Abstract
Objectives
Immunotherapy (IO) has altered the non-small cell lung cancer (NSCLC) therapeutic landscape. However, the majority of patients do not respond to immune-checkpoint blockade, and subsequently either receive further chemotherapy or are referred for clinical trials. Here we examined the outcomes and predictors of response to IO in early phase clinical trials.
Materials and Methods
We analyzed the records of 74 patients with metastatic NSCLC that were enrolled on phase 1 IO trials within MD Anderson Cancer Center from 1/2010 to 7/2017.
Results
The median age was 68, with a median follow-up of 12.3 months. The median lines of prior therapy was three. There were 53 patients who did not receive any IO as a prior line of treatment with a mOS of 8.2 months and mPFS of 3.4 months. There were 21 patients who progressed on a prior IO agent and subsequently went on an IO study with a mOS of 10.5 months and mPFS of 4.3 months, which was similar to patients who did not receive IO HR 0.81 (P=0.51) and PFS HR 0.85 (P=0.59). Royal Marsden Hospital (RMH) prognostic score >1 was predictive of decreased OS HR 3.59 (P=0.014) although PFS was not statistically different. MDACC prognostic score was predictive of both OS HR 3.39 (P= 0.0002) and PFS HR 1.9 (P=0.030). ANC/ALC ratio (NLR) of >6 was predictive of decreased survival mOS 3.2 months compared to NLR <6 mOS 11 months; HR 3.0 (P=0.0023).
Conclusions
In our heavily pretreated patient population with NSCLC, early phase clinical trials with IO demonstrated similar outcomes to those seen in larger clinical studies that also used immune checkpoint inhibitors. The addition of NLR to RMH and MDACC prognostic scores can identify patients with poor overall outcomes treated with early phase IO studies.
Keywords: Immunotherapy, Non-Small Cell Lung Cancer, Predict Response, ANC/ALC ratio
Introduction
Immunotherapy (IO) has altered the landscape of non-small cell lung cancer (NSCLC) therapy. There are multiple immune checkpoint inhibitors approved in the treatment of metastatic NSCLC that have moved the goalpost of median survival well beyond the one-year mark[1–4]. In the first line metastatic setting, pembrolizumab, a programmed cell death -1 (PD-1) inhibitor, is approved as a single agent for programmed cell death ligand - 1 (PD-L1) expression greater than 50% and also approved in combination with chemotherapy regardless of PD-L1 expression[4–6]. Pembrolizumab is also approved for the second-line treatment of advanced NSCLCs with a PD-L1 expression of at least 1%[7]. Nivolumab (PD-1 inhibitor) and atezolizumab (PD-L1 inhibitor) are both approved as second line agents in the metastatic setting[1, 2]. Recently, durvalumab (PD-L1 inhibitor) has been approved as consolidation following definitive concurrent chemo-radiotherapy for Stage IIIb patients [3].
Unfortunately, only a small subset of patients with metastatic NSCLC achieve a response with available immune checkpoint inhibitors. After progression on standard of care IO therapies, patients are often considered for clinical trials. One of the major challenges facing trial enrollment in these patients are the predictors of response or resistance and the determination of prognostic factors. PD-L1 expression is used to predict which patients are more likely to respond to the immune checkpoint inhibitor pembrolizumab[4, 6, 7]. This immuno-histochemical assay has high variability of expression within different regions of the same tumor and is subject to variable interpretation by the pathologist and thus remains a suboptimal predictive marker [8–10]. The majority of NSCLC cases do not over-express PD-L1 and these patients often receive immune checkpoint inhibitors in an unselected manner, with response rates at or below 20% [1, 2].
In the phase 1 setting, there are multiple options for NSCLC patients including immunotherapies, either as a single agent or as combination therapy. In a recent review of IO agents there were over 2000 drugs under investigation with 940 at the clinical stage of development [11]. With more patients receiving standard of care IO, clinical predictors of response are needed to help assess which patients would optimally benefit from early phase IO trials. Both the Royal Marsden Hospital (RMH) scoring system, which includes albumin level, lactate dehydrogenase (LDH) level, and number of metastases, and the MD Anderson Cancer Center (MDACC) prognostic scoring system, which adds Eastern Cooperative Oncology Group (ECOG) performance status (PS) to the variables in the RMH score, have been validated to predict survival inpatients enrolled on phase 1 studies [12, 13]. The Gustave Roussy Immune Score (GRIm-Score) which includes the neutrophil-to-lymphocyte ratio (NLR), albumin and LDH, has recently been demonstrated to predict outcomes in patients who receive IO in the phase 1 setting [14]. However, these scoring systems have not been evaluated specifically for patients with NSCLC receiving IO.
Herein, we investigate the predictors of survival in patients with NSCLC who received IO in phase 1 clinical trials.
Patients and Methods
We conducted a retrospective review of all patients with NSCLC who were enrolled on phase 1 IO trials at MDACC from January 2010 to July of 2017. All clinical trials were approved individually by the institutional review board at UT MD Anderson Cancer Center, which also provided the waiver for this retrospective chart review. Clinical trials using immune checkpoint inhibitors, immune activating cytokines, and immunomodulators were included in analysis. Baseline characteristics at study entrance were: age, histology, ECOG-PS, mutational status, number of prior systemic therapies, sites of metastases, hemoglobin level (g/dL), white blood cell count (k/uL), absolute neutrophil count (k/uL), absolute lymphocyte count (k/uL), platelet count (k/uL), albumin level (g/dL), and LDH level (U/L). Baseline characteristics were presented using percentages for categorical variables and medians with ranges for continuous variables.
The calculation of the RMH score used 3 variables: LDH level (> upper limit of normal [ULN; +1]), albumin level (< 3.5 g/dL [+1]), and number of metastatic sites of disease, ( > 2 [+1]). The calculation of the MDACC score used 4 variables: the 3 RMH variables plus ECOG performance status (≥1 [+1]). The MDACC score + NLR used the additional variable of NLR (>6 [+1]). The GRIm-Score was calculated using LDH level (> upper limit of normal [ULN; +1]), albumin level (< 3.5 g/dL [+1]), and the NLR (>6 [+1]).
Progression-free survival (PFS) was measured from the time of clinical trial enrollment until disease progression defined by imaging using RECIST or death from any cause. Overall survival (OS) was measured from the time of clinical trial enrollment until death from any cause. Median OS (mOS) and median PFS (mPFS) were estimated using the Kaplan-Meier method. Patients were censored at the time of their last follow-up. Univariate Cox proportional hazard analysis was used to compare OS among subgroups of patients. Univariate and multivariate Cox proportional hazards models were fit to assess associations among patient characteristics and clinical outcomes. Cox proportional hazards analysis was used to validate the RMH, MDACC, and GRIm-Score prognostic scores using our data set. We examined the predictive ability of prognostic factors for survival with the Harrell c-statistic; a higher c-statistic indicates greater predictive ability[15]. All statistical tests were 2-sided. In order to minimize the Type I error rate, P values < 0. 01 were considered statistically significant. Statistical analyses were conducted with TIBCO Spotfire S-Plus version 8.2 for Windows.
Results
There were 74 patients (36 [49%] male and 38 [51%] female) with NSCLC who received IO on a phase 1 study at MD Anderson Cancer Center with a median follow-up of 12.3 months. The median age was 68 years with a median of three prior lines of therapy, (range 1–6). The majority of patients had non-squamous histology (84%). There were nine patients with EGFR mutations and one patient with a ROS1 mutation. Twenty-one patients (28%) had received a prior form of IO prior to enrolling in our phase 1 studies, which were all immune checkpoint inhibitors (Table 1).
Table 1.
Patient characteristics of patients with NSCLC enrolled in Immunotherapy trials.
| Characteristic | No. of Patients (%) |
|---|---|
| Age, median | 68 years |
| Male | 36 (49) |
| Female | 38 (51) |
| Histology | |
| Non-squamous | 62 (84) |
| Squamous | 12 (16) |
| ECOG PS | |
| 0 | 12 (16) |
| 1 | 58 (78) |
| 2 | 4 (5) |
| Prior Lines of Therapy | |
| Median | 3 |
| Range | 1–6 |
| Prior Immunotherapy | |
| Nivolumab | 17 (23) |
| Pembrolizumab | 3 (4) |
| Durvalumab | 1 (1) |
| Metastatic Sites | |
| >/=2 | 32 (43) |
| </=2 | 42 (57) |
| Mutation | |
| EGFR | 9 (12) |
| ROS1 | 1 (1) |
| NLR | |
| <=6 | 59 |
| >6 | 15 |
Phase 1 Studies
Fifty-three (72%) patients received an immune checkpoint inhibitor alone or in combination with another agent and 21 (28%) patients received a form of cytokine based therapy. There were 25 (34%) patients who received a Cytotoxic T-Lymphocyte Associated Protein 4(CTLA4) inhibitor, all of which was given in combination with radiation or another agent. Twenty-four (32%) patients received a PD-1 or PD-L1 inhibitor alone or in combination with another agent (Table 2). There were three patients who received anti 4 -1bb and one patient who received an arginase inhibitor.
Table 2.
Immunotherapy agents used in phase 1 clinical trials.
| Therapy | No of Patients |
|---|---|
| CTLA4 Combination | 25 |
| Cytokines | 21 |
| PD1/PD-L1 Combination | 20 |
| PD1/PD-L1 Single Agent | 4 |
| Anti-41-bb | 3 |
| Arginase Inhibitor | 1 |
Clinical Outcomes
The mPFS for all patients was 3.4 months, (95% confidence interval (CI): 2.8–4.1), and a mOS of 9.5 months, (95% CI: 7.1–14.6). Three (4%) patients achieved a partial response,38 (51%) had stable disease, and 33 (45%) had progressive disease as best response. At the time of analysis, there was one patient who maintained an ongoing response while on therapy for 31 months. The mPFS of patients who received prior IO was 4.3 months compared to 3.4 months for patients who received no prior IO( HR 0.85, 95% CI: 0.48–1.52, P= 0.59 ). The mOS for the prior IO group was 10.5 months compared to 8.2 months with no prior IO (HR 0.81, 95% CI: 0.42–1.54, P= 0.51 ). There was one patient who progressed on nivolumab after 2 months and subsequently received CTLA-4 inhibitor in combination with radiation who at time of follow-up (11.4 months ) maintained a partial response. Of the nine patients with EGFR mutations, the mPFS was 2.7 months (95% CI: (1.7,-not reached) and mOS was 10.1 months (95% CI: 6.1 -not reached), with three patients achieving a best response of stable disease.
Predictors of survival
Of the numeric markers, NLR, LDH and Albumin were found to be predictive of OS, with c-index estimates for OS: NLR 0.61, (95% CI: 0.44–0.77), LDH 0.66 (95% CI: 0.49–0.83), and Albumin 0.62 (95% CI: 0.43–0.80). Only Albumin was predictive of PFS with c-index estimate being 0.64 (95% CI: 0.50–0.79). Patients with an NLR of > 6 (15 patients) had a mOS of 3.2 months, while an NLR <=6 (59 patients) had a mOS of 11 months (HR 3.0, 95% CI: 1.6–5.8, P = 0.0023). A lower cutoff of NLR <=4 did not predict survival (HR 1.3, 95% CI: 0.7–2.2, P = 0.36). The presence of soft tissue visceral metastasis was predictive of mOS (HR 2.3, 95% CI: 1.3–4.1, P = 0.0045) with a trend toward predicting PFS (HR 1.7, 95% CI: 1.0–2.0, P= 0.040). Histology, mutational status, brain metastasis, bone metastasis, liver metastasis, and gender were not predictive variables.
Comparison of prognostic scores
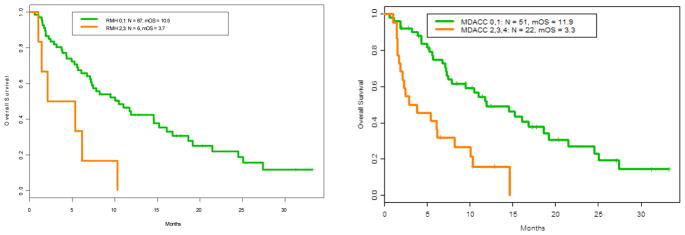
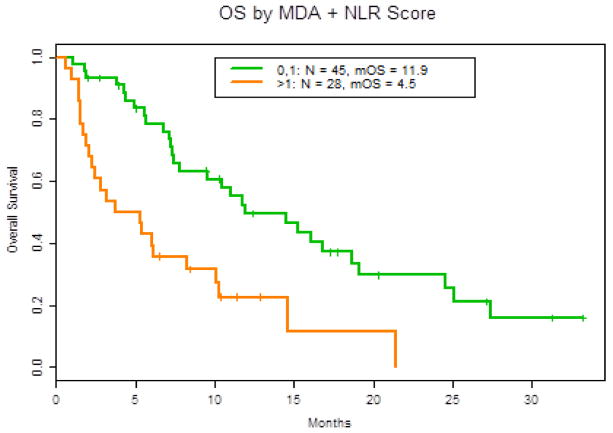
We compared the RMH, GRIm, MDACC, and the MDACC + NLR prognostic scoring systems with respect to survival for 73 patients, with one patient excluded from analysis due to lack of LDH prior to initiation of study drug (Table 3). Sixty-seven patients had a RMH score of 0–1 with a mOS of 10.5 months as compared to 3.7 months for the six patients with a RMH score of 2–3 (HR 3.59, 95% CI: 1.49–8.66, P = 0.014) (Figure 1). The MDACC prognostic score of 0–1 (51 patients) had a mOS of 11.9 months compared to 3.3 months for 22 patients with a score of 2–4 (HR 3.39, 95% CI: 1.83, 6.29 P = 0.0002) (Figure 1). A higher MDACC prognostic score of 2–4 demonstrated a trend toward predicting mPFS, (HR 1.90, 95% CI: 1.09–3.31, P = 0.030). The addition of the NLR (>6 [+1]) as a variable to the MDACC score demonstrated equal predictive value with respect to survival with a score of 0–1 (45 patients) mOS 11.9 months compared to >1 mOS of 4.5 months (HR 2.8, 95% CI: 1.6, 5.1, P = 0.0006) (Figure 2). GRIm-Score of 0–1 (8 patients) as compared to 2–3 (66 patients) did not reach statistical significant in predicting mOS (HR 2.1, 95% CI: 1.0–4.8, P =0.092).
Table 3.
Comparison of predictive scores: RMH, GRIm, MDACC, MDACC + NLR. Low risk score were 0–1 and high risk >1.
| Prognostic Scoring System | No of patient (%) | mOS (Months) | HR (95% CI) | P Value |
|---|---|---|---|---|
| RMH | ||||
| Low risk | 67 (92%) | 10.5 | 3.59 (1.49–8.66) | P = 0.014 |
| High risk | 6 (8%) | 3.7 | ||
| GRIm | ||||
| Low risk | 65 (89%) | 10.3 | 2.1 (1.0–4.8) | P =0.092 |
| High risk | 8 (11%) | 2.6 | ||
| MDACC | ||||
| Low risk | 51 (70%) | 11.9 | 3.39 (1.83–6.29) | P = 0.0002 |
| High risk | 22 (30%) | 3.3 | ||
| MDACC + NLR | ||||
| Low risk | 45 (62%) | 11.9 | 2.8 (1.6–5.1) | P = 0.0006 |
| High risk | 28 (48%) | 4.5 |
Figure 1.
Left: Overall survival based on RMH score. RMH > 1 OS HR 3.59 (1.49, 8.66) P= 0.014, Right: Overall survival based on MDACC score. OS Hazard Ratio for MDACC > 1 = 3.39 (1.83, 6.29) P = 0.0002.
Figure 2.
Overall survival based on MDACC score + NLR > 6, OS: HR = 2.8 (1.6, 5.1) P = 0.0006.
Discussion
Treatment options for NSCLC continue to evolve with increasing availability of IO agents. Nearly all patients with advanced NSCLC are now eligible for US FDA approved PD-1 or PD-L1 inhibitors in the metastatic setting unless they have an auto-immune disease. A subset of our patient population received IO prior to enrolling in a phase 1 IO trial. Our analysis suggests outcomes of patients receiving prior IO were similar to those who had not received prior IO. The prior IO agents received were PD-1 inhibitors. When enrolled on phase 1 studies, these patients subsequently received an immune checkpoint inhibitor combination, a different immune checkpoint i.e. CTLA-4, 4-1BB or cytokine based therapies. This suggests that patients who fail a PD-1 inhibitor should still be considered for a subsequent IO agent on a phase 1 study with a focus on a different pathway or a combination strategy.
In other tumor subtypes such as melanoma and renal cell carcinoma, the NLR is predictive of response to immune checkpoint inhibitors. The actual ratio analyzed in other tumor types have varied from NLR of 4–6, but all demonstrating an elevated NLR correlates with decreased survival [16–20]. Multiple recent studies with NSCLC patients demonstrate that elevated NLR is predictive of decreased survival and response to PD-1 inhibitors, specifically nivolumab [21, 22]. Similarly, our data suggests that the NLR >6 is a strong predictor of poor survival in patients with NSCLC being treated with a variety of IO agents on phase 1 studies.
A recent publication analyzed the predictive value of neutrophils/(leukocytes minus neutrophils) ratio (dNLR) and LDH in patients with metastatic NSCLC [23]. In their analysis, elevated LDH and elevated dNLR had poor outcomes when treated with immunotherapy. However, elevated LDH and dNLR was not prognostic of OS or PFS when patients were treated with chemotherapy. When considering clinical trial options, the NLR could help delineate whether to enroll patients on a chemotherapy or immunotherapy based approach.
The therapeutic targets for our IO studies typically involve the lymphocyte and its interaction with the tumor. Lymphopenia, however, has been demonstrate to not be an independent predictor or response to immunotherapy in the phase 1 setting [24]. The role of the neutrophil and its interaction with the T-cell and tumor microenvironment continues to be investigated. Specific neutrophil phenotypes and granulocytic myeloid-derived suppressor cells, which are thought to be derived from neutrophils, can suppress the effects of the T-cell [25–27]. Further insight is needed to develop therapies to inhibit these immunosuppressive effects.
The RMH and MDACC prognostic scores have been predictive of survival in other tumor types in the phase 1 setting. Our report is the first known analysis to specifically evaluate survival of NSCLC patients receiving IO in phase 1 clinical trials. While both scores were predictive of survival, the MDACC scoring system with the addition of NLR added more patients to the poor risk group while maintaining equal predictive value. There were only six patients (8%) with a high RMH score as compared to 28 patients (38%) with a high MDACC score + NLR. This suggests that MDACC score + NLR identified more NSCLC patients with a poor prognosis. In our analysis, the GRIm-score was not predictive of survival as there were too few patients that were high risk (11%) in our study as compared to 26% that were high risk in the initial study[14]. This could be secondary to our analysis specifically looking at NSCLC while the GRIm-score was validated for all tumor types [14].
As with any retrospective study, limitations exist that include referral bias, single institution data, and lack of standardized biomarkers. Unfortunately, PD-L1 status was not determined in the majority of these patients. Many of these patients were enrolled on study prior to the US FDA approval of PD-L1 testing as a predictive biomarker. Further studies are needed to evaluate the combined predicative value of PD-L1 expression with the MDACC scoring system and NLR.
Conclusions
Our data suggest that patients who progressed on standard of care IO agents for metastatic NSCLC should still be considered for early phase IO trials with non-overlapping agents. The MDACC prognostic score in conjunction with baseline NLR are predictors of survival in patients enrolled on phase 1 IO studies.
Highlights.
Phase 1 IO studies should be considered after prior PD-1/PD-L1 exposure
MDACC score outperformed RMH score in identifying poor risk patients
NLR is an independent predictor of outcomes
MDACC + NLR can be used to predict outcomes for phase 1 IO studies
Acknowledgments
FINANCIAL SUPPORT: The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health Cancer Center Support Grant CA016672. This work was supported in part by Cancer Prevention Research Institute of Texas Grant RP110584 and National Center for Advancing Translational Sciences Grant UL1 TR000371 (Center for Clinical and Translational Sciences).The funding sources had no input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
ABBREVIATIONS
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- EGFR
epidermal growth factor receptor
- HR
hazard ratio
- IO
Immunotherapy
- MDACC
MD Anderson Cancer Center, NSCLC, non-small cell lung cancer
- OS
overall survival
- PD-1
programmed cell death protein-1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- PR
Partial response
- PS
performance status
- RMH
Royal Marsden Hospital
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
Stable disease
Footnotes
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee J-S, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 4.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 5.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JCH, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 2016;387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. Journal of Thoracic Oncology. 12(2):208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Kwon HJ, Park SY, Park E, Chung JH. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget. 2017;8(58):98524–98532. doi: 10.18632/oncotarget.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of pd-l1 expression in non–small-cell lung cancer. JAMA Oncology. 2016;2(1):46–54. doi: 10.1001/jamaoncol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Annals of Oncology. 2017 doi: 10.1093/annonc/mdx755. mdx755-mdx755. [DOI] [PubMed] [Google Scholar]
- 12.Garrido-Laguna I, Janku F, Vaklavas C, Falchook GS, Fu S, Hong DS, Naing A, Tsimberidou AM, Wen S, Kurzrock R. Validation of the royal marsden hospital prognostic score in patients treated in the phase I clinical trials program at the MD Anderson Cancer Center. Cancer. 2012;118(5):1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 13.Livingston JA, Hess KR, Naing A, Hong DS, Patel S, Benjamin RS, Ludwig JA, Conley A, Herzog CE, Anderson P, Meric-Bernstam F, Kurzrock R, Subbiah V. Validation of prognostic scoring and assessment of clinical benefit for patients with bone sarcomas enrolled in phase I clinical trials. Oncotarget. 2016;7(39):64421–64430. doi: 10.18632/oncotarget.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, Angevin E, Armand JP, Ribrag V, Aspeslagh S, Varga A, Bahleda R, Menis J, Gazzah A, Michot JM, Marabelle A, Soria JC, Massard C. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score) European Journal of Cancer. 2017;84(Supplement C):212–218. doi: 10.1016/j.ejca.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Statistics in Medicine. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Martens A, Wistuba-Hamprecht K, Foppen MG, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, Ascierto PA, Di Giacomo AM, Maio M, Schilling B, Sucker A, Schadendorf D, Hassel JC, Eigentler TK, Martus P, Wolchok JD, Blank C, Pawelec G, Garbe C, Weide B. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clinical Cancer Research. 2016;22(12):2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, Machet L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. British Journal of Dermatology. 2016;174(1):146–151. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 18.Martens A, Wistuba-Hamprecht K, Yuan J, Postow MA, Wong P, Capone M, Madonna G, Khammari A, Schilling B, Sucker A, Schadendorf D, Martus P, Dreno B, Ascierto PA, Wolchok JD, Pawelec G, Garbe C, Weide B. Increases in Absolute Lymphocytes and Circulating CD4(+) and CD8(+) T Cells Are Associated with Positive Clinical Outcome of Melanoma Patients Treated with Ipilimumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(19):4848–4858. doi: 10.1158/1078-0432.CCR-16-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, Abe T, Funakoshi T, Yamamoto N, Amagai M, Yamazaki N. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget. 2016;7(47):77404–77415. doi: 10.18632/oncotarget.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandalà M, Simeone E, Valpione S, Altomonte M, Spagnolo F, Cocorocchio E, Gandini S, Giannarelli D, Martinoli C. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Annals of Oncology. 2016;27(4):732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 21.Liu ZL, Zeng TT, Zhou XJ, Ren YN, Zhang L, Zhang XX, Ding ZY. Neutrophil-lymphocyte ratio as a prognostic marker for chemotherapy in advanced lung cancer. The International Journal of Biological Markers. 2016;31(4):0. doi: 10.5301/jbm.5000222. [DOI] [PubMed] [Google Scholar]
- 22.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111(Supplement C):176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncology. 2018 doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun R, Champiat S, Dercle L, Aspeslagh S, Castanon E, Limkin EJ, Baldini C, Postel-Vinay S, Hollebecque A, Massard C, Ammari S, Deutsch E, Soria J-C, Marabelle A, Ferté C. Baseline lymphopenia should not be used as exclusion criteria in early clinical trials investigating immune checkpoint blockers (PD-1/PD-L1 inhibitors) European Journal of Cancer. 2017;84:202–211. doi: 10.1016/j.ejca.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of Clinical Investigation. 2012;122(1):327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cellular and Molecular Life Sciences. 2013;70(20):3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T-t, Zhao Y-l, Peng L-s, Chen N, Chen W, Lv Y-p, Mao F-y, Zhang J-y, Cheng P, Teng Y-s, Fu X-l, Yu P-w, Guo G, Luo P, Zhuang Y, Zou Q-m. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66(11):1900–1911. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]