Abstract
Carbamates are esters of substituted carbamic acids that react with acetylcholinesterase (AChE) by initially transferring the carbamoyl group to a serine residue in the enzyme active site accompanied by loss of the carbamate leaving group followed by hydrolysis of the carbamoyl enzyme. This hydrolysis, or decarbamoylation, is relatively slow, and half-lives of carbamoylated AChEs range from 4 min to more than 30 days. Therefore, carbamates are effective AChE inhibitors that have been developed as insecticides and as therapeutic agents. We show here, in contrast to a previous report, that decarbamoylation rate constants are independent of the leaving group for a series of carbamates with the same carbamoyl group. When the alkyl substituents on the carbamoyl group increased in size from N-monomethyl- to N,N-dimethyl-, N-ethyl-N-methyl-, or N,N-diethyl-, the decarbamoylation rate constants decreased by 4-, 70-, and 800-fold, respectively. We suggest that this relationship arises as a result of active site distortion, particularly in the acyl pocket of the active site. Furthermore, solvent deuterium oxide isotope effects for decarbamoylation decreased from 2.8 for N-monomethylcarbamoyl AChE to 1.1 for N,N-diethylcarbamoyl AChE, indicating a shift in the rate-limiting step from general acid-base catalysis to a likely conformational change in the distorted active site.
Keywords: Acetylcholinesterase, Decarbamoylation, Alzheimer’s disease
1. Introduction
Acetylcholinesterase (AChE) (1) catalyzes the hydrolysis of the neurotransmitter acetylcholine, and rapid hydrolysis of this ester is essential for normal cholinergic synaptic transmission. At neuromuscular junctions, acetylcholine is released by fusion of intracellular vesicles with the presynaptic membrane and hydrolyzed in the synaptic cleft in less than 100 μsec (3). Ligand binding studies (4) and X-ray crystallography (5) of AChE have revealed a narrow active site gorge some 20 Å deep with two separate ligand binding sites. At the base of the gorge is the acylation or A-site where a tryptophan residue binds the trimethylammonium group of acetylcholine and a catalytic triad of glutamate, histidine, and serine residues participate in a triad that catalyzes the transient acylation and deacylation of the serine during each substrate turnover. The peripheral or P-site near the mouth of the gorge is the site of initial binding of acetylcholine in the catalytic pathway (6). Acetylcholine hydrolysis proceeds by transfer of the acetyl group to the active site serine of AChE followed by hydrolysis of the acetyl enzyme. Carbamates are esters of substituted carbamic acids that also react with AChE in a two-step process, with initial transfer of the carbamoyl group to the serine of AChE followed by hydrolysis of the carbamoyl enzyme. This hydrolysis is relatively slow, and half-lives of carbamoylated AChEs range from 4 min to more than 30 days ((7) and data in Table 1 below). Because carbamates are poor, slowly reversible AChE substrates, they are effective AChE inhibitors that have been widely developed as insecticides (8).
Table 1.
Decarbamoylation Rate Constants k21 in the Absence of Carbamate at 25 °C a
| Carbamoyl Group Substituents | Leaving Group b | Carbamate Common Name | nc | k21 × 106 min−1 |
|---|---|---|---|---|
| N-monomethyl | ||||
| HTMA | Monomethyl neostigmine | 8 | 12300 ± 400 | |
| NAP | 4 | 12500 ± 200 | ||
| 1-Napthol | carbaryl | 7 | 11300 ± 600 | |
| M7H | 10 | 12900 ± 500 | ||
| N,N-dimethyl | ||||
| HTMA | neostigmine | 15 | 3600 ± 300 | |
| M7H | 6 | 3000 ± 300 | ||
| F | 4 | 3200 ± 200 | ||
| N-ethyl-N-methyl | ||||
| HTMA | 16 | 150 ± 20 | ||
| NAP | rivastigmine | 19 | 160 ± 10 | |
| M7H | 4 | 200 ± 40 | ||
| N,N-diethyl | ||||
| HTMA | 11 | 16 ± 5 | ||
| NAP | 4 | 23 ± 6 | ||
| M7H | 9 | 14 ± 4 | ||
| F | 2 | 7 ± 1 |
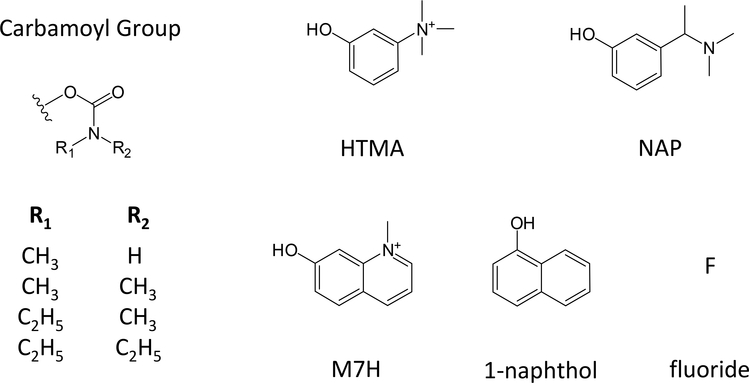
Alzheimer’s disease (AD) is a form of dementia characterized by the presence of amyloid and tau deposits which lead to the loss of cholinergic neurons in the brain. Partial inhibition of AChE preserves residual released acetylcholine and restores some of the cholinergic function lost in AD patients. A small number of AChE inhibitors have been approved by the FDA for this symptomatic treatment of AD, and one of these is rivastigmine (marketed as Exelon), the 3-[( 1-dimethylamino) ethyl] phenyl ester of N-ethyl–N-methylcarbamic acid (see Figure 1 below) (9–11). Of particular interest here, rivastigmine and the smaller N-ethyl–N-methylcarbamoyl chloride (EMCC) form identical carbamoyl AChE intermediates and yet the decarbamoylation rate was reported to be two orders magnitude slower for rivastigmine (12). The crystal structure of rivastigmine-bound TcAChE suggested a structural explanation for this unexpected difference. It revealed that His440 (2) in the catalytic triad had moved out of hydrogen bonding distance to Glu327 and that the N-ethyl–N-methylcarbamoyl group attached to Ser200 and the leaving group cleaved from rivastigmine, (–)-S-3-[1-(dimethylamino)ethyl]phenol (NAP, see Figure 1 below), were trapped inside the distorted A-site (12). One explanation for the survival of this structure during crystallization was that NAP and the N-ethyl–N-methylcarbamoyl group mutually blocked the other’s release from the distorted active site (12). To test this proposed novel mechanism involving synergistic inhibition, we conducted a systematic study of the decarbamoylation rates for a series of carbamates wherein we varied both the N-alkyl- groups on the carbamate and the ester-linked leaving groups. We report here the results of our study.
Figure 1.
Molecular structures of carbamoyl and leaving groups in carbamates examined in this report.
2. Materials and methods
2.1. hAChE
Recombinant hAChE was expressed as a secreted, disulfide-linked dimer in Drosophila S2 cells and purified by affinity chromatography as outlined previously (13). In some experiments, purified hAChE was reductively radiomethylated with [3H]formaldehyde and sodium cyanoborohydride (14). This process methylates primary amino groups on the N-terminus and lysine residues but has essentially no effect on AChE activity.
2.2. Carbamates
N,N-Dimethyl- and N,N-diethylcarbamoyl chlorides were commercial samples from Sigma-Aldrich Chemical Co (St. Louis, MO). Rivastigmine and EMCC were gifts from Dr. Albert Enz, Novartis Institutes for Biomedical Research, Basel, Switzerland. N-methylcarbamoyl chloride was synthesized from N-methylformamide. Other carbamates were synthesized by reacting a slight excess of the appropriate carbamoyl chloride with the appropriate phenol in the presence of either triethylamine or sodium hydride. Synthesis of N-methylcarbamates utilized triethylamine as a base in dichloromethane at room temperature, while synthesis of the other carbamates employed sodium hydride for generation of the phenolate nucleophile in dimethylformamide at 0 °C. Some carbamates were converted to their quaternary ammonium iodides by allowing them to react with methyl iodide overnight. The salts were filtered and recrystallized from an appropriate solvent. The carbamoyl fluorides were prepared by stirring the corresponding chlorides with anhydrous KF in 18-crown-6 ether at room temperature for 24–48 hr. The fluoride was distilled out of the reaction mixture, and the structure was confirmed by 19F NMR. The proton NMR spectra of the synthesized carbamates were consistent with their structures.
2.3. Assay of substrate hydrolysis
Hydrolysis rates v for the substrate acetylthiocholine were measured in a coupled Ellman reaction in which thiocholine generated in the presence of the indicated concentration of DTNB was determined by the formation of the thiolate dianion of DTNB at 412 nm (Δε412 nm = 14,150 M−1cm−1) (15). Total AChE concentrations (Etot) were calculated assuming 450 units/nmol (2) 3 3. Assays were conducted at 25º C in 20 mM sodium phosphate, 60 mM NaCl, and 0.02% Triton X100 (pH 7.0) unless otherwise noted.
2.4. Carbamate reaction with hAChE
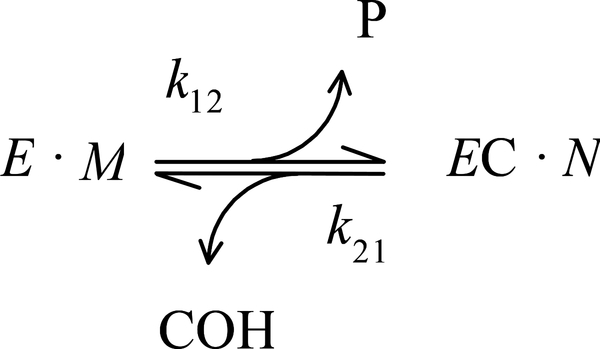
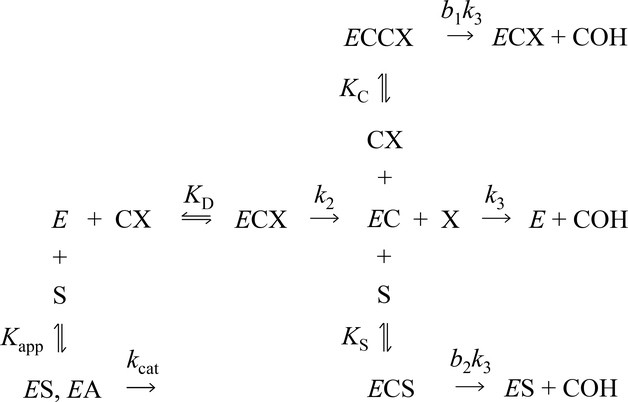
With carbamate substrates, both the formation and hydrolysis of the carbamoylated enzyme (EC), are slow enough to allow equilibrium assumptions. A general approach is given in Scheme 1 (7, 16–18). In this scheme, k12 and k21 are overall carbamoylation and decarbamoylation rate constants that incorporate intrinsic rate constants as described below. The leaving group P and carbamic acid COH are the products released during the carbamoylation and decarbamoylation reactions, respectively. The terms E ·M and EC · N represent all species in equilibrium with E and EC, respectively (i.e., [E] ·M + [EC] · N ) = [E]tot, where [E] and [EC] are the respective concentrations of E and EC, and Etot is the total concentration of enzyme active sites), and M and N are sums of terms (involving equilibrium constants and ligand concentrations) that depend on the details of a selected reaction scheme. The rate equation corresponding to Scheme 1 can be solved explicitly if all ligand concentrations remain essentially constant over the reaction time course, as noted previously (16). For example, the more explicit Scheme 2 is sufficient to analyze the data we present here. In this scheme, carbamate (CX) reaction is coupled to acetylthiocholine (S) hydrolysis. Since a detailed analysis of the carbamoylation reaction is not conducted here, M is simplified to M = 1 + [CX]/KD + [S]/Kapp, while the decarbamoylation term N more explicitly includes additional species that can modify the decarbamoylation rate: N = 1 + [CX]/KC + [S]/Kapp. The corresponding rate constants k12 and k21 are given in Eqs. 1a and 1b:
| (Eqs. 1a & b) |
SCHEME 1.
SCHEME 2.
In the experiments that were analyzed with Eq. 1a here, Kapp was assigned as 0.08 mM (19).
Experimental traces of carbamoylation and decarbamoylation reactions coupled to acetylthiocholine hydrolysis were obtained, and the equation for the general solution used to fit these traces to Schemes 1 and 2 is given in Eq. 2. Data were fitted to Eq. 2 by unweighted nonlinear
| (Eq. 2) |
regression with SigmaPlot (vers. 12.0). In Eq. 2, A412 is the continuously monitored absorbance at 412 nm, t is the time of the reaction, t0 is the time at which carbamate and enzyme were mixed, v0 is the initial substrate hydrolysis rate at t0, R is the fraction of total enzyme that is carbamoylated at the reaction initiation, and A412(t0) is the blank absorbance in the absence of enzyme. We previously reported a similar version of Eq. 2 (7). To maximize accuracy in the stopped flow experiments here, mixing was triggered slightly after the trace time was initiated. This necessitated addition of t0 to the equation.
2.5. Measurements of the decarbamoylation rate constant k21
Two protocols were employed to measure the rate constant k21. One used stopped-flow methods to accurately measure the approach to the carbamoylation steady state following mixing of enzyme and carbamate. A Hi-Tech SFA 20 stopped-flow apparatus thermostatted at 25 °C was used to rapidly mix equal volumes (300 uL) of AChE in one syringe and acetylthiocholine, DTNB, and carbamate in the other. Final concentrations of acetylthiocholine and DTNB were 0.25 mM and 2.0 mM, respectively. This higher than conventional concentration of DTNB was employed to insure that DTNB reaction with thiocholine was not rate limiting. Absorbance at 412 nm was recorded on a Varian Cary 3A spectrophotometer at fixed intervals of 33 msec.
The second protocol for obtaining k21 measured the increase in enzyme activity as carbamoylated AChE slowly decarbamoylated. Since half times for decarbamoylation here were as long as weeks, the key to accurate measurements was precise determination of the fully decarbamoylated enzyme activity. The AChE was radiolabeled to enable this determination. A calibration ratio of radiomethylated AChE activity monitored spectrophotometrically with 0.5 mM acetylthiocholine to radioactivity determined by scintillation counting was established for stock AChE solutions. In a typical assay an AChE sample was inactivated with a carbamate for at least an hour (overnight with N,N-diethylcarbamates), and unreacted carbamate was removed by adding 100 μL of the mix to a 1.5 ml G-50 Sephadex spin column. The columns were prepared freshly and were equilibrated with phosphate buffer prior to addition of the mix. Recovery of enzyme from the columns was monitored by scintillation counting, and an aliquot (50 μL) of the fraction with the highest enzyme concentration was added to 3.0 mL of assay buffer containing 0.1 to 1.0 mM acetylthiocholine. Typically, 0.2 – 8 h of reactivation showed some curvature in the Ellman trace and allowed fitting with Eq. 2, although some reactivation reactions with N,N-diethylcarbamates were extended to 24 h. Data fitting was conducted with k12 and t0 in Eq. 2 set to zero and vf = v0 k21/(k12 + k21) fixed as the predicted final velocity determined from scintillation counting and the calibration ratio. Values listed for k21 are means of n measurements with the standard error of the mean.
2.6. Solvent isotope effects
Decarbamoylation reactions in D2O were paired with reactions in H2O to maximize precision in comparing rates. Ellman assays were conducted at 25º C in 20 mM sodium phosphate and 0.02% Triton X-100 in D2O or H2O, and both buffers were adjusted to pH 7.0. Final assay concentrations of acetylthiocholine and DTNB were 0.25 mM and 0.33 mM DTNB, respectively.
3. Results
3.1. Measurement of decarbamoylation rate constants k21
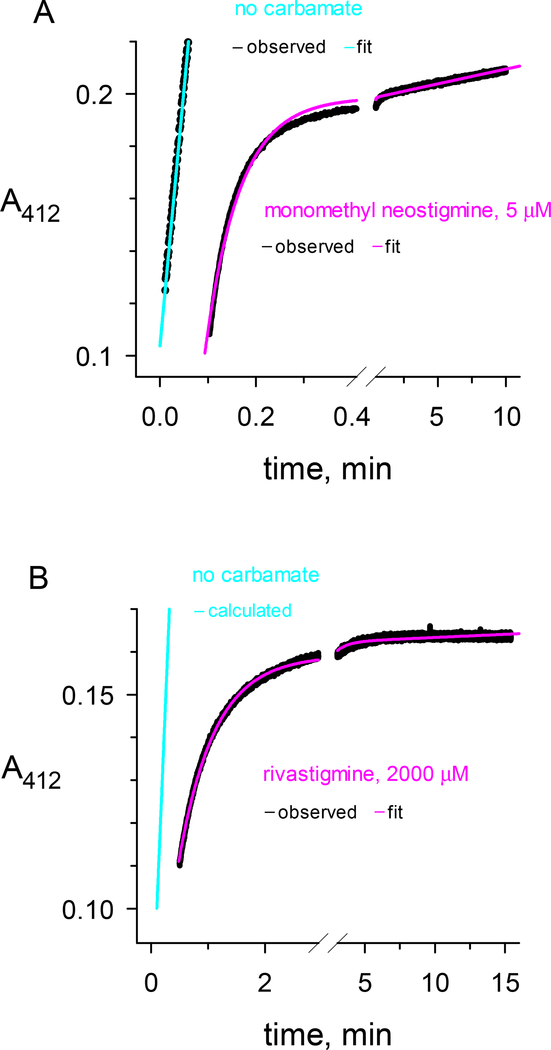
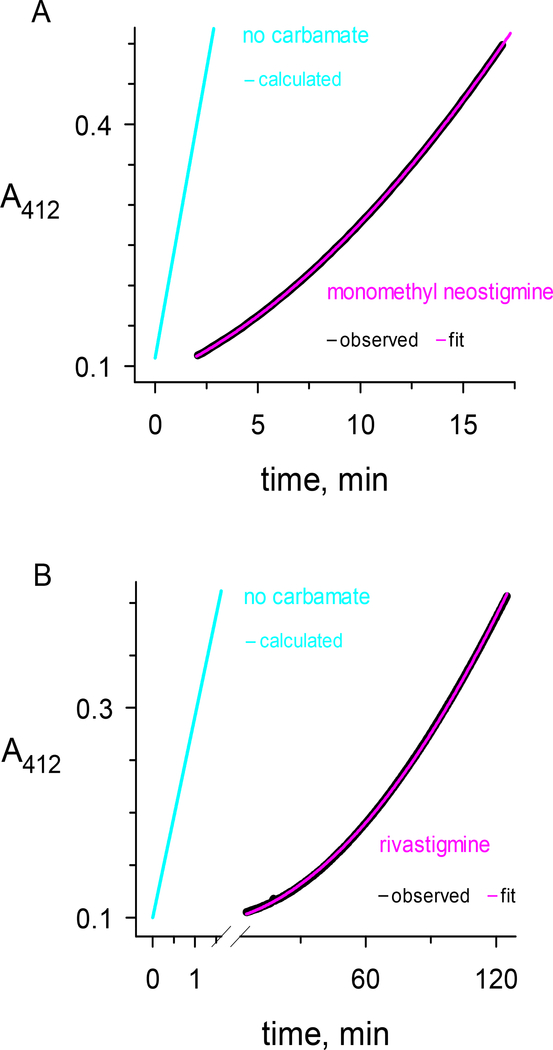
The focus of this study was the measurement of the overall decarbamoylation rate constant k21 for a series of carbamates (CX in Scheme 2) with different leaving groups (X) and with carbamoyl groups (C) of various size (Figure 1). Two protocols were used to measure k21. The first is illustrated in Figure 2, where the approach to the steady state after mixing carbamate and enzyme was monitored. The carbamate in Figure 2a was N-monomethyl neostigmine, one of the most reactive carbamoylating agents of AChE. Although analysis of k12 was given only minor emphasis in this report, the data in Figure 2a combined with additional k12 measurements at higher N-monomethyl neostigmine concentrations and fitted to Eq. 1a gave k2 = 51 ± 2 min−1; KD = 3.2 ± 0.4 μM; and k2/KD = 16 ± 2 min−1μM−1. The steady state in Figure 2a was reached in about one minute, and subsequent substrate turnover was high enough to give an accurate measurement of k21 = 0.013 min−1. Rivastigmine was the carbamate examined in Figure 2b, and it reacted more slowly with AChE. The data in Figure 2b and additional k12 measurements at various rivastigmine concentrations were fitted to Eq. 1a to give k2 = 1.5 ± 0.5 min−1; KD = 130 ± 80 μM; and k2/KD = 12 ± 4 min−1mM−1. This value of k2/KD was about 1000-fold lower than that for N-monomethyl neostigmine. An estimate of k21 = 0.003 min−1 was also obtained from Figure 2b. However, because all data in Figure 2b were fitted simultaneously, the fitted final slope is higher than the observed final slope. The corresponding fitted value of k21 is more than an order of magnitude larger than the more accurate measurements described below. To further compound the problem, any residual nonenzymatic substrate hydrolysis will increase the fitted k21, and for some carbamates the value of b1 in Eq. 1b is greater than 1 (see Discussion). Although the protocol in Figure 2 is useful because it can give simultaneous information about k12 and k21, we conclude that its estimates of k21 values less than 0.01 min−1 are unreliable.
Figure 2.
Reactions of the carbamates a) N-monomethyl neostigmine and b) rivastigmine with hAChE. The carbamates or buffer blanks were mixed with hAChE in a stopped-flow apparatus and reactions were monitored at 412 nm with 0.25 mM acetylthiocholine and 2.0 mM DTNB as outlined in the Experimental Procedures (black traces labeled “observed”). The reaction data for carbamates were fitted to Eq. 2 (pink traces labeled “fit”) with R set to zero, either t0 or A412(t0) fixed at a value determined by inspection or from controls without enzyme, and with v0, A412(t0), k12 and k21 as the fitted parameters. The reaction data for blanks without carbamate gave a linear fit in panel A (cyan trace labeled “fit”) or a linear fit in panel B that was calculated by normalizing panel A data to the AChE concentration (cyan trace labeled “calculated”). a) Final concentrations of N-monomethyl neostigmine and hAChE after mixing were 5.0 μM and 2.0 nM, respectively. Fitting to Eq. 2 with t0 fixed at 0.100 min gave k12 = 14 min−1 and k21 = 13 × 10−3 min−1. b) Final concentrations of rivastigmine and hAChE after mixing were 2.0 mM and 0.33 nM, respectively. Fitting to Eq. 2 with A412(t0) fixed at 0.114 min gave k12 = 1.24 min−1 and k21 = 3.1 × 10−3 min−1. The carbamate trace in panel b was offset by 0.5 min.
The second protocol for determining the decarbamoylation rate constant k21 is shown in Figure 3. This protocol involves radiolabeling the AChE by reductive methylation to allow calculation of the total enzyme concentration and hence the final substrate turnover rate vf that is expected when the enzyme is completely decarbamoylated. Since the half lives of some of the carbamoylated AChEs examined here are on the order of weeks, it is not practical to measure vf directly from an extended reaction trace. The decarbamoylation of AChE after reaction with Nmonomethyl neostigmine is shown in Figure 3a. A k21 = 0.013 min−1 was calculated from these data, in good agreement with the value obtained from Figure 2a. Decarbamoylation of N-ethyl-Nmethylcarbamoyl AChE generated from rivastigmine is shown in Figure 3b. The calculated k21 = 0.00016 min−1 is nearly 100-fold lower than that for N-monomethylcarbamoyl AChE in Figure 3a and also considerably lower than the k21 determined for the same carbamoylated enzyme in Figure 2b, as noted above. Reaction traces similar to those in Figure 3 were obtained when assays were conducted in 0.1, 0.5, and 1.0 mM acetylthiocholine, and no significant differences in k21 values were observed. A similar lack of effect of acetylthiocholine concentration on k21 was observed for carbamoyl AChE produced by carbamoylcholine at acetylthiocholine concentrations up to 30 mM (7). Therefore, we concluded that any binding of acetylthiocholine to the carbamoylated enzymes had no effect on k21 and that, according to Eq. 1b, with this decarbamoylation protocol k21 = k3 (however, see Discussion).
Figure 3.
Decarbamoylation reactions following treatment of hAChE with a) N-monomethyl neostigmine and b) rivastigmine. AChE was radiomethylated, carbamoylated, isolated on a spin column, and subjected to continuous spectrophotometric assay at 412 nm with 0.25 mM acetylthiocholine and 0.33 mM DTNB as outlined in the Experimental Procedures (black traces labeled “observed”). The reaction data were fitted to Eq. 2 (pink traces labeled “fit”) with k12 and t0 set to zero, vf fixed at a predetermined value, and A412(t0), R, and k21 as the fitted parameters. Calculated traces corresponding to vf are shown in cyan (labeled “calculated”). a) Concentrations of N-monomethyl neostigmine and hAChE in the initial mix were 1.0 mM and 1.8 nM, respectively, and 50 μL of the spin column recovery was added to 3.0 mL of assay buffer containing 0.25 mM acetylthiocholine. Fitting to Eq. 2 with vf fixed at 0.143 ΔA/min gave k21 = (12.9 ± 0.02) × 10−3 min−1 and R = 0.90. b) Concentrations of rivastigmine and hAChE in the initial mix were 2.0 mM and 1.8 nM, respectively, and 50 μL of the spin column recovery was added to 3.0 mL of assay buffer containing 0.25 mM acetylthiocholine. Fitting to Eq. 2 with vf fixed at 0.192 ΔA/min gave k21 = (155 ± 0.4) × 10−6 min−1 and R = 0.996. The lower value of R in panel a) relative to panel b) reflects faster reactivation of N-monomethylcarbamoylated AChE during gel filtration and prior to initiation of the reaction trace. Carbamate traces in panels a) and b) were offset by 2.0 and 5.0 min, respectively.
Additional decarbamoylation rate constants k21 measured with the second protocol are listed in Table 1. Carbamates with one of four carbamoyl groups and one of five leaving groups were employed, and Figure 1 gives the structure of these leaving groups. Table 1 shows the carbamoyl group and the leaving group for each carbamate examined. The average number of measurements n of each rate constant are also listed, and in most cases this number is at least four. Values of k21 became progressively smaller as the carbamoyl groups increased in size, but for a given carbamoyl group there were no significant differences in k21 among the five examined leaving groups. Overall mean values of k21 for the N-monomethyl-, N,N-dimethyl-, and N-ethyl-N-methylcarbamoyl groups were 12,100-, 3300-, and 170 × 10−6 min−1, respectively. These values were in good agreement with previously reported decarbamoylation rate constants, as noted in the Discussion. Values of k21 for carbamates with N,N-diethylcarbamoyl groups have not been reported previously. The overall mean value of k21 for these carbamates in Table 1 was 15 × 10−6 min−1, more than an order of magnitude lower than the corresponding k21 for carbamates with N-ethyl-N-methylcarbamoyl groups in Table 1.
3.2. Solvent kinetic isotope effects
Analyses of solvent kinetic isotope effects in D2O relative to H2O have been useful in clarifying details of the AChE reaction pathway with a variety of substrates (20, 21), but we’ve found no reports of these isotope effects on the decarbamoylation of AChE. We examined D2O kinetic isotope effects on k21 for series of carbamoylated AChEs generated from HTMAs with carbamoyl substituents of various size (Table 2). Since the simplest interpretation of Scheme 2 and Eq. 1b under our experimental conditions is that k21 = k3, we anticipated isotope effects of 2 – 3 because proton transfer should be rate limiting for k3 (see Discussion). A value in this range was observed for Nmonomethylcarbamoylated AChE, but isotope effects for larger carbamoylated AChEs were progressively smaller with an isotope effect of 1.1 for N.N-diethylcarbamoylated AChE (Table 2). The basis for this decrease is considered in the Discussion.
Table 2.
Deuterium Oxide Kinetic Isotope Effects on Decarbamoylation Rate Constants k21 at 25 °C a
| Carbamoyl Group Substituents | Leaving Group b | nc | k21 H2O / k21D2O c |
|---|---|---|---|
| N-monomethyl | HTMA | 1 | 2.8 ± 0.2 |
| N,N-dimethyl | HTMA | 4 | 2.4 ± 0.2 |
| N-ethyl-N-methyl | HTMA | 3 | 1.8 ± 0.2 |
| N,N-diethyl | HTMA | 3 | 1.1 ± 0.2 |
Values of k21 obtained as outlined in the Experimental Procedures and Table 2.
Leaving group structures are shown in Figure 1.
Pairs of duplicate or triplicate measurements of k21 in H2O or D2O were collected, and the ratio of the means of k21 in H2O to k21 in D2O (k21 H2O / k21D2O) in each pair comprised one independent measurement. The means and standard errors of n independent measurements are tabulated.
4. Discussion
4.1. Kinetics of decarbamoylation
Our initial goals in this study were 1) to measure the decarbamoylation rate constants k21 for carbamoylated AChEs generated by a series of carbamic acid esters; and 2) to determine whether the ester leaving group had any effect on these rate constants. As discussed below, this second point arose from a report that N-ethyl–N-methylcarbamoylated AChE formed with N-ethyl–N-methylcarbamoyl chloride (EMCC) decarbamoylates more rapidly than that formed with rivastigmine (12). It is clear from our data in Table 1 that the decarbamoylation rate constant is independent of the leaving group for a given carbamoyl group. Regardless of the leaving group, the decarbamoylation rate constants decreased by factors of 4-, 70-, and 800 –fold relative N-monomethylcarbamoylated AChE, respectively, as the size of the N-alkyl- groups in the carbamoyl moiety increased to N,Ndimethyl, N-ethyl-N-methyl and finally to N,N-diethyl. This trend can be extended further by noting that k21 for a carbamoyl AChE with no N-alkyl groups generated from carbamoyl choline is 0.13 min−1 (7), 10-fold larger than that for N-monomethylcarbamoylated AChE. Consistent with the data in Table 1, a k21 value of 2 × 10−2 min−1 was reported for the N-monomethylcarbamoylated AChE formed by physostigmine (22), and a 20–30 fold decrease in k21 was reported in going from the N,Ndimethyl- to the N-ethyl–N-methylcarbamoylated AChE generated by rivastigmine (23).
Clearly, and to our disappointment (15), our work does not support the previous suggestion that the N-ethyl-N-methylcarbamoylated AChE formed with EMCC decarbamoylates more rapidly than that formed with rivastigmine (12). We suspect that this discrepancy results from the fact that aqueous solutions of EMCC are very unstable and carbamoylate AChE poorly. When chloride in EMCC was converted to fluoride (another small leaving group but one that forms a more stable carbamate), the carbamoylation of AChE was more effective. While carbamates with larger carbamoyl groups did decarbamoylate more slowly, we saw no evidence that this involved trapping of the leaving group. In fact, carbamoylation with the N,N-diethylcarbamate of M7H resulted in the immediate release of the fluorescent leaving group at a rate several orders of magnitude larger than k21 for the N,N-diethylcarbamoylated AChE (data not shown).
One novel feature of our report is our data on the largest carbamic acids whose esters were examined here, the N,N-diethylcarbamic acids. The rate constants for decarbamoylation of AChE modified with this carbamoyl group are low and can be difficult to measure. An early effort failed to detect formation of N,N-diethylcarbamoylated AChE from the corresponding N,N-diethylcarbamoyl fluoride or N,N-diethylcarbamoyl choline (24); but, with greater sensitivity and longer time courses for the four N,N-diethylcarbamic acid esters in Table 1, we were able to generate N,Ndiethylcarbamoylated AChE and measure its decarbamoylation rate constant. The accuracy of our rate constant measurements was improved by radiolabeling the AChE. We chose reductive radiomethylation for the labeling, as this modification results in dimethylation of only the N-terminal α-amino and lysine ε-amino groups. These groups are distant from the AChE active site, and the modification has essentially no effect on AChE activity. From the ratio of radioactivity to enzyme activity in the initial AChE preparation, it was possible to calculate the AChE concentration following carbamoylation and removal of excess carbamate by gel filtration and fix the corresponding activity value (vf) in the fitting program. Complete removal of excess carbamate by gel filtration is essential for accurate determination of vf, as simple dilution of the carbamoylation reaction is not sufficient to eliminate continued carbamoylation in the final steady state. We chose to use a continuous spectrophotometric assay of decarbamoylation in the presence of acetylthiocholine substrate (Figure 3). This assay can be justified because we saw no difference in k21 values as the acetylthiocholine concentrations in the assay were varied from 0.1 to 1.0 mM. Furthermore, we previously showed that acetylthiocholine at concentrations up to 30 mM had no effect on the decarbamoylation rate constant k21 for carbamoylated AChE generated from carbamoyl choline (Figure 8b in (7)). This lack of effect indicated that b2 ~= 1 for any acetylthiocholine complex in Scheme 2 and Eq. 1b. We occasionally used the alternative spectrophotometric decarbamoylation assay in Figure 2. This assay is conducted in the presence of the carbamate and has the advantage that it provides estimates of both k12 and k21, but it becomes insensitive to k21 when the k21 value is small. Furthermore, the measured k21, given in Eq. 1b, cannot be converted to k3 unless b in Eq. 1b is known. The measured value of b for carbamoyl choline is 0.14 (7), suggesting that the presence of a carbamate may alter the intrinsic decarbamoylation rate constant k3.
Values of k21 for N,N-diethylcarbamoylated AChE in Table 1 are around 1 – 2 × 10−5 min−1, among the lowest decarbamoylation rate constants yet reported. This value of k21 corresponds to a half time for the N,N-diethylcarbamoylated AChE of nearly 800 hr, about an order of magnitude larger than that for the N-ethyl–N-methylcarbamoylated AChE generated by rivastigmine. We found one previous estimate of this half life from a study that examined the recovery of AChE in rat brain following in vivo treatment with N,N-diethylcarbamoyl fluoride (25). The reported recovery half time of 182 hr is in agreement with our values considering the higher 37 °C in vivo temperature. This agreement, however, may be coincidental, as the half time for AChE itself in the steady state in rat brain is only 68 hr (26). Other carbamates have also been reported to have very low k21 values, particularly N-monoalkyl- or N-alkyl–N-methylcarbamates in which the alkyl group is large (22, 23). The k21 for eptastigmine, an N-monoalkylcarbamate with an N-heptyl group, after carbamoylation of hAChE was reported to be 4 × 10−5 min−1, and that for MF268, another N-monoalkylcarbamate with an N-8-(2,6-dimethylmorpholino)octyl group, was too small to be measured (22). Examination of a series of N-alkyl–N-methylcarbamates clarified this trend (23). We noted earlier that this laboratory reported a 20–30 fold decrease in k21 in going from the N,N-dimethyl- to the N-ethyl–N-methylcarbamoylated AChE. However, k21 did not decrease further as the carbamate was modified from N-ethyl–N-methyl- to N-hexyl–N-methyl-. The trend for hBChE was slightly different (23). The value of k21 for the N,N-dimethylcarbamoylated BChE was similar to that for hAChE, and it decreased only 2–3 fold in going from the N,N-dimethyl to the N-ethyl–N-methyl- and about 2-fold more to the N-hexyl–N-methylcarbamoylated BChE.
4.2. Crystal structures of carbamoylated AChEs
Several crystal structures of carbamoylated AChEs have been reported. We noted in the Introduction the structure of rivastigmine-bound TcAChE, with both its N-ethyl–N-methylcarbamoyl moiety and separated leaving group NAP in the A-site (12). In this structure, the N-ethyl and the N-methyl substituents are in contact with the acyl pocket residues F288 and F290, respectively, although hydrogen bonding in the catalytic triad is disrupted. In addition to this structure, two structures of N-monoalkylcarbamates have been obtained (27, 28). The alkyl groups in these carbamates are large, corresponding to 2-ethylphenyl- in ganstigmine (28) and 8-(cis-2,6dimethylmorpholino)octyl- in MF268 (27). As noted in the previous section, the decarbamoylation rate constants k21 for N-monoalkylcarbamoyl AChEs with large alkyl groups are relatively small. In both structures no substantial conformational changes relative to the native AChE structures are apparent, and after carbamoylation only the carbamoyl group is observed in the active site and no leaving group is evident. The carbamoyl alkyl group in both cases extends along the active site gorge toward the P-site. To rationalize the low decarbamoylation rate constants k21 for these carbamoylated AChEs, despite the absence of significant A-site distortion, the authors noted the relatively short 2.83 Å distance observed between the N atom of the N-H,N-2-ethylphenylcarbamoyl moiety (H donor) and Nε2 of His440 (H acceptor) (2.83 Å) following carbamoylation. This distance corresponds to a strong hydrogen bond, and it could help to explain the small decarbamoylation rate constant if Nε2 is no longer available to a neighboring water molecule that is essential in the decarbamoylation step. This rationalization breaks down, however, when it is noted that the N-alkyl–N-methylcarbamoylated AChEs, for which this strong hydrogen bond cannot form, also have low values of k21 (23). These authors suggest that the increased resistance to reactivation of the conjugates obtained by such compounds may be associated with a substantial change in the orientation of the triad His440 as proposed for rivastigmine (12).
The pronounced decrease in k21 for N,N-diethylcarbamoylated AChE seen in Table 1 suggests even greater distortion of the A-site arising from the shape of this carbamoyl group. In this context, it is of interest to examine the X-ray crystal structure of diethoxyphosphorylated hAChE generated from paraoxon (29), in which the phosphoryl group has some shape similarity to the carbamoyl group in N,N-diethylcarbamoylated AChE. Franklin et al. note that, in this structure, one ethoxy group faces the cationic site (W86) and the other faces the acyl pocket (F295, F297, and F338). Hydrogen bonding within the catalytic triad remains intact (Ser203 Oγ to H477 Nε2 and H477 Nδ1 to E334 Oε1), but the adjacent acyl pocket residues and acyl loop (residue positions 280–297) are significantly perturbed relative to their positions in the ligand-free hAChE structure. Due to its relatively crowded local environment, a side-chain rotation of F295 is insufficient to accommodate the adduct, and instead movements in residue backbone positions are necessary to avert steric clash. The Cα and the sidechain of R296 shift by 4.9 A° and 14.9 A°, respectively, and the sidechain guanidinium group is displaced to a position within the P-site where it is stabilized by π-stacking against the hydroxyphenyl ring of Y72. Large acyl loop backbone rearrangements have also been seen in mAChE and TcAChE inhibited by the organophosphate diisopropylfluorophosphate (30, 31). However, stereoselective inhibition of AChE by organophosphate nerve agents that place a methyl group into the acyl pocket does not affect the acyl loop in the same manner (29, 30, 32).
Additional structures in Franklin et al. (29) indicate that these shifts in the acyl pocket residues and acyl loop in diethoxyphosphorylated hAChE can be largely reversed by cationic ligand binding to the active site. The interaction of cationic oximes with organophosphorylated AChEs is of intense interest because oximes have been shown to reactivate these AChEs by displacing the serine hydroxyl and releasing the organophosphorylated oxime (33, 34). The structure of the complex of diethoxyphosphorylated hAChE with the cationic oxime 2-PAM reveals two bound 2-PAM molecules, one in the P-site and the other stacked against the sidechain of W86, while that with the dicationic HI-6 shows HI-6 in the P-site. In both of these ternary complexes, the backbone conformation of the acyl loop resembles that of the ligand-free state and appears to be stabilized by oxime binding. Therefore, diffusion of these oximes into the crystals of diethoxyphosphorylated hAChE promoted a restoration of the acyl pocket residues and acyl loop to their locations in the unmodified hAChE structure. These observations raise the question of whether the crystal structure of rivastigmine with TcAChE reflects a restoration of a “normal active site” with bound NAP even with the carbamoyl group present.
4.3. Kinetic isotope effects on decarbamoylation rate constants k21 in deuterium oxide
The turnover number kcat for serine hydrolases is a combination of k2 for the acylation step and k3 for the deacylation step. These steps, shown in Scheme 2, typically involve general acid-base catalysis with proton transfer, and as a consequence these rate constants show a D2O kinetic isotope effect (a decrease of 2- to 3-fold when D2O replaces H2O as the solvent) (35). An isotope effect of 2.8 in Table 2 for decarbamoylation of carbamoylated AChE generated by N-monomethyl neostigmine is in this range, suggesting that for this reaction k21 can be equated with k3. The Nmonomethylcarbamoylated AChEs also show the highest k21 values in Table 1. However, as the Nalkyl groups on carbamoylated AChEs increase in size, not only do the k21 values in Table 1 decrease dramatically but also the D2O kinetic isotope effects in Table 2 decrease to the point where the isotope effect on k21 for N,N-diethylcarbamoylated AChE is only slightly above 1. The large size of this carbamoyl group is likely to be an important factor in this shift away from proton transfer in the rate-limiting step for k21. The X-ray crystal structures noted above indicate that AChEs carbamoylated with large carbamoyl groups or phosphorylated with large phosphoryl groups show significant distortion of the acyl pocket as well as the larger active site gorge. One possibility is that the active site is so highly distorted that this N,N-diethylcarbamoylated enzyme is reactivating itself by spontaneous water hydrolysis without any help from the catalytic groups on the enzyme. However, this possibility seems unlikely, as extrapolated rate constants for the hydrolysis of N,Ndialkylcarbamates at neutral pH appear much lower than the average k21 of 1.5 × 10−5 min−1 observed for N,N-diethylcarbamoylated AChEs in Table 1 (36).
A second possibility is that distortion of the active site in AChEs carbamoylated with larger N,N-dialkylcarbamoyl groups results in a change to an enzymatic rate-limiting step for k21 that no longer involves proton transfer. In this regard, it is noteworthy that a similar drop in D2O kinetic isotope effects from 2.4 on kcat to 1.1 – 1.2 on kcat/Kapp was observed for good substrates of AChE like acetylcholine. To interpret this drop, we proposed that proton transfer in the acylation step k2 was rate-limiting for kcat but that an earlier step like substrate binding or an induced-fit conformational change that does not involve proton transfer was rate-limiting for the second order rate constant kcat/Kapp (20). This proposal has gained wide acceptance in the cholinesterase field (21). A similar interpretation here is that an earlier, rate-limiting step that does not involve proton transfer should be incorporated into k21 for AChEs carbamoylated with large carbamoyl groups or phosphorylated with large phosphoryl groups. For decarbamoylation or dephosphorylation of these AChEs to occur, it may be necessary to invoke a transient conformational change that includes a brief conversion to the unmodified or apo AChE active site and its aligned catalytic triad where the k3 hydrolysis reaction can take place. It may be necessary to explore molecular modeling to examine the array of conformational variants available in AChEs with large carbamoyl or phosphoryl groups to obtain support for this proposal. However, it should be emphasized that for this conformational step to be rate limiting, it cannot be equilibrated with the subsequent hydrolysis reaction.
Acknowledgements
This work was supported by grant NS-16577 from the National Institutes of Health, by grant DAMD 17–98-2–8019 from the United States Army Medical Research Acquisition Activity, by grants from the Muscular Dystrophy Association of America to T.L.R, and by NIH NRSA fellowship NS-41828 to J.L.J.
Footnotes
The authors declare no competing financial interests
Abbreviations: AD, Alzheimer’s disease; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; EMCC, N-ethyl–N-methylcarbamoyl chloride; HI-6, 1-(2-hydroxy-iminomethylpyridinium)-1-(4- carboxyamino)-pyridinium dimethylether dichloride; hAChE, human acetylcholinesterase; mAChE, mouse acetylcholinesterase; TcAChE, Torpedo californica acetylcholinesterase; DTNB, 5,5’dithiobis-(2-nitrobenzoic acid); HTMA, 3-hydroxy-N,N,N-trimethylanilinium; M7H, 7-hydroxy-1methylquinolinium; NAP, 3-[1-(dimethylamino)ethyl]phenol; 2-PAM, 2-pyridinealdoxime methiodide;
Throughout this paper we number amino acid residues according to both the hAChE and the TcAChE sequences, with the species designation clearly indicated.
One unit of AChE activity corresponds to 1 μmol of acetylthiocholine hydrolyzed/min under standard pH-stat assay conditions at pH 8 (1, 2). Our conventional spectrophotometric assay at 412 nm is conducted in pH 7.0 buffer. With wild type hAChE and 0.5 mM acetylthiocholine, this assay results in 4.8 ΔA412 nm/min with 1 nM AChE or about 76 % of the pH stat assay standard.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberry TL, and Scoggin DM (1984) Structure of human erythrocyte acetylcholinesterase. Characterization of intersubunit disulfide bonding and detergent interaction, J. Biol. Chem 259, 5643–5652. [PubMed] [Google Scholar]
- 2.De Ferrari GV, Mallender WD, Inestrosa NC, and Rosenberry TL (2001) Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites, J. Biol. Chem 276, 23282–23287. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberry TL (1979) Quantitative simulation of endplate currents at neuro-muscular junctions based on the reactions of acetylcholine with acetylcholine receptor and acetylcholinesterase, Biophys. J 26, 263–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor P, and Lappi S (1975) Interaction of fluorescence probes with acetylcholinesterase.The site and specificity of propidium binding, Biochemistry 14, 1989–1997. [DOI] [PubMed] [Google Scholar]
- 5.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, and Silman I (1991) Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein, Science 253, 872–879. [DOI] [PubMed] [Google Scholar]
- 6.Szegletes T, Mallender WD, Thomas PJ, and Rosenberry TL (1999) Substrate binding to the peripheral site of acetylcholinesterase initiates enzymatic catalysis. Substrate inhibition arises as a secondary effect., Biochemistry 38, 122–133. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberry TL, Johnson JL, Cusack B, Thomas J, Emani S, and Venkatasubban KS (2005) Interactions between the peripheral site and the acylation site in acetylcholinesterase, Chem. Biol. Interact 157–158, 181–189. [DOI] [PubMed] [Google Scholar]
- 8.Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides, Environ Health Perspect 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enz A, Boddeke H, Gray J, and Spiegel R (1991) Pharmacologic and clinicopharmacologic properties of SDZ ENA 713, a centrally selective acetylcholinesterase inhibitor, Ann N Y Acad Sci. 640, 272–275. [DOI] [PubMed] [Google Scholar]
- 10.Rösler M, Retz W, Retz-Junginger P, and Dennler HJ (1998) Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease, Behav Neurol. 11, 211–216. [DOI] [PubMed] [Google Scholar]
- 11.Farlow MR, Grossberg GT, Sadowsky CH, Meng X, and Somogy M (2013) A 24week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6 mg/24 h in severe Alzheimer’s dementia, CNS Neurosci Ther. 19, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, and Silman I (2002) Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine, Biochemistry 41, 3555–3564. [DOI] [PubMed] [Google Scholar]
- 13.Mallender WD, Szegletes T, and Rosenberry TL (1999) Organophosphorylation of acetylcholinesterase in the presence of peripheral site ligands: Distinct effects of propidium and fasciculin, J. Biol. Chem 274, 8491–8499. [DOI] [PubMed] [Google Scholar]
- 14.Haas R, and Rosenberry TL (1985) Quantitative identification of N- terminal amino acids in proteins by radiolabeled reductive methylation and amino acid analysis. Application to human erythrocyte acetylcholinesterase, Analyt. Biochem 148, 154–162. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JL, Cusack B, Davies MP, Fauq A, and Rosenberry TL (2003) Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate, Biochemistry 42, 5438–5452. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberry TL, Sonoda LK, Dekat SE, Cusack B, and Johnson JL (2008) Analysis of the reaction of carbachol with acetylcholinesterase using thioflavin T as a coupled fluorescence reporter, Biochemistry 47, 13056–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberry TL, and Bernhard SA (1971) Studies of catalysis by acetylcholinesterase: Fluorescent titration with a carbamoylating agent, Biochemistry 10, 4114–4120. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberry TL, and Bernhard SA (1972) Studies on catalysis by acetylcholinesterase: Synergistic effects of inhibitors during hydrolysis of acetic acid esters, Biochemistry 11, 4308–4321. [DOI] [PubMed] [Google Scholar]
- 19.Mallender WD, Szegletes T, and Rosenberry TL (2000) Acetylthiocholine binds to Asp74 at the peripheral site of human acetylcholinesterase as the first step in the catalytic pathway, Biochemistry 39, 7753–7763. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberry TL (1975) Catalysis by acetylcholinesterase. Evidence that the rate-limiting step for acylation with certain substrates precedes general acid-base catalysis, Proc. Nat. Acad. Sci. USA 72, 3834–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn DM (1987) Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states, Chem. Rev 87, 955–979. [Google Scholar]
- 22.Perola E, Cellai L, Lamba D, Filocamo L, and Brufani M (1997) Long chain analogs of physostigmine as potential drugs for Alzheimer’s disease: new insights into the mechanism of action in the inhibition of acetylcholinesterase, Biochim Biophys Acta 1343, 41–50. [DOI] [PubMed] [Google Scholar]
- 23.Groner E, Ashani Y, Schorer-Apelbaum D, Sterling J, Herzig Y, and Weinstock M (2007) The kinetics of inhibition of human acetylcholinesterase and butyrylcholinesterase by two series of novel carbamates, Mol. Pharmacol 71, 1610–1617. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IB, Hatch MA, and Ginsburg S (1960) Carbamylation of acetylcholinesterase, J. Biol. Chem 235, 2312–2315. [PubMed] [Google Scholar]
- 25.Myers DK (1956) Studies on cholinesterase. 10. Return of cholinesterase activity in the rat after inhibition by carbamoyl fluorides, Biochem. J 62, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenthold RJ, Mahler HR, and Moore WJ (1974) The half-life of acetylcholinesterase in mature rat brain., J. Neurochem 22, 941–943. [DOI] [PubMed] [Google Scholar]
- 27.Bartolucci C, Perola E, Cellai L, Brufani M, and Lamba D (1999) Back door” opening implied by the crystal structure of a carbamoylated acetylcholinesterase, Biochemistry 38, 5714–5719. [DOI] [PubMed] [Google Scholar]
- 28.Bartolucci C, Siotto M, Ghidini E, Amari G, Bolzoni PT, Racchi M, Villetti G, Delcanale M, and Lamba D (2006) Structural determinants of Torpedo californica acetylcholinesterase inhibition by the novel and orally active carbamate based anti-Alzheimer drug ganstigmine (CHF-2819), J. Med. Chem 49, 5051–5058. [DOI] [PubMed] [Google Scholar]
- 29.Franklin MC, Rudolph MJ, Ginter C, Cassidy MS, and Cheung J (2016) Structures of paraoxon-inhibited human acetylcholinesterase reveal perturbations of the acyl loop and the dimer interface Proteins 84, 1246–1256. [DOI] [PubMed] [Google Scholar]
- 30.Hörnberg A, Tunemalm AK, and Ekström F (2007) Crystal structures of acetylcholinesterase in complex with organophosphorus compounds suggest that the acyl pocket modulates the aging reaction by precluding the formation of the trigonal bipyramidal transition state, Biochemistry 46, 4815–4825. [DOI] [PubMed] [Google Scholar]
- 31.Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, and Sussman JL (1999) Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level, Biochemistry 38, 7032–7039. [DOI] [PubMed] [Google Scholar]
- 32.Millard CB, Koellner G, Ordentlich A, Shafferman A, Silman I, and Sussman JL (1999) Reaction products of acetylcholinesterase and VX reveal a mobile histidine in the catalytic triad, J. Am. Chem. Soc 121, 9883–9884. [Google Scholar]
- 33.Wilson IB (1951) Acetylcholinesterase. XI. Reversibility of tetraethyl pyrophosphate inhibiton, J. Biol. Chem 190, 111–117. [PubMed] [Google Scholar]
- 34.Kovalevsky A, Blumenthal DK, Cheng X, Taylor P, and Radic Z (2016) Limitations in current acetylcholinesterase structure–based design of oxime antidotes for organophosphate poisoning, Ann. N.Y. Acad. Sci 1378, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bender ML, and Hamilton GA (1962) Kinetic isotope effects of deuterium oxide on several α-chymotrypsin-catalyzed reactions J. Am. Chem. Soc 84, 2570–2576. [Google Scholar]
- 36.Christensen I (1964) Alkaline hydrolysis of some carbamic acid esters, Acta Chem. Scand 18, 904–922. [Google Scholar]