought to examine the association between baseline dialysate potassium concentration and all-cause mortality. In secondary analyses, we examined the association between time-dependent dialysate potassium concentration and all-cause mortality, in which we examined repeated measures of time-updated dialysate potassium concentrations.
Socio-demographic, Comorbidity, Dialysis Treatment, and Medication Measures
Baseline socio-demographic, comorbidity, medication, and dialysis treatment data were collected at the time of enrollment and every six months by MADRAD research coordinators. We considered medication data prescribed within one year of study entry (i.e., date of first dialysate potassium concentration assessment) as collected by the MADRAD research coordinators and staff from participating dialysis units.
Body Anthropometry and Nutritional Score Measures
MADRAD research coordinators conducted measurements of body composition surrogates during routine hemodialysis treatments, including body mass index (BMI), subcutaneous fat (determined from biceps and triceps skinfold), visceral fat (determined from waist circumference), lean muscle mass (determined from mid-arm circumference [MAC] and mid-arm muscle circumference [MAMC]), and body fat percentage (measured by near-infrared [NIR] interactance) as has been previously described.[18] Data on Subjective Global Assessment, a nutritional scoring tool, BMI, serum albumin, and total iron binding capacity were also used to estimate the Malnutrition-Inflammation Score as a proxy of protein-energy wasting (scores ranging from 0 to 30 with higher Malnutrition Inflammation Scores reflecting more severe degrees of protein-energy wasting).[21–26]
Outcome Ascertainment
All-cause mortality was the primary outcome of interest. At-risk time began the day after dialysate potassium concentration assessment, and patients were censored for kidney transplantation, transfer to a non-participating dialysis facility, peritoneal dialysis, or at the end of the study period (November 22, 2016). Every six months, information regarding mortality, censoring events, and associated dates from prior study rounds were collected from event forms completed by the MADRAD research coordinators and were reviewed by two MADRAD study nephrologists (CMR and KKZ).
Statistical Methods
Patients’ baseline characteristics stratified by baseline dialysate potassium concentrations were compared with ANOVA, Chi-Square, or Kruskal Wallis tests as appropriate. We first examined the relationship of relevant clinical characteristics with likelihood of being prescribed a low (1mEq/L) dialysate potassium concentration (reference: 2-3mEq/L) using logistic regression.
We then estimated the association between dialysate potassium concentration and all-cause mortality using Cox proportional hazard models. Logistic regression and Cox proportional hazards models were conducted using five incremental levels of covariate adjustment:
Unadjusted model: Included dialysate potassium concentration;
Case-mix model: Covariates in the unadjusted model, as well as age (at the time of dialysate potassium concentration), sex, race (Black vs. Non-Black), and ethnicity (Hispanic vs. Non-Hispanic);
Case-mix+serum potassium model: Covariates in the case-mix adjusted model, as well as serum potassium concentration;
Case-mix+serum potassium+potassium-binding resins model: Covariates in the case-mix+serum potassium model, as well as potassium-binding resins (e.g., sodium polystyrene);
Case-mix+serum potassium+potassium-modifying medications model: Covariates in the case-mix+serum potassium+potassium-binding resin model, plus nonsteroidal anti-inflammatory drugs, potassium sparing diuretics, angiotensin converting enzyme inhibitors, and aldosterone receptor blockers.
We defined the case-mix adjusted model as our primary model, which forced into the model core socio-demographic measures. Analyses incrementally adjusted for serum potassium, potassium-binding resins, and potassium-modifying medications were conducted as secondary analyses. To determine the impact of key confounders upon estimates of the dialysate potassium concentration–mortality association, namely comorbidity burden and nutritional status/dietary compliance, we implemented an expanded case-mix+serum potassium+potassium-modifying medications model that further adjusted for history of diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, any cardiovascular disease, and Malnutrition Inflammation Score[22, 23, 25] as sensitivity analyses. The proportional hazards assumption was confirmed graphically and by Schoenfeld residual function testing. There were no missing covariate data except for Malnutrition Inflammation Score (i.e., 14% of patients with missing data), which was addressed using multiple imputation. Analyses were carried out using the statistical software Stata version 12.0 (StataCorp LP, College Station, TX) and SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Cohort Description
Among 624 patients who met eligibility criteria (Supplementary Figure 1), the mean±SD age of the cohort was 54.5±14.5 years, among whom 43% were women; 35% were of Black race and 47% were of Hispanic ethnicity; and of whom 51% had underlying diabetes. At study entry, 8% (N=47), 72% (N=449), and 21% (N=128) were prescribed 1, 2, and 3mEq/L dialysate concentrations, respectively. Compared to patients who were prescribed 2mEq/L or 3mEq/L dialysate concentrations, those prescribed 1mEq/L dialysate concentrations were more likely to be female and Hispanic; had longer dialysis vintage; and had higher normalized protein catabolic rates (nPCR), serum phosphorus, and serum creatinine levels (Table 1).
Table 1.
Baseline Characteristics according to Dialysate Potassium Concentration.
| DIALYSATE POTASSIUM CONCENTRATION | P* | |||
|---|---|---|---|---|
|
| ||||
| 1mEq/L | 2mEq/L | 3mEq/L | ||
|
| ||||
| No. of patients (%) | 47 (8%) | 449 (72%) | 128 (21%) | N/A |
|
| ||||
| Age, years (Mean±SD) | 50.1±15.5 | 54.1±14.3 | 57.3±14.4 | 0.02 |
|
| ||||
| Female (%) | 58 | 44 | 35 | 0.03 |
|
| ||||
| Black race (%) | 32 | 35 | 38 | 0.69 |
|
| ||||
| Hispanic ethnicity (%) | 64 | 47 | 41 | 0.03 |
|
| ||||
| Vintage, months (Mean±SD) | 71.9 ± 48.3 | 52.2 ± 48.5 | .35.0± 36.2 | <0.001 |
|
| ||||
| Vascular access (%) | ||||
| AVF/AVG | 79 | 79 | 85 | 0.52 |
| Catheter | 21 | 21 | 15 | |
|
| ||||
| Marital status (%) | ||||
| Non-married | 62 | 56 | 52. | 0.28 |
| Married | 38 | 43 | 46 | |
| Unknown/other | 0 | 0 | 2 | |
|
| ||||
| Primary insurance (%) | ||||
| Medicare/Medicaid | 77 | 77 | 67 | 0.18 |
| Private | 11 | 10 | 17 | |
| Other/Unknown | 13 | 13 | 16 | |
|
| ||||
| BMI, kg/m2 (Mean±SD) | 27.5 ± 7.1 | 27.7 ± 6.1 | 28.0 ± 5.5 | 0.46 |
|
| ||||
| COMORBIDITIES | ||||
|
| ||||
| Diabetes (%) | 50 | 52 | 50 | 0.86 |
|
| ||||
| CHF (%) | 13 | 10 | 9 | 0.81 |
|
| ||||
| CAD (%) | 13 | 9 | 13 | 0.34 |
|
| ||||
| CVA/TIA (%) | 2 | 0 | 1 | 0.17 |
|
| ||||
| CVD | 23 | 18 | 19 | 0.64 |
|
| ||||
| MIS (Mean±SD) | 3.9± 2.0 | 4.5±2.9 | 4.6±3.1 | 0.64 |
|
| ||||
| LABORATORY RESULTS (median [IQR]) | ||||
|
| ||||
| Serum Potassium (mEq/L) | 5.21 (4.7, 5.6) | 5.0 (4.6, 5.3) | 4.7 (4.4, 5.0) | <0.001 |
|
| ||||
| Bicarbonate (mEq/L) | 23 (20, 26) | 23 (21, 26) | 23 (21, 25) | 0.80 |
|
| ||||
| spKt/V (single pool) | 1.66 (1.54, 1.93) | 1.63 (1.43, 1.86) | 1.58 (1.41, 1.87) | 0.37 |
|
| ||||
| Serum albumin (g/dL) | 4.1 (3.9, 4.3) | 4.0 (3.8, 4.2) | 4.0 (3.9, 4.2) | 0.24 |
|
| ||||
| nPCR (g/kg/day) | 1.2 (1.0, 1.3) | 1.0 (0.9, 1.2) | 0.9 (0.8, 1.1) | <0.001 |
|
| ||||
| Phosphorus (mg/dL) | 5.2 (4.0, 7.1) | 4.9 (4.1, 5.7) | 4.7 (3.8, 5.3) | 0.01 |
|
| ||||
| Serum creatinine (mg/dL) | 10.8 (9.3, 12.7) | 9.9 (7.9, 12.0) | 8.2 (6.1, 10.4) | <0.001 |
|
| ||||
| Hemoglobin (g/dL) | 10.5 (9.7, 11.2) | 10.7 (10.1, 11.3) | 10.8 (10.3, 11.5) | 0.23 |
|
| ||||
| BODY ANTHROPOMETRY (median [IQR]) | ||||
|
| ||||
| Waist Circumference (cm) | 88 (83, 99) | 96 (85, 107) | 97 (90, 109) | 0.02 |
|
| ||||
| Triceps Skinfold (mm) | 23 (14, 31) | 17 (10, 28) | 19 (12, 28) | 0.12 |
|
| ||||
| Biceps Skinfold (mm) | 14 (8, 21) | 11 (6,19) | 11 (6, 18) | 0.16 |
|
| ||||
| Mid-arm muscle circumference (cm) | 23 (21, 25) | 24 (21, 27) | 25 (22, 28) | 0.25 |
|
| ||||
| Mid-arm circumference (cm) | 31 (27, 34) | 30 (27, 34) | 31 (28, 35) | 0.55 |
|
| ||||
| NIR body fat (%) | 30 (20, 38) | 29 (22, 38) | 30 (21, 39) | 0.99 |
|
| ||||
| MEDICATION USE (%) | ||||
|
| ||||
| Sodium polystyrene | 0 | <1 | <1 | 0.84 |
|
| ||||
| ACEi and ARB | 30 | 37 | 37 | 0.60 |
|
| ||||
| NSAIDs | 19 | 33 | 32 | 0.15 |
|
| ||||
| Potassium Sparing Diuretics | 0 | 0 | <1 | 0.14 |
|
| ||||
| Other (Trimethoprim-Sulfamethoxazole, Heparin, Potassium supplement) | 2 | <1 | 0 | 0.29 |
Note: Categorical variables are given as percentages; continuous variables as mean±SD or median (IQR).
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; CHF, congestive heart failure; MI, myocardial infarction; CAD, coronary artery disease; CVA, cerebrovascular accident; TIA, transient ischemic attack; CVD, cardiovascular disease; nPCR, normalized protein catabolic rate; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
P-value calculated by ANOVA, Chi-square, or Kruskal Wallis tests.
Clinical Characteristics Associated with Dialysate Potassium Concentration
In case-mix adjusted logistic regression models, we observed that patients who were female; Hispanic; of longer dialysis vintage; and with higher nPCR, serum potassium, and serum creatinine levels had higher likelihood of receipt of a low (i.e, 1mEq/L) dialysate potassium concentration. Associations with female sex, Hispanic ethnicity, higher nPCR, and higher serum creatinine levels with low dialysate concentration persisted across all models of covariate adjustment. (Table 2).
Table 2.
Clinical characteristics associated with receipt of 1mEq/L dialysate potassium concentrations (reference: 2–3 mEq/L)
| Unadjusted | Case-mix adjusted | Case-mix + Serum K adjusted | Case-mix + Serum K + potassium-binding resin adjusted | Case-mix + Serum K + modifying medication potassium-adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
|
| ||||||||||
| Age (Δ10 years) | 0.80 (0.65-0.98) | 0.03 | 0.81 (0.66-0.99) | 0.04 | 0.84 (0.68-1.05) | 0.12 | 0.84 (0.68-1.05) | 0.13 | 0.86 (0.69-1.07) | 0.17 |
|
| ||||||||||
| Female (vs. Male) | 1.87 (1.02-3.14) | 0.04 | 2.09 (1.13-3.85) | 0.02 | 1.92 (1.04-3.57) | 0.04 | 1.90 (1.03-3.53) | 0.04 | 2.01 (1.07-3.78) | 0.03 |
|
| ||||||||||
| Black (vs. Non-Black) | 0.84 (0.45-1.60) | 0.60 | 2.47 (0.85-7.20) | 0.10 | 2.66 (0.86-8.18) | 0.09 | 2.66 (0.87-8.17) | 0.09 | 2.72 (0.92-8.02) | 0.07 |
|
| ||||||||||
| Hispanic (vs. Non-Hispanic) | 2.08 (1.12-3.85) | 0.02 | 3.97 (1.41-11.2) | 0.01 | 3.67 (1.24-10.9) | 0.02 | 3.69 (1.24-10.9) | 0.02 | 3.69 (1.3-10.5) | 0.01 |
|
| ||||||||||
| Vintage (∆6 months) | 1.05 (1.02-1.08) | 0.002 | 1.04 (1.01-1.07) | 0.01 | 1.04 (1.00-1.07) | 0.03 | 1.04 (1.00-1.07) | 0.03 | 1.03 (1.00-1.06) | 0.06 |
|
| ||||||||||
| Vascular Access AVF/AVG (vs. Catheter) |
0.91 (0.38-2.17) | 0.83 | 0.86 (0.35-2.10) | 0.74 | 0.83 (0.33-2.06) | 0.68 | 0.84 (0.34-2.09) | 0.71 | 0.78 (0.31-1.96) | 0.60 |
|
| ||||||||||
| Marital status (ref: Married) Non-married |
1.28 (0.69-2.36) | 0.43 | 1.20 (0.62-2.32) | 0.60 | 1.38 (0.71-2.71) | 0.34 | 1.39 (0.71-2.73) | 0.33 | 0.78 (0.31-1.96) | 0.60 |
|
| ||||||||||
| Insurance (ref. Medicare/Medicaid) | ||||||||||
|
| ||||||||||
| Private | 0.88 (0.34-2.32) | 0.80 | 1.13 (0.42-3.05) | 0.82 | 1.33 (0.48-3.68) | 0.58 | 1.36 (0.49-3.77) | 0.56 | 1.33 (0.47-3.71) | 0.59 |
|
| ||||||||||
| Other/Unknown | 0.95 (0.39-2.33) | 0.91 | 0.97 (0.38-2.45) | 0.95 | 1.12 (0.44-2.87) | 0.81 | 1.13 (0.44-2.91) | 0.80 | 1.13 (0.44-2.92) | 0.80 |
|
| ||||||||||
| MIS (Δ1) | 0.92 (0.80-1.05) | 0.22 | 0.92 (0.80-1.05) | 0.21 | 0.91 (0.79-1.05) | 0.19 | 0.91 (0.79-1.05) | 0.19 | 0.91 (0.79-1.05) | 0.20 |
|
| ||||||||||
| Diabetes | 0.90 (0.50-1.63) | 0.72 | 1.02 (0.52-1.99) | 0.96 | 0.96 (0.49-1.89) | 0.91 | 0.96 (0.49-1.90) | 0.91 | 1.10 (0.55-2.21) | 0.79 |
|
| ||||||||||
| CHF | 1.31 (0.54-3.13) | 0.56 | 1.45 (0.57-3.65) | 0.43 | 1.61 (0.63-4.13) | 0.32 | 1.63 (0.64-4.16) | 0.31 | 1.60 (0.62-4.11) | 0.33 |
|
| ||||||||||
| CAD | 1.39 (0.56-3.42) | 0.47 | 1.58 (0.63-3.99) | 0.33 | 1.54 (0.60-3.96) | 0.37 | 1.52 (0.59-3.92) | 0.38 | 1.75 (0.67-4.57) | 0.25 |
|
| ||||||||||
| CVA/TIA | 6.25 (0.56-70.2) | 0.14 | 9.60 (0.81-114) | 0.07 | 16.2 (1.29-203) | 0.03 | 15.9 (1.27-200) | 0.03 | 19.6 (1.52-254) | 0.03 |
|
| ||||||||||
| CVD | 1.39 (0.69-2.82) | 0.36 | 1.55 (0.75-3.21) | 0.23 | 1.61 (0.77-3.35) | 0.21 | 1.60 (0.77-3.35) | 0.21 | 1.71 (0.81-3.60) | 0.16 |
|
| ||||||||||
| Laboratory Data | ||||||||||
|
| ||||||||||
| Serum Potassium (Δ1mEq/L) | 3.71 (1.93-7.11) | <0.001 | 3.16 (1.61-6.21) | 0.001 | 3.16 (1.61-6.21) | 0.001 | 3.16 (1.61-6.21) | 0.001 | 3.24 (1.64-6.42) | 0.001 |
|
| ||||||||||
| Bicarbonate (Δ1mEq/L) | 0.98 (0.90-1.06) | 0.57 | 1.01 (0.92-1.09) | 0.91 | 1.02 (0.94-1.11) | 0.68 | 1.02 (0.93-1.11) | 0.70 | 1.01 (0.93-1.10) | 0.81 |
|
| ||||||||||
| SpKt/V (Δ0.2) | 1.07 (0.90-1.27) | 0.47 | 1.01 (0.83-1.22) | 0.95 | 1.02 (0.83-1.25) | 0.85 | 1.02 (0.83-1.26) | 0.84 | 1.00 (0.82-1.23) | 0.97 |
|
| ||||||||||
| Serum albumin (Δ0.2g/dl) | 1.13 (0.95-1.34) | 0.17 | 1.13 (0.95-1.36) | 0.17 | 1.14 (0.95-1.38) | 0.16 | 1.14 (0.95-1.37) | 0.17 | 1.13 (0.94-1.36) | 0.21 |
|
| ||||||||||
| nPCR (Δ0.2g/kg/day) | 1.40 (1.14-1.71) | 0.001 | 1.39 (1.13-1.71) | 0.002 | 1.32 (1.06-1.64) | 0.01 | 1.32 (1.06-1.63) | 0.01 | 1.31 (1.05-1.63) | 0.02 |
|
| ||||||||||
| Phosphorus (Δ1mg/dl) | 1.22 (1.04-1.45) | 0.02 | 1.19 (1.00-1.42) | 0.05 | 1.12 (0.93-1.34) | 0.22 | 1.12 (0.94-1.35) | 0.21 | 1.13 (0.94-1.36) | 0.18 |
|
| ||||||||||
| Creatinine (Δ1mg/dl) | 1.18 (1.07-1.31) | 0.001 | 1.26 (1.10-1.43) | 0.001 | 1.20 (1.05-1.38) | 0.01 | 1.20 (1.05-1.38) | 0.01 | 1.19 (1.04-1.37) | 0.01 |
|
| ||||||||||
| Hemoglobin (Δ1g/dl) | 0.91 (0.69-1.20) | 0.50 | 0.926 (0.70-1.23) | 0.60 | 0.96 (0.71-1.27) | 0.76 | 0.95 (0.71-1.27) | 0.71 | 0.95 (0.70-1.27) | 0.71 |
|
| ||||||||||
| Body Anthropometry | ||||||||||
| Waist Circumference (Δ5cm) | 0.93 (0.86-1.02) | 0.12 | 0.94 (0.85-1.03) | 0.18 | 0.94 (0.85-1.03) | 0.19 | 0.94 (0.85-1.03) | 0.19 | 0.95 (0.86-1.04) | 0.28 |
| Triceps Skinfold (Δ10mm) | 1.21 (0.95-1.54) | 0.12 | 1.14 (0.88-1.48) | 0.31 | 1.19 (0.92-1.55) | 0.19 | 1.19 (0.92-1.55) | 0.19 | 1.29 (0.98-1.04) | 0.07 |
| Biceps Skinfold (Δ10 mm) | 1.26 (0.94-1.69) | 0.13 | 1.13 (0.83-1.55) | 0.44 | 1.15 (0.84-1.58) | 0.39 | 1.15 (0.84-1.57) | 0.40 | 1.24 (0.89-1.72) | 0.20 |
| Mid-arm muscle circumference (Δ5cm) | 0.83 (0.60-1.16) | 0.28 | 0.91 (0.64-1.28) | 0.58 | 0.88 (0.62-1.25) | 0.48 | 0.88 (0.62-1.24) | 0.46 | 0.87 (0.61-1.23) | 0.43 |
| Mid-arm circumference (Δ5cm) | 1.01 (0.78-1.32) | 0.92 | 1.02 (0.78-1.34) | 0.87 | 1.03 (0.78-1.36) | 0.82 | 1.03 (0.78-1.35) | 0.84 | 1.07 (0.81-1.42) | 0.63 |
| NIR body fat (Δ10%) | 0.97 (0.73-1.29) | 0.84 | 0.77 (0.52-1.13) | 0.18 | 0.77 (0.52-1.13) | 0.18 | 0.77 (0.72-1.19) | 0.17 | 0.79 (0.53-1.18) | 0.25 |
| BMI (Δ 5kg/m2) | 0.97 (0.75-1.25) | 0.81 | 0.95 (0.74-1.23) | 0.71 | 0.93 (0.72-1.19) | 0.55 | 0.92 (0.72-1.19) | 0.54 | 0.95 (0.73-1.22) | 0.68 |
|
| ||||||||||
| Medications | ||||||||||
| ACEi and ARB | 0.72 (0.38-1.38) | 0.32 | 0.68 (0.35-1.31) | 0.25 | 0.64 (0.33-1.24) | 0.18 | 0.64 (0.33-1.24) | 0.19 | 0.71 (0.36-1.41) | 0.33 |
| NSAIDs | 0.49 (0.23-1.03) | 0.06 | 0.48 (0.22-1.02) | 0.06 | 0.47 (0.22-1.02) | 0.05 | 0.47 (0.22-1.03) | 0.06 | 0.51 (0.23-1.11) | 0.09 |
| Other (Trimethoprim-sulfamethoxazole, heparin, potassium supplement) | 4.16 (0.42-40.8) | 0.22 | 6.57 (0.59-73.56) | 0.13 | 5.31 (0.46-61.7) | 0.18 | 5.25 (0.45-61.1) | 0.19 | 6.06 (0.52-70.39) | 0.15 |
Dialysate Potassium Concentration and Mortality
Patients contributed a total of 1867 person-years of follow up during which time 161 death events were observed. Median (IQR) at-risk time was 3.2 (1.8, 4.3) years. In analyses of baseline dialysate potassium concentration in the overall cohort, compared to patients prescribed a 2mEq/L dialysate concentration, those prescribed a 1mEq/L concentration had higher mortality risk in case-mix, case-mix+serum potassium, case-mix+serum potassium+potassium-binding resin, and case mix+serum potassium+potassium-modifying medication adjusted analyses (adjusted HRs [aHRs] [95% CI] 1.70 [1.01-2.88], 1.71 [1.01-2.90], 1.70 [1.00-2.88], and 1.72 [1.01-2.93], respectively), whereas patients prescribed a 3mEq/L bath had similar mortality risk (aHR [95% CI] 0.95 [0.64-1.39], 0.94 [0.63-1.40], 0.94 [0.63-1.39], and 0.91 [0.6-1.36], respectively) (Figure 1 and Supplementary Table 1). In sensitivity analyses adjusted for expanded case-mix+serum potassium+potassium-modifying medications covariates, point estimates for receipt of a 1mEq/L dialysate potassium concentration suggested higher mortality risk, although associations narrowly missed statistical significance: aHR (95%CI) 1.70 (0.97-2.96), p=0.06 (Supplementary Table 1).
Figure 1.
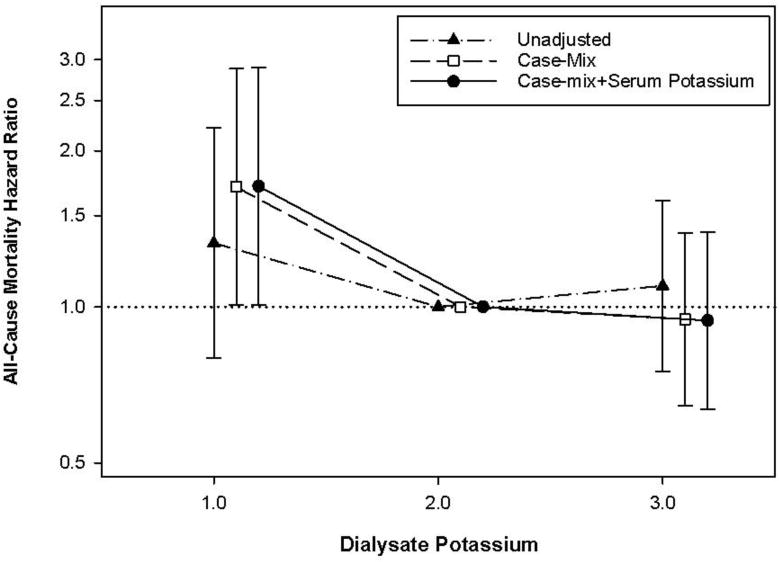
Baseline dialysate potassium concentration and all-cause mortality risk in the overall cohort using case-mix adjusted Cox models.
We observed that pre-dialysis serum potassium level was an effect modifier of the association between dialysate potassium concentration and mortality risk. In analyses stratified by serum potassium, patients prescribed a 1mEq/L bath had higher mortality among those with serum potassium levels ≥5mEq/L in case-mix adjusted analyses (reference: 2mEq/L): aHR (95% CI) 2.87 (1.51-5.46). However, we did not observe a significant association between patients prescribed a 1mEq/L bath and mortality among those with serum potassium levels <5mEq/L: aHR (95% CI) 0.74 (0.27-2.07), p-interaction=0.04. These patterns of association persisted in models incrementally adjusted for case-mix, case-mix+serum potassium, case-mix+serum potassium+potassium-binding resin, case-mix+serum potassium+potassium-modifying medication covariates (Figure 2 and Supplementary Table 2). Sensitivity analyses adjusted for expanded case-mix+serum potassium+potassium-modifying medications covariates also showed that a 1mEq/L dialysate potassium concentration was associated with higher mortality in patients with serum potassium levels ≥5mEq/L but not in those with serum potassium <5mEq/L: aHRs (95% CI) 2.51 (1.24-5.06) and 0.76 (0.26-2.23), respectively; p-interaction=0.04 (Supplementary Table 2).
Figure 2.
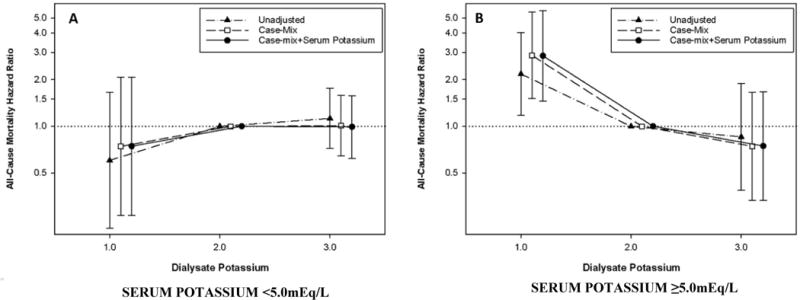
Baseline dialysate potassium concentration and all-cause mortality, stratified according to pre-dialysis serum potassium level, using case-mix adjusted Cox models: Serum potassium <5mEq/L (Panel A) vs. serum potassium ≥ 5 mEq/L (Panel B).
In analyses of time-dependent dialysate and serum potassium concentrations adjusted for case-mix covariates, there was a trend between receipt of 1mEq/L dialysate potassium concentrations and higher mortality risk, although associations did not achieve statistical significance (Supplementary Table 3). Similar patterns were observed when analyses of time-dependent dialysate potassium concentration and mortality were stratified by time-dependent serum potassium level (Supplementary Table 4).
Discussion
In a prospective, multi-center cohort of maintenance hemodialysis patients across Southern California, we observed that patients prescribed a dialysate potassium concentration of 1mEq/L had higher mortality risk compared to those receiving a 2 or 3mEq/L dialysate potassium concentration bath. However, in a priori defined analyses stratified by serum potassium level, we found that low dialysate potassium concentrations of 1mEq/L were associated with higher mortality risk only among patients with high pre-dialysis serum potassium levels (≥5mEq/L) but not in those with low serum potassium levels (<5mEq/L). These observations persisted across sensitivity analyses with incremental levels of multivariable adjustment for case-mix covariates, serum potassium levels, potassium-binding resins, and potassium-modifying medications.
There has been considerable debate regarding the safety of low dialysate potassium concentrations particularly in the setting of high pre-dialysis serum potassium levels.[27] An early report by Karnik et al. and a more recent study by Pun et al. have both shown that receipt of low dialysate potassium concentrations (defined as 0-1mEq/L and <2mEq/L, respectively) were associated with higher risk of cardiac arrest among in-center hemodialysis patients.[11, 12] However, a study of 81,013 prevalent dialysis patients from a large national dialysis organization by Kovesdy et al. observed that higher dialysate potassium concentrations (>3mEq/L) were associated with higher mortality among those with elevated pre-dialysis serum potassium levels (>5mEq/L),[2] whereas an analysis of 55,183 patients from the Dialysis Outcomes and Practice Patterns Study (DOPPS) cohort who were administered a dialysate potassium concentration of 2mEq/L vs. 3mEq/L showed no associations with all-cause mortality or cardiovascular events.[10] Most recently, Brunelli et al. examined hemodialysis patients from a national LDO and found an incremental association between higher serum-to-dialysate potassium gradients ≥3meq/L with higher risk of hospitalizations and emergency department visits.[17]
To our knowledge, our study is the first to show that low dialysate potassium concentrations are associated with higher mortality risk among patients with high serum potassium concentrations (≥5mEq/L) but not in those with normal serum potassium (<5mEq/L), supporting findings from the recent Brunelli et al. study suggesting that high serum-to-dialysate potassium gradients may be harmful.[14, 15] While the majority of total body potassium exists in intracellular compartments, potassium removal during dialysis is largely driven by the extracellular serum-to-dialysate-potassium concentration gradient, which is highest in the first hour of dialysis, dissipates over treatment, and may be further attenuated post-dialysis due to a rebound effect (i.e., post-dialysis extracellular potassium movement).[8] It has been suggested that rapid changes in serum potassium levels, primarily during the first hour of dialysis, can lead to de-stabilization of cell membranes, QTc interval dispersion, ventricular ectopy, and arrhythmogenicity.[28–30]
The heterogeneous findings across studies may potentially be explained by a differential relationship between the serum-to-dialysate-potassium gradient with short-term vs. long-term risk.[17] While a small serum-to-dialysate-potassium gradient among patients with higher serum potassium levels may bear long-term risk (i.e., potassium accumulation/overload leading to eventual death), it is also possible that a large gradient may lead to malignant arrhythmias, sudden cardiac death, and hence short-term risk. Based on these collective data, further studies are needed to determine whether alternative methods of dialysis prescription (i.e., potassium profiling), pharmacotherapies (e.g., sodium zirconium cyclosilicate, patiromer), and dietary interventions that prevent inter-dialytic accumulation of serum potassium can improve the survival of hemodialysis patients.
The strengths of our study include its examination of a prospective cohort of hemodialysis patients who underwent protocolized collection of socio-demographic, comorbidity, laboratory, and medication data; longitudinal information on dialysis prescription characteristics; and rigorous adjudication of mortality events. However, there are several limitations of the study that should be noted. First, while we observed a robust association between baseline dialysate potassium and serum potassium levels with mortality, these findings do not reflect how changes in dialysate and serum potassium concentrations over time influence mortality. However, we found that the vast majority (~73%) of our study maintained the same dialysate potassium concentration during follow up. Second, as we do not know the clinical indications for which specific dialysate potassium concentrations were prescribed, it is possible that those receiving 1mEq/L dialysate potassium concentrations were those who were less compliant with dialysis and dietary prescriptions and with worse underlying ill health status. While we observed robust associations between receipt of a 1mEq/L dialysate potassium concentration and higher mortality among patients with serum potassium levels ≥5mEq/L but not in those with serum potassium <5mEq/L after accounting for differences in pre-dialysis serum potassium levels, multiple comorbidities and nutritional status, we cannot exclude confounding by indication on this basis. Third, our study population’s observed pre-dialysis serum potassium levels were largely within a narrow range (4 to 6mEq/L; only 8 patients had serum potassium levels ≥6mEq/L), which may limit generalizability to patients with very low or high serum potassium levels. However, our findings do apply to the vast majority of patients in clinical practice whose serum potassium generally ranges between 4 and 6mEq/L. Fourth, we lacked data on cause-specific death in providing insights into underlying pathways towards mortality. Lastly, as with all observational studies, we cannot exclude the possibility of residual confounding, and our findings do not confirm a causal association of dialysate and serum potassium with mortality risk.
In conclusion, our study is the first to show that the prescription of low (1mEq/L) potassium dialysate concentrations is associated with higher mortality risk, particularly among hemodialysis patients with high pre-dialysis serum potassium concentrations, which may relate to large serum-to-dialysate-potassium gradients leading to rapid changes in potassium during the dialysis procedure. Further studies are needed to verify the risk associated with rapid potassium removal during dialysis, as well as the potential role for interventions (i.e., potassium binding resins) obviating the need and risk of excessive potassium removal during dialysis.
Supplementary Material
Supplementary Figure 1. Study cohort creation.
Acknowledgments
Portions of these data were presented as an abstract at the 2018 National Kidney Foundation Spring Clinical Meeting, April 10-14, 2018, Austin, TX.
Funding/Support:
The authors are supported by the research grants from the NIH/NIDDK including K23-DK102903 (CMR), K24-DK091419 (KKZ), R01-DK096920 (CPK, KKZ), U01-DK102163 (KKZ, CPK), and philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, and Dr. Joseph Lee.
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Iseki K, Uehara H, Nishime K, Tokuyama K, Yoshihara K, Kinjo K, Shiohira Y, Fukiyama K. Impact of the initial levels of laboratory variables on survival in chronic dialysis patients. Am J Kidney Dis. 1996;28(4):541–8. doi: 10.1016/s0272-6386(96)90465-5. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(5):999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 3.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15(5):458–82. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 4.Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480–90. doi: 10.1152/advan.00121.2016. [DOI] [PubMed] [Google Scholar]
- 5.Palmer BF, Clegg DJ. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017;84(12):934–42. doi: 10.3949/ccjm.84a.17056. [DOI] [PubMed] [Google Scholar]
- 6.Palmer BF, Clegg DJ. Hyperkalemia across the Continuum of Kidney Function. Clin J Am Soc Nephrol. 2018;13(1):155–7. doi: 10.2215/CJN.09340817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrier RW. Renal and electrolyte disorders. Eighth. Philadelphia: Wolters Kluwer; 2018. [Google Scholar]
- 8.Agar BU, Culleton BF, Fluck R, Leypoldt JK. Potassium kinetics during hemodialysis. Hemodial Int. 2015;19(1):23–32. doi: 10.1111/hdi.12195. [DOI] [PubMed] [Google Scholar]
- 9.Hung AM, Hakim RM. Dialysate and serum potassium in hemodialysis. Am J Kidney Dis. 2015;66(1):125–32. doi: 10.1053/j.ajkd.2015.02.322. [DOI] [PubMed] [Google Scholar]
- 10.Karaboyas A, Zee J, Brunelli SM, Usvyat LA, Weiner DE, Maddux FW, Nissenson AR, Jadoul M, Locatelli F, Winkelmayer WC, Port FK, Robinson BM, Tentori F. Dialysate Potassium, Serum Potassium, Mortality, and Arrhythmia Events in Hemodialysis: Results From the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2017;69(2):266–77. doi: 10.1053/j.ajkd.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79(2):218–27. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 12.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60(1):350–7. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 13.Jadoul M, Thumma J, Fuller DS, Tentori F, Li Y, Morgenstern H, Mendelssohn D, Tomo T, Ethier J, Port F, Robinson BM. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol. 2012;7(5):765–74. doi: 10.2215/CJN.08850811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee CM. Serum Potassium and the Long Interdialytic Interval: Minding the Gap. Am Journal of Kidney Dis. 2017;70(1):4–7. doi: 10.1053/j.ajkd.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum Potassium and Short-term Clinical Outcomes Among Hemodialysis Patients: Impact of the Long Interdialytic Interval. Am J Kidney Dis. 2017;70(1):4–7. doi: 10.1053/j.ajkd.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. US Department of Health and Human Services; Atlanta: 2014. [Google Scholar]
- 17.Brunelli SM, Spiegel DM, Du Mond C, Oestreicher N, Winkelmayer WC, Kovesdy CP. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant. doi: 10.1093/ndt/gfx241. Epub August 9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of Adiponectin With Body Composition and Mortality in Hemodialysis Patients. Am J Kidney Dis. 2015;66(2):313–21. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee CM, You AS, Nguyen DV, Brunelli SM, Budoff MJ, Streja E, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Thyroid Status and Mortality in a Prospective Hemodialysis Cohort. J Clin Endocrinol Metab. 2017;102(5):1568–77. doi: 10.1210/jc.2016-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You AS, Kalantar-Zadeh K, Lerner L, Nakata T, Lopez N, Lou L, Veliz M, Soohoo M, Jing J, Zaldivar F, Gyuris J, Nguyen DV, Rhee CM. Association of Growth Differentiation Factor 15 with Mortality in a Prospective Hemodialysis Cohort. Cardiorenal Med. 2017;7(2):158–68. doi: 10.1159/000455907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–63. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19(6):1507–19. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 24.Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Current opinion in clinical nutrition and metabolic care. 2015;18(3):254–62. doi: 10.1097/MCO.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53(2):298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ujszaszi A, Czira ME, Fornadi K, Novak M, Mucsi I, Molnar MZ. Quality of life and protein-energy wasting in kidney transplant recipients. Int Urol Nephrol. 2012;44(4):1257–68. doi: 10.1007/s11255-012-0122-3. [DOI] [PubMed] [Google Scholar]
- 27.Pun PH, Middleton JP. Dialysate Potassium, Dialysate Magnesium, and Hemodialysis Risk. J Am Soc Nephrol. 2017;28(12):3441–51. doi: 10.1681/ASN.2017060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buemi M, Aloisi E, Coppolino G, Loddo S, Crasci E, Aloisi C, Barilla A, Cosentini V, Nostro L, Caccamo C, Floccari F, Romeo A, Frisina N, Teti D. The effect of two different protocols of potassium haemodiafiltration on QT dispersion. Nephrol Dial Transplant. 2005;20(6):1148–54. doi: 10.1093/ndt/gfh770. [DOI] [PubMed] [Google Scholar]
- 29.Santoro A, Mancini E, London G, Mercadal L, Fessy H, Perrone B, Cagnoli L, Grandi E, Severi S, Cavalcanti S. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant. 2008;23(4):1415–21. doi: 10.1093/ndt/gfm730. [DOI] [PubMed] [Google Scholar]
- 30.Redaelli B, Locatelli F, Limido D, Andrulli S, Signorini MG, Sforzini S, Bonoldi L, Vincenti A, Cerutti S, Orlandini G. Effect of a new model of hemodialysis potassium removal on the control of ventricular arrhythmias. Kidney Int. 1996;50(2):609–17. doi: 10.1038/ki.1996.356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study cohort creation.


