Abstract
OBJECTIVE
To measure total tumour volume (TTV) and dominant TV (DTV) in radical prostatectomy (RP) specimens from patients predicted to have low-volume, low-grade (LV/LG) prostate cancer, as this entity can be predicted from biopsy findings and prostate-specific antigen (PSA) level, but tumour under-sampling remains a challenge in active surveillance programmes.
PATIENTS AND METHODS
This was a retrospective study from an academic centre, of men with prostate cancer treated from 2000 to 2007, with a PSA level of <10 ng/mL and one core of cancer from an extended scheme showing either Gleason score (GS) 3 + 3 of <3.0 mm or 3 + 4 of <2.0 mm. All men had RP, and the TTV, DTV, tumour location, pathological GS and stage were measured.
RESULTS
Of 3055 RPs, 66 (2.1%) met the inclusion criteria. The core with cancer was from a sextant and alternative site in 26 (39%) and 40 (61%) patients, respectively. A pathological GS 3 + 3 or 3 + 4 was assigned to 94%, while 6% were GS ≥ 4 + 3; all 66 tumours were organ-confined. The median (range) TTV and DTV were 0.15 (0.0008–5.06) and 0.14 (0.0008–5.04) mL, respectively. The median number of tumour foci was 3 (1–7), being unifocal in 17/66 (26%) and multifocal in 49/66 (74%). The transition zone was involved in 29% of unifocal and 71% of multifocal tumours. Of all 66 patients, the TTV was <0.5 mL in 47 (71%), and of 59 patients with biopsy GS 3 + 3, 33 (56%) had a TTV of <0.5 mL and pathological GS 3 + 3. Of 19 patients with a TTV of ≥0.5 mL, the median TTV was 1.06 (0.51–5.05) mL, with tumour foci of transition zone origin in 16 (84%). The study was limited by its retrospective design and small sample size.
CONCLUSIONS
Using conservative selection criteria for predicting LV/LG cancer, RP specimens showed organ-confined disease in all cases, upgrading to GS ≥ 4 + 3 in 6%, and TTV <0.5 mL in 71% of cases. The transition zone is a common location of under-sampled disease.
Keywords: prostate cancer, active surveillance, radical prostatectomy
INTRODUCTION
Watchful waiting is an accepted management option for localized prostate cancer [1], and in the present era of PSA screening might have an expanding role with the increased detection of low-volume, low-grade (LV/LG) tumours. Active surveillance has become the term for forgoing curative therapy until the development of additional clinical findings such as an increase in PSA kinetics and/or progression of biopsy findings. Implementation of active surveillance (AS) is challenged by a lack of consensus on: (i) selection criteria that define non-lethal disease; (ii) a reliable, anticipatory surveillance strategy for disease progression; and (iii) widespread acceptance of monitoring among patients and clinicians. Furthermore, potentially all disease might be lethal given enough time.
The absence of molecular tests and imaging techniques capable of reliably identifying LV/LG disease makes dependence on clinicopathological variables necessary [2]. Prostate tumour volume (TV) correlates with biological behaviour [3–5] and tumours of <0.5 mL and Gleason score (GS) 3 + 3 might remain clinically insignificant, i.e. organ-confined [6]. The pathological stage of prostate cancer worsens as TVs increase to 0.5–1.5 mL and to >1.5 mL [7].
Therefore, we undertook a retrospective study to evaluate the pathological features of radical prostatectomy (RP) specimens in patients who would meet the selection criteria for LV/LG tumour used for our prospective AS protocol currently underway at our tertiary cancer centre.
PATIENTS AND METHODS
This retrospective study was approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center. The inclusion criteria were: (i) tumour stage of cT1c-cT2a-c; (ii) a PSA level of <4 ng/mL (group A) or 4–10 ng/mL (group B); (iii) extended-biopsy scheme of at least 10 cores; (iv) one positive biopsy core with a GS of 3 + 3 and <3.0 mm or 3 + 4 and <2.0 mm. Biopsies were most commonly taken outside of the institution, and the specimens were reevaluated by institution genitourinary pathologists. To classify as an extended-biopsy scheme, the referred ultrasonography/biopsy procedure notes and pathological materials must have identified sextant biopsies (parasagittal right/left base, right/left mid, right/left apex) and any four or more biopsies from alternative sites including the far laterals, transition zone (TZ), anterior zone, and midline. Exclusion criteria included a PSA level of >10 ng/mL, fewer than 10 cores taken, two or more positive cores, dominant Gleason pattern 4, and tumour length greater than the inclusion criteria. Histopathological features of the biopsy specimens were obtained from the pathology reports. The RP specimens (51 open, four laparoscopic, and 11 robotic) were analysed according to our institutional protocol [8–10]. The specimens were submitted in their entirety for histopathological evaluation. The cross sections were subdivided into portions to fit standard-size cassettes. The blocks from each cross-section were diagrammed in a manner that permitted the reconstruction of the entire cross-sections and allowed us to record individual tumour foci. An area of carcinoma was considered a different focus if it was separated by a low-power field diameter (4.5 mm) from the nearest adjacent focus [8]. Each tumour focus was outlined on the histological sections and the zonal origin and GS of each tumour focus were recorded in the specimen diagram. The GS assigned to the specimen was that of the tumour focus with the highest GS. The volume of the five largest tumour foci was determined using the three-dimensional volume estimation method [9]. The largest focus is considered the dominant tumour.
Data extracted included pretreatment data (clinical stage, PSA level, biopsy/ultrasonography, demographics), and RP data, i.e. pathological stage, GS, surgical margin status, total TV (TTV), dominant TV (DTV), and tumour foci zonal origin. Cases were classified as clinically insignificant disease (TTV <0.5 mL of GS 3 + 3) by TTV. Furthermore, cases were classified by zonal origin of all and dominant tumour foci, correlated for the side of positive biopsy to the side of dominant tumour focus. Biopsy and RP GSs were compared to determine the incidences of upgrading and downgrading.
Descriptive statistics, such as median (range) for continuous variables and percentage measures for categorical variables, were used to summarize the data. The Wilcoxon rank-sum test was used to assess the difference between the two PSA groups in terms of TRUS-based prostate volume measurement.
RESULTS
From 2000 to 2007, 3055 RPs were performed at M.D. Anderson Cancer Center, and 66 (2.1%) met the inclusion criteria, 36 in group A and 30 in group B. Many potential cases in the earlier years were excluded as there were fewer than <10 biopsies, and therefore the sample size is not a true estimate of incidence. Table 1 summarizes the demographic and clinical characteristics of these 66 patients. Group B had higher average TRUS volumes than group A (40.4 vs 32.7 mL, P = 0.008). Further comparisons of groups A and B showed no significant differences (not shown) and the remaining data are presented as a single group. Table 1 also summarizes the extended-biopsy characteristics for the 66 patients. The biopsy core with carcinoma was identified at a sextant site in 26 (39%) and at an alternative site in 40 (61%).
TABLE 1.
The characteristics of 66 patients treated with RP and meeting specific criteria for AS, and the characteristics of the biopsy and RP specimens
| Characteristic | Median (range) or n (%) |
|---|---|
| Age, years | 58 (35–70) |
| Race | |
| White | 49 (79) |
| Black | 8 (12) |
| Hispanic | 6 (3) |
| Asian | 3 (5) |
| Clinical Stage | |
| cT1c | 58 (88) |
| cT2 | 8 (12) |
| PSA level, ng/mL | 3.9 (0.3–9.7) |
| <4 (Group A) | 2.7 (0.3–3.9), 36 (55) |
| 4–10 (Group B) | 6.1 (4.0–9.7), 30 (45) |
| TRUS volume, mL* | 35.5 (18.1–99.3) |
| Group A | 32.7 (18.1–54.5) |
| Group B | 40.4 (22–99.3) |
| Biopsy characteristics | |
| N biopsy cores | 12 (10–22) |
| Sextant locations | 66 (100) |
| Alternative locations | |
| Anterior horn/lateral | 66 (100) |
| TZ | 13 (20) |
| Midline | 10 (15) |
| Location of core with carcinoma | |
| Sextant site | 26 (39) |
| Alternative site | 40 (61) |
| Anterior horn/lateral | 38 (58) |
| TZ | 2 (2) |
| GS | |
| 3 + 3 | 59 (89) |
| 3 + 4 | 7 (11) |
| Extent of carcinoma, mm | |
| ≤1.0 | 36 (55) |
| 1–<2.0 | 14 (21) |
| ≥2.0–<3.0 | 16 (24) |
| RP specimens | |
| GS | |
| 3 + 3 | 41 (62) |
| 3 + 4 | 21 (32) |
| 4 + 3 | 2 (3) |
| ≥4 + 4 | 2 (3) |
| Pathological stage | |
| pT2, MOR − | 59 (89) |
| pT2, MOR + | 7 (11) |
| N0 | 24 (36) |
| Nx | 42 (64) |
| TTV, mL | 0.15 (0.0008–5.06) |
| <0.5 | 47 (71) |
| <0.5 + biopsy GS 3 + 3 | 33/59 (56) |
| DTV | 0.14 (0.0008–5.04) |
TRUS volume, group A vs B, P = 0.008; MOR, margins of resection.
Table 1 also summarizes the RP pathology results; a GS of 3 + 3 or 3 + 4 was assigned to 94% of RP specimens, while four (6%) were upgraded to a GS of ≥4 + 3. All tumours were organ-confined, with negative surgical margins in 89%. Of the 59 patients with a biopsy GS of 3 + 3, 33 (56%) had a final TV of <0.5 mL. The median TTV and DTV were small for both (<0.2 mL), but with a wide range of 0.0008–5.06 and 0.0008–5.04 mL, respectively.
The median number of cancer foci in all cases was 3 (1–7). The cancer was unifocal in 17/66 (26%) and multifocal in 49/66 (74%). Of 17 unifocal cancers, 12 (71%) were in the PZ and five (29%) in the TZ. Of 49 multifocal cancers, 14 (29%) were in the PZ only, the TZ alone in one (2%), the PZ and TZ in 29 (59%) and all zones (PZ, TZ, central) in five (10%). The dominant tumour focus was more commonly in the PZ than TZ, at 43 (65%) vs 23 (35%) (P = 0.04).
The pathological correlation of biopsy to RP findings is shown in Table 2. The TTV was <0.5 mL in 71% of patients, and the PZ TV was <0.5 mL in 85% of patients. Among the 19 patients with a biopsy GS of 3 + 3 who were upgraded at RP, nine had a TTV of <0.5 mL. Among the seven patients with a biopsy GS of 3 + 4, five had the same score at RP; one was upgraded to 8 (4 + 4), with a TV of >0.5 mL, and one was downgraded to GS 3 + 3, with a TTV of <0.5 mL.
TABLE 2.
Correlation of biopsy with RP findings by GS and TV
| Biopsy GS (n) | RP GS | n (%) | n, TTV, mL | n, PZ TV, mL | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| <0.5 | ≥0.5 | <0.5 | ≥0.5 | |||
| 3 + 3 (59) | 3 + 3 | 40 (68) | 33 | 7 | 37 | 3 |
| >3 + 3 | 19 (32) | 9 | 10 | 14 | 5 | |
| 3 + 4 (7) | 3 + 4 | 5 | 4 | 1 | 4 | 1 |
| >3 + 4 | 1 | 0 | 1 | 0 | 1 | |
| 3 + 3 | 1 | 1 | 0 | 1 | 0 | |
| All patients | > Biopsy | 20 (30) | ||||
| All patients | 66 | 47 (71) | 19 (29) | 56 (85) | 10 (15) | |
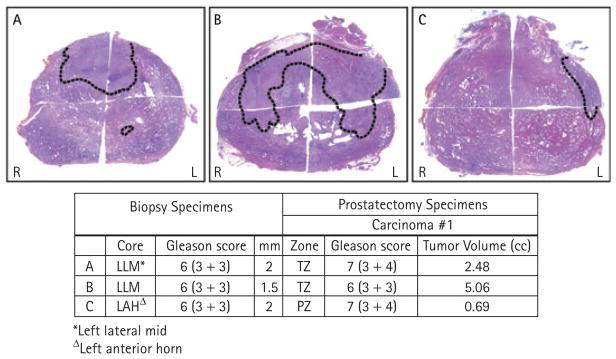
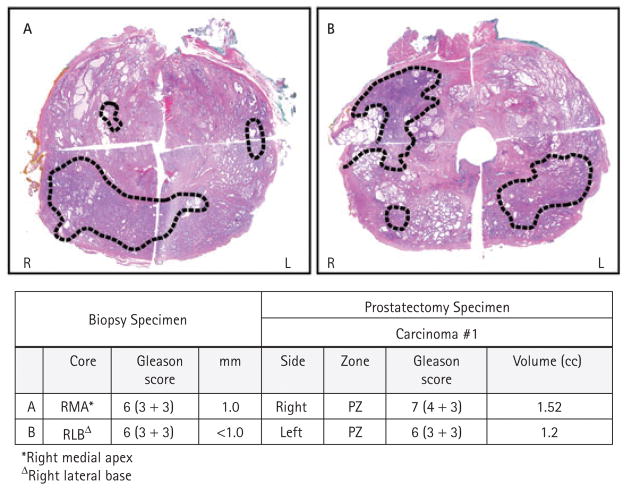
Overall, 19 patients had a TTV of ≥0.5 mL; the median TV in these patients was 1.06 (0.51–5.05) mL, and the median number of tumour foci was 4 (1–7). Table 3 shows the zonal origin of the tumour foci and dominant tumour focus. Figures 1 and 2 illustrate cases in which patients with one biopsy core showed significantly larger RP TV at both anterior and posterior locations, respectively. Table 4 shows the correlation between the side of the positive biopsy with the site of the dominant tumour focus; three (5%) were bilateral, 45 (68%) ipsilateral and 18 (27%) contralateral.
TABLE 3.
Characteristics of RP specimens from 19 patients with a TTV of ≥0.5 mL
| Variable | Median (range) or n (%) |
|---|---|
| No. tumour foci | 4 (1–7) |
| TTV | 1.06 (0.51–5.05) |
| Zonal distribution of tumour foci | |
| PZ + TZ | 13 |
| PZ | 3 |
| PZ + TZ + CZ* | 2 |
| TZ | 1 |
| Dominant tumour focus | |
| PZ | 10 |
| TZ | 9 |
| DTV, mL | |
| ≥0.5 | 17 (PZ 8; TZ 9) |
| <0.5 | 2 (PZ 2) |
CZ, central zone.
FIG. 1.
Three illustrative examples of RP specimens from patients with one positive biopsy core of GS 3 + 3 and <3 mm. In these cases, the DTV was located in the anterior half of the prostate including in the TZ (cases A, B) or anterolateral PZ (case C), resulting in under-sampling of the TV.
FIG. 2.
Two illustrative examples of RP specimens from patients with one positive biopsy core of GS 3 + 3 and <3 mm. In these cases the DTV was in the posterior half of the prostate including in the right PZ (case A) or left PZ (case B), and was under-represented in extended-core biopsies.
TABLE 4.
Correlation of side of positive biopsy with dominant tumour focus
| Biopsy side (n) |
n or n (%)
|
||
|---|---|---|---|
| Bilateral | Ipsilateral | Contralateral | |
| Right (35) | 0 | 25 | 10 |
| PZ 6 | |||
| TZ 4 | |||
| Left (31) | 3 | 20 | 8 |
| PZ 6 | |||
| TZ 2 | |||
| Total (66) | 3 (5) | 45 (68) | 18 (27) |
| PZ 12 | |||
| TZ 6 | |||
DISCUSSION
Active surveillance for localized prostate cancer is a standard option for patients to consider [1]. At our centre, patients with a high probability of having LV/LG disease are encouraged to enrol in a prospective protocol designed to monitor their disease and to further our understanding of ideal patient selection, monitoring methods, and adherence. Previous studies showed that a prediction of the presence of LV/LG disease is possible using the detection of one positive core from extended biopsies, length of tumour focus, PSA density, and patient age [2]. The purpose of the present study was to apply similar criteria to a retrospective cohort of patients after RP and to conduct a detailed analysis of the range of pathology findings in this group.
We searched a dataset of 3055 RPs and identified 66 (2%) cases meeting our prospective study inclusion criteria for AS. However, because cases with <10 cores were excluded, the prevalence of cases with potential clinically insignificant disease on biopsies is underestimated. Overall, 47/66 (71%) had a TTV of <0.5 mL. Of the 59 patients with a biopsy GS of 3 + 3, 33 (56%) had a TTV of <0.5 mL and no upgrading. We hypothesise that such patients are unlikely to have morbidity/mortality from prostate cancer and are the ideal candidates for AS. Nevertheless, careful follow-up is recommended to identify any progression, and it is notable that the ‘<0.5 mL of GS 3 + 3’ criteria for LV/LG disease has not been rigorously validated with long-term survival outcomes.
A critical question for our study and others of similar design is the fate of patients not found to have LV/LG disease. In our study we identified 12 (18%) patients with a RP GS of 3 + 4 and a TTV of <0.5 mL, and seven (11%) with a GS of 3 + 3 and a TTV of >0.5 mL (Table 2). Such patients do not meet the criteria for LV/LG disease but AS might nevertheless be appropriate. The challenge remains to define the upper limit of TTV that is GS 3 + 3 or 3 + 4 and that will not develop rapid progression. In our study, only 6% were upgraded to ≥GS 4 + 3, and none to pT3. Thus, our study suggests that the chance of significant biopsy under-sampling is low using these conservative selection criteria and extended-core biopsies.
What lessons can be learned from the 19 (29%) patients with a TTV of ≥0.5 mL? The PZ was the most common location of tumour foci. As Table 3 shows, the presence of additional TV in the TZ might be significant; 16/19 (84%) had tumour foci of TZ origin, and in nine (47%) the dominant tumour focus originated in the TZ. Table 4 shows that the dominant tumour focus was contralateral to the positive biopsy side in 18/66 (27%) patients, including both PZ (12) and TZ (six) sites. Figure 1A,B shows possible tumour configurations in which extended PZ biopsies might underestimate the volume of disease in the posterior or anterior regions. A possible solution that merits further study would be a more aggressive biopsy scheme to include the TZ, but this would probably be associated with more complications after biopsy. However, Jack et al. [11] reported that predominantly TZ tumours can have significantly higher TVs while maintaining lower rates of pT3 stage and positive margins. Acceptance of AS would certainly improve if refinements to selection and monitoring were less dependent on biopsy and more dependent on new molecular markers such as PCA3 [12] and/or better imaging tools.
Other studies have been reported that compared biopsy findings to RP findings. Noguchi et al. [13] reported a detailed comparison between patients biopsied with a sextant technique and RP. In the series of 222 RPs unselected for LV/LG disease, the incidence of the same was 10%. Biopsy features significantly predicted TV, but the regression analysis showed a very weak correlation of <10% (linear). In the lowest-risk patients with one positive core with no Gleason 4/5 pattern, 81% had a TTV of >0.5 mL, and if the single-core tumour focus was <3 mm, 71% had a TTV of >0.5 mL. Griffin et al. [14] retrospectively examined a single-surgeon series to identify potential candidates for AS, using the criteria of one or two biopsy cores with a GS of 3 + 3 but not using a length criterion. Among 664 patients, 292 (44%) met these criteria. There was upgrading in 78 (27%); 58 were upgraded to 3+ 4, 16 to 4 + 3, three to 4 + 4, and one to 5 + 4. There was extraprostatic extension in 16%, and insignificant disease in 24/282 (8%). Chun et al. [15] studied another single-surgeon series of 1180 RPs from 1992 to 2003, during which time sextant biopsies were taken in 77.5%, eight-core in 9.1% and 10-core in 13.4%. They selected 209 patients with inclusion criteria of a GS of ≤6 in only one core (any PSA level, any tumour length). At RP, insignificant cancer was found in 13.4% (15.4% of 117 with cT1c and PSA level of ≤10 ng/mL). Unfavourable cancer, defined as GS 7–10, pT3a-b and N+, was present in 33.5%, and if GS 7 was excluded, the proportion was 11%.
These series, using variable selection criteria, show that the rate of insignificant disease varied from half or more in our study to 8% and 13.4% in the studies by Griffen et al. and Chun et al., respectively. The rate of extraprostatic disease varied from none in the present series to 16% and 11%, respectively. The study of Chun et al. had relatively few cases with 10-core biopsies, but they estimated a mean TTV of 1.8 (0.3–5.7) mL for 10 cores, vs 3.0 (0.1–23.4) mL for six cores. These three series show that differences in selection criteria and biopsy techniques can make significant differences in RP pathological findings and, by inference, significant differences in the rates of disease progression during AS. Such differences might explain the concern with the AS programme reported by Klotz et al. [16], in which 64% of enrolled patients who had clinical, biochemical or grade progression had extraprostatic disease at RP.
Nomograms are available to predict minimal-volume disease. Nakanishi et al. [17] from our institution and Steyerberg et al. [18] have validated nomograms using PSA levels and biopsy characteristics. Raaijmakers et al. [19] reported that hK2 and free PSA levels might be useful additions to models that predict LV disease. Van As et al. [20] showed that the free PSA to total PSA ratio and the clinical T stage were independent predictors of patients who progressed beyond pre-set values and had radical treatment. Additional biomarkers and/or imaging techniques are needed, and another report from our institution suggests that the PCA3 urine marker [12] might be used to further refine selection criteria for LV disease. Validation studies are being planned.
Potential limitations of our study are its retrospective design and small sample size. Biopsies were taken mostly outside the institution, and therefore with nonstandardized biopsy schemes. The strengths of the study include careful analysis of RP specimens by one genitourinary pathologist according to an established protocol for estimated TTV and DTV. This study is not a ‘stand-alone’ retrospective exercise, but rather a background study with which we can compare our current recommendation of repeat extended biopsies before a patient enters AS. Since implementing this recommendation, ≈ 15% of patients who are eligible for AS after one biopsy have significantly different pathology on the second biopsy and are excluded (data not shown). Indeed, the series from Carter et al. [21] had significantly fewer patients treated in the first 3 years of AS when the entry criteria shifted from sextant to extended biopsies.
In conclusion, in this retrospective study of 66 patients meeting recently defined criteria for AS and who had RP, all cases were organ-confined and 56% met the criteria for clinically insignificant disease. Upgrading to ≥GS 4 + 3 occurred in 6%, and the TTV was ≥0.5 mL in 29%. If TV in the PZ is considered, then 15% of the patients had a TV of ≥0.5 mL. For patients who had GS 6 or 7 on biopsy, 32% and 14% had upgrading on RP, respectively. Tumour foci of TZ origin contributed to a significant number of cases of an underestimated TTV. Future studies might define the contributions of repeat extended-core biopsies at baseline, molecular markers, and imaging, to enhance our selection of candidates for AS.
Abbreviations
- AS
active surveillance
- (T)(D)TV
(total) (dominant) tumour volume
- TZ
transition zone
- PZ
peripheral zone
- RP
radical prostatectomy
- LV/LG
low-volume, low-grade
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: update 2007. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Ochiai A, Trpkov K, Yilmaz A, Donnelly B, Babaian RJ. Validation of a prediction model for low volume/low grade cancer: application in selecting patients for active surveillance. J Urol. 2007;177:907–10. doi: 10.1016/j.juro.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 3.McNeal JE, Bostwick DG, Kindrachuk RA, Redwine EA, Freiha FS, Stamey TA. Patterns of progression in prostate cancer. Lancet. 1986;11:60–3. doi: 10.1016/s0140-6736(86)90715-4. [DOI] [PubMed] [Google Scholar]
- 4.McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225–33. doi: 10.1002/1097-0142(19900915)66:6<1225::aid-cncr2820660624>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am J Surg Pathol. 1990;14:240–7. doi: 10.1097/00000478-199003000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71:933–8. doi: 10.1002/1097-0142(19930201)71:3+<933::aid-cncr2820711408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Babaian RJ, Troncoso P, Steelhammer LC, Lloreta-Trull J, Ramirez EI. Tumor volume and prostate specific antigen: implications for early detection and defining a window of curability. J Urol. 1995;154:1808–12. doi: 10.1016/s0022-5347(01)66790-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen ME, Johnston DA, Tang K, Babaian RJ, Troncoso P. Detailed mapping of prostate carcinoma foci: biopsy strategy implications. Cancer. 2000;89:1800–9. doi: 10.1002/1097-0142(20001015)89:8<1800::aid-cncr21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Chen ME, Johnston D, Reyes AO, Soto CP, Babaian RJ, Troncoso P. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Path. 2003;27:1291–301. doi: 10.1097/00000478-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Ortiz RF, Troncoso P, Babaian RJ, Lloreta J, Johnston DA, Pettaway CA. African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched White men. Cancer. 2006;107:75–82. doi: 10.1002/cncr.21954. [DOI] [PubMed] [Google Scholar]
- 11.Jack GS, Cookson MS, Coffey CS, et al. Pathological parameters of radical prostatectomy for clinical stages T1c versus T2 prostate adenocarcinoma: decreased pathological stage and increased detection of transition zone tumors. J Urol. 2002;168:519–24. [PubMed] [Google Scholar]
- 12.Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804–9. doi: 10.1016/j.juro.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi M, Stamey TA, McNeal JE, Yemoto CM. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with no palpable prostate cancer. J Urol. 2001;166:104–10. [PubMed] [Google Scholar]
- 14.Griffin CR, Yu X, Loeb S, Desireddi VN, Han M, Graif T, Catalona WF. Pathological features after radical prostatectomy in potential candidates for active monitoring. J Urol. 2007;178:860–3. doi: 10.1016/j.juro.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Chun FK, Suardi N, Capitanio U, et al. Assessment of pathological prostate cancer characteristics in men with favorable biopsy features on predominantly sextant biopsy. Eur Urol. 2009;55:617–28. doi: 10.1016/j.eururo.2008.04.099. [DOI] [PubMed] [Google Scholar]
- 16.Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23:8165–9. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi H, Wang X, Ochiai A, et al. A nomogram for predicting low-volume/low-grade prostate cancer: a tool in selecting patients for active surveillance. Cancer. 2007;110:2441–7. doi: 10.1002/cncr.23055. [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HG, Schröder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107–12. doi: 10.1016/j.juro.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 19.Raaijmakers R, deVries SH, Blijenberg BG, et al. hK2 and free PSA, a prognostic combination in predicting minimal prostate cancer in screen-detected men within the PSA range 4–10 ng/ml. Eur Urol. 2007;52:1358–64. doi: 10.1016/j.eururo.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localized prostate cancer managed by active surveillance. Eur Urol. 2008;54:1297–305. doi: 10.1016/j.eururo.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins Experience. J Urol. 2007;178:2359–65. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]