Abstract
Antimicrobial peptide (AMP) production and melanization are two key humoral immune responses in insects. Induced synthesis of AMPs results from Toll and IMD signal transduction whereas melanization depends on prophenoloxidase (PPO) activation system. During invasion, pathogens produce toxins and other virulent factors to counteract host immune responses. Here we show that the pathways leading to PPO activation and AMP synthesis in the silkworm Bombyx mori are affected by a metalloprotease, named elastase B, secreted by Pseudomonas aeruginosa (PAO1). The metalloprotease gene (lasB) was expressed shortly after PAO1 cells had been injected into the larval silkworm hemocoel, leading to an increase of elastase activity. Injection of the purified PAO1 elastase B into silkworm hemolymph compromised PPO activation. In contrast, the protease caused a level increase of gloverin, an AMP in the hemolymph. To verify our results obtained using the purified elastase B, we infected B. mori with PAO1 ΔlasB mutant and found that PO activity in hemolymph of the PAO1 ΔlasB-infected larvae was significantly higher than that in the wild type-infected. The mutant-inhabited hemolymph had lower levels of gloverin and antimicrobial activity. PAO1 ΔlasB showed a decreased viability in the silkworm hemolymph whereas the host had a lower mortality. In addition, the effects caused by the ΔlasB mutant were restored by a complementary strain. These data collectively indicated that the elastase B produced by PAO1 is an important virulent factor that manipulates the silkworm immune system during infection.
Keywords: Bombyx mori, Pseudomonas aeruginosa, immune, elastase B, phenoloxidase, antimicrobial peptide
1. INTRODUCTION
Insects rely on an innate immune system to defend themselves against pathogenic bacteria. This system consists of cellular and humoral responses. The former is mediated by hemocytes including phagocytosis, nodulation and encapsulation (Strand, 2008) and the latter includes melanization and antimicrobial peptide (AMP) synthesis. Melanization is catalyzed by phenoloxidase (PO) produced in a prophenoloxidase (PPO) activation system. AMP synthesis is induced by NF-kB signaling pathways (Vallet-Gely et al., 2008). Similar to other insects, the silkworm Bombyx mori employs POs and AMPs to cope with bacterial infection (Tanaka et al., 2008; Huang et al., 2009; Yang et al., 2011).
To avoid the attacks by the host immune system, bacteria may hide from detection or recognition (Hurst et al., 2003). In other cases, bacteria may suppress the host defense responses in various manners. For instance, an entomopathogenic bacterium Photorhabdus luminescens produces an antibiotic (E)-1,3-dihydroxy-2-(isopropyl)-5-(2-phenylethenyl) benzene that inhibits phenoloxidase (PO) of the tobacco hornworm Manduca sexta (Eleftherianos et al., 2007). Live Xenorhabdus hominickii ANU101 infection reduces PO activity and inhibits AMP expression in the beet armyworm Spodoptera exigua (Sadekuzzaman et al., 2017). Serratia marcescens infection induces apoptosis of the silkworm hemocytes (Ishii et al., 2012). A metalloprotease serralysin secreted by S. marcescens disrupts the adhesive properties of hemocytes and suppresses would healing, leading to a massive loss of hemolymph in the silkworm (Ishii et al., 2014a, 2014b).
The Gram-negative bacterium P. aeruginosa is a widespread opportunistic pathogen of plants, insects, nematodes and human. Several insect species have been used to study P. aeruginosa pathogenesis and virulence. These include the greater waxworm Galleria mellonella (Jander et al., 2000; Miyata et al., 2003; Andrejko et al., 2009; Koch et al., 2014; Doberenz et al., 2017), fruit fly Drosophila melanogaster (Apidianakis et al., 2005; Lutter et al., 2012; Haller et al., 2014; Martínez et al., 2016), silkworm (Kaito et al., 2002; Iiyama et al., 2007; Okuda et al., 2010; Chieda et al., 2011), and mealworm Tenebrio molitor (Park et al., 2014). In the present study, we attempted to identify secreted virulent factors of P. aeruginosa by using larval silkworm as the host. Through biochemical and bacterial mutagenesis studies, we obtained results showing that P. aeruginosa secretes a metalloprotease (elastase B) as a virulent factor into the hemolymph to manipulate the host PPO activation and AMP production. Possible molecular mechanisms for its effects on the humoral responses are explored and discussed.
2. MATERIALS AND METHODS
2.1. Identification of major extracellular proteins secreted by P. aeruginosa (PAO1)
The secreted proteins of PAO1 were isolated as described previously (Wu et al., 2008; Altindis et al., 2015) with some modifications. Briefly, PAO1 was cultured in Luria-Bertani medium at 37°C overnight and cell growth was monitored by taking absorbance readings at 600 nm until the A600nm reached about 2.3. The cells were removed by centrifugation at 8,000g for 20 min at 4°C, and the supernatant was filtered through a 0.22 μm pore-size filter. In 100 ml of the filtered supernatant, 20 ml of 100% trichloroacetic acid (TCA) was added and incubated on ice overnight before centrifugation at 16,000g at 4°C for 60 min. The resulting protein pellet was washed three times with 100% ice-cold acetone. Then the pellet was resuspended in SDS-PAGE sample buffer, incubated at 95°C for 5 min, and separated by SDS-PAGE. The major bands were excised and digested using In-Gel Tryptic Digestion Kit (Thermo, USA) and analyzed by ion trap LC-MS/MS on Thermo Scientific LTQ XL.
2.2. Purification of P. aeruginosa elastase B
The PAO1 culture supernatant was prepared as above, filtered through a 0.22 μm pore-size filter, and concentrated 30 fold by ultra-filtration in a centrifugal device (Amicon Ultra-15, NMWL: 10 kDa, Merck Millipore, America). The concentrate was dialyzed against phosphate buffered saline (PBS, 0.27 g/L KH2PO4, 1.42 g/L Na2HPO4, 8 g/L NaCl and 0.2 g/L KCl) overnight. Proteins were then precipitated by ammonium sulfate at 75% of saturation. After centrifugation at 16,000g for 30 min at 4°C, the protein pellet was dissolved in 50 mM Tris-HCl, pH 8.3 and dialyzed against the same buffer overnight. The dialyzed elastase B was loaded onto an anion-exchange Q-Sepharose Fast-Flow column (2 ml bed volume, GE Healthcare Life Sciences, Sweden) and eluted sequentially with 3 ml of 50 mM Tris-HCl, pH 8.3, containing 100 mM, 250 mM and 1M NaCl. The fractions containing elastase B were concentrated 5 fold in a centrifugal filter (Amicon Ultra-0.5, NMWL: 10 kDa, Merck Millipore, America), dialyzed against 50 mM Tris-HCl, pH 8.3, and fractionated on the Q-Sepharose column under the same condition. The concentrated elastase B fractions were loaded onto an HPLC gel filtration column (TSKgel SuperSW2000, TOSOH, Japan) running with 50 mM sodium phosphate buffer (pH 8.0) containing 150 mM NaCl at a flow rate of 0.5 ml/min. The purified elastase B fractions were pooled, concentrated, dialyzed against PBS, and stored at −80°C.
2.3. Preparation of PAO1 lasB deletion (ΔlasB) and ΔlasB complemented (ΔlasB-C) mutants
As described previously (Lin et al., 2015), the 866 bp upstream and 668 bp downstream fragments of the lasB gene were amplified by overlapping PCR using Pfu DNA polymerase and the primer pairs (lasB up F/R and lasB down F/R)(Table 1). The product was inserted into the BamHI and HindIII sites of pK18mobsacB, a suicide vector. The gentamicin resistance gene cassette from p34S-Gm was subsequently inserted into the HindIII site to yield the mutation plasmid pK-B. After mating an E. coli S17–1 derivative carrying pK-B with P. aeruginosa (PAO1) on LB plates at 37°C for 48 h, the cells were suspended in LB at appropriate dilutions and spread on LB plates containing chloramphenicol (to select against the donor strain) and gentamicin (to select for recipient with non-replicating plasmid integrated into its genome). Several colonies were transferred to LB medium and incubated at 37°C overnight before appropriate dilutions were spread on LB plates containing 12% sucrose for counter-selection against single cross-over mutants. Double cross-over mutants resulting in the nonpolar deletion of lasB were verified by PCR using external primer pair lasB up F and lasB down R and DNA sequencing. The resulting lasB deletion mutant contains the first 79 codons fused in frame with the last 65 codons.
Table 1.
Oligonucleotide primers used in this study
| Primers | sequence (5’-3’) |
|---|---|
| ΔlasB | |
| P.a lasB up F | CCTGGGATCCGGGCTGATCAGCA A GTTC |
| P.a lasB up R | GGTTGTACACGAGTTTGGACACGTCGATC |
| P.a lasB down F | GTCCAAACTCGTGTACAACCGTGCGTTC |
| P.a lasB down R | CCTGAAGCTTGAAACGGGTGATGCGATG |
| ΔlasB-C | |
| P.a lasB full F | CCTGAAGCTTGCGTCGGCCGAGTACTTC |
| P.a lasB full R | CCTGGGATCCGAGCTTACAACGCGCTCG |
| lasB expression | |
| P.a lasB F | CAACCAGAAGATCGGCAAGTA |
| P.a lasB R | CGAATTGGCCAACAGGTAGA |
| P.a GAPDH F | GCAGGTAGTGGCGATCAAT |
| P.a GAPDHR | GGTTCTGGTCGTTGGTGTAG |
| Quantitative real-time-PCR | |
| B.m gloverin2 F | ACGGACCTTCTGATTACGC |
| B.m gloverin2 R | CATTCTTGTTCGCCCAGT |
| B.m gloverin3 F | GACACGAGAATGGGAGGAG |
| B.m gloverin3 R | AAGACCCTGGTGCCGTAA |
| B.m gloverin4 F | CTTGACAAGAACACCCGCCT |
| B.m gloverin4 R | GTCTTGAAGGGATCTTCTGGAT |
| B.m IF4A F | TCTGGCATCATACCTTCTACAA |
| B.m IF4A R | TCTGTGTCATCTTTTCCCTGTT |
| P.a aprA F | AGTTCCAAGCTGGTGTTCTC |
| P.a aprA R | CCTTCTCGTTGAGGTTGATCTT |
| P.a lasA F | CGCTGAATGACGACCTGTT |
| P.a lasA R | CTTTCGGGTTGATGCTGTAGTA |
| P.a lepA F | AGGACTGGTCGGATACAGTT |
| P.a lepA R | TTTACGTTGAGGCCGATGAG |
| P.apiv F | ATACCCTGACCGTCGAACT |
| P.a piv R | GGAGTCGGCGAAATACGATAC |
| P.a GAPDH F(Q) | GCTGGTGTCGGTGGATTT |
| P.a GAPDH R(Q) | AGCATGCGGTTGGAGAAG |
The restriction enzyme sites BamHI and HindIII are underlined
To generate lasB deletion-complemented mutant, a 1549 bp DNA fragment containing the entire lasB opening reading frame was amplified by PCR using Pfu DNA-polymerase with lasB full F/R primer pairs (Table 1). After DNA sequence verification, the PCR product was inserted into the BamHI and HindIII sites of pBBR1MCS-5. The recombinant plasmid was electro-transfected into competent PAO1-ΔLasB recipient cells. The complemented strain was selected on gentamicin-kanamycin LB plate and DNA sequence verification.
2.4. Growth rate assay of PAO1 strains in vitro
To compare the growth rate of different strains of PAO1 in vitro, the bacteria were cultured till the A600nm = 0.6. 400 μl of these suspensions were added into 50 ml of Luria-Bertani medium respectively and cultured at 37°C. The A600 was measured by spectrophotometer every 2 h.
2.5. Silkworm rearing and bacteria preparation
The silkworm B. mori (Nistari strain) was reared on fresh mulberry leaves at 27°C in 70% relative humidity and a photoperiod of 13 : 11 (light : dark). Day 3, 5th instar silkworm larvae were used in all experiments. The PAO1 wide type (WT), lasB deletion mutant (ΔlasB), ΔlasB complemented (ΔlasB-C), and ΔlasB-C control (ΔlasB-pBBR1MCS-5) strains were respectively cultured at 37°C in LB medium till A600nm reached 0.8. The bacteria cells were collected by centrifugation at 8,000g for 20 min at 4°C. The cells were washed for three times with sterilized PBS and resuspended to 1×108 CFU/50 μl.
2.6. Phenoloxidase (PO) activity assay and prophenoloxidase (PPO) cleavage
After anesthetization on ice for 20 min, day 3, 5th instar silkworm larvae were injected with 10 μl of PBS, 10 μl of 30 μg/ml bovine serum albumin (BSA, BIO BASIC, Canada) in PBS 10 μl of 30 μg/ml purified elastase B or 50 μl of PBS, and 50 μl bacterial preparations (1×108 CFU/50 μl PBS). At different time points, the hemolymph was collected and centrifuged immediately at 16,000g for 30 s at 4°C to remove hemocytes and bacterial cells. Aliquots (2 μl) of plasma sample were added to wells of a 96-wells plate, 100 μl of 2 mM dopamine in 50 mM sodium phosphate was then added and mixed immediately. The absorbance at 490 nm was recorded every 30 s for 15 min on a microplate reader (Tecan, Pro200, Switzerland), and the maximum slope was determined.
Fifty μl of fresh plasm samples were collected as described above, added simultaneously to 1.5 ml Eppendorf tubes that contained 2 μl of 1 mg/ml curdlan from Alcaligenes faecalis (InvivoGen, USA), and incubated at room temperature for 30 min. These mixed samples (2 μl) were taken to measure the PO activity as described above.
For PPO activation assay in hemolymph of protein- or bacteria-injected silkworm, 50 μl aliquots of the plasma samples freshly collected from protein-injected larvae at 24 h or bacteria-injected at 6 h post injection were transferred to 1.5 ml Eppendorf tubes containing 2 μl of 1 mg/ml curdlan and incubated at room temperature for 30 min. Then, each sample was mixed with 52 μl of 2×SDS loading buffer and boiled immediately at 100°C for 5 min. After centrifugation at 10,000g for 3 min, the samples were separated by 8% SDS-PAGE, electro-transferred onto PVDF membrane, and subjected to immunoblot analysis using 1:10,000 diluted PPO1 and PPO2 antisera (gifts from Dr. Michael Strand) as the primary antibodies. The proteolytic processing of PPO1 and PPO2 to PO1 and PO2 was visualized using western blotting detection kit (Advansta, USA) on a Chemiluminescence imaging system (ChemiScope Mini2950, Clinx, China).
2.7. Melanotic encapsulation of Sepharose beads
Encapsulation of Sepharose beads in silkworm hemolymph was carried out as described before (Li et al., 2016). The QAE Sepharose Fast Flow chromatography beads (50−150 μm in diameter, Sigma, USA) were fully stained with 0.1% Congo-Red for 2 h and then washed three times with 50 mM Tris-HCl (pH 8.3). The washed beads (50 μl containing 100 beads) were incubated in 1 ml of purified elastase B or BSA (6 μg/ml in 50 mM Tris-HCl, pH 8.3) for 1 h at room temperature with gentle rotation. Fifty μl aliquots of the bead suspensions were injected into larval hemocoel. After 4 h, the larvae were dissected to recover the beads for observation under microscope.
2.8. Expression analysis of PAO1 lasB gene in the hemocoel of B. mori
Bacteria preparations of wild-type, ΔlasB, ΔlasB-C and ΔlasB-pBBR1MCS-5 PAO1 cells were separately injected into hemocoel of B. mori larvae. Total RNA was extracted from the PAO1 bacteria cells and hemocytes harvested from hemolymph of the infected silkworm at 3, 6 and 12 hpi using TriPure Isolation Reagent (Roche, Basel, Switzerland) and purified by Direct-zol™ RNA Miniprep (Zymo Research, California, USA). cDNA was synthesized from the purified RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) with random hexamers. Resulting cDNA was used as a template for PCR amplification by lasB F/R primer pair with GAPDH as an internal control, and the primers used in this experiment were listed in Table 1.
2.9. Elastolytic activity assay
Elastolytic activity of elastase B produced by PAO1s in hemolymph of PAO1-infected silkworm was determined in an ECR (Elastin-Congo-Red, Sigma, USA) assay (Pearson et al., 1997) with some modifications. At 4, 8, 12 and 24 h post infection, fresh hemolymph samples were quickly collected into tubes containing 10 μl of 1% 1-phenyl-2-thiourea (PTU) per 100 μl hemolymph. The samples were centrifuged at 10,000g at 4°C for 1 min and the supernatants were filtered through 0.22 μm pore-size membrane to remove hemocytes and bacteria. Fifty μl plasma was added to tubes containing 20 mg of ECR and 1 ml of 100 mM Tris-HCl, 1mM CaCl2 and incubated at 37°C for 24 h with rotation. Ten μl of 100 mM EDTA was added to stop the reaction. Insoluble ECR was removed by centrifugation, and A495nm was measured on a spectrophotometer, corrected by subtracting the A495nm of each sample that had been incubated in the absence of ECR. The assay was repeated independently with three biological replications.
2.10. Antimicrobial activity assay and antibacterial peptide genes expression analysis
Hemolymph from five silkworm larvae was collected at 24 h after injection of PBS, BSA and elastase B, or 12 h and 24 h after injection of PAO1. After heating at 100°C for 5 min, the supernatant was harvested after centrifugation. Zone of inhibition in a thin layer of LB agar plate assay was used to determine antibacterial activity of these samples as described previously (Hoffmann et al., 1981). In brief, 2 μl wild type PAO1 culture suspension in log-phase (A600nm ≈ 0.8) was mixed with 10 ml melt LB agar and poured into sterile Petri dishes. Holes with a diameter of 1.5 mm were made using a cut 1 ml pipet tip. Two μl of the supernatants were added to each hole and the zone of inhibition diameter was measured after overnight incubation at 37°C. The supernatants were also treated with 4× SDS loading buffer at 100 °C for 5 min and separated on a 16% Tricine-SDS- PAGE (Schagger, 2006). The bands were excised and digested using In-Gel Tryptic Digestion Kit (Thermo, USA), and subjected to ion trap LC-MS/MS analysis on Thermo Scientific LTQ.
For AMP genes expression analysis, fat body tissues from five silkworm larvae were collected at 6, 12 and 24 h after injection of PBS, BSA and elastase B, or 12 h after injection of PAO1. Total RNA of the collected fat body was extracted and expression of AMP genes was analyzed by quantitative real-time PCR performed on a Rotor Q (Qiagen, Hilden, Germany) with IF4A as an internal control. The primers were listed in Table 1. The experiment was performed with three biological replicates and the results were calculated using a relative quantitative method (2-ΔΔCt).
2.11. Determination of colony forming units (CFU) of PAO1 in the hemolymph of silkworm and silkworm survival assay
Day 3, 5th instar silkworm larvae were injected with freshly bacteria preparations. After 12, 24 and 36 h, hemolymph of 8 infected larvae from each group was collected in 1.5 ml Eppendorf tubes containing 2 μl of 1% PTU on ice. The samples were serially diluted with sterile PBS and spread on LB agar plate containing kanamycin sulfate (50 μg/ml). Colonies on the plates were counted after overnight incubation at 37°C.
Day 3, 5th instar larvae were anaesthetized on ice for 20 min and injected with 50 μL fresh bacteria preparations. Each group consisted of at least twenty larvae and survival was monitored every 12 h.
2.12. Statistical analyses
Graphpad Prism 5.0 was used to plot the data. The student’s test was used to analyze the statistical significance of PO activity, beads melanization assay, antimicrobial activity, CFUs and the fold change of gene transcripts. Log-rank (Mantel-Cox) test was used to analyze statistical significance.
3. RESULTS
3.1. Elastase B from PAO1 secretion suppresses PPO activation but stimulates AMP production in B. mori
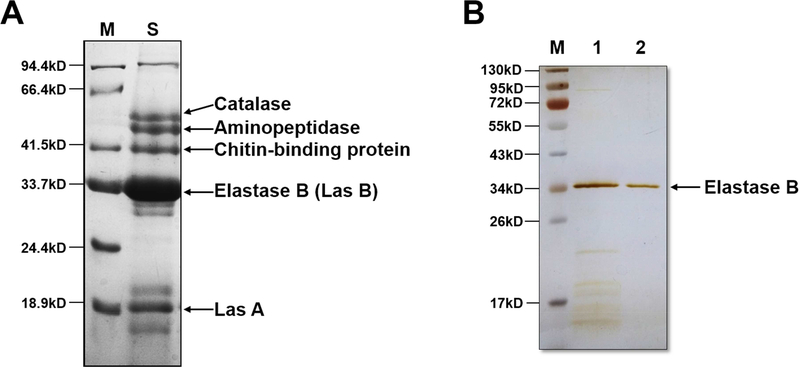
To identify the proteins PAO1 secretes, we collected the conditioned culture medium and separated the TCA-precipitated proteins by SDS-PAGE. The major bands on the gel were identified as catalase, aminopeptidase, chitin-binding protein, elastase B and Las A by MALDI-TOF/TOF (Figure 1A). Elastase B is the most abundant protein in the secretion. After separation by ion exchange and gel filtration chromatography, we obtained about 120 μg of purified elastase B (Figure 1B) from 3 liters of the PAO1 culture broth.
Figure 1. Identification of the PAO1 secreted proteins and purification of elastase B.
(A) The secreted proteins of PAO1 was separated by SDS-PAGE, visualized by Coomassie Brilliant Blue R-250, followed by in-gel trypsin digestion and mass spectrometry analysis. The identified proteins are indicated. Lane M, marker proteins; lane S, PAO1 secreted proteins. (B) Lane 1, fraction collected from 100 mM NaCl elution on the second run of Q-Fast Flow Sepharose column. Lane 2, purified elastase B from the HPLC gel filtration column. The protein bands were visualized by silver staining.
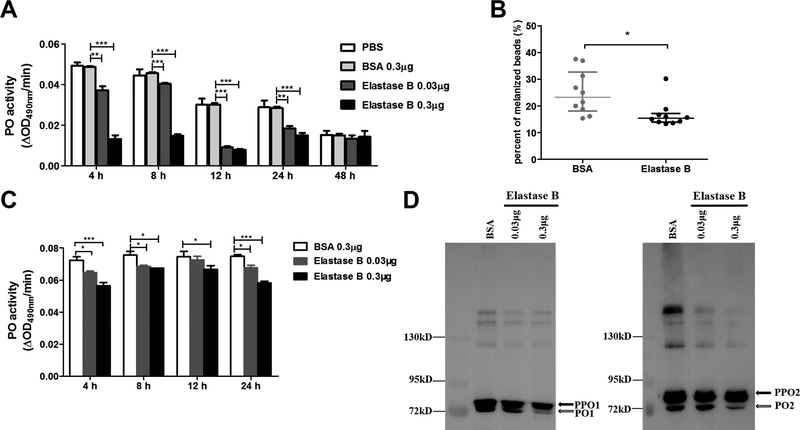
In order to investigate the effect of elastase B on the silkworm immune system, we injected the purified elastase B into the hemolymph of the tested larvae. The hemolymph samples were collected and subjected to PPO activation and PO activity assays. Within 24 h after injection of elastase B, PO activity decreased in an elastase B dosage-dependent manner (Figure 2A). Melanized beads in the elastase B-injected larvae were fewer than those in the BSA-injected larvae (Figure 2B), suggesting that elastase B had suppressed melanotic encapsulation. To examine the cleavage activation of PPO, we incubated the hemolymph samples with curdlan. PO activity of the curdlan-activated hemolymph was lower after elastase B treatment (Figure 2C). Immunoblot results revealed that cleavage of PPO1 and PPO2 was hindered by elastase B (Figure 2D). These data together indicate that PAO1 elastase B somehow inhibits the silkworm PPO activation.
Figure 2. PAO1 elastase B reduces silkworm phenoloxidase (PO) activity and melanization.
(A) The hemolymph PO activity was measured after injection of purified PAO1 elastase B into the larval silkworm, with phosphate-buffered saline (PBS) and bovine serum albumin (BSA) as controls. (B) Q-Fast Flow Sepharose beads were incubated with purified elastase B or BSA and then injected into the larval hemocoel. The beads were recovered from dissected larvae for melanization inspection under a microscope 4 h later. Each dot represents an individual larva. (C) PO activity of hemolymph from the larvae injected with purified PAO1 elastase B, activated by incubation with curdlan for 30 min. (D) The hemolymph samples were collected from larvae at 24 h post injection of PAO1 elastase B or BSA, activated by curdlan, and then separated on 8% SDS-PAGE followed by immunoblotting using silkworm PPO-1 and −2 antibodies.
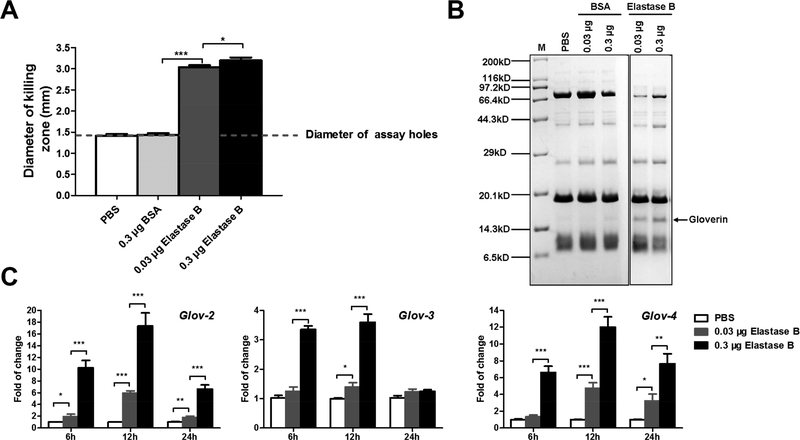
Along with PPO activation and melanization, induced production of antimicrobial peptides is another immune response. Therefore, we measured antibacterial activity of the silkworm hemolymph. In the samples from larvae that were injected with elastase B, we observed higher antimicrobial activity than in the samples from PBS- and BSA- injected larvae (Figure 3A). This observation prompted us to examine the change of AMP protein levels in the hemolymph after elastase B injection. We separated low molecular weight protein fraction of the heated hemolymph by Tricine-SDS-PAGE and detected a new band at about 16 kDa in the elastase B-injected hemolymph samples, and a higher dosage of the enzyme resulted in a higher intensity of the band (Figure 3B). Mass spectrometry identified this band as silkworm gloverins (data not shown). Quantitative PCR results confirmed that gloverin genes expression in the silkworm fat body were up-regulated after elastase B injection (Figure 3C). All these data suggest that PAO1 elastase B somehow stimulates silkworm AMP production.
Figure 3. PAO1 elastase B induces the production of silkworm gloverins.
(A) The silkworm hemolymph samples from larvae at 24 h after injection of PAO1 elastase B, PBS and BSA were subjected to bacterial growth inhibition assays. PAO1 was used as the indicating bacterium on the plates. The data was from five individual larvae. (B) Boiled hemolymph samples from the silkworm larvae 24 h post injection were separated on a 16% Tricine-SDS-PAGE and the bands were digested with trypsin and analyzed by mass spectrometry. The band corresponded to silkworm gloverin is indicated by an arrow. (C) Gloverin-2, −3, and −4 relative mRNA levels in the fat body collected from larvae at 12 h post injection were determined by quantitative real-time PCR. The samples from PBS-injected larvae were used as control. All analyses were performed with five biological replicates.
3.2. PAO1 secretes elastase B into the silkworm hemolymph after invasion
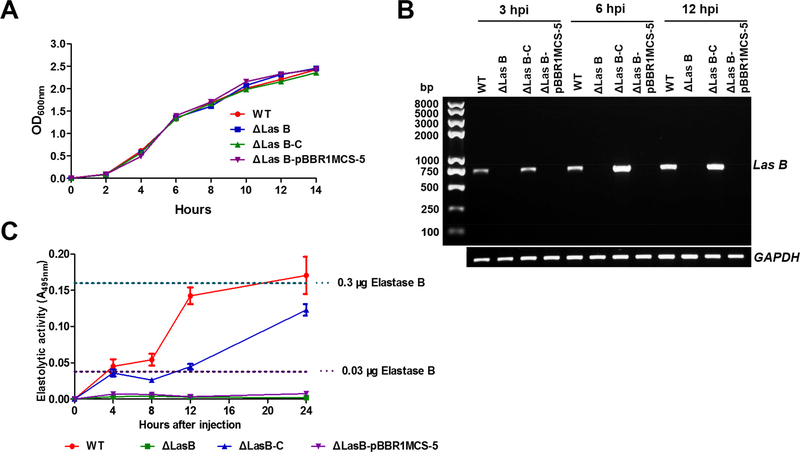
To validate above the results obtained using the purified elastase B, we made a deletion in the elastase B gene (ΔlasB) and its corresponding complemented (ΔlasB-C) PAO1 strains. Elastolytic activity assays confirmed our successful construction of the deletion and complemented strains (Supplemental Figure 1). These strains grew in LB medium with no apparent difference (Figure 4A), indicating that the deletion of lasB does not impair the growth of PAO1. The deletion did not affect expression of PAO1 extracellular proteases aprA, lasA, lepA, and protease IV (Supplemental Figure 2). We detected the expression of lasB after injection of wild-type and lasB complemented PAO1 cells into the hemolymph of the silkworm. Our results demonstrated that PAO1 lasB is expressed shortly (3 h) in the silkworm after injection (Figure 4B). Since the PAO1 elastase B antibody is not available for detection of elastase B protein, we measured elastolytic activity in the silkworm hemolymph after bacterial injection. A rapid increase of the activity was observed at 4 h after infection by the wild type (WT) and ΔlasB-C PAO1 (Figure 4C). Of note, at 12 h after injection of WT, the elastolytic activity was equivalent to that in hemolymph from silkworm that had been injected with 0.3 μg purified elastase B. This assured consistency of our data obtained from live bacterial cells and purified elastase B protein.
Figure 4. PAO1 lasB expresses in the hemolymph of silkworm larvae after infection.
(A) The growth curves of different PAO1 strains in Luria-Bertani broth. WT, PAO1 wild type; ΔlasB, lasB deletion mutant; ΔlasB-C, ΔlasB complemented; ΔlasB-pBBR1MCS-5, ΔlasB-C control. (B) PAO1 lasB expression after injection into the larval silkworm. The gene expression was analyzed by semi-quantitative PCR with GAPDH acting as an internal control. (C) Elastolytic activity in the hemolymph of PAO1 infected-silkworm larvae. The dash lines represent activity in the hemolymph after injection of purified elastase B.
3.3. Deletion of lasB results in partial loss of the ability of PAO1 in PPO activation inhibition and to AMP induction in the silkworm
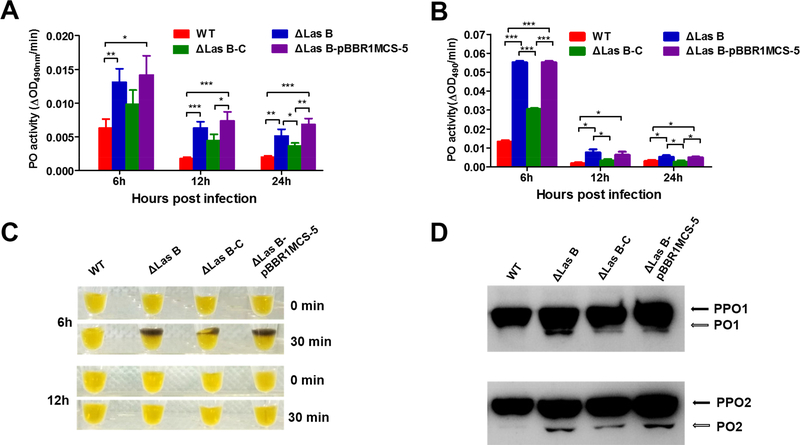
We next examined PO activity and PPO activation in the hemolymph collected from PAO1 infected larval silkworm. Compared with WT infected, ΔlasB infected samples had higher PO activity with (Figure 5A) or without activation by curdlan (Figure 5B), suggesting that PAO1 elastase B contributes to the inhibition of PO activity in the silkworm. After 6 h infection, ΔlasB-infected samples showed obvious melanization but WT- infected sample did not (Figure 5C). Complementation of ΔlasB partially restored the inhibition of PO activity and melanization (Figure 5A, 5B and 5C), implying that other PAO1 factors contribute to the host PPO activation inhibition along with elastase B during the infection. Immunoblotting revealed the proteolytic activation of PPO in samples from larvae at 6 h post infection with different PAO1 strains (Figure 5D) was consistent with the PO activity data and hemolymph melanization.
Figure 5. Deletion of lasB causes an increase in PO activity of hemolymph from PAO1 infected silkworm larvae.
(A) PO activity of the hemolymph from larvae injected with PAO1 cells. (B) PO activity of hemolymph collected from larvae injected with PAO1 cells and activated by curdlan. (C) Melanization of hemolymph samples collected from larvae at 6 and 12 h after injection with PAO1 cells and activated by curdlan. These samples were placed on bench and photographed immediately (0 min) after activation and 30 min later. (D) Proteolytic processing of PPO in the hemolymph at 6 h post infection. The hemolymph samples were collected from larvae 6 h post injection with PAO1 cells and activated by curdlan. The samples were separated by 8% SDS-PAGE and visualized by immunoblotting with silkworm PPO antibodies.
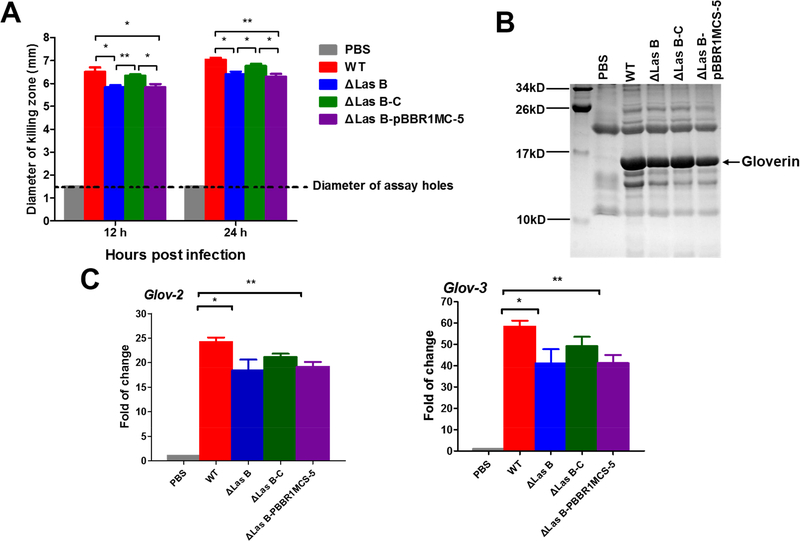
We compared the antimicrobial activity of silkworm hemolymph after PAO1 infection. Overall, PAO1 infection induced strong antibacterial activity in the hemolymph (Figure 6A). Hemolymph from PAO1 ΔlasB-infected larvae displayed slightly lower activity than that from the WT-infected larvae, and PAO1 ΔlasB-C-infected hemolymph had an activity between the WT- and ΔlasB-infected samples. Gloverin protein levels (Figure 6B) and mRNA levels (Figure 6C) in the silkworm infected with different PAO1 strains exhibited similar patterns as the antibacterial activity. These results confirm that elastase B is one of the factors that induce AMP production when PAO1 infects the silkworm.
Figure 6. Deletion of lasB decreases the expression of the silkworm gloverin genes after PAO1 infection.
(A) Anti-PAO1 activity of the hemolymph from PAO1-infected larval silkworm. (B) Comparison of the gloverin levels in the hemolymph from PAO1-infected larvae at 24 hpi after Tricine-SDS-PAGE. The band corresponded to gloverin was confirmed by mass spectrometry. (C) Quantitative real-time PCR analysis of expression of gloverin-2 and −3 in fat body from larvae at 12 h post infection.
3.4. Deletion of lasB results in higher susceptibility of PAO1 to the silkworm defense
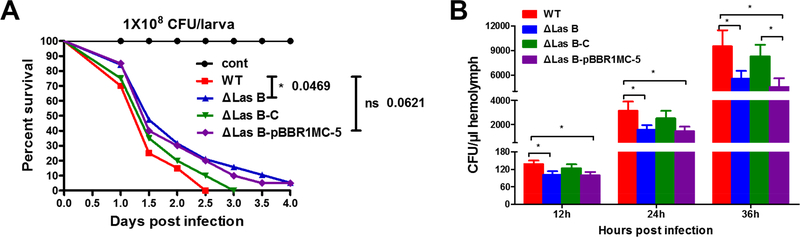
We finally compared the survival of silkworm larvae and proliferation of the bacteria in the silkworm hemolymph after PAO1 infection. We injected 1×108 cells of the four PAO1 strains into each larvae at the dosage used in the assays of lasB expression, elastolytic activity, silkworm PO activity, PPO activation, and antibacterial activity. No larvae survived over 2.5 days in the group of WT infection, whereas about 5% survived at 4 days in the group of ΔlasB-infected group (Figure 7A). Bacterial colony counting indicted that PAO1 propagated fast in the silkworm hemolymph 12 h after injection (Figure 7B). PAO1 ΔlasB grew slower than the WT in the hemolymph, indicating that deletion of ΔlasB caused PAO1 to be less virulent and more susceptible to the host immune reactions. If we pre-injected the silkworm with PO inhibitor 1-phenyl-2-thiourea (PTU), the survival of infected larvae and bacterial CFU in the larval hemolymph had no significant difference between the WT- and ΔlasB-infected groups (Supplemental Figure 3). This result reinforced the critical role of PO in killing of bacterium PAO1 and the potential role of PAO1 elastase B in counteracting of silkworm PO pathway.
Figure 7. Deletion of lasB reduces the pathogenicity of PAO1 to the silkworm larvae.
(A) Survival curve of the silkworm larvae infected with 1×108 CFU PAO1 per larva. (B) CFU of PAO1 in hemolymph from the infected larvae.
4. DISCUSSION
Bacteria produce proteases during invasion and infection of their hosts. For instance, the honey bee pathogenic bacterium Paenibacillus larvae produces a metalloprotease in the host midgut after it orally infects the bees (Antúnez et al., 2011). The insect pathogen P. luminescens produces an M4 metalloprotease PrtS to induce melanization in the hemolymph of M. sexta and Drosophila during infection (Held et al., 2007). Pseudomonas entomophila produces an alkaline zinc metalloprotease AprA to suppress the IMD pathway of Drosophila (Liehl et al., 2006), and cellular and humoral immune responses of bean bug Riptortus pedestris (Lee et al., 2018). In the present study, we showed that PAO1 produces an M4 metalloprotease elastase B in the hemolymph of silkworm during infection (Figure 4B and 4C). Serralysin, an M12 metalloprotease secreted by S. marcescens, may degrade a serine protease homolog, SPH-1, which is involved in melanization and nodulation in the silkworm (Tokura et al., 2014) and thus suppress the immune responses (Ishii et al., 2014a). In mammalian infection models, elastase B enhance the virulence of P. aeruginosa strains through directly destructing tissue and damaging cell functions, or indirectly interfering with host-defense (Nicas and Iglewski, 1985; Adekoya and Sylte, 2009). Elastase B protects P. aeruginosa cells against phagocytosis by degrading the collectin which is a strong inducer of plant, insect and mammalian immune responses (Samakovlis et al., 1992; Casilag et al., 2015; Hajam et al., 2017; Cui et al., 2018) and it disrupts human vascular cells and endothelial barrier (Beaufort et al; 2011, 2013). Biofilm formation is the predominant growth mode for bacteria in various environments and provides a way to escape host immunity systems (Fux et al., 2003). Elastase B can also increase P. aeruginosa attachment, microcolony and biofilm formation to escape host immune responses (Yu et al., 2014). In the greater wax moth larvae, injection of thermolysin, an M4 metalloprotease, induced the PO and antibacterial activities in the hemolymph (Griesch et al., 2000; Altincicek et al., 2007). Similarly, challenge with live P. aeruginosa cells or injection with elastase B protein up-regulated PO and antibacterial activities in the larvae of G. mellonella (Andrejko et al., 2011, 2014). A recent study in Drosophila showed that a P. aeruginosa mutant strain of lasB caused less expression of drosomycin, an AMP induced by the Toll pathway (Issa et al., 2018). In our study, by using the purified protein and mutant bacterial strains, we clearly demonstrated that PAO1 elastase B plays a role in AMP induction but PPO activation suppression in the silkworm. Regarding the effect of metalloproteases on the insect PPO activation system, we speculate that the difference between the results from us and others might arise from the differences in bacteria strains (Andrejko et al., 2014), hosts, and experimental procedures.
AMP generation and PO-catalyzed melanization are two critical defense responses of insects. In general, AMP gene transcription is mediated through the Toll or IMD pathways upon recognition of invading pathogens or parasites by the host. Activation of the Toll pathway and PPO is mediated by an extracellular clip-domain serine protease cascade (Kambris et al., 2006; Tang et al., 2006; An et al., 2009; Kanost and Jiang, 2015), which is tightly regulated by serine protease inhibitors in the serpin superfamily (Meekins et al., 2016). To find how the PAO1 elastase B affects silkworm PPO activation and AMP production, we examined proteins in the elastase B-injected hemolymph of larval silkworm and tobacco hornworm (Supplemental Figure 4). Injection of elastase B resulted in decrease of silkworm inter-alpha-trypsin inhibitor H4-like protein, hemocyte aggregation inhibitor protein (HAIP), serpin-1A and serpin-9 (Supplemental Figure 4A). Inter-alpha-trypsin inhibitor plays a major role in extracellular matrix stability and integrity (Bost et al., 1998). In our study, we found that a high dosage of elastase B caused a breakup of the silkworm gut tissue (data not shown). Human vascular cells and endothelial barrier were also susceptible to elastase B (Beaufort et al., 2011, 2013). HAIPs were suggested to serves in Drosophila hemolymph clotting and epithelial defense against pathogens (Kucerova et al., 2015; Pesch et al., 2015). Among the decreased proteins, serpin-1A and −9 are of particular interest. We previously identified serpin-5 as a negative regulator of PPO activation and AMP induction pathways in the silkworm (Li et al., 2016). We demonstrated in this study that injection of elastase B resulted in a decrease of serpin-1A and −9 and an increase of AMP production. This implies that these two serpins might be involved in regulation of silkworm AMP induction. To take the advantage of the antibodies against serine proteases, serine protease homologs (SPHs) and serpins in M. sexta hemolymph, we injected elastase B into hemocoel of the tobacco hornworm and examined the changes of hemolymph protein by immunoblotting (Supplemental Figure 4B). Injection of elastase B into tobacco hornworm strongly inhibited PO activity in the hemolymph (Supplemental Figure 5A and 5B), as that did to the silkworm (Figure 2). Among about twenty proteins we examined, activation of SPH-1, −2 and −33 were somewhat hampered by elastase B (Supplemental Figure 4B). The interactions among SPH-1/2, PPOs and PPO-activating protease (PAP) known to be required for generating active PO (Yu et al., 2003; Gupta et al., 2005). It is possible that elastase B impaired SPH-1 and −2 cleavage activation by an unknown mechanism and thus reduced hemolymph PPO activation in the hornworm. Given the finding that silkworm SPH-1 is involved in melanization (Tokura et al., 2014), it is possible that elastase B suppresses silkworm PPO activation in a similar manner.
It has been reported that another protease secreted by PAO1, protease IV, interferes with AMP production but activates PPO and that elastase B induces generation of AMPs in the mealworm (Park et al., 2014). We found that injection of elastase B into Tenebrio molitor larvae strongly reduced PPO activation (Supplemental Figure 5C), as observed in the silkworm and tobacco hornworm. These results raise an interesting question: why a simple bacterium needs different secreted proteases to modulate the immune pathways of its insect host in opposite ways? As a matter of fact, bacteria produce an array of proteases as virulent factors to interfere with host defense responses. These proteases may destruct components of the immune pathways, interfere intracellular immune signaling, inactivate AMPs, impair phagocytosis, and so on (Potempa and Pike, 2009). From P. aeruginosa KU2 strain, a large exoprotease (LepA) was shown to activate NF-κB-driven promotors (Kida et al., 2008). LepA acts in concert with hemolytic phospholipase C (PlcH) for the virulence and growth of KU2 in mice (Kida et al., 2011). A recent work showed that, in addition to alkaline protease AprA, elastase B can degrade exogenous flagellin to prevent flagellin-mediated immune recognition. It is thought that production of the two proteases with flagellin-degrading activity provides an infallible mechanism for P. aeruginosa to evade recognition by the host (Casilag et al., 2015). The findings in our current study and made by others (Park et al., 2014) suggest that a more complicated scenario about the interplay of bacterial virulent factors during invasion and infection of the host.
In summary, we presented evidence that PAO1 secretes elastase B as a virulent factor during its infection of a lepidopteran insect, B. mori. Elastase B inhibits PPO activation but somehow induces AMP production in the silkworm hemolymph. Our study suggests more complex interactions between virulent factors of bacterial pathogens and host immune systems.
Supplementary Material
Supplementary figure 1. Elastolytic activity assays of PAO1 wild type (WT), ΔlasB, ΔlasB-C and ΔlasB-pBBR1MCS-5. The activity was determined by ECR assay (30) with some modification. These PAO1 strains were cultured in LB medium at 37°C overnight and the cell growth was monitored by taking absorbance readings at 600 nm until A600 reached about 2.3. Then samples were centrifuged at 8,000g at 4°C for 20 min and the supernatants were filtered through 0.22 μm pore-size membrane to remove residual bacteria. Fifty μl supernatant was added to 1.5 ml tubes containing 20 mg of ECR and 1 ml of 100 mM Tris-HCl, 1mM CaCl2 and incubated at 37°C for 24 h with rotation. Ten μl of 100 mM EDTA was added to stop the reaction. Insoluble ECR was spun down, and the result was recorded by photography (A). Absorbance at 495 nm of the supernatants from above treatment was measured on a spectrophotometer, corrected by subtracting A495nm of samples that was incubated in the absence of ECR (B).
Supplementary figure 2. Effect of deletion of lasB on the expression of other secreted protease genes, including aprA, lasA, lepA, and protease IV of PAO1. The PAO1 wild type (WT), ΔlasB, ΔlasB-C and ΔlasB-pBBR1MCS-5 were cultured as above and harvested till the A600nm=1.0. The total RNA was extracted using TriPure Isolation Reagent (Roche, Basel, Switzerland) and purified by Direct-zol RNA Miniprep (Zymo Research, California, USA). cDNA was synthesized from the purified RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) with random hexamers. The synthesized cDNA was used as template for aprA, lasA, lepA and piv expression analysis by quantitative real-time PCR on a Rotor Q thermocycler (Qiagen, Hilden, Germany) with GAPDH as an internal control. The primers used in quantitative real-time PCR were listed in Table 1. The experiment was performed with three biological replicates and the results were calculated using a relative quantitative method (2-ΔΔCt). The expression of aprA (A), lasA (B), lepA (C), and protease IV (D) of these PAO1 strains were no difference.
Supplementary figure 3. (A) Survival of silkworm larvae infected with 1×108 CFU PAO1 per larva. (B) CFU of PAO1 in hemolymph from the infected larvae. Note: Twenty μl of PO inhibitor, 20 mM PTU was injected into the tested larvae before injection of bacteria.
Supplementary figure 4. Injection of PAO1 elastase B caused change of hemolymph proteins of the silkworm (A) and tobacco hornworm (B). (A) Hemolymph collected from elastase B-injected silkworm larvae (24 h after injection) was separated by SDS-PAGE, and the bands showing changes between treatments were analyzed by mass spectrometry. (B) Hemolymph samples were collected at 6 and 10 h after day 2, 5th instar M. sexta larvae had been injected with 50 μl PBS of elastase B (1 μg in 50 μl PBS) per larva. After hemocyte removal by centrifugation at 16,000g for 1 min, plasma (1 μl) was incubated with 9 μl 20 mM Tris-HCl, pH 7.5, 0.001% Tween-20, 5 mM CaCl2, 0.001% PTU, and 1 μl Micrococcus luteus (1 μg/μl) for 20 min at room temperature prior to 10% SDS-PAGE and immunoblot analysis using 1:2000 diluted antisera against SPH1, SPH2 and SPH33 as the primary antibodies, respectively.
Supplementary figure 5. PO activity decreased in hemolymph from the tobacco hornworm (A and B) and mealworm (C) after treatment of PAO1 elastase B. (A) Hemolymph samples were collected at 6 and 10 h after day 2, 5th instar M. sexta larvae had been injected with 50 μl PBS or elastase B (1 μg in 50 μl PBS) per larva. After hemocyte removal by centrifugation, plasma (1 μl) was mixed with 9 μl 20 mM Tris-HCl, pH 7.5, 0.001% Tween-20, 5 mM CaCl2 for 2 h at room temperature prior to PO activity assay. (B) Hemolymph samples were collected as above. After hemocyte removal by centrifugation, plasma (1 μl) was mixed with 1 μg M. luteus and 9 μl 20 mM Tris-HCl, pH 7.5, 0.001% Tween-20, 5 mM CaCl2 for 20 min at room temperature prior to PO activity assay. (C) Tenebrio molitor larvae (body length: about 20 mm) were injected with 10 μl of PBS or 10 μl of purified elastase B (10 ng/μl or 1 ng/μl). After 1 and 4 h, hemolymph samples were collected and centrifuged immediately at 16,000g for 1 min to remove hemocytes. PO activity of the plasma was measured.
Highlights.
Pseudomonas aeruginosa expresses and releases elastase B in the hemolymph after infection of larval silkworm.
Elastase B compromises prophenoloxidase activation and melanization of the silkworm hemolymph.
Elastase B upregulates expression of antimicrobial peptide gloverin in the silkworm.
Elastase B contributes to the growth of P. aeruginosa in the silkworm and pathogenicity of P. aeruginosa to the host.
ACKNOWLEDGMENT
This study was supported by the National Science Foundation of China under grants 31272497 and 31772530 to ZL; and by the National Institutes of Health under grants AI112662 and GM58634 to HJ. The authors would like to thank Dr. Michael Strand at University of Georgia for providing the antisera to the silkworm PPO-1 and −2.
Abbreviations:
- AMP
antimicrobial peptide
- BSA
bovine serum albumin
- CFU
colony forming units
- HPLC
high-performance liquid chromatography
- IMD
immune deficiency
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LB
Luria-Bertani
- PBS
phosphate-buffered saline
- PO
phenoloxidase
- PPO
prophenoloxidase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TCA
trichloroacetic acid
Footnotes
DECLARATION OF INTEREST STATEMENT
The authors declare that there are no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adekoya OA, Sylte I, 2009. The thermolysin family (M4) of enzymes: therapeutic and biotechnological potential. Chem. Biol. Drug Des 73, 7–16. [DOI] [PubMed] [Google Scholar]
- Altincicek B, Linder M, Linder D, Preissner KT, Vilcinskas A, 2007. Microbial metalloproteinases mediate sensing of invading pathogens and activate innate immune responses in the lepidopteran model host Galleria mellonella. Infect. Immun 75, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altindis E, Dong T, Catalano C, Mekalanos J, 2015. Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair. MBio 2, e00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Ishibashi J, Ragan EJ, Jiang H, Kanost MR, 2009. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem 284, 19716–19726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejko M, Mizerska-Dudka M, 2011. Elastase B of Pseudomonas aeruginosa stimulates the humoral immune response in the greater wax moth, Galleria mellonella. J. Invertebr. Pathol 107, 16–26. [DOI] [PubMed] [Google Scholar]
- Andrejko M, Mizerska-Dudka M, Jakubowicz T, 2009. Antibacterial activity in vivo and in vitro in the hemolymph of Galleria mellonella infected with Pseudomonas aeruginosa. Comp. Biochem. Physiol. B Biochem. Mol. Biol 152, 118–123. [DOI] [PubMed] [Google Scholar]
- Andrejko M, Zdybicka-Barabas A, Cytryńska M, 2014. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J. Invertebr. Pathol 115, 14–25. [DOI] [PubMed] [Google Scholar]
- Antúnez K, Arredondo D, Anido M, Zunino P, 2011. Metalloprotease production by Paenibacillus larvae during the infection of honeybee larvae. Microbiology 157, 1474–1480. [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL, Davis RW, Rahme LG, 2005. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc. Natl. Acad. Sci. U. S. A 102, 2573–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufort N, Corvazier E, Hervieu A, Choqueux C, Dussiot M, Louedec L, Cady A, de Bentzmann S, Michel JB, Pidard D, 2011. The thermolysin-like metalloproteinase and virulence factor LasB from pathogenic Pseudomonas aeruginosa induces anoikis of human vascular cells. Cell Microbiol. 13, 1149–1167. [DOI] [PubMed] [Google Scholar]
- Beaufort N, Corvazier E, Mlanaoindrou S, Bentzmann SD, Pidard D, 2013. Disruption of the endothelial barrier by proteases from the bacterial pathogen Pseudomonas aeruginosa: implication of matrilysis and receptor cleavage. PLoS One 8, e75708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Diarra-Mehrpour M, Martin JP, 1998. Inter-α-trypsin inhibitor proteoglycan family. Eur. J. Biochem 252, 339–346. [DOI] [PubMed] [Google Scholar]
- Casilag F, Lorenz A, Krueger J, Klawonn F, Weiss S, Häussler S, 2015. The LasB elastase of Pseudomonas aeruginosa acts in concert with alkaline protease AprA to prevent Flagellin-mediated immune recognition. Infect. Immun 84, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieda Y, Iiyama K, Lee JM, Kusakabe T, Yasunaga-Aoki C, Shimizu S, 2011. Virulence of an exotoxin A-deficient strain of Pseudomonas aeruginosa toward the silkworm, Bombyx mori. Microb. Pathog 51, 407–414. [DOI] [PubMed] [Google Scholar]
- Cui B, Liu X, Fang Y, Zhou P, Zhang Y, Wang Y, 2018. Flagellin as a vaccine adjuvant. Expert Rev. Vaccines 17, 335–349. [DOI] [PubMed] [Google Scholar]
- Doberenz S, Eckweiler D, Reichert O, Jensen V, Bunk B, Spröer C, Kordes A, Franggipani E, Luong K, Korlach J, Heeb S, Overmann J, Kaever V, Haussler S, 2017. Identification of a Pseudomonas aeruginosa PAO1 DNA methyltransferase, its targets, and physiological roles. MBio 8, e02312–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos I, Boundy S, Joyce SA, Aslam S, Marshall JW, Cox RJ, Simpson TJ, Clarke DJ, ffrench-Constant RH, Reynolds SE, 2007. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc. Natl. Acad. Sci. U. S. A 104, 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW, 2003. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti-Infect. Ther 1, 667–683. [DOI] [PubMed] [Google Scholar]
- Griesch J, Wedde M, Vilcinskas A, 2000. Recognition and regulation of metalloproteinase activity in the haemolymph of Galleria mellonella: a new pathway mediating induction of humoral immune responses. Insect Biochem. Mol. Biol 30, 461–472. [DOI] [PubMed] [Google Scholar]
- Gupta S, Wang Y, Jiang H, 2005. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem. Mol. Biol 35, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam IA, Dar PA, Shahnawaz I, Jaume JC, Lee JH, 2017. Bacterial flagellin-a potent immunomodulatory agent. Exp. Mol. Med 49, e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Limmer S, Ferrandon D, 2014. Assessing Pseudomonas virulence with a nonmammalian host: Drosophila melanogaster. Methods Mol. Biol 1149, 723–740. [DOI] [PubMed] [Google Scholar]
- Held KG, LaRock CN, D’Argenio DA, Berg CA, Collins CM, 2007. A metalloprotease secreted by the insect pathogen Photorhabdus luminescens induces melanization. Appl. Environ. Microb 73, 7622–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Hultmark D, Boman HG, 1981. Insect immunity: Galleria mellonella and other lepidoptera have cecropia-P9-like factors active against gram-negative bacteria. Insect Biochem. 11, 537–548. [Google Scholar]
- Huang L, Cheng T, Xu P, Cheng D, Fang T, Xia Q, 2009. A genome-wide survey for host response of silkworm, Bombyx mori during pathogen Bacillus bombyseptieus infection. PLoS One 4, e8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GD, Anbutsu H, Kutsukake M, Fukatsu T, 2003. Hidden from the host: Spiroplasma bacteria infecting Drosophila do not cause an immune response, but are suppressed by ectopic immune activation. Insect Mol. Biol 12, 93–97. [DOI] [PubMed] [Google Scholar]
- Iiyama K, Chieda Y, Lee JM, Kusakabe T, Yasunaga-Aoki C, Shimizu S, 2007. Effect of superoxide dismutase gene inactivation on virulence of Pseudomonas aeruginosa PAO1 toward the silkworm, Bombyx mori. Appl. Environ. Microbiol 73, 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Adachi T, Hamamoto H, Sekimizu K, 2014a. Serratia marcescens suppresses host cellular immunity via the production of an adhesion-inhibitory factor against immunosurveillance cells. J. Biol. Chem 289, 5876–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Adachi T, Hara T, Hamamoto H, Sekimizu K, 2014b. Identification of a Serratia marcescens virulence factor that promotes hemolymph bleeding in the silkworm, Bombyx mori. J. Invertebr. Pathol 117, 61–67. [DOI] [PubMed] [Google Scholar]
- Ishii K, Adachi T, Imamura K, Takano S, Usui K, Suzuki K, Hamamoto H, Watanabe T, Sekimizu K, 2012. Serratia marcescens induces apoptotic cell death in host immune cells via a lipopolysaccharide- and flagella-dependent mechanism. J. Biol. Chem 287, 36582–36592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa N, Guillaumot N, Lauret E, Matt N, Schaeffer-Reiss C, Van Dorsselaer A, Reichhart JM, Veillard F, 2018. The Circulating Protease Persephone Is an Immune Sensor for Microbial Proteolytic Activities Upstream of the Drosophila Toll Pathway. Mol. Cell 69, 539–550 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Rahme LG, Ausubel FM, 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol 182, 3843–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C, Akimitsu N, Watanabe H, Sekimizu K, 2002. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog 32, 183–190. [DOI] [PubMed] [Google Scholar]
- Kambris Z, Brun S, Jang IH, Nam HJ, Romeo Y, Takahashi K, Lee WJ, Ueda R, Lematitre B, 2006. Drosophila immunity: A large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr. Biol 16, 808–813. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, 2015. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect Sci 11, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y, Higashimoto Y, Inoue H, Shimizu T, Kuwano K, 2008. A novel secreted protease from Pseudomonas aeruginosa activates NF-κB through protease-activated receptors. Cell Microbiol. 10, 1491–1504. [DOI] [PubMed] [Google Scholar]
- Kida Y, Shimizu T, Kuwano K, 2011. Cooperation between LepA and PlcH contributes to the in vivo virulence and growth of Pseudomonas aeruginosa in mice. Infect. Immun 79, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Nadal-Jimenez P, Cool RH, Quax WJ, 2014. Assessing Pseudomonas virulence with nonmammalian host: Galleria mellonella. Methods Mol. Biol 1149, 681–688. [DOI] [PubMed] [Google Scholar]
- Kucerova L, Broz V, Arefin B, Maaroufi HO, Hurychova J, Strnad H, Zurovec M, Theopold U, 2015. The Drosophila Chitinase-like protein IDGF3 Is involved in protection against nematodes and in wound healing. J. Innate Immun 8, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Jang SH, Kim BH, Shibata T, Yoo J, Jung Y, Kawabata SI, Lee BL, 2018. Insecticidal activity of the metalloprotease AprA occurs through suppression of host cellular and humoral immunity. Dev. Comp. Immunol 81, 116–126. [DOI] [PubMed] [Google Scholar]
- Li J, Ma L, Lin Z, Zou Z, Lu Z, 2016. Serpin-5 regulates prophenoloxidase activation and antimicrobial peptide pathways in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol 73, 27–37. [DOI] [PubMed] [Google Scholar]
- Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B, 2006. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2, e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Cheng J, Chen K, Guo C, Zhang W, Yang X, Ding W, Ma L, Wang Y, Shen X, 2015. The icmF3 locus is involved in multiple adaptation- and virulence-related characteristics in Pseudomonas aeruginosa PAO1. Front. Cell. Infect. Microbiol 5, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter EI, Purighalla S, Duong J, Storey DG, 2012. Lethality and cooperation of Pseudomonas aeruginosa quorum-sensing mutants in Drosophila melanogaster infection models. Microbiology 158, 2125–2132. [DOI] [PubMed] [Google Scholar]
- Martínez E, Campos-Gómez J, 2016. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat. Commun 7, 13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins DA, Kanost MR, Michel K, 2016. Serpins in arthropod biology. Semi. Cell Dev. Biol 62, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Casey M, Frank DW, Ausubel FM, Drenkard E, 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun 71, 2404–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T, Iglewski BH, 1985. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can. J. Microbiol 31, 387–392. [DOI] [PubMed] [Google Scholar]
- Okuda J, Hayashi N, Okamoto M, Sawada S, Minagawa S, Yano Y, Gotoh N, 2010. Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na,K-ATPase regulator, FXYD3. Infect. Immun 78, 4511–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Kim SK, So YI, Park HY, Li XH, Yeom DH, Lee MN, Lee BL, Lee JH, 2014. Protease IV, a quorum sensing-dependent protease of Pseudomonas aeruginosa modulates insect innate immunity. Mol. Microbiol 94, 1298–1314. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Pesci EC, Iglewski BH, 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol 179, 5756–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch YY, Riedel D, Patil KR, Loch G, Behr M, 2015. Chitinases and imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci. Rep 6, 18340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Pike RN, 2009. Corruption of innate immunity by bacterial proteases. J. Innate Immun 1, 70–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadekuzzaman M, Park Y, Lee S, Kim K, Jung JK, Kim Y, 2017. An entomopathogenic bacterium, Xenorhabdus hominickii ANU101, produces oxindole and suppresses host insect immune response by inhibiting eicosanoid biosynthesis. J. Invertebr. Pathol 145, 13–22. [DOI] [PubMed] [Google Scholar]
- Samakovlis C, Asling B, Boman HG, Gateff E, Hultmark D, 1992. In vitro induction of cecropin genes--an immune response in a Drosophila blood cell line. Biochem. Biophys. Res. Commun 188, 1169–1175. [DOI] [PubMed] [Google Scholar]
- Schagger H, 2006. Tricine-SDS-PAGE. Nat. Protoc 1, 16–22. [DOI] [PubMed] [Google Scholar]
- Strand MR, 2008. The insect cellular immune response. Insect Sci. 15, 1–14. [Google Scholar]
- Tanaka H, Ishibashi J, Fujita K, Nakajima Y, Sagisaka A, Tomimoto K, Suzuki N, Yoshiyama M, Kaneko Y, Iwasaki T, Sunagawa T, Yamaji K, Asapka A, Mita K, Yamakawa M, 2008. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem. Mol. Biol 38, 1087–1110. [DOI] [PubMed] [Google Scholar]
- Tang H, Kambris Z, Lemaitre B, Hashimoto C, 2006. Two proteases defining a melanization cascade in the immune system of Drosophila. J. Biol. Chem 281, 28097–28104. [DOI] [PubMed] [Google Scholar]
- Tokura A, Fu GS, Sakamoto M, Endo H, Tanaka S, Kikuta S, Tabunoki H, Sato R, 2014. Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J. Insect Physiol 60, 40–49. [DOI] [PubMed] [Google Scholar]
- Vallet-Gely I, Lemaitre B, Boccard F, 2008. Bacterial strategies to overcome insect defences. Nat. Rev. Micro 6, 302–313. [DOI] [PubMed] [Google Scholar]
- Wu HY, Chung PC, Shih HW, Wen SR, Lai EM, 2008. Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens. J. Bacteriol 190, 2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Cheng T, Ye M, Deng X, Yi H, Huang Y, Tan X, Han D, Wang B, Xiang Z, Cao Y, Xia Q, 2011. Functional divergence among silkworm antimicrobial peptide paralogs by the activities of recombinant proteins and the induced expression profiles. PLoS One 6, e18109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, He X, Xie W, Xiong J, Sheng H, Guo S, Huang C, Zhang D, Zhang K, 2014. Elastase LasB of Pseudomonas aeruginosa promotes biofilm formation partly through rhamnolipid-mediated regulation. Can. J. Microbiol 60, 227–235. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Jiang H, Wang Y, Kanost MR, 2003. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol 33, 197–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Elastolytic activity assays of PAO1 wild type (WT), ΔlasB, ΔlasB-C and ΔlasB-pBBR1MCS-5. The activity was determined by ECR assay (30) with some modification. These PAO1 strains were cultured in LB medium at 37°C overnight and the cell growth was monitored by taking absorbance readings at 600 nm until A600 reached about 2.3. Then samples were centrifuged at 8,000g at 4°C for 20 min and the supernatants were filtered through 0.22 μm pore-size membrane to remove residual bacteria. Fifty μl supernatant was added to 1.5 ml tubes containing 20 mg of ECR and 1 ml of 100 mM Tris-HCl, 1mM CaCl2 and incubated at 37°C for 24 h with rotation. Ten μl of 100 mM EDTA was added to stop the reaction. Insoluble ECR was spun down, and the result was recorded by photography (A). Absorbance at 495 nm of the supernatants from above treatment was measured on a spectrophotometer, corrected by subtracting A495nm of samples that was incubated in the absence of ECR (B).
Supplementary figure 2. Effect of deletion of lasB on the expression of other secreted protease genes, including aprA, lasA, lepA, and protease IV of PAO1. The PAO1 wild type (WT), ΔlasB, ΔlasB-C and ΔlasB-pBBR1MCS-5 were cultured as above and harvested till the A600nm=1.0. The total RNA was extracted using TriPure Isolation Reagent (Roche, Basel, Switzerland) and purified by Direct-zol RNA Miniprep (Zymo Research, California, USA). cDNA was synthesized from the purified RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) with random hexamers. The synthesized cDNA was used as template for aprA, lasA, lepA and piv expression analysis by quantitative real-time PCR on a Rotor Q thermocycler (Qiagen, Hilden, Germany) with GAPDH as an internal control. The primers used in quantitative real-time PCR were listed in Table 1. The experiment was performed with three biological replicates and the results were calculated using a relative quantitative method (2-ΔΔCt). The expression of aprA (A), lasA (B), lepA (C), and protease IV (D) of these PAO1 strains were no difference.
Supplementary figure 3. (A) Survival of silkworm larvae infected with 1×108 CFU PAO1 per larva. (B) CFU of PAO1 in hemolymph from the infected larvae. Note: Twenty μl of PO inhibitor, 20 mM PTU was injected into the tested larvae before injection of bacteria.
Supplementary figure 4. Injection of PAO1 elastase B caused change of hemolymph proteins of the silkworm (A) and tobacco hornworm (B). (A) Hemolymph collected from elastase B-injected silkworm larvae (24 h after injection) was separated by SDS-PAGE, and the bands showing changes between treatments were analyzed by mass spectrometry. (B) Hemolymph samples were collected at 6 and 10 h after day 2, 5th instar M. sexta larvae had been injected with 50 μl PBS of elastase B (1 μg in 50 μl PBS) per larva. After hemocyte removal by centrifugation at 16,000g for 1 min, plasma (1 μl) was incubated with 9 μl 20 mM Tris-HCl, pH 7.5, 0.001% Tween-20, 5 mM CaCl2, 0.001% PTU, and 1 μl Micrococcus luteus (1 μg/μl) for 20 min at room temperature prior to 10% SDS-PAGE and immunoblot analysis using 1:2000 diluted antisera against SPH1, SPH2 and SPH33 as the primary antibodies, respectively.
Supplementary figure 5. PO activity decreased in hemolymph from the tobacco hornworm (A and B) and mealworm (C) after treatment of PAO1 elastase B. (A) Hemolymph samples were collected at 6 and 10 h after day 2, 5th instar M. sexta larvae had been injected with 50 μl PBS or elastase B (1 μg in 50 μl PBS) per larva. After hemocyte removal by centrifugation, plasma (1 μl) was mixed with 9 μl 20 mM Tris-HCl, pH 7.5, 0.001% Tween-20, 5 mM CaCl2 for 2 h at room temperature prior to PO activity assay. (B) Hemolymph samples were collected as above. After hemocyte removal by centrifugation, plasma (1 μl) was mixed with 1 μg M. luteus and 9 μl 20 mM Tris-HCl, pH 7.5, 0.001% Tween-20, 5 mM CaCl2 for 20 min at room temperature prior to PO activity assay. (C) Tenebrio molitor larvae (body length: about 20 mm) were injected with 10 μl of PBS or 10 μl of purified elastase B (10 ng/μl or 1 ng/μl). After 1 and 4 h, hemolymph samples were collected and centrifuged immediately at 16,000g for 1 min to remove hemocytes. PO activity of the plasma was measured.