INTRODUCTION
In the United States, children living with congenital heart disease (CHD) are outnumbered by adults with CHD (ACHD). Due to surgical and medical advances, it is now estimated that there are more than 1 million adults with CHD in the United States.1,2 Advances in surgical technique, trans-catheter intervention, imaging modalities, and focus on high-quality multidisciplinary care teams has contributed to improved CHD survival. Recent studies have shown that the median age of patients with severe CHF has increased from 11 years in 1985 to 17 years in 2000, and the overall age at death increased from 37 years in 2002 to 57 years in 2007.3 Improved survival to adult age and late adulthood translates to a population with both cardiac and extracardiac disease, and specialized care needs. In particular, heart failure (HF) is common in the adult patient with CHD. Adults with CHD experience more hospitalizations, episodes of decompensation, and ultimately have higher mortality than non-CHD cohorts.4–6 Therefore, it is critical to understand the heterogenetic nature of HF in ACHD population, assessment and management across the spectrum of CHD, and treatment options, as well as current gaps in treatments available to this unique group.
THE DISTINCT NATURE OF HEART FAILURE IN ADULT CONGENITAL HEART DISEASE
As CHD patients live to older age, the population becomes even more diverse because it includes patients who survived several percutaneous and surgical procedures in childhood, and adults who presented and were diagnosed later in life. Residual anatomic and hemodynamic lesions accompanied by acquired heart disease lead to an increasingly complex group of patients with varied presentation. HF, along with arrhythmia, sudden death, and late vascular complications are the most common late cardiac presentations in adults.7
Bolger and colleagues8 described CHD as the original HF syndrome as “…characterized by a triad comprising cardiac abnormality, exercise limitation, and neurohormonal activation.” The Heart Failure Society of America guidelines’ define HF as “…a syndrome characterized by either or both pulmonary and systemic venous congestion or inadequate peripheral oxygen delivery, at rest of during stress caused by cardiac dysfunction.”9,10 In the CHD population, it is often difficult to stratify patients into common categories such as left-sided failure or right-sided failure. Standard functional class categorization is also difficult because it based on the premise that patients do not have structural abnormalities at baseline. For instance, the 2005 American College of Cardiology and American Heart Association guidelines recommend HF be divided into 4 subtypes: A, at risk for HF; B, structural heart disease without signs or symptoms; C, structural heart disease with previous or current symptoms; and D, refractory heart disease requiring advanced therapies.11 One may wonder how CHD patients fit into this classification, which, despite updating in 2013, still did not account for the CHD patient with an underlying congenital cardiac defect. Guideline-level documents such as this are often not particularly helpful in the management of CHD patients because they amass data in the acquired HF population, which is often significantly different if not less well-studied than CHD patients.9,12,13
From an epidemiologic perspective, CHD patients do not fare as well as patients with HF from acquired forms of heart disease. Hospitalization rates in CHD patients with HF are higher (214 admissions/1000 adults) and the mean length of stay is longer (11.5 days in complex CHD vs 8 days in the acquired HF cohort).14 The underlying anatomic defect and prior surgical interventions have been identified as independent risk factors for HF admission in CHD. When admitted for HF, CHD patients have a 5-fold higher risk of in-hospital mortality; death at 1 and 3 years post-HF admission was exceptionally high at 24% and 35%, respectively.15
Predictors of death due to HF include endocarditis, supraventricular tachycardia, ventricular tachyarrhythmia, conduction disturbances, pulmonary arterial hypertension, and myocardial infarction (hazard ratio 2–5; P<.05).7 In 2 different European cohorts, HF has been shown to be the most common cause of mortality with the average age of death reported between 47 to 50 years of age.7,16 As the CHD population continues to age, both outpatient and inpatient care for HF will continue to become among the most important aspects of managing these patients.17–19
ANATOMY DICTATES HEART FAILURE PHENOTYPE
HF in CHD is a broad topic that is often difficult to understand. It is easy to see why this may be the case, given the broad spectrum of CHD. Clinically, CHD is subdivided into categories based on the complexity of the structural lesions. Defects are classified as simple CHD, moderately complex CHD, or severely complex CHD. Published guidelines in the treatment of ACHD have indicated follow-up intervals for continued care based on the severity of underlying CHD20 (Table 1). Prior interventions, including cardiac surgery, also play a role in the development of HF and late CVD risk; therefore, they are crucial to consider in caring for the ACHD patient. Given the heterogenous nature of CHD and palliative or surgical repair, the cause and presentation of HF in CHD is diverse. Some common themes in describing HF in this population include the side of the HF (subpulmonic ventricular vs subsystemic ventricular dysfunction),21 cyanotic versus acyanotic HF, single ventricular failure, and pressure versus volume-mediated HF, among others.
Table 1:
Complexity of adult congenital heart disease
| Simple CHD | Moderate CHD | Severely Complex CHD |
|---|---|---|
|
|
|
|
|
|
Abbreviations: AI, aortic insufficiency; AS, aortic stenosis; ASD, atrial septal defect; AVSD, atrioventricular septal defect; CoA, coarctation of the aorta; MV, mitral valve; NOS, not otherwise specified; PDA, patent ductus arteriosus; PS, pulmonic stenosis; RVOTO, right ventricular outflow tract obstruction; TV, tricuspid valve; VSD, ventricular septal defect.
Modified from Connelly MS, Webb GD, Somerville J, et al. Canadian consensus conference on adult congenital heart disease, 1996. Can J Cardiol 1998;14:395–452; with permission.
ETIOLOGIC FACTORS OF CONGENITAL HEART DISEASE–HEART FAILURE
The mechanisms leading to HF in CHD are numerous and variable. Some potential causes include abnormal pressure or volume-loading of either the morphologic right ventricle (RV) or left ventricle, myocardial ischemia from either a supply demand mismatch or coronaries anomalies, ventricular hypertrophy, and constriction from prior sternotomy. Myocardial architecture must be considered in patients with CHD. Data suggest embryologic development of the right ventricular myocardium may be different from the left ventricle and more susceptible to dysfunction in lesions where the RV is the systemic ventricle.9,22 Perfusion also seems to also be important as evidenced by the high prevalence of RV systolic function following the atrial switch operation in complete transposition.23,24 Reduced right ventricular myocardial microvascular density of the septal wall is seen in complete transposition and in RV hypertrophy in tetralogy of Fallot patients. This seems to be related to a reduced myocardial perfusion reserve and impaired right ventricular systolic function.25 It is likely that myocardial perfusion defects are a sensitive predictor of systemic ventricular impairment.26 Similar to acquired heart disease, activation of natriuretic peptides, the sympathoadrenergic system, and the endothelin and renin angiotensin aldosterone system have been implicated to play an important role in CHD-HF.27 Fibrosis and aberrant remodeling are also important deleterious processes important to HF in CHD.28–30
CLINICAL PRESENTATION AND EVALUATION
Presentation of HF in CHD can vary depending on anatomic and physiologic factors related to underlying anatomy and prior repair. Systolic function may not necessarily be the primary cause of HF in these patients. Typical HF symptoms, such as exertional dyspnea, fatigue, exercise intolerance, and signs of volume overload, although present may not always be dramatic. A comprehensive history and examination is critical in evaluation of this population. Special testing often involves imaging, such as transthoracic echocardiogram or cardiac MRI–computed tomography, to provide information about anatomic detail and function. Cardiopulmonary stress testing is an invaluable tool to assess functional capacity and other vital information, such as chronotropic competence and oxygenation level with exercise. Patients with CHD exhibit lower than normal oxygen consumption per unit time (VO2) in comparison with age-matched healthy cohorts31,32 and, given that New York Heart Association status is not accurate in CHD, trends in peak Vo2 and functional capacity are valuable. A rhythm monitor is also very important in CHD because arrhythmia and requirement for pacemaker are more common than in other forms of acquired heart disease.
MANAGEMENT OF HEART FAILURE IN CONGENITAL HEART DISEASE
In acquired HF, medical treatment is the cornerstone of management; however, the same therapy is not well-studied in CHD patients. Treatment typically focuses on correcting structural abnormalities and potentially reversing abnormalities. The management of arrhythmia is also critically important in the ACHD patient because it occurs in 15% of CHD patients and more than doubles the risk of HF.33 Common late anatomic, physiologic, or arrhythmogenic problems, as well as available interventions in select moderate and severely complex ACHD patients, are reviewed in Table 2.
Table 2:
Common procedural and surgical interventions in moderate-severe adult congenital heart disease-heart failure patients
| Late (Adult) Anatomic or Physiologic Problem | Intervention | |
|---|---|---|
| Tetralogy of Fallot |
|
|
| DTGA with atrial switch |
|
|
| CCTGA |
|
|
| Ebstein anomaly |
|
|
| Fontan palliation |
|
|
Abbreviations: CCTGA, congenitally corrected transposition of the great arteries; DTGA, dextro-transposition of the great arteries; EPS, electrophysiology study; FALD, Fontan-associated liver disease; IART, intra-atrial reentrant tachycardia; ICD, implantable cardiac defibrillator; PI, pulmonic insufficiency; PLE, protein-losing enteropathy; RFA, radiofrequency ablation; SCD, sudden cardiac death; Side arrows, leads to; upward arrow, increased; VT, ventricular tachycardia.
Medical Therapy
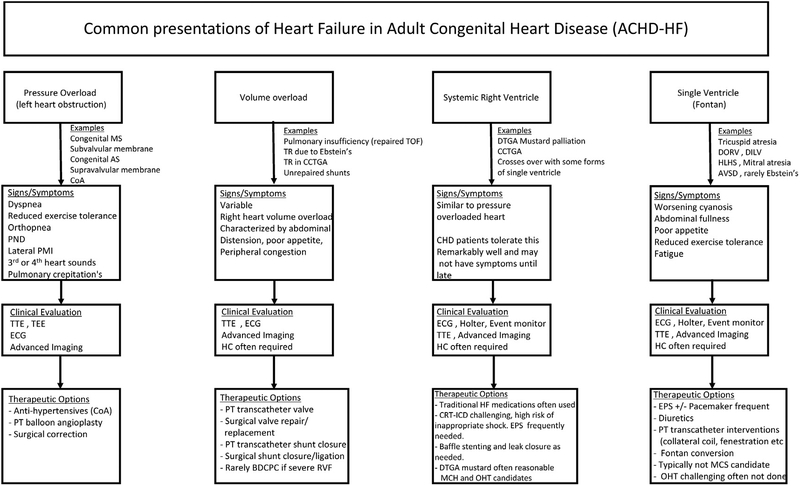
Traditional HF medications are not well-studied in the ACHD population (Table 3). Even with the lack of substantial data, there may be a role for these treatments because some of the mechanisms for HF overlap.34 In general, one must consider all the indications for cardiac medications in these patients because there are often multiple reasons to use medications such as beta-blockers, diuretics, and angiotensin-converting enzyme inhibitors. Typically, patients with significant HF and ACHD are followed by both congenital heart specialists and HF specialists. This allows each team to provide expertise on the nature of CHD and recommended HF therapies, respectively. A reasonable approach to the clinical evaluation of some common ACHD-HF phenotypes is outlined in Fig. 1 with potential treatment considerations and strategies.
Table 3:
Heart failure medication use in congenital heart disease patients
| Diagnosis | Medications (Reference) | Important Findings |
|---|---|---|
| TGA, systemic RV | Beta blockers: Giardini et a I,47 2007; Shaddyetal,48 2007; Doughan et aI,49 2007; Bouallal et al,50 2010; Khairy et al,41 2017 | |
| ACE inhibitors: Therrien et al,51 2008; Hechteret al,52 2001; Robinson et al,53 2001; Tutarel etal,542012 | ||
| Angiotensin receptor blockers: Van der Bom et al,55 2013; Dore et al,56 2005; Lester et al,57 2001 | ||
| Aldosterone receptor blockers: Dos etal,582013 |
|
|
| Tetralogy of Fa Not, subpulmonic RV | Beta blockers: Norozi et al,59 2007 |
|
| ACE inhibitors: Babu-Narayan etal,602012 |
|
|
| Angiotensin receptor blockers: Bokma etal (REDEFINE),61 2018 |
|
|
| Single ventricle circulation | Beta blockers: Ishibashi et al,62 2011 |
|
| ACE inhibitors: Hsu et al,63 2010; Kouatli etal,64 1997 | ||
| Aldosterone receptor blockers: Mahle etal,65 2009 |
|
|
| Phosphodiesterase inhibitors: Goldberg etal,66 2011 Endothelin receptor blockers: Hebert et al (TEMPO),67 2014; Schuuring etal,68 2013 |
Abbreviations: ACE, angiotensin converting enzyme; APPROPRIATE, Ace Inhibitors for Potential Prevention of the Deleterious Effects of Pulmonary Regurgitation in Adults with Repaired Tetralogy of Fallot; BNP, brain natriuretic peptide; CTB, collagen turnover biomarker; LVEF, left ventricular ejection fraction; NT-pro (BNP), N-terminal pro (brain natriuretic peptide); NYHA, New York Heart Association; QOL, quality of life; RVEDV, right ventricular end diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular systolic; SVR, systemic vascular resistance; TGA, transposition of the great arteries (here includes DTGA with atrial switch and CCTGA); TR, tricuspid regurgitation.
Fig 1.
Approach to managing HF in ACHD. Common presentations of ACHD-HF are outlined inclusive of example CHD lesions, signs, or symptoms; reasonable clinical evaluation or special testing; and potential therapeutic options. +/−, with or without; AS, aortic stenosis; AVSD, atrioventricular septal defect; BDCPC, bidirectional cavopulmonary connection; CCTGA, congenitally corrected transposition of the great arteries; CoA, coarctation of the aorta; CRT-ICD, cardiac resynchronization therapy–implantable cardiac defibrillator; DILV, double inlet left ventricle; DORV, double-outlet RV; DTGA, dextrotransposition of the great arteries; ECG, electrocardiogram; EPS, electrophysiology study; HC, hemodynamic catheterization; HLHS, hypoplastic left heart syndrome; MCS, mechanical circulatory support; MS, mitral stenosis; OHT, orthotopic heart transplant; PMI, point of maximum impulse; PND, paroxysmal nocturnal dyspnea; PT, percutaneous; RVF, RV failure; TEE, transesophageal echocardiogram; TOF, tetralogy of Fallot; TR, tricuspid regurgitation; TTE, transthoracic echocardiogram.
Mechanical Circulatory Support and Cardiac Transplantation
Advanced therapies for HF, which include the use of mechanical circulatory support (MCS), total artificial heart, and organ transplantation, are challenging in the CHD population owing to prior surgical repair, repeat sternotomy, nontraditional anatomy, elevated panel reactive antibodies, bleeding risk, hypercoagulability, and extracardiac disease secondary to CHD. Several studies have shown that up-front mortality is higher in the CHD population; however, long-term outcomes (>1 year posttransplant) are favorable.35,36 The current organ allocation system is predicated on the sickest patients receiving listing preference. Unfortunately for many CHD patients, their anatomy may not be suitable for traditional measures for HF decompensation. In particular, this is true of the patient with a single functioning ventricle or Fontan that may have preserved systolic function but presents with congestive right-sided failure symptoms. There are only a handful of centers that have the resources and multidisciplinary teams required to care for these unique patients when MCS and transplant are considered.
SUMMARY
As the CHD population continues to age, managing late HF will continue to be an important part of long-term care. Important first steps in evaluating these patients are to consider prior anatomy and procedures, and to identify potentially reversible or treatable lesions. Device therapy, MCS, and transplant have a limited role in this population, and require evaluation by an experienced and specialized team.
KEY POINTS:
Children with congenital heart disease (CHD) are outnumbered by adults with CHD (ACHD).
CHD–heart failure (HF) presentation differs based on anatomy and prior surgical repair.
HF medical therapy is less well studied in CHD and needs to be considered in the context of CHD-related anatomy or physiology.
The first step in evaluation of the adult CHD patient with HF is to examine the underlying anatomy for lesions with the possibility for intervention.
Mechanical circulatory support and heart transplant in CHD is more complex secondary to anatomic limitations. These patients are at a disadvantage in the current allocation system.
Footnotes
Disclosures: The authors have nothing to disclose.
REFERENCES
- 1.Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007;115:163–72. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J 2004;147: 425–39. [DOI] [PubMed] [Google Scholar]
- 3.van der Bom T, Zomer AC, Zwinderman AH, et al. The changing epidemiology of congenital heart disease. Nat Rev Cardiol 2011;8:50–60. [DOI] [PubMed] [Google Scholar]
- 4.Wren C, O’Sullivan JJ. Survival with congenital heart disease and need for follow up in adult life. Heart 2001;85:438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison DA, Connelly M, Harris L, et al. Sudden cardiac death in the adult with congenital heart disease. Can J Cardiol 1996;12:1161–3. [PubMed] [Google Scholar]
- 6.Oechslin EN, Harrison DA, Connelly MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111–6. [DOI] [PubMed] [Google Scholar]
- 7.Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220–9. [DOI] [PubMed] [Google Scholar]
- 8.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: the original heart failure syndrome. Eur Heart J 2003;24:970–6. [DOI] [PubMed] [Google Scholar]
- 9.Stout KK, Broberg CS, Book WM, et al. , American Heart Association Council on Clinical Cardiology CoFG, Translational B, council on cardiovascular R and imaging. Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation 2016;133: 770–801. [DOI] [PubMed] [Google Scholar]
- 10.Heart Failure Society of America. Executive summary: HFSA 2006 Comprehensive heart failure practice guideline. J Card Fail 2006;12:10–38. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, American College of Cardiology, American Heart Association Task Force on Practice Guidelines. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46: e1–82. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. 2013. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 13.Acker MA, Pagani FD, Stough WG, et al. Statement regarding the pre and post market assessment of durable, implantable ventricular assist devices in the United States: executive summary. Circ Heart Fail 2013;6:145–50. [DOI] [PubMed] [Google Scholar]
- 14.Mackie AS, Pilote L, Ionescu-Ittu R, et al. Health care resource utilization in adults with congenital heart disease. Am J Cardiol 2007;99:839–43. [DOI] [PubMed] [Google Scholar]
- 15.Zomer AC, Vaartjes I, van der Velde ET, et al. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol 2013;168:2487–93. [DOI] [PubMed] [Google Scholar]
- 16.Diller GP, Kempny A, Alonso-Gonzalez R, et al. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation 2015;132:2118–25. [DOI] [PubMed] [Google Scholar]
- 17.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol 2009;54:460–7. [DOI] [PubMed] [Google Scholar]
- 18.Briston DA, Bradley EA, Sabanayagam A, et al. Health Care costs for adults with congenital heart disease in the United States 2002 to 2012. Am J Cardiol 2016;118:590–6. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt AB, Foster E, Kuehl K, et al. Association Council on Clinical C. Congenital heart disease in the older adult: a scientific statement from the American Heart Association. Circulation 2015;131:1884–931. [DOI] [PubMed] [Google Scholar]
- 20.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714–833. [DOI] [PubMed] [Google Scholar]
- 21.Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 2018;137:e578–622. [DOI] [PubMed] [Google Scholar]
- 22.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol 2007;18: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puley G, Siu S, Connelly M, et al. Arrhythmia and survival in patients >18 years of age after the mustard procedure for complete transposition of the great arteries. Am J Cardiol 1999;83:1080–4. [DOI] [PubMed] [Google Scholar]
- 24.Kirjavainen M, Happonen JM, Louhimo I. Late results of Senning operation. J Thorac Cardiovasc Surg 1999;117:488–95. [DOI] [PubMed] [Google Scholar]
- 25.Rutz T, de Marchi SF, Schwerzmann M, et al. Right ventricular absolute myocardial blood flow in complex congenital heart disease. Heart 2010;96: 1056–62. [DOI] [PubMed] [Google Scholar]
- 26.Lubiszewska B, Gosiewska E, Hoffman P, et al. Myocardial perfusion and function of the systemic right ventricle in patients after atrial switch procedure for complete transposition: long-term follow-up. J Am Coll Cardiol 2000;36:1365–70. [DOI] [PubMed] [Google Scholar]
- 27.Bolger AP, Sharma R, Li W, et al. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 2002;106:92–9. [DOI] [PubMed] [Google Scholar]
- 28.Babu-Narayan SV, Goktekin O, Moon JC, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation 2005; 111:2091–8. [DOI] [PubMed] [Google Scholar]
- 29.Broberg CS, Prasad SK, Carr C, et al. Myocardial fibrosis in Eisenmenger syndrome: a descriptive cohort study exploring associations of late gadolinium enhancement with clinical status and survival. J Cardiovasc Magn Reson 2014;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathod RH, Prakash A, Powell AJ, et al. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol 2010;55:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredriksen PM, Veldtman G, Hechter S, et al. Aerobic capacity in adults with various congenital heart diseases. Am J Cardiol 2001;87:310–4. [DOI] [PubMed] [Google Scholar]
- 32.Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828–35. [DOI] [PubMed] [Google Scholar]
- 33.Bouchardy J, Therrien J, Pilote L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009;120:1679–86. [DOI] [PubMed] [Google Scholar]
- 34.McMurray JJ, Adamopoulos S, Anker SD, et al. , Avrupa Kardiyoloji Derneği (ESC) Akut ve Kronik Kalp Yetersizliği Tani ve Tedavisi 2012 Görev Grubu, ESC Kalp Yetersizliği Birliğinin Işbirliğiyle hazirlan miştir, Heart Failure Association. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Turk Kardiyol Dern Ars 2012; 40(Suppl 3):77–137 [in Turkish]. [PubMed] [Google Scholar]
- 35.Davies RR, Russo MJ, Yang J, et al. Listing and transplanting adults with congenital heart disease. Circulation 2011;123:759–67. [DOI] [PubMed] [Google Scholar]
- 36.Bradley EA, Pinyoluksana KO, Moore-Clingenpeel M, et al. Isolated heart transplant and combined heart-liver transplant in adult congenital heart disease patients: insights from the united network of organ sharing. Int J Cardiol 2017;228:790–5. [DOI] [PubMed] [Google Scholar]
- 37.Park SC, Neches WH, Mathews RA, et al. Hemodynamic function after the Mustard operation for transposition of the great arteries. Am J Cardiol 1983;51: 1514–9. [DOI] [PubMed] [Google Scholar]
- 38.Bradley EA, Cai A, Cheatham SL, et al. Mustard baffle obstruction and leak - how successful are percutaneous interventions in adults? Prog Pediatr Cardiol 2015;39:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley EA, Zaidi AN, Morrison J, et al. Effectiveness of early invasive therapy for atrial tachycardia in adult atrial-baffle survivors. Tex Heart Inst J 2017;44:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khairy P Sudden cardiac death in transposition of the great arteries with a Mustard or Senning baffle: the myocardial ischemia hypothesis. Curr Opin Cardiol 2017;32:101–7. [DOI] [PubMed] [Google Scholar]
- 41.Khairy P, Harris L, Landzberg MJ, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol 2008;1:250–7. [DOI] [PubMed] [Google Scholar]
- 42.Diller GP, Dimopoulos K, Okonko D, et al. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol 2006;48:1250–6. [DOI] [PubMed] [Google Scholar]
- 43.Diller GP, Okonko DO, Uebing A, et al. Impaired heart rate response to exercise in adult patients with a systemic right ventricle or univentricular circulation: prevalence, relation to exercise, and potential therapeutic implications. Int J Cardiol 2009;134:59–66. [DOI] [PubMed] [Google Scholar]
- 44.Kaltman JR, Ro PS, Zimmerman F, et al. Managed ventricular pacing in pediatric patients and patients with congenital heart disease. Am J Cardiol 2008; 102:875–8. [DOI] [PubMed] [Google Scholar]
- 45.Huhta JC, Maloney JD, Ritter DG, et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation 1983;67:1374–7. [DOI] [PubMed] [Google Scholar]
- 46.Piran S, Veldtman G, Siu S, et al. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002;105: 1189–94. [DOI] [PubMed] [Google Scholar]
- 47.Giardini A, Lovato L, Donti A, et al. A pilot study on the effects of carvedilol on right ventricular remodelling and exercise tolerance in patients with systemic right ventricle. Int J Cardiol 2007;114: 241–6. [DOI] [PubMed] [Google Scholar]
- 48.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 2007;298:1171–9. [DOI] [PubMed] [Google Scholar]
- 49.Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol 2007;99:704–6. [DOI] [PubMed] [Google Scholar]
- 50.Bouallal R, Godart F, Francart C, et al. Interest of beta-blockers in patients with right ventricular systemic dysfunction. Cardiol Young 2010;20:615–9. [DOI] [PubMed] [Google Scholar]
- 51.Therrien J, Provost Y, Harrison J, et al. Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebo-controlled study. Int J Cardiol 2008;129: 187–92. [DOI] [PubMed] [Google Scholar]
- 52.Hechter SJ, Fredriksen PM, Liu P, et al. Angiotensin-converting enzyme inhibitors in adults after the Mustard procedure. Am J Cardiol 2001;87: 660–3. a11. [DOI] [PubMed] [Google Scholar]
- 53.Robinson B, Heise CT, Moore JW, et al. Afterload reduction therapy in patients following intraatrial baffle operation for transposition of the great arteries. Pediatr Cardiol 2002;23:618–23. [DOI] [PubMed] [Google Scholar]
- 54.Tutarel O, Meyer GP, Bertram H, et al. Safety and efficiency of chronic ACE inhibition in symptomatic heart failure patients with a systemic right ventricle. Int J Cardiol 2012;154:14–6. [DOI] [PubMed] [Google Scholar]
- 55.van der Bom T, Winter MM, Bouma BJ, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation 2013;127:322–30. [DOI] [PubMed] [Google Scholar]
- 56.Dore A, Houde C, Chan KL, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation 2005; 112:2411–6. [DOI] [PubMed] [Google Scholar]
- 57.Lester SJ, McElhinney DB, Viloria E, et al. Effects of losartan in patients with a systemically functioning morphologic right ventricle after atrial repair of transposition of the great arteries. Am J Cardiol 2001;88: 1314–6. [DOI] [PubMed] [Google Scholar]
- 58.Dos L, Pujadas S, Estruch M, et al. Eplerenone in systemic right ventricle: double blind randomized clinical trial. The evedes study. Int J Cardiol 2013; 168:5167–73. [DOI] [PubMed] [Google Scholar]
- 59.Norozi K, Bahlmann J, Raab B, et al. A prospective, randomized, double-blind, placebo controlled trial of beta-blockade in patients who have undergone surgical correction of tetralogy of Fallot. Cardiol Young 2007;17:372–9. [DOI] [PubMed] [Google Scholar]
- 60.Babu-Narayan SV, Uebing A, Davlouros PA, et al. Randomised trial of ramipril in repaired tetralogy of Fallot and pulmonary regurgitation: the APPROPRIATE study (Ace inhibitors for Potential PRevention of the deleterious effects of Pulmonary Regurgitation in Adults with repaired TEtralogy of Fallot). Int J Cardiol 2012;154:299–305. [DOI] [PubMed] [Google Scholar]
- 61.Bokma JP, Winter MM, van Dijk AP, et al. Effect of losartan on RV dysfunction: results from the double-blind, randomized REDEFINE trial in adults with repaired tetralogy of Fallot. Circulation 2018; 137(14):1463–71. [DOI] [PubMed] [Google Scholar]
- 62.Ishibashi N, Park IS, Waragai T, et al. Effect of carve-dilol on heart failure in patients with a functionally univentricular heart. Circ J 2011;75:1394–9. [DOI] [PubMed] [Google Scholar]
- 63.Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 2010;122:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kouatli AA, Garcia JA, Zellers TM, et al. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation 1997;96:1507–12. [DOI] [PubMed] [Google Scholar]
- 65.Mahle WT, Wang A, Quyyumi AA, et al. Impact of spironolactone on endothelial function in patients with single ventricle heart. Congenit Heart Dis 2009;4:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation 2011;123:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (treatment with Endothelin receptor antagonist in fontan patients, a randomized, placebo-controlled, double-blind study measuring peak oxygen consumption) study. Circulation 2014;130:2021–30. [DOI] [PubMed] [Google Scholar]
- 68.Schuuring MJ, Vis JC, van Dijk AP, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail 2013;15:690–8. [DOI] [PubMed] [Google Scholar]