Summary
Anaemia or decreased blood haemoglobin is the most common blood disorder often characterized by reduced red blood cell (RBC) numbers. RBCs are produced from differentiation and commitment of haematopoietic stem cells to the erythroid lineage by a process called erythropoiesis. Coordination of erythropoietin receptor signalling with several erythroid transcription factors including GATA1 is essential for this process. A number of additional players that are critical for RBC production have been identified in recent years. Major technological advances, such as the development of RNA interference, genetically modified animals, including zebrafish, and imaging flow cytometry have led to these discoveries; the emergence of -omics approaches in combination with the optimization of ex vivo erythroid cultures have also produced a more comprehensive understanding of erythropoiesis. Here we summarize studies describing novel regulators of erythropoiesis that modulate erythroid cell production in the context of human erythroid disorders involving hypoxia, iron regulation, immune-related molecules, and the transcription factor FOXO3.
Keywords: erythropoiesis, mechanisms, homeostasis
Introduction
Erythropoiesis is the process through which red blood cells (RBCs) are produced from haematopoietic stem and progenitor cells. On average, the bone marrow of a healthy adult generates two million erythrocytes per second (Palis, 2014). Production at this scale requires an intricate coordination between intrinsic and extrinsic erythroid programmes. Erythropoietin (EPO) and EPO receptor (EPOR) signalling are essential for erythroid cell production (Wu et al, 1995). EPO production is a highly regulated process that is mainly stimulated by tissue oxygen tension levels. As such, EPO production can increase by a thousand fold when the haematocrit is decreased and patients are severely anaemic (Erslev & Besarab, 1997). The development of new tools and approaches in the last two and half decades have led to the discovery of an array of regulators that control EPO production and the response to EPOR signalling. The novel techniques include the widespread use of genetically modified animals including zebrafish, highly improved in vitro RBC differentiation systems, and RNA interference technology along with flow cytometry methods to identify and sort distinct erythroid populations based on high affinity antibody recognition of specific cell surface receptors (Socolovsky et al, 2001; Zhang et al, 2003; Galloway et al, 2008; Chen et al, 2009; England et al, 2011; Hu et al, 2013; Liu et al, 2013). Furthermore, next generation sequencing has improved the feasibility of –omics approaches in combination with other high-throughput methods including imaging flow cytometry (McGrath et al, 2008; An et al, 2014). Here we sought to summarize studies of novel regulators of erythropoiesis that stimulate, inhibit or fine-tune erythroid cell production and have been tested in the context of erythroid disorders.
Hypoxia, HIF, and the regulation of Erythropoiesis
Recent evidence challenges the classical view of the haematopoietic lineage commitment hierarchy. Single-cell lineage tracing of human and mice haematopoietic stem and progenitors revealed heterogeneity within adult oligopotent progenitor populations thought to be homogenous. Further analysis of oligopotent progenitors, such as common myeloid progenitors (CMPs), suggests CMPs consist of distinct lineage-committed unipotent progenitors. This is in line with evidence showing erythroid lineages directly derive from haematopoietic stem cells without intermediate progenitor steps (Adolfsson et al, 2005; Guo et al, 2013; Notta et al, 2016; Paul et al, 2015). These findings reinforce the idea that under high erythropoietic demand, as in the case of haemorrhage or severe anaemia, RBC production can increase rapidly because lineage commitment occurs at an early stage without the need to proceed through various oligopotent progenitors. As such, erythropoiesis is a dynamic process that can respond to the physiological needs of the body. Erythroid-committed progenitors consist of burst-forming unit-erythroid (BFU-E) cells and colony-forming unit-erythroid (CFU-E) cells. Human BFU-E and CFU-E cells are identified though functional colony forming assays that lead to the formation of at least 16 clusters of 8–32 cells each for BFU-E cells around 15 days in culture, and up to 64 mature erythroblasts in one to two clusters for CFU-E cells detected around day 7 of culture (Gregory, 1976; Gregory & Eaves, 1978). Committed progenitors differentiate into erythroblasts that mature through three to five divisions that ultimately produce RBCs. In adults, normal oxygen tension in arterial blood ranges from 80–100 mmHg, compared to 30–40 mmHg in venous blood, and from 30–40 mmHg within the bone marrow (Carreau et al, 2011). Under conditions of lower than normal tissue oxygen levels (<26 mmHg O2), or hypoxia, EPO is produced and released into the circulation mainly from the kidney and also from the liver (Figure 1) (Beru et al, 1986, 1987). While not required for producing BFU-E and CFU-E cells from stem cells per se, EPO is essential for RBC production from CFU-Es and acts as a strong stimulator by inducing CFU-E survival and proliferation, subsequently enhancing the capacity of blood to transport oxygen (Wu et al, 1995). On a systemic level, O2 detection involves communication between multiple tissues and cell types, and becomes even more intricate on a molecular level, working through a family of transcription factors called hypoxia inducible factors (HIFs).
Figure 1. Systemic regulation of erythropoiesis through EPO.

Under hypoxic conditions resulting from low red blood cell (RBC) numbers, the kidney will produce erythropoietin (EPO), which is carried through the blood to the bone marrow (BM) and spleen to enhance proliferation and survival of erythroid progenitors, including burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) cells. Expansion of the erythroid-committed progenitor pool leads to increased RBC numbers, ablates the hypoxic conditions and reduces EPO production back to normal levels.
The HIF family of proteins includes the O2 sensitive HIF1α (HIF1A), and HIF2α (EPAS1), and the constitutively expressed HIF1β (ARNT). HIF1 protein is continually produced and degraded at normal O2 concentrations, initiated through hydroxylation of proline residues 402 and/or 564 by prolyl hydroxylase (PHD1-3) (Takeda et al, 2008). This leads to recruitment of the von Hippel-Lindau protein (VHL), and subsequent recognition by a group of proteins (Elongin C, Elongin B, RBX1, CUL2, and E2 ubiquitin-conjugating enzyme) that form the E3 ubiquitin ligase complex (Schofield & Ratcliffe, 2004; Kaelin, 2002). The hydroxylation of proline residues catalysed by PHD is dependent on various substrates, such as α-ketoglutarate, iron, reactive oxygen species (ROS) and O2), which translates to a protein highly responsive to global tissue metabolic and oxygen demands. Mainly, as tissue pO2 levels decrease, HIF1 protein levels increase as a result of decreased PHD activity. As HIFα accumulates due to hypoxic conditions, it forms heterodimers with HIF1β within the nucleus and activates the transcription of a set of genes that promote adaptation to limiting O2 concentrations, such as glycolytic enzymes that function in the anaerobic energy production pathway (Lendahl et al, 2009). Furthermore, in kidney and liver cells HIFα/HIF2β highly induces transcription of EPO to boost the production of RBCs (Wojchowski et al, 2010). Together, HIFs are critical transcription factors that maintain oxygen homeostasis throughout the body and regulate erythropoiesis under hypoxia (Semenza, 2009).
Although HIF1α and HIF2α are regulated in an identical manner and are responsive to O2 concentration with overlapping targets, they also have many distinct functions. Multiple studies provide evidence that HIF1α is crucial for the formation of vasculature and erythropoiesis during embryonic development, while HIF2α is more critical during adult stages in response to stress and hypoxia. HIF2α expression is mostly restricted to the liver, kidney, retina and endothelial cells, while HIF1α expression is ubiquitous, suggesting specialized tissue specific roles for HIF2a (Iyer et al, 1998; Scortegagna et al, 2005; Rankin et al, 2007; Yamashita et al, 2008; Yoon et al, 2006). Indeed, HIF1α ablation in mice is embryonic lethal at E10.5, due to decreased yolk-sac erythropoiesis and a lack of fully formed vasculature (Yoon et al, 2006). Mice with HIF2α knocked out in certain isogeneic strains are able to survive to adulthood, but display ineffective erythropoiesis during terminal stages of erythroblast maturation and fail to induce EPO in hepatic and renal cells under hypoxia and anaemia (Rankin et al, 2007; Yamashita et al, 2008; Kapitsinou et al, 2010; Tojo et al, 2015). HIF2α is also demonstrated to serve different roles dependent on the cell type: in renal, hepatic and even glial cells, HIF2α is required for EPO induction and systemic stimulation of erythropoiesis, while in endothelial cells HIF2α activates VCAM-1 expression, a membrane protein that promotes erythroid maturation in haematopoietic niches of the bone marrow and spleen (Yamashita et al, 2008; Rankin et al, 2007; Kapitsinou et al, 2010; Weidemann et al, 2009). HIF2α also has a distinct role in enterocytes of the duodenum to promote iron uptake and release into the blood (discussed in more detail below). Conversely, ablation of VHL or PHD function that lead to inappropriate HIFα accumulation cause erythrocytosis partially due to increased levels of EPO, and is characterized by significant increases in haematocrit and associated with pulmonary hypertension (Hickey et al, 2007; Franke et al, 2013; Rankin et al, 2005; Takeda et al, 2008). These defects can only be rescued by subsequent knock down of HIF2α, but with little or no effect with HIF1α knock down. Taken together, HIF1α appears more critical for primitive erythropoiesis during embryonic development, while HIF2α is potentially more critical for the regulation of definitive erythropoiesis, beginning as early as the fetal liver.
Multiple mutations in proteins regulating the oxygen sensing HIF pathway have also been discovered and shown to cause a variety of polycythaemias (Semenza, 2009). All of the pathogenic mutations lead to aberrant HIFα accumulation, and specifically due to HIF2α activity. Mutations in the EGLN1 (also termed PHD2) gene (P317R, R371H), encoding PHD2 (EGLN1), the first negative regulator of HIFα in the oxygen-sensing pathway, lead to loss of function potentially through an inability to bind HIFα. Individuals heterozygous for these mutations display erythrocytosis, with higher than normal levels of haemoglobin and haematocrit. Similar defects are observed in Egln1 (Phd2) knockout mice, with no phenotype in heterozygotes suggesting a dominant negative effect of the human P317R and R371H mutations (Percy et al, 2006). A mutation in the downstream VHL gene, (R200W, M54I), encoding the VHL protein that recognizes hydroxylated HIFα and marks it for subsequent degradation, is associated with Chuvash polycythaemia (Bartels et al, 2015; Kaelin, 2002). The mutation lies within the HIF binding domain of VHL and in mice was shown to specifically disrupt HIF1β binding (Hickey et al, 2007). Patients with Chuvash polycythaemia also display with abnormal changes in cardiac and vascular systems, especially when exposed to hypoxic stress, which highlights the non-erythropoiesis targets of HIF (Smith et al, 2006). Lastly, multiple gain of function mutations in EPAS1 (HIF2α, HIF2A; G537W, G537R, M535V, M535I) that prevent degradation through loss of recognition by VHL or PHD are known to be associated with erythrocytosis (Percy et al, 2008; Zhuang et al, 2012). Some of these mutations have been replicated in mice and the accumulation of HIF2α was shown to be the molecular mechanism causing aberrant EPO production followed by excessive RBC production (Tan et al, 2013).
Interestingly, non-pathogenic mutations in the EGLN1 gene (D4E, C127S) have also been identified as an adaptation to living in hypoxic environments at high altitude (Lorenzo et al, 2014). These mutations were found in a large proportion of Tibetans, who originate from conditions of extremely high elevation with lower than normal atmospheric O2. Mutant PHD2 (EGLN1) was found to have a lower Km for O2, which translates to an ability of PHD2 to stay active even under low O2 conditions and leads to degradation of HIFα. Although the precise function of HIF is to allow cells to survive under hypoxic conditions, long-term exposure to hypoxia can lead to the pathological accumulation of HIFα. As seen with the many EPAS1 gain of function mutations, this can lead to erythrocytosis and polycythaemia (Semenza, 2009). Therefore, the regulation of erythropoiesis requires an intricate oxygen-sensing HIF system. The HIF pathway maintains a delicate balance between activation and repression based on the integration of multiple environmental and physiological cues that in turn regulates a multi-organ effort to enhance erythropoiesis.
Iron Regulation and Erythropoiesis
Iron is another major component of erythropoiesis, required to form the oxygen-carrying haem centres of haemoglobin. Therefore, physiological signals and molecular mechanisms that maintain proper levels of iron can concurrently affect erythropoiesis. Specifically, iron and HIF systems interconnect at a systemic and cellular level. On a systemic level, the hormones hepcidin (HAMP) and the recently identified erythroferrone (ERFE, FAM132B) limit and enhance, respectively, plasma iron concentrations to meet the iron demand required for RBC production (Figure 2) (Kautz et al, 2015; Ganz & Nemeth, 2012). At the cellular level, a second system through iron regulatory proteins and iron regulatory elements (IRP/IRE) respond to fluctuating iron concentrations, which affects translation of key iron import and export genes (Anderson S.A. et al, 2013; Meyron-Holtz et al, 2004; Wilkinson & Pantopoulos, 2014).
Figure 2. Systemic cross-talk between iron regulation and erythropoiesis.
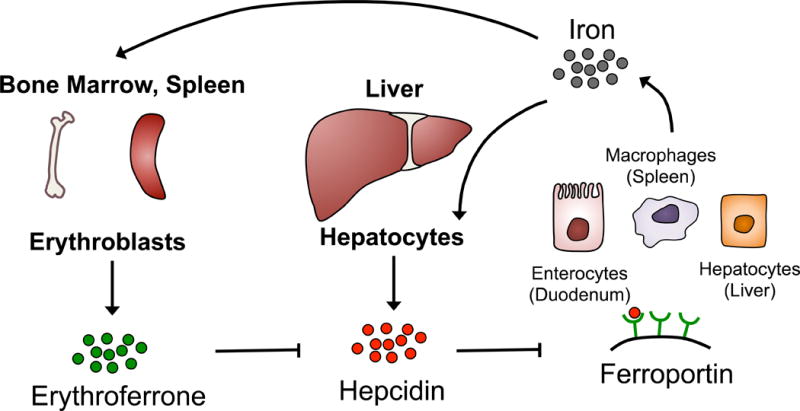
During iron-replete conditions, hepatocytes can directly sense transferrin-bound iron, which stimulates production of the hormone hepcidin to limit any further release of iron. Hepcidin directly binds and induces the endocytosis and proteolysis of Ferroportin, a transmembrane exporter of iron found mainly on enterocytes, splenic macrophages, and hepatocytes, which are the main reservoirs of iron in the body. Alternatively, when the body is in need of iron during increased erythropoiesis rate, differentiating erythroblasts produce the hormone erythroferrone, which suppresses hepcidin production. In turn, ferroportin accumulates on iron reservoir cells leading to increased plasma iron concentrations to feed the production of haemoglobin in erythroblasts.
Hepcidin/Erythroferrone
Hepcidin (HAMP) is a small 25 amino acid long hormone produced primarily by hepatocytes in the liver, but is also expressed at low levels by macrophages and adipocytes. Under iron-replete conditions, hepcidin levels in the blood are high, which signals to various iron storing organs to suppress the release of plasma iron (Ganz & Nemeth, 2012; Ginzburg & Rivella, 2011). Ferroportin is a transmembrane protein responsible for transporting intracellular iron from enterocytes (duodenum), macrophages (spleen), and hepatocytes (liver) into the bloodstream mainly to be used by erythroid precursors for haeme synthesis. Hepcidin binds directly to ferroportin and triggers its endocytosis and subsequent degradation limiting the amount of available circulating iron in the blood (Figure 2) (Nemeth et al, 2004).
Due to the reactive nature of iron and its ability to generate ROS through the Fenton reaction, free iron in the blood is bound to the protein transferrin, termed holo-transferrin when saturated with iron or apo-transferrin when free of iron. To limit ROS generation within cells, intracellular iron is sequestered by the protein ferritin. As such, insufficient levels of hepcidin can lead to iron overload through the release of intracellular iron stores combined with greater iron absorption from the diet leading to excessive plasma iron unbound by transferrin. This disorder can arise from mutations in genes regulating hepcidin expression and is also one of the major symptoms seen in β-thalassaemia patients (Li et al, 2010). Normally, hepcidin induction is regulated through multiple signalling pathways, including direct feedback by plasma iron concentrations in addition to BMP2/4, BMP6/HJV (HFE2), and IL6 signalling pathways. Iron-bound transferrin from the blood signals through transferrin receptor 1 and 2 (TFR1 [also termed TFRC], TRF2) and hereditary haemochromatosis protein (HFE) to stimulate hepcidin production in hepatocytes (Schmidt et al, 2008; Gao et al, 2009). However in β-thalassaemia, hepcidin is aberrantly suppressed even under conditions of high plasma iron concentration (Li et al, 2010). In β-thalassaemia patients a mutation in the β globin gene (HBB) causes an imbalance between α and β globin leading to accumulating α globin – haem aggregates called haemichromes (Rachmilewitz, 1969; Rachmilewitz & Thorell, 1972; Ginzburg & Rivella, 2011; Arlet et al, 2014). These aggregates damage the RBC membrane and cause excessive ROS generation, which decreases the lifespan of RBCs, leading to anaemia. A high level of apoptosis is also observed in erythroid precursors, which together with decreased RBC lifespan feedback to continually stimulate erythropoiesis through enhanced EPO production (Yuan et al, 1993; Centis et al, 2000). The high rate of erythropoiesis suppresses hepcidin production, as the generation of RBCs increases the demand for iron. However, the mutant erythroid progenitors and RBCs have shorter lifespans, leading to the release of their intracellular iron and contributing to aberrantly high plasma iron levels. High levels of plasma iron can overwhelm the amount of non-iron bound apo-transferrin available in the blood preventing proper delivery to developing RBCs and also cause oxidative stress. Together these pathogenic processes continue the cycle of defective RBCs, erythropoiesis stimulation, and subsequent hepcidin suppression (Li et al, 2010). As such, enhanced expression of hepcidin or treatment with transferrin was found to decrease the burden of iron overload and be beneficial to β-thalassaemic erythropoiesis (Gardenghi et al, 2010). This data raises the possibility of potential hepcidin- or transferrin-enhancing drugs as therapy for this disorder.
Although in mouse models, transferrin injections were shown to rescue many defects of β-thalassaemia through decreasing the burden of iron overload and enhancing hepcidin expression, it had been proposed that a direct signal from proliferating erythroid progenitors or erythroblasts is responsible for directly suppressing hepcidin (Li et al, 2010; Ganz & Nemeth, 2012). This erythroid signal would mediate the crosstalk between erythropoiesis and iron metabolism. As a result, microarray screening for highly upregulated erythroid specific genes from mouse bone marrow cells after phlebotomy identified Fam132b (Kautz et al, 2014). The authors termed the secreted factor erythroferrone and found its exclusive expression within differentiating erythroblasts especially after stimulation by EPO (Kautz et al, 2014). Erythroferrone was necessary and sufficient to suppress hepatocyte expression of hepcidin. In β-thalassaemic mice, it is also clear that erythroferrone contributes to the aberrant hepcidin suppression as ablation of Fam132b restored hepcidin expression in β-thalassaemia (Kautz et al, 2015). However, the anaemia did not improve, as the iron overload was only partially relieved. These data suggest that other hepcidin-independent mechanisms of iron accumulation are activated, such as HIF2α accumulation (discussed below). Interestingly, Hamp overexpression in β-thalassaemia mice is able to correct iron-overload, improve erythropoiesis and, strikingly, even improve the imbalance between the alpha and beta chains (Gardenghi et al, 2010). One potential explanation for the differing degrees of rescue between Famb132b ablation and Hamp overexpression is the supra-physiological levels of hepcidin overexpression in these experiments, which was 2- to 4-fold higher than basal hepcidin expression (Gardenghi et al, 2010). Collectively, these findings also suggest that hepcidin may be suppressed through erythroferrone-independent mechanisms that are also deregulated in β-thalassaemia. Potentially, drugs mimicking inhibition of erythroferrone could be combined with transferrin therapy to reduce the iron overload while enhancing erythroid progenitor cell survival and RBC lifespan, but this remains to be tested. Other recently discovered negative regulators of hepcidin include twisted gastrulation (TWSG1) and growth differentiation factor 15 (GDF15). TWSG1 is a cytokine that inhibits human hepcidin production via inhibition of BMP2/4 and is highly upregulated in the bone marrow, spleen and liver of thalassaemic mice (Tanno et al, 2009). GDF15, another cytokine shown to repress hepcidin, was found in the context of thalassaemia. Levels of GDF15 were highly upregulated in thalassaemic patients’ sera and expressed from human erythroid progenitors cells (Tanno et al, 2007).
HIF regulation of Iron Homeostasis
HIF2α is also an important regulator of iron metabolism. Although early studies had originally demonstrated direct transcriptional repression of hepcidin expression by HIFα, more recent studies provide evidence of indirect regulation of hepcidin, which requires HIF2α- and not HIF1α-mediated induction of EPO (Peyssonnaux et al, 2007; Volke et al, 2009). Potentially, stimulation of erythropoiesis by EPO then leads to increased production of erythroferrone by maturing erythroblasts, which in turn suppresses hepcidin (Kautz et al, 2014; Liu et al, 2012). On a cellular level, HIF2α regulates iron homeostasis through direct transcriptional regulation of multiple iron transport and release genes in duodenum enterocytes (Mastrogiannaki et al, 2009, 2012). These genes include Slc11a2 (also termed divalent metal transporter 1; Dmt1), ferroportin (Slc40a1), and duodenal cytochrome b (Cybrd1), which act together to absorb iron from the diet and transport it through the cell to be released as plasma iron within the bloodstream. Intestinal specific deletion of HIF2α results in decreased serum and liver iron levels and indirectly induces a compensatory reduction in the levels of hepcidin (Mastrogiannaki et al, 2009). Furthermore, conditional HIF2α deletion in the intestine can partially rescue iron overload in hepcidin-deficient mice by decreasing liver and pancreatic iron levels, but not serum iron levels (Mastrogiannaki et al, 2012; Das et al, 2015). The partial rescue suggests other sources of iron release, such as macrophages, that hepcidin normally acts upon to suppress. When tested in mouse models of β-thalassaemic and sickle cell anaemia, HIF2α deletion in intestine alleviated multiple symptoms of iron overload (Anderson, E.R et al, 2013; Das et al, 2015). Additionally, oxidative stress was reduced through decreasing overall levels of iron in plasma, spleen and liver. On a functional level, RBC lifespan as well as RBC numbers, haemoglobin concentration and haematocrit increased, while EPO levels decreased relative to sickle cell mutant mice (Das et al, 2015).
In turn, changing intercellular iron levels can feedback to regulate HIF2α. One mechanism occurs through PHD-mediated degradation of HIF2α, which is partially dependent on iron as a co-factor. Therefore, PHD can be suppressed not only under hypoxia but also under conditions of iron deficiency, leading to HIF2α accumulation (Schofield & Ratcliffe, 2004). The IRP/IRE system further fine-tunes HIF2α levels, by suppressing HIF2α translation under iron deficient conditions to limit erythropoiesis, which conserves iron for other critical cellular processes that require iron (Anderson, S.A. et al, 2013). Specifically, this occurs through IRP1, and not IRP2, as IRP1 knock out mice display HIF2α hyperactivity leading to polycythaemia and extramedullary erythropoiesis. Together, PHD and IRP2 can sense intracellular iron levels to adjust the concentration of HIF2α by regulating the synthesis and degradation of HIF2α (Figure 3).
Figure 3. Fine-tune regulation of HIF2α.
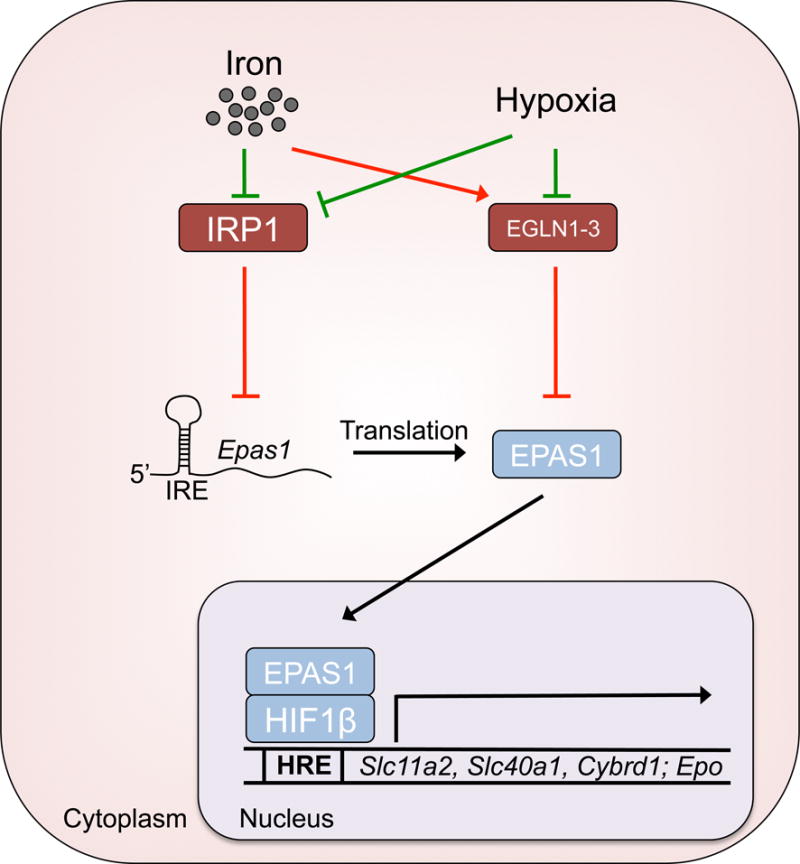
The translation and degradation of hypoxia inducible factor 2α (HIF2α, also termed EPAS1)are highly regulated by intracellular iron and oxygen level. Under iron-replete conditions, iron regulatory protein 1 (IRP1) is suppressed from binding the iron response element (IRE) of Epas1 (also termed Hif2a) mRNA. This allows for normal rates of HIF2α translation and the induction of iron transport genes and Epo by HIF2α, but the protein levels of HIF2α are still kept in check by prolyl hydroxylase domain (PHD)-mediated degradation under normoxic conditions. When iron is limited, IRP1 is able to bind Epas1 mRNA and inhibit translation, preventing release of limited iron stores and the restricting the erythropoietic demand. Under hypoxic conditions, IRP1 and PHD become suppressed, allowing for rapid induction and accumulation of HIF2α. Subsequently, HIF2α translocates to the nucleus and binds HIF1β, which transactivates a gene programme to adapt to hypoxia and also restore oxygen tension levels. HRE or hypoxia-response element, is the consensus sequence found within promoter regions of HIF target genes, which are transcribed when bound by HIF.
Ineffective Erythropoiesis
Role of GDF11
Ineffective erythropoiesis is a condition seen in certain genetic disorders of the blood and often associated with β-thalassaemia. It is characterized by apoptosis of erythroid precursors and a blockade to terminal erythroid maturation that together lead to an insufficient number of RBCs and decreased oxygen carrying capacity of blood (Yuan et al, 1993; Pootrakul et al, 2000; Gardenghi et al, 2007). The apoptosis that is characteristic of ineffective erythropoiesis is tightly linked to erythroid expansion, which may lead to extramedullary haematopoiesis (Yuan et al, 1993; Pootrakul et al, 2000; Camaschella & Nai, 2015; Ribeil et al, 2013). Due to the pathogenic mechanisms of ineffective erythropoiesis, the lack of mature RBCs stimulates erythropoiesis and triggers the overproduction of EPO. Even with increased concentrations of EPO, the death of erythroid progenitors and terminal maturation arrest occurs (Figure 4A). Therefore, the anaemia arising from ineffective erythropoiesis seen in β-thalassaemia or some myelodysplastic syndromes (MDS) are not treatable by EPO and remain resistant to EPO (Dussiot et al, 2014; Suragani et al, 2014). Recent studies have identified a class of drugs known as activin receptor IIA ligand traps (ActIIA) that relieve the blockade in terminal maturation due to ineffective erythropoiesis in mouse models of MDS and β-thalassaemia. The target of ActIIA is GDF11 (also known as bone morphogenic protein 11, a member of the TGF-β superfamily), which is a secreted factor that signals through the TGF-β pathway specifically through SMAD2/3 in erythroid progenitors. These studies revealed that GDF11 plays a major role in potentiating ineffective erythropoiesis by promoting the expansion of the committed erythroid progenitor pool, while preventing terminal maturation through an autocrine signalling loop (Dussiot et al, 2014). The precise gene expression programme and molecular mechanism downstream of GDF11/SMAD2/SMAD3 remain unknown. However, in β-thalassaemic cells, GDF11 expression was shown to increase in response to ROS as treatment with an antioxidant could reduce levels of GDF11 expression (Dussiot et al, 2014). Furthermore, inhibition of GDF11 signalling with ActIIA also enhanced expression of hepcidin, corresponding to a reduction of iron concentrations, while restricting alpha globin aggregate formation corresponding to lower levels of ROS in β-thalassaemic mice (Dussiot et al, 2014). Together, these results suggest that increased ROS in erythroid progenitors due to iron overload and alpha-globin aggregates can lead to increased expression of GDF11, which in turn promotes ineffective erythropoiesis. These studies also raise the possibility of using compounds that reduce ROS as therapies to improve ineffective erythropoiesis, especially as oxidative stress is already known to contribute to defects in erythropoiesis (Figure 4A) (Marinkovic et al, 2007).
Figure 4. Mechanisms of ineffective erythropoiesis in β-Thalassaemia.
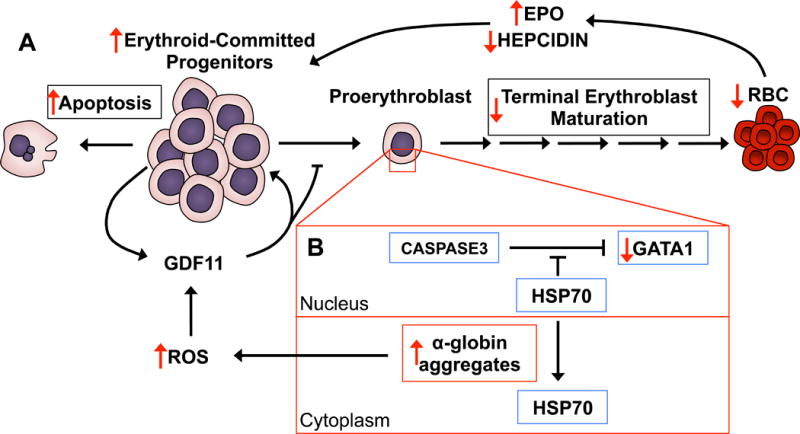
A) In β-thalassaemia patients and mice, the erythroid-committed progenitor pool is abnormally high with a significant increase in the frequency of apoptosis compared to normal cells. Growth differentiation factor 11 (GDF11) feeds the cycle of aberrant erythroid progenitor expansion and also inhibits terminal differentiation, which leads to an overall decrease in mature red blood cells (RBCs). The lower RBC numbers result in lower tissue oxygenation, subsequent induction of erythropoietin (EPO) expression and suppression of hepcidin production. Together, high levels of EPO and low levels of hepcidin further contribute to stimulating proliferation of erythroid progenitors and the iron overload phenotype, respectively. B) Due to the imbalance between α and β globin levels, α-globin misfolds and forms harmful α-globin aggregates. Normally HSP70 localizes within the nucleus of erythroid committed progenitors and early erythroblasts to protect GATA1 from caspase 3 cleavage. However, in β-thalassaemic erythroid cells, α-globin aggregates sequester HSP70 to the cytoplasm, leading to degradation of GATA1 and a blockade to terminal erythroblast maturation. α-globin aggregates also produce increased levels of reactive oxygen species (ROS), which can also induce GDF11 production in erythroid-committed progenitors. (Red arrows denote significant differences in β-thalassaemia relative to normal erythropoiesis.)
EPO, erythropoietin; RBC, red blood cell; GDF11, growth differentiation factor 11; ROS, reactive oxygen species; GATA1, GATA binding protein 1; HSP70, heat shock protein 70.
Interestingly, the enhancement of β-thalassaemia erythroid maturation by ActIIA was exclusive to splenic erythroblasts and also enhanced terminal erythroid maturation of normal mice (Dussiot et al, 2014; Suragani et al, 2014). Based on these results, in normal individuals GDF11 may function as a transient factor activated under conditions of stress that require an acute demand for RBCs. GDF11 could act to quickly expand the erythroid progenitor pool, and shortly after become degraded or downregulated to allow for terminal maturation in response to another signal. GDF11 is also implicated in aging and muscle rejuvenation, although these findings have been recently challenged (Sinha et al, 2014; Egerman et al, 2015).
BMPs are also implicated in stress erythropoiesis, which is a physiological response triggered by acute anaemia and leads to rapid production of new erythrocytes. Studies in the past decade have shown differences in the microenvironment and repertoire of regulators between stress erythropoiesis and normal homeostatic erythropoiesis. Stress erythropoiesis occurs mainly in mouse spleen and fetal liver but also in adult liver. In addition to BMPs, other factors, including hypoxia, stem cell factor and Hedgehog signalling, are also involved in regulating stress erythropoiesis. Studies into stress erythropoiesis highlight the impact of tissue context or microenvironment in the regulation of erythropoiesis (Paulson et al, 2011).
HSP70 and Caspase3 Regulation of GATA1
Another potential mechanism causing the ineffective erythropoiesis in B-thalassaemia was demonstrated to occur through GATA1 cleavage by the apoptosis effector protein caspase 3 (CASP3) (Figure 4B)(Arlet et al, 2014). GATA1 is a master transcription factor essential for inducing transcription of gene programmes required for erythroblast maturation (Cantor & Orkin, 2002). In vitro cultured human erythroblasts express death receptors including both FAS and FAS ligand (FASLG) (De Maria et al, 1999). Elegant studies by De Maria et al (1999) showed that late stage erythroblasts express and secrete FASLG, which in turn binds FAS on primitive erythroblast precursors. Stimulation of FAS activates CASP3 and subsequent GATA1 cleavage. The authors further demonstrated that CASP3 directly binds GATA1 through a caspase recognition sequence and targets GATA1 for proteolytic cleavage. Expansion and differentiation of erythroblasts transduced with caspase-resistant GATA1 mutant was restored despite death receptor stimulation (De Maria et al, 1999). The authors proposed this mechanism to act as a negative feedback loop that holds erythroid expansion in check. Additional data suggested that CASP3 is also required for downstream terminal erythroblast maturation through poorly understood mechanisms (Zermati et al, 2001). This latter role for CASP3 appears to be preceded by mitochondria depolarization, which can be an initiation event for mitophagy, a critical cellular process that erythroblasts undergo in order to mature (Zermati et al, 2001). Furthermore, there is some evidence that the requirement for CASP3 activation during erythroblast maturation is related to promoting cell cycle progression (Boehm et al, 2013).
However, how exactly erythroblasts are protected from caspase-mediated proteolytic degradation during erythroid differentiation and maturation remained an open question. This was directly addressed by Ribeil and colleagues’ studies that demonstrated the chaperone Heat Shock Protein 70 (HSP70) protects GATA1 from CASP3 cleavage during human erythroid maturation, but not during apoptosis induction (Ribeil et al, 2007). In committed erythroid progenitors and early stage erythroblasts, HSP70 co-localizes with GATA1 inside the nucleus. HSP70 is required to protect GATA1 from cleavage, as knockdown of HSP70 in erythroid progenitors led to GATA1 degradation and prevented erythroblast maturation (Figure 4B). The HSP70 mediated protection of GATA1 was only observed in EPO-fed and not in EPO-starved cells, where HSP70 is translocated to the erythroblast cytosol exposing GATA1 to CASP3 cleavage (Ribeil et al, 2007). These data suggest the balance between differentiation and apoptosis of human erythroblasts may depend at least partly on HSP70 abundance and nuclear localization.
Given the importance of apoptosis as a contributor to ineffective β-thalassaemic erythropoiesis, Ribeil et al (2007) also analysed the status of HSP70 in β-thalassaemic erythroblasts. They found that HSP70 does not localize with GATA1 inside the nucleus both from freshly isolated β-thalassaemic erythroblasts nor in vitro erythroblasts cultured in the presence of EPO. Instead, HSP70 is sequestered by α-globin aggregates, leaving GATA1 vulnerable to CASP3 cleavage (Figure 4B) (Arlet et al, 2014). These studies suggest that HSP70 modulation has therapeutic potential.
However, the regulation of CASP3 through FAS and FASLG appears to be dissimilar in human versus mouse erythropoiesis (De Maria et al, 1999; Socolovsky et al, 2007). In fact there are key stage- and species-specific differences throughout terminal erythroid differentiation as recent transcriptomic studies comparing freshly isolated pure populations of mouse and human erythroblasts at distinct developmental stages revealed (An et al, 2014). The disparity between mouse and human erythroblast CASP3 regulation might be explained by differences in the expression of Fas and FASLG in mouse erythroblasts versus human erythroblasts. In mouse spleen and fetal liver, FAS and FASLG are expressed on immature erythroblasts at similar stages of differentiation, which is in contrast to human erythroblasts (De Maria et al, 1999; Socolovsky et al, 2007); this provides a negative regulation of erythropoiesis by promoting apoptosis and regulating erythroid cell output. The mouse system has also lent itself to mathematical modelling of erythroid development that reveals its robustness in face of environmental perturbations (Socolovsky et al, 2007). Although differing in the precise FAS-FASLG mechanism of regulation of erythroid cell homeostasis through apoptosis, these studies are overall in agreement with the findings of De Maria et al (1999) in human erythropoiesis.
Aberrant repression of GATA1 protein levels also contributes to Diamond-Blackfan anaemia, where mutations in either ribosomal proteins or directly within GATA1 lead to diminished GATA1 translation and subsequent defects in erythropoiesis (Ludwig et al, 2014). Therefore, a lack of GATA1 activity can be a major pathogenic mechanism that contributes to anaemia, and provides potential therapeutic targets for enhancing GATA1 expression to ameliorate anaemia.
Potential Role of Immune-Related genes in Erythroid Cells/Expression of Immune-Related Genes by Erythroid Cells
A surprising and relatively unexplored aspect of erythroblast maturation and RBC biology is the potential immune modulatory effects of erythroblasts and RBCs. The majority of studies have focused on how during infection, pro-inflammatory cytokines, such as IL6, can signal hepatocytes to induce expression of hepcidin, limiting the absorption and release of iron (Schmidt, 2015). Iron restriction during infection is used as a weapon against bacterial pathogens because of its role as a co-factor for many critical bacterial enzymes. As such, anaemia is often associated with infection due to the increased hepcidin levels and iron restrictions. A few recent studies have implicated immune-related genes in haematopoietic development, either directly through interferon signalling or indirectly through TNF-α signalling (Li et al, 2014; Espín-Palazón et al, 2014). Additionally, immune regulation by terminally maturing erythroblasts or RBCs have also been demonstrated (Morera et al, 2011; Greenfest-Allen et al, 2013; Liang et al, 2015). These studies have identified upregulation of immune-related genes involved in the response to pathogenic infection, including the STAT family, interferon, and other genes that are part of the innate immune response, in the trancriptome of maturing erythroblasts of mammalian and avian species (Morera et al, 2011; Greenfest-Allen et al, 2013). Clearly immune-related genes are expressed and functional in immature erythroblasts as discussed above (De Maria et al, 1999; Socolovsky et al, 2007). In addition, fetal CD71+, CD235+ cells representing early erythroblasts, were shown to possess immunosuppressive functions as a mechanism of inducing tolerance and allowing for commensal bacteria colonization in neonates; although certain aspects of this finding are currently being challenged (Elahi et al, 2013; Wynn et al, 2015). Our recent findings through transcriptomic and Western blot analysis of maturing erythroblasts are consistent with this group of studies by showing that a cluster of immune-related genes becomes progressively upregulated as murine erythroblasts mature (Liang et al, 2015). No known function in erythroblasts has been described for these genes, but their relative high expression raises the possibility that they may have some potential function during erythroblast maturation. Based on these studies and the ubiquity of RBCs throughout the body, mature erythroblasts serving a potential immune-related function remain an attractive hypothesis to explore.
Transcriptional regulation of erythropoiesis and forkhead box O3 (FOXO3)
The complete mechanisms that orchestrate the coordination of different subsets of erythroid programmes remain to be elucidated. The current research suggests that multi-subunit complexes of transcription factors and nuclear adapters function together to control erythroid gene expression programs. These complexes consist of the transcription factors GATA1, KLF1, TAL1, E2A, and nuclear adapters LMO2 and LDB1 (Tsiftsoglou et al, 2009; Love et al, 2014). Recent evidence suggests the transcription factor FOXO3 operates similarly to these complexes to promote erythroid gene expression. A critical role for FOXO3 in the terminal maturation of mouse erythroblasts is well established (Liang et al, 2015), but has not yet been demonstrated in human erythroblasts. However, the pattern of increasing FOXO3 expression during erythroblast maturation is similar between mice and humans, which suggests human FOXO3 may function in an analogous manner as mouse FOXO3 in the physiological regulation of erythropoiesis (Bakker et al, 2007; Marinkovic et al, 2007; Yu et al, 2010; Kang et al, 2012; McIver et al, 2014; An et al, 2014; Zhang et al, 2014; Liang et al, 2015).
Loss of FOXO3 leads to deregulated expression of over one third of genes differentially expressed at distinct stages of terminal erythroblast maturation (Liang et al, 2015). FOXO3 regulation of erythroid gene expression potentially occurs through enhancing the function of other erythroid transcription factors (Kang et al, 2012; Eijkelenboom et al, 2013; McIver et al, 2014; Zhang et al, 2014; Liang et al, 2015; Li et al, 2013). FOXO3-dependent genes represent a number of critical cellular processes in erythroid cells, including regulation of oxidative stress, autophagy, metabolism, enucleation, mitochondrial removal (mitophagy), and the cell cycle (Figure 5). Mice with FOXO3 ablation acquire a compensated anaemia associated with haemolysis and RBCs with a shortened lifespan due to oxidative stress (Castrillon et al, 2003; Marinkovic et al, 2007). FOXO3 activity is also partially regulated by EPOR signalling through AKT phosphorylation in erythroid progenitors and early stage erythroblasts, which further supports FOXO3 as a critical factor during erythropoiesis (Kashii et al, 2000; Uddin et al, 2000; Mahmud et al, 2002; Ghaffari et al, 2003; Bakker et al, 2004). Additional mechanisms of FOXO3 regulation specific to haematopoietic and erythroid progenitors, such as FOXO3 acetylation and deacetylation, may also be involved (Mahmud et al, 2002; Rimmelé et al, 2014; Liang et al, 2016).
Figure 5. FOXO3 regulation of terminal erythroblast maturation.
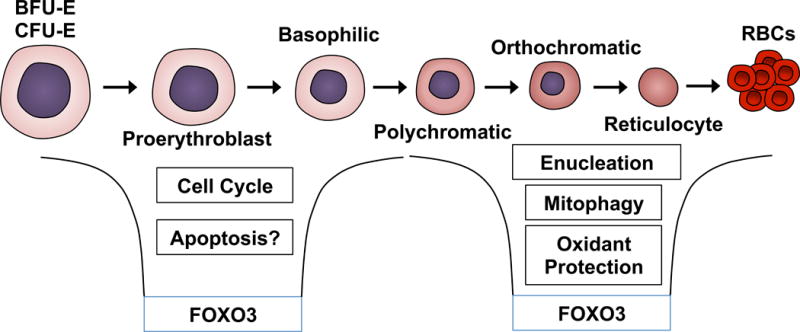
The transcription factor forkhead box O3 (FOXO3) serves diverse functions dependent on the stage of murine erythroblast maturation. FOXO3 is responsible for regulation of a third of genes that are differentially expressed during normal post progenitor cell maturation. In early stage of bone marrow erythroblasts, FOXO3 regulates cell cycle progression and potentially has a role in modulating apoptosis. Furthermore, in late stage erythroblasts, FOXO3 regulates genes responsible for processes of terminal erythropoiesis including erythroblast maturation, enucleation, mitophagy and antioxidant defence.
BFU-E, burst-forming unit-erythroid cells; CFU-E colony-forming unit-erythroid cells.
FOXO3 is also implicated in erythroid disorders, such as β-thalassaemia and sickle cell disease, where the exact regulation and function of FOXO3 remain to be established (Pourfarzad et al, 2013; Sheehan et al, 2013; Zhang et al, 2014; Franco et al, 2014). These collective results raise the possibility that modulations of FOXO3 might contribute to enhancing the in vitro production of RBCs, which has remained relatively impractical due to the inefficiency of in vitro erythroblast enucleation. Continued research into FOXO3 function in the context of disease may reveal abnormal FOXO3 roles that contribute to pathogenicity not seen under homeostatic conditions.
Summary
Future challenges will include validating data derived from mouse studies in human erythropoiesis where these results have not been tested. This will require developing improved in vitro systems for precise monitoring of human erythroid cell maturation as well as robust in vivo chimeric systems for investigating mechanisms of erythroid disorders. The current understanding of erythropoiesis raises the hope that adjuvant therapies might be within reach for at least some forms of erythroid disorders and may supplement gene therapy approaches.
Acknowledgments
We apologize to those authors whose work could not be included due to space limitations. R.L. is partially supported by an American Heart Association fellowship. S.G. is supported by NIH R01 HL116365 and an ASH Bridge Award.
Footnotes
Author contributions:
R.L. and S.G. wrote the paper. R.L. produced the figures.
Bibliography
- Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LAM, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SEW. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- An X, Schulz VP, Li J, Wu K, Liu J, Xue F, Hu J, Mohandas N, Gallagher PG. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood. 2014;123:3466–77. doi: 10.1182/blood-2014-01-548305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, Xie L, Bredell BX, Gardenghi S, Rivella S, Shah YM. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4922–30. doi: 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Nizzi CP, Chang Y, Deck KM, Schmidt PJ, Galy B, Damnernsawad A, Broman AT, Kendziorski C, Hentze MW, Fleming MD, Zhang J. The IRP1-HIF-2a Axis Coordinates Iron and Oxygen Sensing with Erythropoiesis and Iron Absorption. Cell Metabolism. 2013;17:282–290. doi: 10.1016/j.cmet.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlet JB, Ribeil JA, Guillem F, Negre O, Hazoume A, Marcion G, Beuzard Y, Dussiot M, Moura IC, Demarest S, de Beauchêne IC, Belaid-Choucair Z, Sevin M, Maciel TT, Auclair C, Leboulch P, Chretien S, Tchertanov L, Baudin-Creuza V, Seigneuric R, Fontenay M, Garrido C, Hermine O, Courois G. HSP70 sequestration by free α-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature. 2014;514:242–6. doi: 10.1038/nature13614. [DOI] [PubMed] [Google Scholar]
- Bakker WJ, Blázquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, Coffer PJ, Löwenberg B, von Lindern M, van Dijk TB. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. The Journal of cell biology. 2004;164:175–84. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, Kolbus A, Yamamoto K, Steinlein P, Verhaak RGW, Mak TW, Beug H, Löwenberg B, von Lindern M. Differential regulation of Foxo3a target genes in erythropoiesis. Molecular and cellular biology. 2007;27:3839–3854. doi: 10.1128/MCB.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, van der Zalm MM, van Oirschot BA, Lee FS, Giles RH, Kruip MJHA, Gitz-Francois JJJM, Van Solinge WW, Bierings M, van Wijk R. Novel Homozygous Mutation of the Internal Translation Initiation Start Site of VHL is Exclusively Associated with Erythrocytosis: Indications for Distinct Functional Roles of von Hippel-Lindau Tumor Suppressor Isoforms. Human mutation. 2015;36:1039–42. doi: 10.1002/humu.22846. [DOI] [PubMed] [Google Scholar]
- Beru N, McDonald J, Lacombe C, Goldwasser E. Expression of the erythropoietin gene. Molecular and cellular biology. 1986;6:2571–5. doi: 10.1128/mcb.6.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beru N, McDonald J, Goldwasser E. Expression of the erythropoietin gene. Blood cells. 1987;13:263–8. [PubMed] [Google Scholar]
- Boehm D, Mazurier C, Giarratana MC, Darghouth D, Faussat AM, Harmand L, Douay L. Caspase-3 is involved in the signalling in erythroid differentiation by targeting late progenitors. PloS one. 2013;8:e62303. doi: 10.1371/journal.pone.0062303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. British journal of haematology. 2015 doi: 10.1111/bjh.13820. [DOI] [PubMed] [Google Scholar]
- Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21:3368–76. doi: 10.1038/sj.onc.1205326. [DOI] [PubMed] [Google Scholar]
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. Journal of cellular and molecular medicine. 2011;15:1239–53. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Science. Vol. 301. New York, N.Y: 2003. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a; pp. 215–8. [DOI] [PubMed] [Google Scholar]
- Centis F, Tabellini L, Lucarelli G, Buffi O, Tonucci P, Persini B, Annibali M, Emiliani R, Iliescu A, Rapa S, Rossi R, Ma L, Angelucci E, Schrier SL. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with beta-thalassemia major. Blood. 2000;96:3624–9. [PubMed] [Google Scholar]
- Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17413–8. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Xie L, Ramakrishnan SK, Campbell A, Rivella S, Shah YM. Intestine specific disruption of HIF-2alpha improves anemia in sickle cell disease. The Journal of biological chemistry. 2015;290:23523–23527. doi: 10.1074/jbc.C115.681643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U, Peschle C. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–93. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Dussiot M, Maciel TT, Fricot A, Chartier C, Negre O, Veiga J, Grapton D, Paubelle E, Payen E, Beuzard Y, Leboulch P, Ribeil JA, Arlet JB, Coté F, Courtois G, Ginzburg YZ, Daniel TO, Chopra R, Sung V, Hermine O, Moura IC. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nature medicine. 2014;20:398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metabolism. 2015;22:164–74. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelenboom A, Mokry M, Smits LM, Nieuwenhuis EE, Burgering BMT. FOXO3 selectively amplifies enhancer activity to establish target gene regulation. Cell reports. 2013;5:1664–78. doi: 10.1016/j.celrep.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher Ka, Kalfa Ta, Shaaban AF, Way SS. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–62. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England SJ, McGrath KE, Frame JM, Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–17. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erslev AJ, Besarab A. Erythropoietin in the pathogenesis and treatment of the anemia of chronic renal failure. Kidney International. 1997;51:622–630. doi: 10.1038/ki.1997.91. [DOI] [PubMed] [Google Scholar]
- Espín-Palazón R, Stachura DL, Campbell CA, García-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, Traver D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–85. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SS, De Falco L, Ghaffari S, Brugnara C, Sinclair DA, Matte’ A, Iolascon A, Mohandas N, Bertoldi M, An X, Siciliano A, Rimmelé P, Cappellini MD, Michan S, Zoratti E, Anne J, De Franceschi L. Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2014;99:267–75. doi: 10.3324/haematol.2013.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Kalucka J, Mamlouk S, Singh RP, Muschter A, Weidemann A, Iyengar V, Jahn S, Wieczorek K, Geiger K, Muders M, Sykes AM, Poitz DM, Ripich T, Otto T, Bergmann S, Breier G, Baretton G, Fong GH, Greaves DR, Bornstein S, Chavakis T, Fandrey J, Gassmann M, Wielockx B. HIF-1α is a protective factor in conditional PHD2-deficient mice suffering from severe HIF-2α-induced excessive erythropoiesis. Blood. 2013;121:1436–45. doi: 10.1182/blood-2012-08-449181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Combinatorial regulation of novel erythroid gene expression in zebrafish. Experimental hematology. 2008;36:424–32. doi: 10.1016/j.exphem.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochimica et biophysica acta. 2012;1823:1434–43. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell metabolism. 2009;9:217–27. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, Liu Y, Amariglio N, Rechavi G, Rachmilewitz EA, Breuer W, Cabantchik ZI, Wrighting DM, Andrews NC, de Sousa M, Giardina PJ, Grady RW, Rivella S. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–35. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, Muirhead K, Rao N, Roy CN, Andrews NC, Nemeth E, Follenzi A, An X, Mohandas N, Ginzburg Y, Rachmilewitz EA, Giardina PJ, Grady RW, Rivella S. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. The Journal of clinical investigation. 2010;120:4466–77. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6523–8. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg Y, Rivella S. B-Thalassemia: a Model for Elucidating the Dynamic Regulation of Ineffective Erythropoiesis and Iron Metabolism. Blood. 2011;118:4321–30. doi: 10.1182/blood-2011-03-283614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfest-Allen E, Malik J, Palis J, Stoeckert CJ. Stat and interferon genes identified by network analysis differentially regulate primitive and definitive erythropoiesis. BMC systems biology. 2013;7:38. doi: 10.1186/1752-0509-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CJ. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. Journal of cellular physiology. 1976;89:289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- Gregory CJ, Eaves AC. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978;51:527–537. [PubMed] [Google Scholar]
- Guo G, Luc S, Marco E, Lin TW, Peng C, Kerenyi MA, Beyaz S, Kim W, Xu J, Das PP, Neff T, Zou K, Yuan GC, Orkin SH. Mapping cellular hierarchy by single-cell analysis of the cell surface repertoire. Cell stem cell. 2013;13:492–505. doi: 10.1016/j.stem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel – Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2α signaling and splenic erythropoiesis. 2007;117:3879–3889. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Liu J, Xue F, Halverson G, Reid M, Guo A, Chen L, Raza A, Galili N, Jaffray J, Lane J, Chasis JA, Taylor N, Mohandas N, An X. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–53. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes & development. 1998;12:149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nature reviews Cancer. 2002;2:673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- Kang YA, Sanalkumar R, O’Geen H, Linnemann AK, Chang CJ, Bouhassira EE, Farnham PJ, Keles S, Bresnick EH. Autophagy driven by a master regulator of hematopoiesis. Molecular and cellular biology. 2012;32:226–39. doi: 10.1128/MCB.06166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–48. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashii Y, Uchida M, Kirito K, Tanaka M, Nishijima K, Toshima M, Ando T, Koizumi K. A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase-Akt activation pathway in erythropoietin signal transduction. 2000;96:941–949. [PubMed] [Google Scholar]
- Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nature genetics. 2014;46:678–84. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, Nemeth E, Ganz T. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126:2031–7. doi: 10.1182/blood-2015-07-658419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nature reviews Genetics. 2009;10:821–32. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, Cabantchik ZI, Bouhassira EE, Fabry ME, Ginzburg YZ. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nature medicine. 2010;16:177–82. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- Li L, Freudenberg J, Cui K, Dale R, Song SH, Dean A, Zhao K, Jothi R, Love PE. Ldb1-nucleated transcription complexes function as primary mediators of global erythroid gene activation. Blood. 2013;121:4575–85. doi: 10.1182/blood-2013-01-479451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW, Tober J, Dzierzak E, Orkin SH, Tan K, North TE, Speck NA. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes & development. 2014;28:2597–612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Campreciós G, Kou Y, McGrath K, Nowak R, Catherman S, Bigarella CL, Rimmelé P, Zhang X, Gnanapragasam MN, Bieker JJ, Papatsenko D, Ma’ayan A, Bresnick E, Fowler V, Palis J, Ghaffari S. A Systems Approach Identifies Essential FOXO3 Functions At Key Steps of Terminal Erythropoiesis. PLoS Genetics. 2015;10:e1005526. doi: 10.1371/journal.pgen.1005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Rimmelé P, Bigarella CL, Yalcin S, Ghaffari S. Evidence for AKT-Independent Regulation of FOXO1 and FOXO3 in Hematopoietic Stem and Progenitor Cells. Cell cycle. 2016 doi: 10.1080/15384101.2015.1123355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang J, Ginzburg Y, Li H, Xue F, De Franceschi L, Chasis JA, Mohandas N, An X. Quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood. 2013;121:e43–9. doi: 10.1182/blood-2012-09-456079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. The Journal of clinical investigation. 2012;122:4635–44. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain Da, Khan TM, Koul Pa, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nature genetics. 2014;46:951–6. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love PE, Warzecha C, Li L. Ldb1 complexes: the new master regulators of erythroid gene transcription. Trends in genetics : TIG. 2014;30:1–9. doi: 10.1016/j.tig.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig LS, Gazda HT, Eng JC, Eichhorn SW, Thiru P, Ghazvinian R, George TI, Gotlib JR, Beggs AH, Sieff CA, Lodish HF, Lander ES, Sankaran VG. Altered translation of GATA1 in Diamond-Blackfan anemia. Nature medicine. 2014;20:748–53. doi: 10.1038/nm.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud DL, G-Amlak M, Deb DK, Platanias LC, Uddin S, Wickrema A. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene. 2002;21:1556–62. doi: 10.1038/sj.onc.1205230. [DOI] [PubMed] [Google Scholar]
- Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. The Journal of clinical investigation. 2007;117:2133–44. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. The Journal of clinical investigation. 2009;119:1159–66. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Delga S, Deschemin JC, Vaulont S, Peyssonnaux C. Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood. 2012;119:587–90. doi: 10.1182/blood-2011-09-380337. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: Where flow meets morphology. Journal of Immunological Methods. 2008;336:91–97. doi: 10.1016/j.jim.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SC, Kang YA, DeVilbiss AW, O’Driscoll CA, Ouellette JN, Pope NJ, Camprecios G, Chang CJ, Yang D, Bouhassira EE, Ghaffari S, Bresnick EH. The exosome complex establishes a barricade to erythroid maturation. Blood. 2014;124:2285–97. doi: 10.1182/blood-2014-04-571083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyron-Holtz EG, Ghosh MC, Rouault TA. Science. Vol. 306. New York, N.Y: 2004. Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo; pp. 2087–90. [DOI] [PubMed] [Google Scholar]
- Morera D, Roher N, Ribas L, Balasch JC, Doñate C, Callol A, Boltaña S, Roberts S, Goetz G, Goetz FW, MacKenzie SA. RNA-Seq reveals an integrated immune response in nucleated erythrocytes. PloS one. 2011;6:e26998. doi: 10.1371/journal.pone.0026998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Science. Vol. 306. New York, N.Y: 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization; pp. 2090–3. [DOI] [PubMed] [Google Scholar]
- Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, Kaufmann KB, McLeod J, Laurenti E, Dunant CF, McPherson JD, Stein LD, Dror Y, Dick JE. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;(6269) doi: 10.1126/science.aab2116. aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J. Primitive and definitive erythropoiesis in mammals. Frontiers in physiology. 2014;5:3. doi: 10.3389/fphys.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul F, Arkin Y, Giladi A, Jaitin DA, Kenigsberg E, Keren-Shaul H, Winter D, Lara-Astiaso D, Gury M, Weiner A, David E, Cohen N, Lauridsen FKB, Haas S, Schlitzer A, Mildner A, Ginhoux F, Jung S, Trumpp A, Porse BT, Tanay A, Amit I. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell. 2015;163:1663–1677. doi: 10.1016/j.cell.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Current opinion in hematology. 2011;18:139–45. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TRJ, Maxwell PH, McMullin MF, Lee FS. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:654–9. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, Rainey MG, van Wijk R, Wood M, Lappin TRJ, McMullin MF, Lee FS. Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood. 2008;111:5400–2. doi: 10.1182/blood-2008-02-137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) The Journal of clinical investigation. 2007;117:1926–32. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, Wasi P, Ma L, Schrier SL. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in thai patients with thalassemia. Blood. 2000;96:2606–12. [PubMed] [Google Scholar]
- Pourfarzad F, von Lindern M, Azarkeivan A, Hou J, Kia SK, Esteghamat F, van Ijcken W, Philipsen S, Najmabadi H, Grosveld F. Hydroxyurea responsiveness in β-thalassemic patients is determined by the stress response adaptation of erythroid progenitors and their differentiation propensity. Haematologica. 2013;98:696–704. doi: 10.3324/haematol.2012.074492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz EA. FORMATION OF HEMICHROMES FROM OXIDIZED HEMOGLOBIN SUBUNITS. Annals of the New York Academy of Sciences. 1969;165:171–184. doi: 10.1111/j.1749-6632.1969.tb27787.x. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz EA, Thorell B. Hemichromes in single inclusion bodies in red cells of beta thalassemia. Blood. 1972;39:794–800. [PubMed] [Google Scholar]
- Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Molecular and cellular biology. 2005;25:3163–72. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, Coulon S, Moura IC, Zeuner A, Kirkegaard-Sørensen T, Varet B, Solary E, Garrido C, Hermine O. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–5. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- Ribeil JA, Arlet JB, Dussiot M, Moura IC, Courtois G, Hermine O. Ineffective erythropoiesis in β -thalassemia. TheScientificWorldJournal. 2013;2013:394295. doi: 10.1155/2013/394295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelé P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, Ghaffari S. Aging-like Phenotype and Defective Lineage Specification in SIRT1-Deleted Hematopoietic Stem and Progenitor Cells. Stem Cell Reports. 2014;3:1–16. doi: 10.1016/j.stemcr.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ. Regulation of Iron Metabolism by Hepcidin under Conditions of Inflammation. The Journal of biological chemistry. 2015;290:18975–83. doi: 10.1074/jbc.R115.650150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell metabolism. 2008;7:205–14. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nature Reviews Molecular Cell Biology. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–40. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–9. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]
- Sheehan VA, Crosby JR, Sabo A, Howard TA, Muzny DM, Reid JG, Aygun B, Boerwinkle E, Gibbs RA, Ware RE. FOXO3 Variants Are Associated With Lower Fetal Hemoglobin Levels In Children With Sickle Cell Disease. Blood. 2013;122:778. [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Science. Vol. 344. New York, N.Y: 2014. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle; pp. 649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, Percy MJ, Pugh CW, Ratcliffe PJ, Talbot NP, Treacy M, Robbins PA. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS medicine. 2006;3:e290. doi: 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Murrell M, Liu Y, Pop R, Porpiglia E, Levchenko A. Negative autoregulation by FAS mediates robust fetal erythropoiesis. PLoS biology. 2007;5:e252. doi: 10.1371/journal.pbio.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suragani RNVS, Cawley SM, Li R, Wallner S, Alexander MJ, Mulivor AW, Gardenghi S, Rivella S, Grinberg AV, Pearsall RS, Kumar R. Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine β-thalassemia. Blood. 2014;123:3864–72. doi: 10.1182/blood-2013-06-511238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–35. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, Christofidou-solomidou M, Lappin TRJ, Lee FS. Erythrocytosis and Pulmonary Hypertension in a Mouse Model of Human HIF2A Gain of Function Mutation*. 2013;288:17134–17144. doi: 10.1074/jbc.M112.444059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NLC, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nature medicine. 2007;13:1096–101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, Paulson RF, Miller JL. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–6. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo Y, Sekine H, Hirano I, Pan X, Souma T, Tsujita T, Kawaguchi S, Takeda N, Takeda K, Fong GH, Dan T, Ichinose M, Miyata T, Yamamoto M, Suzuki N. Hypoxia Signaling Cascade for Erythropoietin Production in Hepatocytes. Molecular and cellular biology. 2015;35:2658–72. doi: 10.1128/MCB.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB life. 2009;61:800–30. doi: 10.1002/iub.226. [DOI] [PubMed] [Google Scholar]
- Uddin S, Kottegoda S, Stigger D, Platanias LC, Wickrema A. Activation of the Akt/FKHRL1 pathway mediates the antiapoptotic effects of erythropoietin in primary human erythroid progenitors. Biochemical and biophysical research communications. 2000;275:16–9. doi: 10.1006/bbrc.2000.3266. [DOI] [PubMed] [Google Scholar]
- Volke M, Gale DP, Maegdefrau U, Schley G, Klanke B, Bosserhoff AK, Maxwell PH, Eckardt KU, Warnecke C. Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors. PloS one. 2009;4:e7875. doi: 10.1371/journal.pone.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, Simon MC, Kleinfeld D, Johnson RS. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson N, Pantopoulos K. The IRP/IRE system in vivo: insights from mouse models. Frontiers in pharmacology. 2014;5:176. doi: 10.3389/fphar.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojchowski DM, Sathyanarayana P, Dev A. Erythropoietin receptor response circuits. Current opinion in hematology. 2010;17:169–76. doi: 10.1097/MOH.0b013e328338008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Wynn JL, Scumpia PO, Stocks BT, Romano-Keeler J, Alrifai MW, Liu JH, Kim AS, Alford CE, Matta P, Weitkamp JH, Moore DJ. Neonatal CD71+ Erythroid Cells Do Not Modify Murine Sepsis Mortality. Journal of immunology (Baltimore, Md: 1950) 2015;195:1064–70. doi: 10.4049/jimmunol.1500771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ohneda O, Sakiyama A, Iwata F, Ohneda K, Fujii-Kuriyama Y. The microenvironment for erythropoiesis is regulated by HIF-2alpha through VCAM-1 in endothelial cells. Blood. 2008;112:1482–92. doi: 10.1182/blood-2007-11-122648. [DOI] [PubMed] [Google Scholar]
- Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. The Journal of biological chemistry. 2006;281:25703–11. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, Dore LC, Yao Y, D’Souza J, Zhang Z, Ghaffari S, Choi J, Friend S, Tong W, Orange JS, Paw BH, Weiss MJ. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes & development. 2010;24:1620–33. doi: 10.1101/gad.1942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Angelucci E, Lucarelli G, Aljurf M, Snyder LM, Kiefer CR, Ma L, Schrier SL. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe beta-thalassemia (Cooley’s anemia) Blood. 1993;82:374–7. [PubMed] [Google Scholar]
- Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase Activation Is Required for Terminal Erythroid Differentiation. Journal of Experimental Medicine. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–46. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- Zhang X, Campreciós G, Rimmelé P, Liang R, Yalcin S, Mungamuri SK, Barminko J, D’Escamard V, Baron MH, Brugnara C, Papatsenko D, Rivella S, Ghaffari S. FOXO3-mTOR Metabolic Cooperation in the Regulation of Erythroid Cell Maturation and Homeostasis. American journal of hematology. 2014;00:1–10. doi: 10.1002/ajh.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. The New England journal of medicine. 2012;367:922–30. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]


