Abstract
TGF beta is a multifunctional cytokine that regulates alveolar epithelial cells as well as immune cells and fibroblasts. TGF beta inhibits surfactant protein A, B and C expression in fetal human lung and can inhibit type II cell proliferation induced by FGF7 (KGF). However, little is known about direct effects of TGF beta on adult human type II cells. We cultured alveolar type II cells under air/liquid interface conditions to maintain their state of differentiation with or without TGF beta. TGF beta markedly decreased expression of SP-A, SP-B, SP-C, fatty acid synthase, and the phospholipid transporter ABCA3. However, TGF beta increased protein levels of SPD with little change in mRNA levels, indicating that it is regulated independently from other components of surfactant. TGF beta is a negative regulator of both the protein and the phospholipid components of surfactant. TGF beta did not induce EMT changes in highly differentiated human type II cells. SP-D is an important host defense molecule and regulated independently from the other surfactant proteins. Taken together these data are the first report of the effect of TGF beta on highly differentiated adult human type II cells. The effects on the surfactant system are likely important in the development of fibrotic lung diseases.
Keywords: ABCA3, surfactant proteins, pulmonary fibrosis, lung
Introduction
TGF beta is a critical cytokine for the development of pulmonary fibrosis [1, 2]. TGF beta increases expression of smooth muscle actin and extracellular matrix proteins in lung fibroblasts, and these effects have been studied extensively. However, TGF beta also has effects on the alveolar epithelium. Most studies of the effect of TGF beta on alveolar type II cells have focused on the surfactant system in the developing lung. In the fetal lung, TGF beta decreases the expression of SP-A, SP-B and SP-C [3, 4]. Using antibodies to endogenous TGFB1, McDevitt and colleagues showed that TGF beta alters a variety of other genes in epithelial cells from fetal human lung, but the largest effects were the decreased expression of SP-A, SP-B, and SP-C [5]. In mice, TGF beta delivered by an adenoviral vector produces fibrosis, increases surface tension, and decreases expression of SP-B and SP-C [6]. Less is known about the effects of TGF beta on regulation of SP-D and the lipid components of pulmonary surfactant.
The inhibitory effect of TGF beta on the surfactant system may contribute to the pathophysiology of pulmonary fibrosis [7]. TGF beta is expressed in many cell types and there are several routes for release and activation [1, 2]. Alveolar type II cells in the IPF lung appear to be a rich source of this cytokine [8, 9]. There is also significant impairment of pulmonary surfactant in IPF [10]. Surfactant recovered from patients with IPF fails to produce low surface tension and has altered protein and phospholipid composition [10]. The increased surface tension is thought to cause atelectasis, hypoxia, and ultimately appositional atelectasis or collapse induration with loss of alveolar units [11, 12]. In addition, mutations in surfactant proteins and ABCA3 cause familiar forms of pulmonary fibrosis and interstitial disease [13–17]. Similarly, in bleomycin induced pulmonary fibrosis, the fibrotic lung has reduced expression of SP-A, SP-B and SP-C, and the isolated surfactant has altered phospholipid composition and impaired ability to lower surface tension [18, 19]. Importantly from a therapeutic perspective, surfactant replacement prevents collapse induration and loss of alveolar units in rats instilled with bleomycin [20]. In addition, TGF beta inhibits keratinocyte growth factor (KGF, FGF7) induced type II cell proliferation [21], and inhibition of type II cell proliferation would be expected to contribute to the fibrotic response based on the classic studies of Witschi and Adamson [22–25].
Another property of TGF beta suggested to contribute to pulmonary fibrosis is the induction of epithelial to mesenchymal cell transition (EMT) [26–29]. However, the role of EMT in the pathogenesis remains controversial [30–32]. TGF beta induces EMT of alveolar epithelial cells in vitro when they are cultured on tissue culture plastic, dedifferentiate, spread on the surface and cease to express the surfactant proteins [27]. However, there is little evidence that TGF beta will induce EMT in highly differentiated cuboidal type II cells [28].
Although there are multiple potential effects of TGF beta on alveolar type II cells that have important implications for human disease, direct effects of TGF beta on adult human alveolar type II cells have not been reported. In this report, we demonstrate that TGF beta inhibits the expression of surfactant proteins SP-A, SP-B, and SP-C, fatty acid synthase, and the phospholipid transporter ABCA3 but not SP-D. These results are important for our understanding of how TGF beta may impair surfactant function in pulmonary fibrosis and lead to collapse induration and loss of alveolar units.
Methods
Type II cell isolation and culture
Primary alveolar type II cells were isolated from human lungs from de-identified organ donors whose lungs were not suitable for transplantation. The Committee for the Protection of Human Subjects at National Jewish Health deemed this research as non-human subject research. The lung was perfused, lavaged, and digested with elastase as described previously [33]. The lung was minced, and the cells were partially purified by centrifugation on a discontinuous density gradient made of Optiprep (Accurate Chemical Scientific Corp., Westbury, NY) with densities of 1.085 and 1.040. The type II cells were then isolated by nonadherence to IgG coated petri dishes. The isolated cells were suspended in Advanced DMEM/F12 medium (Life Technologies, Grand Island, NY) or regular DMEM (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 μg/ml penicillin G (GIBCO BRL, Life Technologies Inc., Rockville, MD), and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO) or frozen down in 90% FBS and 10% DMSO to be used for culture at a later date.
For air liquid interface (ALI) cultures, the epithelial cells were plated on gels composed of 80% rat-tail collagen and 20% Matrigel (Corning Inc, Corning, NY) at a density of 1.5M cells/cm2. The gels were formed on Corning Costar six well 0.4 uM polycarbonate inserts. After 48 hours the nonaherent cells were removed, the gel was rimmed so that it could contract, and culture medium was changed to DMEM with 1% charcoal-stripped FBS supplemented with 10 ng/ml KGF, and 10 nM dexamethasone Corningwith a small amount of fluid on the apical surface. Twenty four hours later the apical fluid was removed and the cells were cultured under ALI conditions. TGF beta (5 ng/ml) was added on day 4 and 6 and the cultures were harvested on day 8.
Immunocytochemistry
The cultures were fixed with 4% paraformaldehyde (proSP-B, proSP-C, Muc1 beta catenin, and E-cadherin) or acid alcohol(SP-A, SP-D, vimentin, smooth muscle actin) [34] and paraffin embedded. The paraformaldehyde fixed specimens were processed by microwave antigen retrieval in10 mM citrate buffer, pH 6.O. The slices were incubated with the primary antibodies overnight. Primary antibodies were SP-A (PE-10 mouse monoclonal antibody, a gift from Professor Yoshio Kuroki, Sapporo, Japan), proSP-B (Seven Hills, Cincinnati, OH (WRAB-55522), proSP-C (Seven Hills, WRAB-9337), SP-D (1G-11 mouse monoclonal antibody, a gift from Professor Yoshio Kuroki, Sapporo, Japan), Muc1 (Millipore, Burlington, MA, 05–652 clone 214D4), E-cadherin (Abcam, Cambridge, MA, 40772), and beta catenin (BD Biosciences, San Jose, CA, #610153). The secondary antibodies were anti-Mouse IgG Alexa Fluor 594 (Molecular Probes, Eugene, OR, A21–203), and anti-Rabbit IgG Alexa Fluor 488 (Molecular Probes, A21206).
Real-Time RT-PCR (qPCR)
RNA isolation was done using Qiagen RNeasy Kits, according to the manufacturer’s instructions. For real-time RT-PCR, the expression levels of genes were expressed as a ratio to the expression of the constitutive probe GAPDH [33, 35]. The specific verified primers and probes were purchased from Applied Biosystems (Foster City, CA).
Western Blotting
For the western blotting analysis, polyacrylamide gradient gels (8–16%; Invitrogen Corporation) were run in tris glycine buffer to separate the proteins. Protein loading was normalized to beta actin. The primary antibodies were SP-A (PE-10 mouse monoclonal antibody, a gift from Professor Yoshio Kuroki, Sapporo, Japan); mature SP-B (Seven Hills, WRAB-48604), proSP-B (Seven Hills, WRAB-55522), mature SP-C (Seven Hills, WRAB-76694) proSP-C (Seven Hills, WRAB-9337) and SP-D (1G-11 mouse monoclonal antibody, a gift from Professor Yoshio Kuroki, Sapporo, Japan). All proteins were run under reduced conditions except proSP-B and mature SP-B, which were run under non-reduced conditions. The images were quantified using the ImageJ 64 analysis.
Statistical analysis
Paired and unpaired comparisons were performed using the Student’s t test. All statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, CA). P-values less than 0.05 were considered significant.
Results
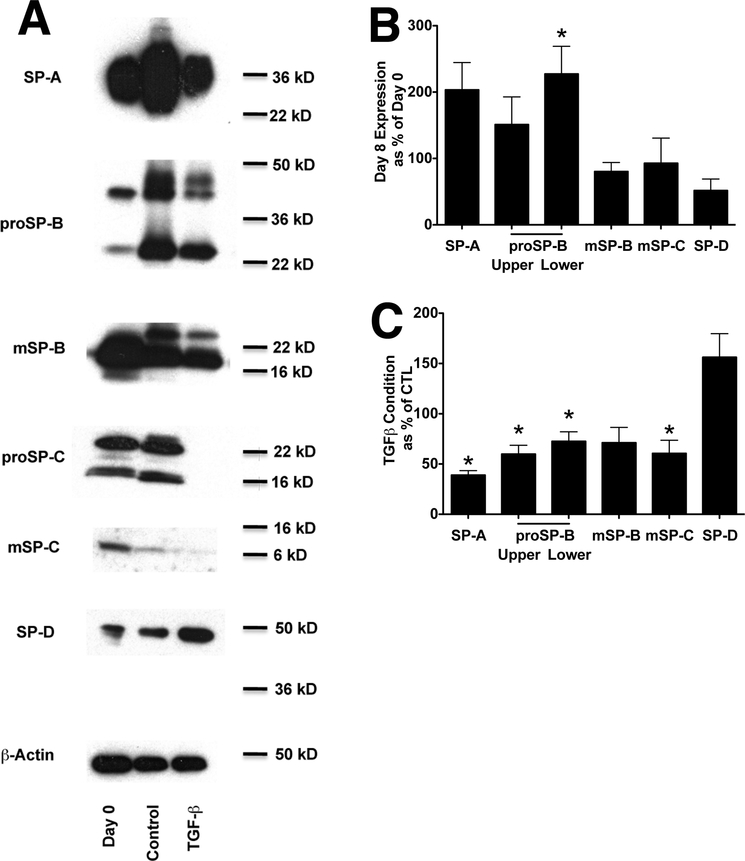
To evaluate the effects of TGF beta on components of the surfactant system, we cultured type II cells under ALI conditions in order to have a relatively high level of expression of the surfactant proteins [33]. The expression of the surfactant proteins was comparable to the levels in the freshly isolated type II cells (Figure 1A,B). Since the expression of all of the surfactant proteins drops at the beginning of culture and then recovers by day four, TGF beta was added on day four and reduced protein levels of SP-A, proSP-B, proSP-C, mature SP-C, but not SP-D (Figure 1C).
Figure 1. TGF beta inhibits the expression of SP-A, SP-B, and SP-C but increases the expression of SP-D.
Panel A. Protein expression under air/liquid conditions. Western analyses of surfactant protein levels in freshly isolated cells and cultured with or without TGF beta for the final 4 days. The columns for the Western are Day 0 (freshly isolated type II cells), control (cells cultured for eight days and under air/liquid conditions for the last 6 days), and TGF beta (cells cultured for eight days and in the presence of 5 ng/ml TGF-B for the last 4 days. Representative of four independent experiments
Panel B. Protein expression for comparing the level of expression on day 8 in culture to the freshly isolated type II cells. Westerns from four separate experiments were quantitated by ImageJ 64 software. * indicates p<0.05 compared to day 0 control.
Panel C. Data for protein expression in the presence of TGF beta is compared to the control value on day 8. Results are from four separate experiments. * indicates p<0.05
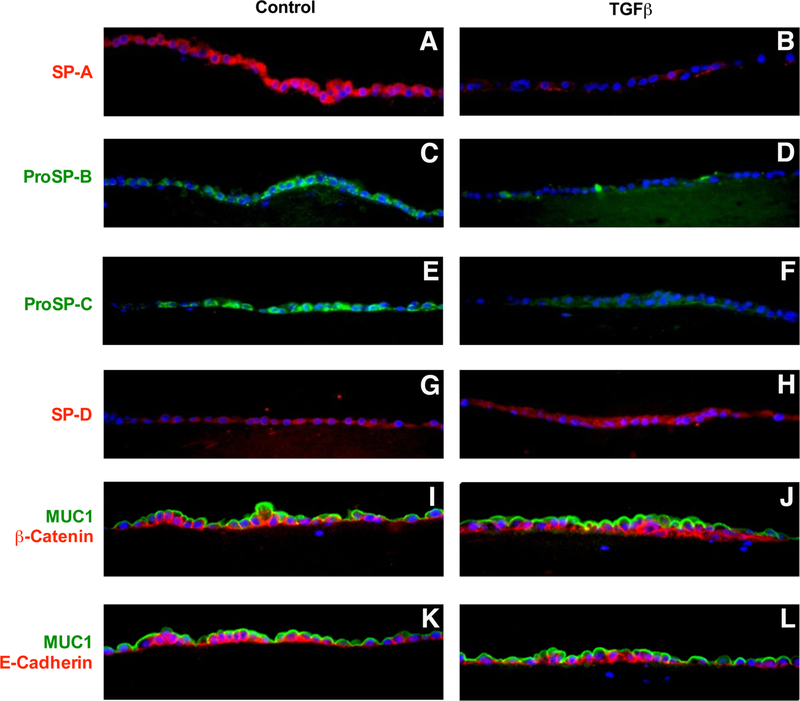
To examine the state of cells after treatment with TGF beta, we used immunocytochemistry to confirm and expand these results. TGF beta markedly reduced expression of SP-A, proSP-B, and proSP-C and slightly increased SP-D (Figure 2). The cells were well polarized with Muc1 on the apical surface and beta catenin and E-cadherin on the basolateral surfaces. There was no evidence of EMT in response to TGF beta under these conditions, as determined by no loss of cell polarity, no shift of beta-catenin to the nucleus, and no loss of E-cadherin. In addition, no staining for vimentin or smooth muscle actin was observed in the epithelial cells under these conditions (data not shown).
Figure 2. Immunocytochemistry of the effect of TGF beta on SP-A, proSP-B, proSP-C, and SP-D.
Type II cells were cultured under ALI conditions with and without TGF beta for last four days as in Figure 1. The exposures were set for the ALI control conditions, and the TGF beta condition was photographed at the same exposure and there was no computer enhancement of the images. Panel A SP-A, Panel B SP-A with TGF beta, Panel C proSP-B, Panel D proSP-B with TGF beta, Panel E proSP-C, Panel F proSP-C with TGF beta, Panel G SP-D, Panel H SP-D with TGF beta, Panel I Muc 1 (green) and beta catenin (red), Panel J Muc 1 (green) and beta catenin (red) with TGF beta, Panel K Muc 1 (green) and E-cadherin (red), Panel L Muc 1 (green) and E-cadherin (red) with TGF beta. These results are representative of four independent experiments
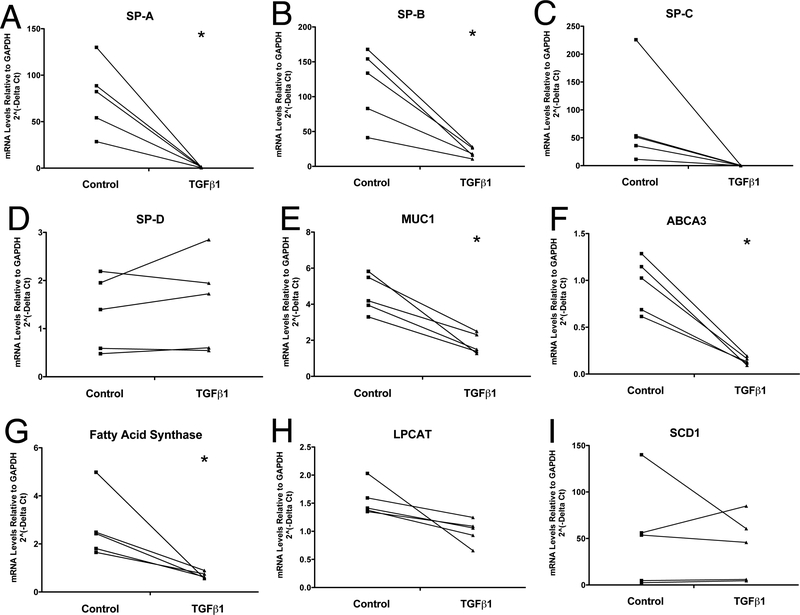
TGF beta markedly reduced the mRNA levels of SP-A and SP-B. The inhibition of SPC did not achieve statistical significance because of the one high control value. Similarly, there was a significant reduction mRNA levels of fatty acid synthase and the phospholipid transporter ABCA3, important for synthesizing and transporting the phospholipid components of surfactant into the lamellar body (Figure 3). There was a slight reduction in expression of Muc 1. TGF beta did not alter the mRNA levels of SP-D.
Figure 3. Effect of TGF beta on mRNA levels for the surfactant proteins and selected lipogenic proteins.
Type II cells were cultured under ALI conditions with and without TGF beta for last four days as in Figure 1 and Figure 2. mRNA levels were determined as stated in the methods section. Panel A, SP-A; Panel B, SP-B; Panel C, SP-C; Panel D, SP-D; Panel E, Muc1; Panel F, ABCA3; Panel G, fatty acid synthase; Panel H, LPCAT1; Panel I, SCD1 These are the results of five independent experments. * indicates p<0.05 compared to control.
Discussion
TGF-beta treatment significantly reduces alveolar type II cell expression of SP-A, SBB, SP-C, ABCA3, and fatty acid synthase without changing the expression of SP-D. The implication of these findings is that in pulmonary fibrosis where TGF-beta is expressed and functional, one would expect TGF-beta to impair the production of pulmonary surfactant. An increase in surface tension in the alveoli and small airways would increase the work of breathing and promote atelectasis and hypoxemia. The long-term effect would be the development of collapse induration or appositional atelectasis, which are thought to be the cause for the loss of alveolar units in pulmonary fibrosis [11, 20]. Collapse induration is the process of alveolar atelectasis, epithelial damage, apposition of alveolar surfaces, fusion of alveolar walls and basement membranes, and loss of alveolar units. These adverse effects of TGF-beta on the surfactant system provide additional rationale for evaluating anti-TGF-beta strategies for the treatment of pulmonary fibrosis [2]. Another implication of these findings is that increasing endogenous surfactant production, which has been a long-term goal in the treatment of ARDS and respiratory distress of the newborn, would also be beneficial in the treatment of IPF.
SP-A is the one surfactant protein that is readily maintained in human type II cell cultures. This protein is important for both host defense and formation of tubular myelin but not critical for lowering surface tension in the alveolus or small airways. SP-B is critical for surfactant function and is the only surfactant protein that shows a gene dose effect. Heterozygote deficient mice have a decreased lung compliance and airway collapse at low inflation pressures [36]. SP-C is the protein that is most difficult to maintain in adult human type II cells in vitro. It is specific for type II cells but probably not critical for lowering surface tension in the alveoli and not necessary for formation of tubular myelin. SP-C deficient animals depending on the strain live normally [37]. The reduction ABCA3 and fatty acid synthase would be expected to severely reduce the assembly of the phospholipids of surfactant. LPCAT1 (lysophosphatidyl acyl transferase) is important for the production of surfactant [38]. SCD1(stearoyl CoA desaturase) is a lipogenic enzyme highly expressed in differentiated type II cells [39]. Based on the evidence above, inhibition of SP-B, ABCA3, and fatty acid synthase expression would severely impair surfactant production.
The effects of TGF beta on adult human type II cells are consistent with observations made by others with other experimental approaches. Beers et al. demonstrated that TGF beta decreased SP-A, B, and C as well as fatty acid synthase and ABCA3 in human fetal lung [4]. Qui et al. showed that TGF beta inhibited SP-A, B and C, but not SP-D in fetal pigs [40]. The mechanism of how TGF beta inhibits SP-A, SP-B, and SP-C expression has been studied in detail. TGF beta inhibits expression of NKX2.1 (TTF1) regulated genes [3, 41]. Smad3 binds NKX2.1, sequesters it in the cytoplasm, and prevents it from activating NKX2.1 regulated genes [6, 42].
The observation that SP-D is not regulated by TGF-beta is consistent with previous observations. In the studies of fetal lung, the focus has been on SP-A, SB-B and SP-C, which are regulated predominately by NKX2.1. Alterations of NKX2.1 expression in the fetal human lung alters SP-B and SB-C but apparently not SP-D [5, 41]. Although SP-D can be regulated by NKX2.1, the major regulators of SP-D are C/EBP alpha, C/EBP delta, and nuclear factor of activated T cells (NFAT) [43]. In adult rat type II cells TGF beta decreases SP-A secretion without changing SP-D secretion [21]. Our results support the concept that SP-D is not an integral surfactant protein. SP-D binds the lipids of surfactant proteins poorly and is not found in lamellar bodies or tubular myelin [44]. SP-D is physiologically a host defense protein that binds and agglutinates a variety of viruses, bacteria, and fungi through its calcium dependent carbohydrate recognition domain. SP-D is particularly important as a host defense molecule against influenza [45–47]
We found no indication that TGF-beta induces EMT changes in the highly differentiated type II cells. TGF-beta can induce EMT changes in type II cells that are spreading on tissue culture plastic, dedifferentiating, and have a very low level of surfactant protein expression. Type II cells normally spread to repair an injured alveolar surface and develop to type I cells. In this process, these cells would be expected to express proteins that regulate cell motility. In addition, there is little evidence that type II cells can be transformed into myofibroblasts [30].
In summary TGF beta inhibits mRNA levels of SP-A, SP-B, SP-C, fatty acid synthase and ABCA3 in adult human alveolar type II cells. The implication of these in vitro findings is that TGF beta mediated decreases in pulmonary surfactant would contribute to collapse induration. If this occurred in vivo, one would hypothesize that exogenous surfactant would decrease fibrosis and increase lung volumes, and this is what has been observed in rats instilled with bleomycin [20]. Focusing on means of inhibiting collapse induration is a promising strategy for the treatment of progressive pulmonary fibrosis.
Supplementary Material
BBRC Highlights.
TGF beta inhibits expression of SP-A, SP-B, SP-C, Fatty acid synthase, and ABCA3.
TGF beta does not inhibit the expression of SP-D
TGF beta does not induce EMT changes in highly differentiated type II cells
Acknowledgements:
We thank the families of the de-identified organ donors and IIAM, NDRI, and Donors Alliance, who made this research possible. Sarah Murrell helped prepare this manuscript for publication. This work was supported by a grant from Gilead Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Leask A, Abraham DJ, TGF-beta signaling and the fibrotic response, FA SEB J, 18 (2004) 816–827. [DOI] [PubMed] [Google Scholar]
- [2].Fernandez IE, Eickelberg O, The impact of TGF-beta on lung fibrosis: from targeting to biomarkers, Proc Am Thorac Soc, 9 (2012) 111–116. [DOI] [PubMed] [Google Scholar]
- [3].Kumar AS, Gonzales LW, Ballard PL, Transforming growth factor-beta(1) regulation of surfactant protein B gene expression is mediated by protein kinase-dependent intracellular translocation of thyroid transcription factor-1 and hepatocyte nuclear factor 3, Biochim Biophys Acta, 1492 (2000) 45–55. [DOI] [PubMed] [Google Scholar]
- [4].Beers MF, Solarin KO, Guttentag SH, Rosenbloom J, Kormilli A, Gonzales LW, Ballard PL, TGF-beta1 inhibits surfactant component expression and epithelial cell maturation in cultured human fetal lung, Am J Physiol, 275 (1998) L950–960. [DOI] [PubMed] [Google Scholar]
- [5].McDevitt TM, Gonzales LW, Savani RC, Ballard PL, Role of endogenous TGFbeta in glucocorticoid-induced lung type II cell differentiation, Am J Physiol Lung Cell Mol Physiol, 292 (2007) L249–257. [DOI] [PubMed] [Google Scholar]
- [6].Lopez-Rodriguez E, Boden C, Echaide M, Perez-Gil J, Kolb M, Gauldie J, Maus UA, Ochs M, Knudsen L, Surfactant dysfunction during overexpression of TGFbeta1 precedes profibrotic lung remodeling in vivo, Am J Physiol Lung Cell Mol Physiol, 310 (2016) L1260–1271. [DOI] [PubMed] [Google Scholar]
- [7].Gunther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P, Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis, Eur Respir Rev, 21 (2012) 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH, Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis, Am J Respir Cell Mol Biol, 5 (1991) 155–162. [DOI] [PubMed] [Google Scholar]
- [9].Lomas NJ, Watts KL, Akram KM, Forsyth NR, Spiteri MA, Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers, Int J Clin Exp Pathol, 5 (2012) 58–71. [PMC free article] [PubMed] [Google Scholar]
- [10].Gunther A, Schmidt R, Nix F, Yabut-Perez M, Guth C, Rosseau S, Siebert C, Grimminger F, Morr H, Velcovsky HG, Seeger W, Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis, Eur Respir J, 14 (1999) 565–573. [DOI] [PubMed] [Google Scholar]
- [11].Lutz D, Gazdhar A, Lopez-Rodriguez E, Ruppert C, Mahavadi P, Gunther A, Klepetko W, Bates JH, Smith B, Geiser T, Ochs M, Knudsen L, Alveolar derecruitment and collapse induration as crucial mechanisms in lung injury and fibrosis, Am J Respir Cell Mol Biol, 52 (2015) 232–243. [DOI] [PubMed] [Google Scholar]
- [12].Leslie KO, Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis, Arch Pathol Lab Med, 136 (2012) 591–600. [DOI] [PubMed] [Google Scholar]
- [13].Whitsett JA, Wert SE, Weaver TE, Diseases of pulmonary surfactant homeostasis, Annu Rev Pathol, 10 (2015) 371–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC, Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort, Am J Respir Crit Care Med, 182 (2010) 1419–1425. [DOI] [PubMed] [Google Scholar]
- [15].Campo I, Zorzetto M, Mariani F, Kadija Z, Morbini P, Dore R, Kaltenborn E, Frixel S, Zarbock R, Liebisch G, Hegermann J, Wrede C, Griese M, Luisetti M, A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant, Respir Res, 15 (2014) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, Roldan J, Scott TA, Blackwell TS, Phillips JA 3rd, Loyd JE, du Bois RM, Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF, Thorax, 59 (2004) 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ono S, Tanaka T, Ishida M, Kinoshita A, Fukuoka J, Takaki M, Sakamoto N, Ishimatsu Y, Kohno S, Hayashi T, Senba M, Yasunami M, Kubo Y, Yoshida LM, Kubo H, Ariyoshi K, Yoshiura K, Morimoto K, Surfactant protein C G100S mutation causes familial pulmonary fibrosis in Japanese kindred, Eur Respir J, 38 (2011) 861–869. [DOI] [PubMed] [Google Scholar]
- [18].Horiuchi T, Mason RJ, Kuroki Y, Cherniack RM, Surface and tissue forces, surfactant protein A, and the phospholipid components of pulmonary surfactant in bleomycin-induced pulmonary fibrosis in the rat, Am Rev Respir Dis, 141 (1990) 1006–1013. [DOI] [PubMed] [Google Scholar]
- [19].Horiuchi T, Ikegami M, Cherniack RM, Mason RJ, Increased surface tension of the lung and surfactant in bleomycin-induced pulmonary fibrosis in rats, Am J Respir Crit Care Med, 154 (1996) 1002–1005. [DOI] [PubMed] [Google Scholar]
- [20].Steffen L, Ruppert C, Hoymann HG, Funke M, Ebener S, Kloth C, Muhlfeld C, Ochs M, Knudsen L, Lopez-Rodriguez E, Surfactant replacement therapy reduces acute lung injury and collapse induration-related lung remodeling in the bleomycin model, Am J Physiol Lung Cell Mol Physiol, 313 (2017) L313–L327. [DOI] [PubMed] [Google Scholar]
- [21].Zhang F, Nielsen LD, Lucas JJ, Mason RJ, Transforming growth factor-beta antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor, Am J Respir Cell Mol Biol, 31 (2004) 679–686. [DOI] [PubMed] [Google Scholar]
- [22].Witschi H, Responses of the lung to toxic injury, Environ Health Perspect, 85 (1990) 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Adamson IY, Young L, Bowden DH, Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis, Am J Pathol, 130 (1988) 377–383. [PMC free article] [PubMed] [Google Scholar]
- [24].Adamson IY, Hedgecock C, Bowden DH, Epithelial cell-fibroblast interactions in lung injury and repair, Am J Pathol, 137 (1990) 385–392. [PMC free article] [PubMed] [Google Scholar]
- [25].Uhal BD, Nguyen H, The Witschi Hypothesis revisited after 35 years: genetic proof from SP-C BRICHOS domain mutations, Am J Physiol Lung Cell Mol Physiol, 305 (2013) L906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wolters PJ, Collard HR, Jones KD, Pathogenesis of idiopathic pulmonary fibrosis, Annu Rev Pathol, 9 (2014) 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z, Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis, Am J Pathol, 166 (2005) 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA, Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix, Proc Natl Acad Sci U S A, 103 (2006) 13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin L, Han Q, Xiong Y, Li T, Liu Z, Xu H, Wu Y, Wang N, Liu X, Krupple-likefactor 4 Attenuates Lung Fibrosis via Inhibiting Epithelial-mesenchymal Transition, Sci Rep, 7 (2017) 15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL, Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition, Proc Natl Acad Sci U S A, 108 (2011) E1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chapman HA, Epithelial-mesenchymal interactions in pulmonary fibrosis, Annu Rev Physiol, 73 (2011) 413–435. [DOI] [PubMed] [Google Scholar]
- [32].Yamada M, Kuwano K, Maeyama T, Hamada N, Yoshimi M, Nakanishi Y, Kasper M, Dual-immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis, Histochem Cell Biol, 129 (2008) 453462. [DOI] [PubMed] [Google Scholar]
- [33].Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ, Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro, Am J Respir Cell Mol Biol, 36 (2007) 661668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuhn C, Mason RJ, Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis, Am J Pathol, 147 (1995) 1759–1769. [PMC free article] [PubMed] [Google Scholar]
- [35].Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ, Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus, Respir Res, 13 (2012) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Clark JC, Weaver TE, Iwamoto HS, Ikegami M, Jobe AH, Hull WM, Whitsett JA, Decreased lung compliance and air trapping in heterozygous SP-B-deficient mice, Am J Respir Cell Mol Biol, 16 (1997) 46–52. [DOI] [PubMed] [Google Scholar]
- [37].Weaver TE, Conkright JJ, Function of surfactant proteins B and C, Annu Rev Physiol, 63 (2001) 555–578. [DOI] [PubMed] [Google Scholar]
- [38].Bridges JP, Ikegami M, Brilli LL, Chen X, Mason RJ, Shannon JM, LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice, J Clin Invest, 120 (2010) 1736–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mason RJ, Pan T, Edeen KE, Nielsen LD, Zhang F, Longphre M, Eckart MR, Neben S, Keratinocyte growth factor and the transcription factors C/EBP alpha, C/EBP delta, and SREBP-1c regulate fatty acid synthesis in alveolar type II cells, J Clin Invest, 112 (2003) 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Qiu L, Deng C, Fu Z, Guo C, The role of transforming growth factors beta1 and beta3 in pre- and post-natal pulmonary surfactant development, Cell Biol Int, 35 (2011) 287–292. [DOI] [PubMed] [Google Scholar]
- [41].Kolla V, Gonzales LW, Gonzales J, Wang P, Angampalli S, Feinstein SI, Ballard PL, Thyroid transcription factor in differentiating type II cells: regulation, isoforms, and target genes, Am J Respir Cell Mol Biol, 36 (2007) 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Minoo P, Hu L, Zhu N, Borok Z, Bellusci S, Groffen J, Kardassis D, Li C, SMAD3 prevents binding of NKX2.1 and FOXA1 to the SpB promoter through its MH1 and MH2 domains, Nucleic Acids Res, 36 (2008) 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dave V, Childs T, Whitsett JA, Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells, J Biol Chem, 279 (2004) 34578–34588. [DOI] [PubMed] [Google Scholar]
- [44].Mason RJ, Greene K, Voelker DR, Surfactant protein A and surfactant protein D in health and disease, Am J Physiol, 275 (1998) L1–13. [DOI] [PubMed] [Google Scholar]
- [45].Reading PC, Morey LS, Crouch EC, Anders EM, Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice, J Virol, 71 (1997) 8204–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hillaire ML, Haagsman HP, Osterhaus AD, Rimmelzwaan GF, van Eijk M, Pulmonary surfactant protein D in first-line innate defence against influenza A virus infections, J Innate Immun, 5 (2013) 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qi L, Kash JC, Dugan VG, Jagger BW, Lau YF, Sheng ZM, Crouch EC, Hartshorn KL, Taubenberger JK, The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding, Virology, 412 (2011) 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.