Abstract
Previous studies have shown that people with PD benefit from a variety of exercise modalities with respect to symptom management and function. Among possible exercise modalities, speedwork has been identified as a promising strategy, with direct implications for the rate and amplitude of nervous system involvement. Considering that previous speed-based exercise for PD has often been equipment, personnel and/or facility dependent, and often time intensive, our purpose was to develop a population specific exercise program that could be self-administered with equipment that is readily found in fitness centers or perhaps the home. Fourteen individuals with PD (Hoehn-Yahr stage of 3.0 or less) participated in 12, 30-minute sessions of low-resistance interval training on a stationary recumbent bicycle. Motor examination section of the UPDRS, 10-meter walk, timed-up-and-go, functional reach, 4-square step test, 9-hole peg test, and simple reaction time scores all exhibited significant improvements (p < 0.05). These results add further support to the practice of speedwork for people with PD and outline a population-amenable program with high feasibility.
Keywords: motor function, exercise, symptom management, quickness, neurological diseases, mobility
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and the number of individuals diagnosed with PD is expected to increase by twofold over the next two decades (Dorsey et al, 2007). The most common motor symptoms of PD include: bradykinesia (i.e. reductions in the speed and amplitude of movement); rigidity; postural instability; and tremor (Berardelli, Rothwell, Thompson, and Hallet, 2001). Although there is no known cure for PD, pharmaceutical and neurosurgical treatment methods have been used for symptom management. While these methods are beneficial, they are known to lose effectiveness over time and have severe side effects (e.g. Levodopa-induced dyskinesia or impulse control disorder). As an alternative or supplement to medical treatment, exercise has recently been evaluated as a neurorehabilitative tool that could slow down the disease progression and improve PD related symptoms (Fisher et al, 2004).
Various exercise routines aimed towards improving: strength (Corcos et al, 2013; Dibble et al, 2009); aerobic capacity (Bergen et al, 2002); balance (Herman, Giladi, Gruendlinger, and Hausdorff, 2007; Li et al, 2012; Rose, Lokkegaard, Sonne-Holm, and Jensen, 2013); and speed (Herman, Giladi, Gruendlinger, and Hausdorff, 2007; Rose, Lokkegaard, Sonne-Holm, and Jensen, 2013) have been shown to be effective in the improvement of PD related symptoms. Among these routines, speed (or quickness) training has recently been identified as a valuable training method addressing bradykinesia. PD patients who accomplished incremental speed-dependent treadmill training showed improvement in: gait related parameters (Cakit et al, 2007; Herman, Giladi, Gruendlinger, and Hausdorff, 2007); disease severity (as assessed by the motor section of Unified Parkinson’s Disease Rating Scale (UPDRS-III)) (Herman, Giladi, Gruendlinger, and Hausdorff, 2007); and postural stability (Cakit et al, 2007). Similarly, both manual dexterity and motor functions were improved in a study involving PD patients pedaling on a tandem bike with an assistant pedaling in front to augment the pedaling rate (Ridgel, Peacock, Fickes, and Kim, 2012; Ridgel, Vitek, and Alberts, 2009).
The high-speed training modes used in the previous research usually required either a: clinical setting with a harness system (Cakit et al, 2007; Herman, Giladi, Gruendlinger, and Hausdorff, 2007); an able bodied cycling partner on a tandem bike (Alberts et al, 2016; Beall et al, 2013; Ridgel, Vitek, and Alberts, 2009); or a motorized bicycle (Ridgel, Peacock, Fickes, and Kim, 2012). However, these factors affect the cost of and access to this type of exercise and hinder its use in home or in community based settings. Considering the promising results for “speedwork” in PD, there is a need for speed training that is more accessible and feasible as compared to existing training modalities. Therefore, we designed a high speed-low resistance (HS-LR) cycling program that could be self-administered for individuals at Hoehn-Yahr (H-Y) stage three or less (Uygur et al, 2015). An exercise session consists of 30 minutes of pedaling at a preferred comfortable speed including 20 bouts of 15-second high speed pedaling, nested within the middle 20 min of exercise. We preferred to use a stationary recumbent bicycle to reduce the risk of falls compared to exercise modes such as treadmill or upright cycle. The resistance of the bike was set at the lowest possible level in order to keep musculoskeletal loading minimal.
The aim of this study was to evaluate the effects of a six-week high speed-low resistance recumbent bicycling on the physical and cognitive functional performance in people with PD. We hypothesized that the disease severity along with mobility and balance related functions would improve after six weeks of HS-LR training. In addition, since this type of training is expected to elicit neural adaptations through increasing neurotropic proteins and the neurotransmitters (Alberts et al, 2011), we also hypothesized that the improvements would be present in dexterity and cognition related function.
METHODS
Participants
Fourteen people with idiopathic Parkinson’s disease were recruited through local PD support groups to participate in the study (Table 1). The whole data collection was completed in 11 months and 16 days. The participants read and signed an institutionally approved informed consent document. A physician’s clearance for exercise was provided if participants answered yes to one or more questions in the physical activity readiness questionnaire (Adams, 1999), or if they were 75 years old or older. Exclusion criteria included: recent (< 3 months) myocardial infarction; muscle or joint conditions that could be worsened by the exercise or testing; insulin dependent diabetes; inability to communicate with investigators; and inability to walk without assistance from another person but may use a cane or walker. None of the subjects who participated in the study required any assistance during walking. Subjects were instructed not to change their medications during the study. We valued testing in the ‘on-meds’ condition because it represented the actual conditions under which PD patients would exercise. Participants completed all training sessions and assessments at the same time of the day to minimize the effects of timing of medication on the motor function.
Table 1.
Descriptive of study participants
| Descriptive (mean (SD)) | |
|---|---|
| Age (y) | 62.64 (8.81) |
| Gender (men/women) | 10/4 |
| Height (cm) | 176.9 (12.12) |
| Weight (kg) | 73.73 (14.28) |
| Disease duration (months) | 40.14 (28.91) |
| Medication (Levodopa equivalent daily dose in mg) | |
| Mean (SD) | 883.71 (645.92) |
| Range (min - max) | 200 – 2400 |
Pre- and Post- Training Assessments
Pre- and post-assessments were completed within three days before the start and after completion of six weeks HS-LR intervention, respectively. In each assessment session, participants were asked to practice the functional tests until they felt comfortable performing the task. The order of functional tests was randomized. Subjects self-reported their “more affected side” which was then tested in unilateral functional tests. Unless otherwise noted, each functional test was performed three times within both sessions and thirty-second rest time was allowed between the trials. In all of the functional tests, subjects were instructed to perform at their best or fastest possible level while being safe. Two experienced researchers timed the tests (manually), and the average value of the two researchers was recorded for each trial. Since the subjects were instructed to perform at their maximum ability, we used the trials with the best performance in further analyses. Pre- and post-assessments included a clinical assessment, questionnaires, and functional tests.
Clinical Assessment
An experienced neurologist conducted the 14-item motor examination of UPDRS (UPDRS-III), scores ranged from 0 to 56 (Brusse, Zimdars, Zalewski, and Steffen, 2005; Fahn et al, 1987; Li et al, 2012).
Questionnaires
Participants completed the Activities-specific Balance Confidence (ABC) which has been used as a subjective measure of balance confidence in performing various ambulatory activities (Mak and Pang, 2009) and the Short Form-36 Health Survey (SF-36) that assesses a person’s perceived status related to physical, social, mental and emotional health (Ware and Sherbourne, 1992).
Functional Tests
Participants performed the following tests: Ten meter walk test (10mW) measures the time and number of steps (Steps) taken by an individual to walk the middle 6 meters of the 10 meter walk (Lam, Lau, Chan, and Sykes, 2010). Timed-up-and-go test (TUG) measures the time taken by an individual to stand up from a chair, walk a distance of 3 meters, turn, walk back to the chair, and sit down (Podsiadlo and Richardson, 1991). Both TUG and 10mW tests are tests of functional mobility. Four square step test (4SST) is a dynamic balance test that requires participants to rapidly change direction while stepping forward, backward, and sideways directions over a low obstacle (Duncan and Earhart, 2013). Functional reach test (FRT) is a static balance test that measures the maximum distance one can reach in the forward direction while his/her base of support remains fixed on the ground (Duncan, Weiner, Chandler, and Studenski, 1990). The Nine-hole peg test (9HPT) is a test of dexterity and upper extremity function in which subjects were required to place and remove nine pegs in a peg board (Earhart et al, 2011). Cognitive function was assessed by using simple (SRT) and choice (CRT) reaction time tests (Kutukcu, Marks, Goodin, and Aminoff, 1999). In SRT, seated subjects placed the index finger of their more affected side on a sensor on the reaction time board (Lafayette Instrument Co, Lafayette, IN) with an indicator light directly above it. The subjects were asked to remove their index finger as fast as possible from the sensor as soon as the light was lit. The time from the onset of the light to the removal of the finger was recorded as the SRT. For CRT, subjects placed their index and middle fingers of both left and right hands on four sensors. Relative to the midline of the reaction time board, two sensors were located to the left and the other two were located to the right. Each sensor had a corresponding light. Subjects were asked to lift the finger corresponding with the light that was illuminated as fast as possible while keeping the other fingers on the sensors. The time between onset of the light and removal of the finger was recorded. Grip Strength (Grip) was a test of strength which was found to be highly correlated to knee extensor strength (Bohannon et al, 2012) and it was measured as subjects squeezed a handheld dynamometer (Model 78010) as hard as possible.
Exercise Intervention
Participants were trained on a stationary recumbent bike under the supervision of an experienced trainer two days a week for six weeks (12 sessions). The time of day was kept the same across the six weeks and training days were separated by at least two days. Each training session lasted 30 minutes. For the first and last five minutes of training, participants were instructed to pedal at a preferred rate to warm-up and cool-down, respectively. Preferred rate was instructed as the rate at which they could pedal for at least an hour without getting tired. After a five-minute warm-up, participants were asked to pedal as fast as possible for the first 15s and slow down to their preferred rate for the remaining 45s of every minute for 20 minutes. Heart rate was monitored throughout the training, and a longer recovery was given between the high-speed bouts if their heart rate reached 80% of their maximum heart rate (maximum HR=220-age). The resistance of the recumbent bike was set at the lowest possible level at which subjects typically produced less than 100 Watts of power at their fastest pedaling rate. The maximum rate during the 15s fast pedaling and the average rate during preferred pedaling were recorded manually and they were averaged in each session to represent fast and preferred rates. A subjective rating of perceived exertion (RPE) was collected by using BORG scale (Borg, 1982).
Statistical Analysis
Two-tailed paired sample t-tests were used to compare the measurements taken before and after six weeks of HSLR training. Test-retest reliability of each test was quantified by using ICC (2,1). Together with the standard deviation values obtained from pre testing results, ICC values were used to calculate the standard error of the measurement (SEM= sd x √(1-ICC)). We also calculated the minimum detectable change at the 95% confidence level (MDC95) (MDC95= SEM x 1.96 × √2) (Portney and Watkins, 2008). To analyze the improvements in pedaling rate, we divided 6-weeks training (or 12 training sessions) into three blocks of 2-weeks (or 4 training sessions). This decision was made a posteriori after visually observing the trend obtained from pedaling rate data. Comparisons among blocks were made by using two repeated measures of ANOVAs separately for preferred and fast pedaling rates. All statistical analyses were performed in SPSS (version 22, IBM, SPSS Statistics, Armonk, NY) and p-value was set at 0.05.
RESULTS
All subjects completed the study with no adverse effects. The average fast pedaling cadence changed across the three blocks (F (1.24, 12.36) = 12.57, p < 0.01), with a faster pedaling rate in the second and third blocks compared to first block (p < 0.05). Fast pedaling rate in the second and third blocks were not different (p > 0.05). There were no differences in the preferred pedaling rate across three blocks (F (2, 20) = 0.13, p > 0.05) (Figure 1).
Figure 1.
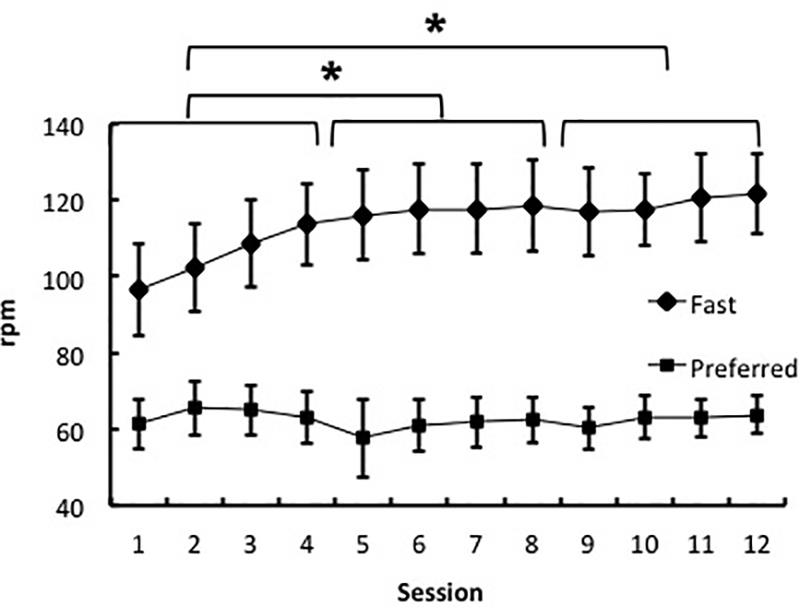
Progression of fast pedaling rate (rpm) from session 1 to session 12. * denotes significant improvements in fast rpm during first 4 sessions (p < 0.05).
Table 2 compares the assessments completed before and after six weeks of HS-LR training. Compared to pre-assessments, disease severity (i.e. UPDRS-III; p=0.001, UPDRS_Brady; p < 0.05), the tests of functional mobility (i.e. 10mW; p = 0.001, Steps; p < 0.001, and TUG; p < 0.001), and balance (i.e. FRT; p < 0.01, and 4SST; p < 0.01) improved after six weeks of HS-LR training. Similar to improvements in tests of balance, ABC scores also had a trend towards improvement after training (t = 1.95, p = 0.073). Regarding the cognitive function measures, SRT showed 13.1% improvement (p < 0.05) while there was a non-significant trend towards improvement (12.6%) in CRT (t = 2.06, p = 0.06).
Table 2.
Effects of high speed-low resistance (HS-LR) intervention on clinical and functional tests.
| Pre | Post | % change | Statistics | ||||
|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | t-value | p-value | ||
| UPDRS-III | 17.53 | 6.43 | 14.00 | 5.62 | −20.14 | 4.35 | < 0.001 |
| UPDRS_Brady | 7.19 | 2.52 | 6.08 | 1.77 | −15.01 | 2.19 | 0.049 |
| H&Y Scale | 2.54 | 0.61 | 2.38 | 0.63 | −6.24 | 0.77 | 0.455 |
| ABC | 79.95 | 16.10 | 88.59 | 10.80 | 10.81 | −1.95 | 0.073 |
| SF36 | 58.15 | 23.46 | 62.00 | 22.09 | 6.62 | −1.55 | 0.147 |
| 10mW (s) | 3.42 | 0.88 | 2.88 | 0.66 | −15.59 | 5.51 | < 0.001 |
| Steps (#) | 8.10 | 1.21 | 7.08 | 0.95 | −12.59 | 6.87 | < 0.001 |
| TUG (s) | 7.29 | 1.60 | 6.19 | 1.51 | −15.06 | 6.59 | < 0.001 |
| FRT (cm) | 27.12 | 4.95 | 33.28 | 6.10 | 22.71 | −3.98 | 0.002 |
| 4SST (s) | 9.11 | 4.51 | 7.56 | 3.08 | −17.04 | 3.07 | 0.009 |
| 9HPT (s) | 25.38 | 5.95 | 23.36 | 5.98 | −7.96 | 3.61 | 0.003 |
| SRT (s) | 0.285 | 0.077 | 0.247 | 0.055 | −13.15 | 2.62 | 0.021 |
| CRT (s) | 0.412 | 0.081 | 0.360 | 0.071 | −12.55 | 2.06 | 0.060 |
| Grip (kg) | 32.71 | 10.3 | 32.43 | 9.9 | −0.87 | 0.18 | 0.872 |
UPDRS-III: motor section of the Unified Parkinson’s Disease Rating Scale; UPDRS_Brady: UPDRS bradykinesia subsection (total score of the items #23 (finger taps), 24 (hand movements), 25 (rapid alternating movements of hands, 26 (leg agility), and 31 (body bradykinesia and hypokinesia); H&Y: Hoehn-Yarn; ABC: Activities-Specific Balance Confidence; SF36: Short Form-36 Health Survey; 10mW: 10-meter Walk Test; Steps: Steps taken in 10mW; TUG: Timed-UP-and-Go; FRT: Functional Reach Test; 4SST: Four Square Step Test; 9HPT: Nine Hole Peg Test; SRT: Simple Reaction Time; CRT: Choice Reaction Time; Grip: Grip Strength
Table 3 presents the SEM, ICC, and MDC95 for each selected measure. MDC% ranged between 12.92–44.65 for functional tests and 11.73–50.02 for clinical assessments while SEM% ranged between 4.66–16.11 for functional tests and 4.23–18.04 for clinical assessments.
Table 3.
Standard error of the measure (SEM), SEM as the percent of pre-intervention (SEM%), intra-class correlation coefficient (ICC(2,1)) with 95% confidence interval, minimal detectable change (MDC0.95) and minimum detectable change as percentage of pre-intervention condition (MDC%).
| Outcome Measure | SEM | SEM % | ICC(2,1) | MDC0.95 | MDC% |
|---|---|---|---|---|---|
| UPDRS-III* | 1.96 | 11.16 | 0.90 (0.69–0.97) | 5.42 | 30.94 |
| UPDRS_Brady* | 1.30 | 18.04 | 0.65 (0.11–0.88) | 3.60 | 50.02 |
| H&Y Scale | 0.11 | 4.23 | 0.97 (0.90–0.99) | 0.30 | 11.73 |
| SF36 | 6.32 | 10.86 | 0.92 (0.77–0.98) | 17.50 | 30.10 |
| ABC | 11.70 | 14.63 | 0.27 (−0.28–0.69) | 32.42 | 40.55 |
| 10mW (s)* | 0.26 | 7.50 | 0.89 (0.70–0.96) | 0.71 | 20.79 |
| Steps (#)* | 0.38 | 4.66 | 0.88 (0.65–0.96) | 1.05 | 12.92 |
| TUG (s)* | 0.44 | 6.04 | 0.92 (0.77–0.97) | 1.22 | 16.74 |
| FRT (cm)* | 4.02 | 14.81 | 0.48 (−0.75–0.81) | 11.13 | 41.05 |
| 4SST (s)* | 1.34 | 14.70 | 0.88 (0.67–0.96) | 3.71 | 40.75 |
| 9HPT (s)* | 1.49 | 5.85 | 0.94 (0.82–0.98) | 4.12 | 16.23 |
| SRT (s)* | 0.038 | 13.29 | 0.68 (0.26–0.89) | 0.10 | 36.83 |
| CRT (s) | 0.066 | 16.11 | 0.24 (−0.32–0.67) | 0.18 | 44.65 |
| Grip (kg) | 4.19 | 12.79 | 0.83 (0.55–0.94) | 11.60 | 35.46 |
denotes outcome measures with significant improvements after intervention.
DISCUSSION
This study was designed to evaluate the effects of six weeks of high speed-low resistance (HS-LR) recumbent cycling on the disease severity and functional and cognitive performance in people with mild to moderate PD (H-Y scale ≤ 3). We found that six weeks of HS-LR training was effective in improving physical and cognitive function in people with mild to moderate PD. Specifically, significant decreases were observed in disease severity as well as improvements in the tests that assess mobility, static and dynamic balance, dexterity, and cognitive function after completion of “speedwork” training.
The average RPE of 13.72 indicates that the participants found the high speed interval training to be “somewhat hard”. All subjects completed all 12 training sessions and there was no self-reported adverse effect of training. Moreover, subjects improved their fast pedaling cadence within the first two weeks, and maintained their improved fast cadence for the remaining four weeks of the training program (Figure 1). Altogether, these findings indicate that the HS-LR training was well-received by study participants. The brevity and feasibility of the proposed training method suggest the inclusion of this training program into the current standard exercise recommendations that includes cardiorespiratory fitness, flexibility, and strength (Carr and Shepherd, 1998).
In line with our interest in improving the speed of movement in people with PD through HS-LR training, in all of the selected functional tests, participants were instructed to perform as fast as possible and we reported the best performance. We assessed functional mobility through the standard clinical tests including10mW and TUG. Regarding 10mW test, results indicate that both walking speed and step length increased after HS-LR training. Together with improvements observed in bradykinesia subsection of UPDRS-III, results indicate the efficacy of HS-LR training in counteracting the PD related decrease in the speed and amplitude of a movement. Similar improvements were also seen in TUG. One should note that, unlike 10mW, TUG consists of three main components that include walking, turning, and standing up and sitting down on a chair (Wall, Bell, Campbell, and Davis, 2000). Although we did not measure the individual times to complete each component within TUG, increased walking speed (i.e. 10mW) and dynamic balance (i.e. 4SST) suggest that the improvement in TUG was mainly due to the improvements in walking and dynamic balance components rather than the strength component. Similar improvements in functional mobility were also reported in the previous research that studied the effects of high speed gait training on a treadmill in people with PD (Cakit et al, 2007; Herman, Giladi, Gruendlinger, and Hausdorff, 2007; Miyai et al, 2000; Miyai et al, 2002). Although the treadmill training modalities have shown promising results in PD related symptoms involving mobility, cautionary remarks have been made about how commonalities among interventions and testing procedures may limit the potential for mechanistic conclusions involving supraspinal centers (Ridgel, Vitek, and Alberts, 2009). While some tests used in our study were not anatomically independent from our HS-LR training, the overall improvements seen in both upper and lower extremity tests support central adaptations rather than a simple practice effect.
Both the performance of static (e.g. functional reach test) and dynamic balance (i.e. four square step test) improved after the completion of our HS-LR training. Moreover, this improvement was also partly supported by the non-significant (p=0.07) positive trend shown in the subjective balance confidence score (i.e. ABC), which is a tool that predicts future falls in PD (Mak and Pang, 2009). Overall improvements in balance indicate that HS-LR training could also be an effective strategy for fall prevention in PD. This improvement is quite promising when one considers the high incidence rate (Li et al, 2012) and detrimental consequences of falls in people with PD.
Results of our study indicated that people with PD improved their dexterity and simple reaction time after completing 6-weeks HS-LR training. Similar to our findings, Ridgel, Vitek and Alberts (2009) showed an improvement in manual dexterity in PD patients who are forced assisted by a trainer co-pedaling on a tandem bike to cycle at a rate that is 30% higher than their preferred rate for a period of eight weeks. Although the present reaction time measures do not point specifically to improved executive processing or information processing rates, they are consistent with the cognitive improvements observed after a single session of high speed passive cycling (Ridgel et al, 2011). Improvements seen in the dexterity and cognitive functions after completing a lower extremity training program could indicate the presence of the higher level neural adaptations following speed-based exercise.
The percent changes of the all of the selected outcome measures that improved significantly after the completion of HS-LR training were smaller than the MDC% estimates but larger than the SEM% estimates (the only exception was UPDRS_Brady: 15.01% improvement, SEM% = 18,04). Therefore, we question the use of MDC% in clinical and rehabilitation research regarding its conservative nature and suggest the use of SEM% as a more accurate estimate of the observed changes that were determined to be significant.
Although the exact mechanisms responsible for improved disease severity and physical and cognitive function after completing HS-LR training is unknown, we would like to speculate on several neural factors that could explain improvements in those functions. It has been demonstrated that PD patients have impaired peripheral afferent feedback (Abbruzzese and Berardelli, 2003; Klockgether et al, 1995) which affects corticomotor excitability (Coxon, Stinear, and Byblow, 2005). The repetitive nature of our HS-LR training may have resulted in either an increase in the activity of proprioceptors or in the efficiency of utilizing the feedback information from those proprioceptors. This increase in afferent information could in turn, improve the corticomotor excitability in PD (Christensen et al, 2000; Fisher et al, 2008). Recent findings also suggest increased activation within the cortical areas of PD patients after completing one session of “forced-exercise” during which voluntary cycling cadence of PD patients were augmented by an abled body on a tandem bicycle (Alberts et al, 2011; Alberts et al, 2016; Beall et al, 2013). This facilitation could be amplified even more with the interval nature of our training program which required switching pedaling pace between high and low rates (Armstrong, 1988). Finally, animal models of PD have shown that high intensity exercise facilitates neuroplasticity through increasing the synaptic availability of dopamine (Petzinger et al, 2007) and improving the dopamine receptor activity within the basal ganglia (Vuckovic et al, 2010). Those improvements would elicit a higher efficiency in neurotransmission and, therefore, improve motor function (Petzinger et al, 2010).
Study Limitations
There are few limitations to consider: 1) The results of this study could only be generalized to people with PD who are at Hoehn-Yahr stage of 3.0 or less; 2) We did not include a time control since previous research has shown stable motor function within similar intervention periods (Lauhoff, Murphy, Doherty, and Horgan, 2013; Rose, Lokkegaard, Sonne-Holm, and Jensen, 2013) and across a two-week period within our own laboratory (Uygur et al, 2015). Moreover, given the progressive nature of the disease, the PD related symptoms could only get worse without any intervention; 3) Although most of the improvements after six weeks of HS-LR training were substantial, we do not know how long these improvements would last since we did not have a follow-up assessment. However, considering the progressive nature of the PD and what is known about aging and inactivity, one could assume that people with PD would not continue to improve or maintain function without adherence to favorable activity and exercise behaviors; 4) One can claim that the improvements in the functional tasks could be attributable to mere exposure to functional tests. However, prior work from our laboratory has shown that the performance of the PD patients for the assessed functional tasks did not change over the course of three different days (Uygur et al, 2015); and 5) Finally, one can argue that the improvements seen after completing HS-LR training could be due to a placebo effect of training. Therefore, there is a need for a study that compares HS-LR training to a more traditional cycling training (e.g. moderate intensity, continuous cycling) to distinguish the effect of HS-LR training from the placebo effect of any type of training.
CONCLUSION
The results of this present study suggest that high speed-low resistance recumbent cycling is a useful training modality to improve disease severity, functional mobility, balance, cognitive and upper extremity function in people with mild to moderate PD. Future neurophysiological studies are needed to understand the neural adaptations associated with HS-LR training. There is also a need for future studies that could be designed to assess the minimum required dose of HS-LR. This could be important when one considers the time requirements of a comprehensive exercise program that includes strength, endurance, balance, and flexibility.
ACKNOWLEDGEMENT
The authors acknowledge Dr. S. Radosavljevic Jaric for clinical evaluation of PD patients. This project was supported by the Delaware INBRE program, with a grant from the National Institute of General Medical Sciences - NIGMS (8 P20 GM103446–13) from the National Institutes of Health and financial support from Shake It Off, Inc. (West Chester, PA)
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
REFERENCES
- Abbruzzese G, Berardelli A 2003. Sensorimotor integration in movement disorders. Movement Disorders 18: 231–240. [DOI] [PubMed] [Google Scholar]
- Adams R 1999. Revised physical activity readiness questionnaire. Canadian Family Physician 45: 1004–1005. [PMC free article] [PubMed] [Google Scholar]
- Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M 2011. It is not about the bike, it is about the pedaling: Forced exercise and Parkinson’s disease. Exercise and Sport Sciences Reviews 39: 177–186. [DOI] [PubMed] [Google Scholar]
- Alberts JL, Phillips M, Lowe MJ, Frankemolle A, Thota A, Beall EB, Feldman M, Ahmed A, Ridgel AL 2016. Cortical and motor responses to acute forced exercise in Parkinson’s disease. Parkinsonism and Related Disorders 24: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM 1988. The supraspinal control of mammalian locomotion. Journal of Physiology 405: 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EB, Lowe MJ, Alberts JL, Frankemolle AM, Thota AK, Shah C, Phillips MD 2013. The effect of forced-exercise therapy for Parkinson’s disease on motor cortex functional connectivity. Brain Connectivity 3:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallet M 2001. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124: 2131–2146. [DOI] [PubMed] [Google Scholar]
- Bergen JL, Toole T, Elliott RG, Wallace B, Robinson K, Maitland CG 2002. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson’s disease patients. Neurorehabilitation 17: 161–168. [PubMed] [Google Scholar]
- Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC 2012. Grip and knee extension muscle strength reflect a common construct among adults. Muscle and Nerve 46: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G 1982. Ratings of perceived exertion and heart-rates during short-term cycle exercise and their use in a new cycling strength test. International Journal of Sports Medicine 3: 153–158. [DOI] [PubMed] [Google Scholar]
- Brusse KJ, Zimdars S, Zalewski KR, Steffen TM 2005. Testing functional performance in people with Parkinson disease. Physical Therapy 85: 134–141. [PubMed] [Google Scholar]
- Cakit BD, Saracoglu M, Genc H, Erdem HR, Inan L 2007. The effects of incremental speed-dependent treadmill training on postural instability and fear of failing in Parkinson’s disease. Clinical Rehabilitation 21: 698–705. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB 1998. Neurological Rehabilitation: Optimizing Motor Performance. Oxford: Butterworth-Heinemann. [Google Scholar]
- Christensen LO, Johannsen P, Sinkjaer T, Petersen N, Pyndt HS, Nielsen JB 2000. Cerebral activation during bicycle movements in man. Experimental Brain Research 135: 66–72. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Rafferty MR, Kohrt WM, Comella CL 2013. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Movement Disorders 28: 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Stinear JW, Byblow WD 2005. Amplitude of muscle stretch modulates corticomotor gain during passive movement. Brain Research 1031:109–117. [DOI] [PubMed] [Google Scholar]
- Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC 2009. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson’s disease: A preliminary study. Parkinsonism and Related Disorders 15: 752–757. [DOI] [PubMed] [Google Scholar]
- Dorsey E, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM 2007. Projected number of people with Parkinson’s disease in the most populous nations, 2005–2030. Neurology 68: 384–386. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Weiner DK, Chandler J, Studenski S 1990. Functional reach - A new clinical measure of balance. Journals of Gerontology 45: M192–M197. [DOI] [PubMed] [Google Scholar]
- Duncan RP, Earhart GM 2013. Four Square step test performance in people with Parkinson disease. Journal of Neurologic Physical Therapy 37: 2–8. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Cavanaugh JT, Ellis T, Ford MP, Foreman KB, Dibble L 2011. The 9-hole peg test of upper extremity function: Average values, test-retest reliability, and factors contributing to performance in people with Parkinson disease. Journal of Neurologic Physical Therapy 35: 157–163. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, UPDRS-Development-Committee 1987. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds), Recent Developments in Parkinson’s Disease, Volume 2, p. 153–164. Florham Park, NJ: Macmillan Health Care [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW 2004. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. Journal of Neuroscience Research 77: 378–390. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, Cen S, Gordon J, Jakowec M, Petzinger G 2008. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Archives of Physical Medicine and Rehabilitation 89: 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T, Giladi N, Gruendlinger L, Hausdorff JM 2007. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: A pilot study. Archives of Physical Medicine and Rehabilitation 88:1154–1158. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J 1995. A defect of kinesthesia in Parkinsons-disease. Movement Disorders 10: 460–465. [DOI] [PubMed] [Google Scholar]
- Kutukcu Y, Marks WJ, Goodin DS, Aminoff MJ 1999. Simple and choice reaction time in Parkinson’s disease. Brain Research 815: 367–372. [DOI] [PubMed] [Google Scholar]
- Lam HS, Lau FW, Chan GK, Sykes K 2010. The validity and reliability of a 6-Metre Timed Walk for the functional assessment of patients with stroke. Physiotherapy Theory and Practice 26: 251–235. [DOI] [PubMed] [Google Scholar]
- Lauhoff P, Murphy N, Doherty C, Horgan NF 2013. A controlled clinical trial investigating the effects of cycle ergometry training on exercise tolerance, balance and quality of life in patients with Parkinson’s disease. Disability and Rehabilitation 35: 382–387. [DOI] [PubMed] [Google Scholar]
- Li FZ, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G, Batya SS 2012. Tai Chi and postural stability in patients with Parkinson’s disease. New England Journal of Medicine 366: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak MK, Pang MY 2009. Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: A 1-year prospective study. Journal of Neurology 256: 1689–1695. [DOI] [PubMed] [Google Scholar]
- Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, Saito T, Kang J 2000. Treadmill training with body weight support: Its effect on Parkinson’s disease. Archives of Physical Medicine and Rehabilitation 81: 849–852. [DOI] [PubMed] [Google Scholar]
- Miyai I, Fujimoto Y, Yamamoto H, Ueda Y, Saito T, Nozaki S, Kang J 2002. Long-term effect of body weight-supported treadmill training in Parkinson’s disease: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 83: 1370–1373. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW 2010. Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Movement Disorders 25: S141–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW 2007. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Journal of Neuroscience 27: 5291–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S 1991. The Timed up and Go - A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society 39: 142–148. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP 2008. Foundations of Clinical Research: Applications to Practice (3rd ed). Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- Ridgel AL, Kim CH, Fickes EJ, Muller MD, Alberts JL 2011. Changes in executive function after acute bouts of passive cycling in Parkinson’s disease. Journal of Aging and Physical Activity 19: 87–98. [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Peacock CA, Fickes EJ, Kim CH 2012. Active-assisted cycling improves tremor and bradykinesia in Parkinson’s disease. Archives of Physical Medicine and Rehabilitation 93: 2049–2054. [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Vitek JL, Alberts JL 2009. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabilitation and Neural Repair 23: 600–608. [DOI] [PubMed] [Google Scholar]
- Rose MH, Lokkegaard A, Sonne-Holm S, Jensen BR 2013. Improved clinical status, quality of life, and walking capacity in Parkinson’s disease after body weight-supported high-intensity locomotor training. Archives of Physical Medicine and Rehabilitation 94: 687–692. [DOI] [PubMed] [Google Scholar]
- Uygur M, Bellumori M, LeNoir K, Poole K, Pretzer-Aboff I, Knight CA 2015. Immediate effects of high-speed cycling intervals on bradykinesia in Parkinson’s disease. Physiotherapy Theory and Practice 31: 77–82. [DOI] [PubMed] [Google Scholar]
- Vuckovic MG, Li QZ, Fisher B, Nacca A, Leahy RM, Walsh JP, Mukherjee J, Williams C, Jakowec MW, Petzinger GM 2010. Exercise elevates dopamine d2 receptor in a mouse model of Parkinson’s disease: In vivo imaging with [F-18]Fallypride. Movement Disorders 25: 2777–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JC, Bell C, Campbell S, Davis J 2000. The timed get-up-and-go test revisited: Measurement of the component tasks. Journal of Rehabilitation Research and Development 37: 109–113. [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD 1992. The MOS 36-Item Short-Form Health Survey (SF-36) .1. Conceptual-Framework and Item Selection. Medical Care 30: 473–483. [PubMed] [Google Scholar]


