Abstract
Comparative analyses of the four known anthraquinone-fused enediynes biosynthetic gene clusters identified four genes, tnmE6, tnmH, tnmL, and tnmQ, unique to the tnm gene cluster. Larger scale fermentation of both the S. sp. CB03234 wild-type and the ΔtnmH and ΔtnmL mutant strains resulted in the characterization of 20 new tiancimycin (TNM) congeners, including five enediynes. These findings enabled a proposal for the late stage of TNM biosynthesis featuring an intermediate possibly common for all anthraqui-none-fused enediynes.
Graphical Abstract
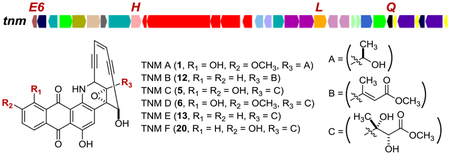
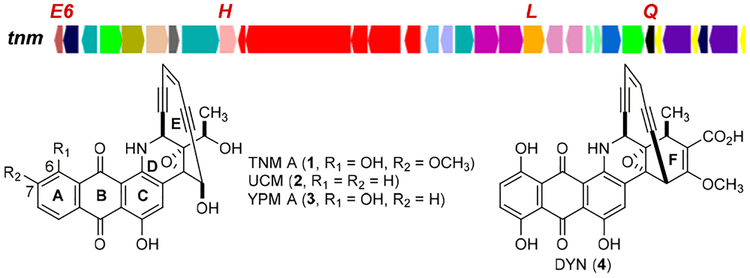
The enediyne natural products have been classified into 9-or 10-membered subcategories according to the size of the enediyne cores.1–3 The 10-membered enediynes can be further divided into two subfamilies: the calicheamicin (CAL)-like enediynes and the anthraquinone-fused enediynes, including tiancimycin A (TNM A, 1) from Streptomyces sp. CB03234,4 uncialamycin (UCM, 2) from Streptomyces uncialis,5 yangpumicin A (YPM A, 3) from Micromonospora yangpuensis,6 and dynemicin (DYN, 4) from Micromonospora chersina7 (Figure 1). While biosynthesis and engineering of the 9-membered enediynes have been greatly facilitated by the genetic amenability of selected producers with high enediyne titers,8–13 biosynthesis of the 10-membered enediynes remains poorly understood, mainly due to the lack of a model system with expedient genetic amenability, high enediyne titer, or combination of both.11,12
Figure 1.
Structures of the four known anthraquinone-fused enediynes, TNM A (1), UCM (2), YPM (3), and DYN (4), and the tnm biosynthetic gene cluster from S. sp. CB03234 with the four genes inactivated in this study highlighted in red.
Biosynthesis of anthraquinone-fused enediynes has received considerable attention since the discovery of 4 in 1989.7 Early isotope labeling studies in M. chersina revealed that both the enediyne core and the anthraquinone halves of 4 were of polyketide origin.13 A recent study suggested that the enediyne polyketide synthase (PKS) DynE8 may play a dual role in the biosynthesis of both halves of 4.14,15 Both 3 and 4 are produced by Micromonospora species, which, in general, lack expedient genetic tools for in vivo studies, and 2 is produced by a Streptomyces species, but only on solid media with extremely low titer.5 We recently demonstrated that S. sp. CB03234 is genetically amenable4 and can produce 1 with high titer (~22 mg/L) in liquid fermentation,16 making it an ideal model system to study anthraquinone-fused enediyne biosynthesis.
The similarity in both structures and gene clusters of 1−4 (Figure 1, Figure S1) suggests a common pathway for anthraquinone-fused enediyne biosynthesis. The dyn and ypm gene clusters are similar to each other in both gene content and organization,6 harboring multiple genes that are absent in the tnm and ucm gene clusters.4 The tnm cluster contains all 28 genes in the ucm cluster, with four additional genes, tnmE6, tnmH, tnmL, and tnmQ (Figure 1, Figure S1), which encode a flavin reductase (TnmE6), a SAM-dependent methyltransferase (TnmH), a P450 hydroxylase (TnmL), and a protein of unknown function (TnmQ), respectively.4 Considering 1 as a 6-hydroxyl and 7-methoxyl congener of 2 (Figure 1), we initially proposed 2 as a biosynthetic intermediate en route to 1 in S. sp. CB03234. However, inactivation of tnmH in CB03234 afforded the ΔtnmH mutant strain SB20002 that produced TNM C (5), an enediyne with an unexpected side chain appended to the enediyne core (Figure 2),4 suggesting a more complicated pathway for 1 biosynthesis.
Figure 2.
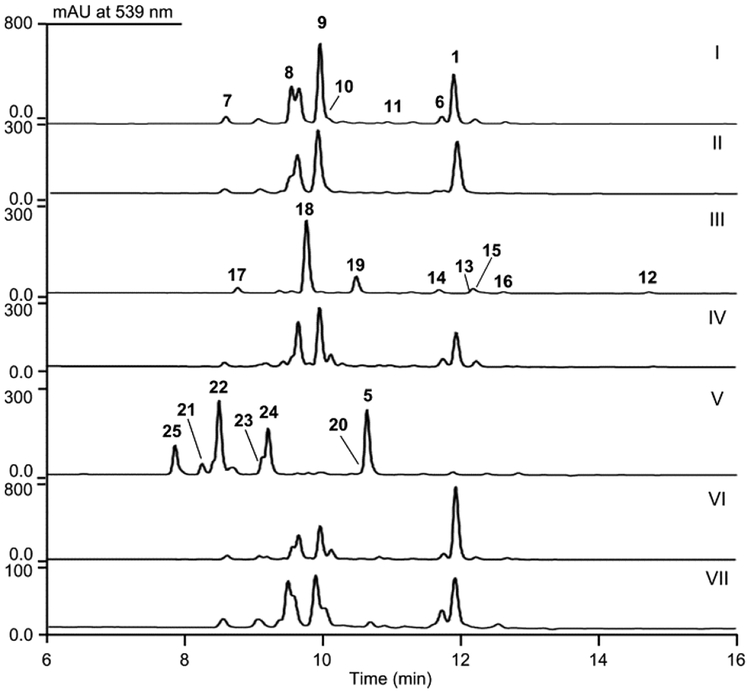
Metabolite profiles of the S. sp. CB03234 WT and mutant strains: (I) CB03234 WT; (II) SB20019 (ΔtnmE6); (III) SB20020 (ΔtnmL), (IV) SB20023 (ΔtnmL + tnmL); (V) SB20002 (ΔtnmH);(VI) SB20022 (ΔtnmH + tnmH); (VII) SB20021 (ΔtnmQ). See Figure 3 for structures of 1 and 5−25.
In this study, we inactivated tnmE6, tnmL, and tnmQ individually in S. sp. CB03234 to afford the mutant strains SB20019 (i.e., ΔtnmE6), SB20020 (i.e., ΔtnmL), and SB20021(i.e., ΔtnmQ), respectively (Figures S2−S4). Fermentation of SB20019, SB20020, and SB20021, together with SB20002,4 as well as CB03234 as a control, showed that production of 1 was not affected in SB20019 and SB20021, indicative of functional redundancy, but abolished in SB20002 and SB20020, with concomitant accumulation of new metabolites (Figures 2, S5−S7). Complementation of the ΔtnmH or ΔtnmL mutation, by expressing a functional copy of tnmH (i.e., SB20022) or tnmL (i.e., SB20023) in trans, restored 1 production, excluding potential polar effects in the two mutants (Figure 2). We subjected the CB03234 wild-type (WT) and the SB20002 and SB20020 mutant strains to large-scale fermentation (14 L each) and isolated, in addition to 1 and 5, 20 new TNM congeners (6−25); their structures were established based on a combination of high-resolution MS and 1D and 2D NMR analyses (Tables S4−S8 and Figures S8−S156).4–7
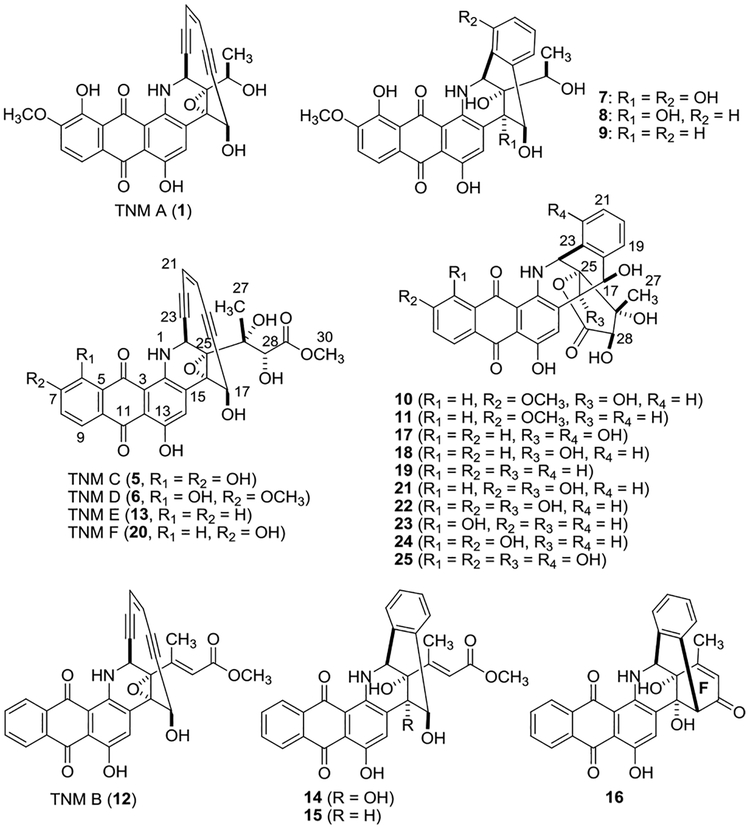
Six new TNM congeners (6−11), in addition to 1, were isolated from the CB03234 WT (Figures 2, 3). Compound 6, named TNM D, was characterized as a new enediyne with a side chain identical to TNM C (5)4 (Tables S4 and Figure S8). Compounds 7−11 were isolated as the cycloaromatized products, of which 7−9 were from 1, while the enediyne form of 10 and 11 has yet to be isolated (Table S5 and Figure S13, S15). For 10 and 11, the configuration of C-26 (R) and C-28 (R) was established through ROESY correlations between H-17 and CH3-27 and between H-24 and H-28, respectively (Figures S11). Since cycloaromatization does not affect the stereochemistry at C-26 and C-28 (Figure S15), the stereochemistry of 6 could also be assigned as 26R and 28R based on the same biosynthetic origin (Figures 3, 4, S8).
Figure 3.
Structures of TNM A (1), TNM C (5), and the 20 new congeners (6−25) isolated from the S. sp. CB03234 WT and the SB20020 (ΔtnmL) and SB20002 (ΔtnmH) mutant strains.
Figure 4.
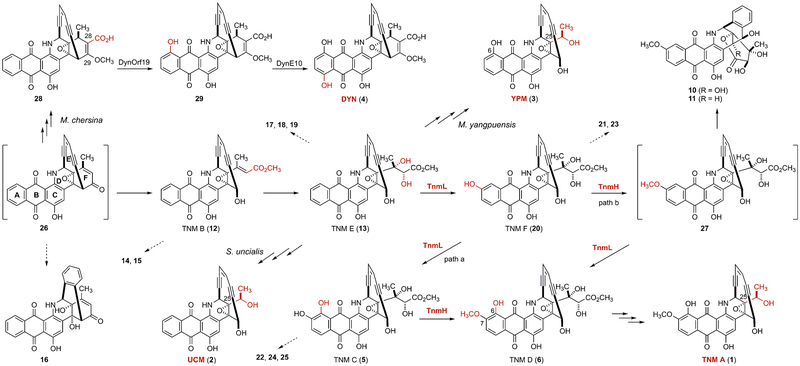
Post-enediyne PKS tailoring steps for TNM A (1) biosynthesis in S. sp. CB03234 enabled the formulation of a unified biosynthetic pathway for all members of the family of anthraquinone-fused enediynes, DYN (4) in M. chersina, TNM (1), UCM (2) in S. uncialis, and YPM (3) in M. yangpuensis, featuring 26 as a key common intermediate. Groups transformed in each of the tailoring steps are highlighted in red. Intermediates in brackets are not isolated. Dashed arrows indicate shunt metabolites isolated.
Eight new TNM congeners (12−19) were isolated from the ΔtnmL mutant strain SB20020 (Figures 2, 3, Tables S4, S6−S7, and Figures S8−S11), including two enediynes, named TNM B (12) and TNM E (13), both of which also possess a side chain attached to the enediyne core. The side chain of 12 features an α,β-unsaturated double bond, the trans configuration of which was assigned based on the absence of ROESY correlation between H-27 and H-28 (Figure S8). Compounds 14 and 15 are the cycloaromatized products of 12 (Figure S14). The side chain of 13 is identical to that of 5 and 6 (Table S4, Figure S14), and 17−19 are the cycloaromatized products of 13 (Figure S15). The stereochemistry of 13 can be similarly assigned as 26R and 28R on the basis of ROESY correlations between H-17 and CH3-27 and between H-24 and H-28, respectively, observed for 17−19 (Figure S10). Compound 16 was unique among all the compounds isolated due to the presence of an additional F-ring, which is reminiscent of the structure of 4 (Figures 1A, S12, Table S8). The corresponding enediyne form of 16 was not isolated, however, in spite of extensive effort (Figures 4, S16). Since all the compounds isolated from SB20020 lack the hydroxyl group at C-6 and C-7, TnmL most likely catalyzes hydroxylation at both positions (Figure 4).
Six new TNM congeners (20−25), in addition to 5,4 were isolated from the ΔtnmH mutant strain SB20002 (Figures 2, 3,Tables S4, S7), including a new enediyne named TNM F (20) and five cycloaromatized products 21−25. Compound 20, which bears the same side chain as 5, 6, and 13, was readily determined to be the 6-deshydroxyl congener of 5 (Table S4, Figure S8). Compounds 21 and 23 are the cycloaromatized products of 20, and 22, 24, and 25 are the cycloaromatized products of 5 (Figure S15). The stereochemistry at C-26 and C-28 of both 20 and 5 can be similarly assigned as 26R and 28R on the basis of ROESY correlations between H-17 and CH3-27 and between H-24 and H-28, respectively, observed for 22−25 (Figure S11). Since all congeners isolated from SB20002 feature a free hydroxyl group at C-7, TnmH most likely catalyzes O-methylation of the C-7 hydroxyl group. The fact that all congeners isolated possess a C-7 hydroxyl group would suggest that hydroxylation at C7 occurs before that at C6 (Figure 4)
Isolation and structural elucidation of the TNM congeners from the S. sp CB03234 WT and the SB20020 and SB20002 mutant strains enabled us to propose the post-PKS tailoring steps for 1 biosynthesis (Figure 4). Thus, the tailoring steps start from a hexacyclic intermediate 26, the identity of which was supported by the isolation of its cycloaromatized form 16 from the ΔtnmL mutant of SB20020 (Figure S16). Since no other compounds isolated possess an intact F-ring, 26 must have first undergone F-ring cleavage to afford 12, the α,β-unsaturated methyl ester side chain of which is subsequently dihydroxylated to yield 13, as supported by the isolation of 12 and 13, and their cycloaromatized products 14 and 15, and 17−19, respectively, from SB20020 (Figures S14, S15). Oxidation and O-methylation of the A-ring of 13 must have proceeded before the processing of its side chain, as evidenced by the isolation of 20, and 5, and their cycloaromatized products 21 and 23, and 22−25, respectively, from the ΔtnmH mutant of SB20002 and 6 and 27, as its cycloaromatized forms 10 and 11, from the WT strain of CB03234 (Figure S15). TnmL and TnmH next serve as the candidates, catalyzing sequential hydroxylations at C-7 and C-6, and O-methylation of the hydroxyl group at C-7, respectively, although it is unclear if hydroxylation at C-6 (path a) or O-methylation of the hydroxyl group at C-7 (path b) occurs first, since both 5 and 27, in its cycloaromatized form, have been isolated. Both paths converge to afford 6, which, with its fully modified A-ring, undergoes the final steps of side chain processing to yield 1 (Figure 4).
In contrast, for biosynthesis of 2 in S. uncialis or 3 in M. yangpuensis, 26 would first undergo the same F-ring cleavage as 1 in S. sp. CB03234 to afford 13. Subsequent processing of the side chain of 13, however, would vary according to the substitutions on the A-ring, as exemplified by none, C-6 hydroxylation, or C-6 hydroxylation and C-7 methoxylation, respectively, en route to 2, 3, or 1 (Figure 4). Examination of the four gene clusters indeed revealed three sets of genes, tnmI/ucmI/ypmI encoding an oxidoreductase, tnmK1/ucmK1/ypmK1 encoding a protein of unknown function, and tnmM/ucmM/ypmM encoding a Rieske iron−sulfur protein, that are absolutely conserved among the tnm, ucm, and ypm clusters but are absent from the dyn cluster (Figure S1).4,6,12 These enzymes serve as the candidates that catalyze the conversion of 26 to 13 and subsequently 13 to 1, 2, and 3, respectively (Figure 4). The exact timing for each of the steps and the substrate specificity of these enzymes from each of the pathways, however, will have to be determined by in vitro characterizations.
Finally, the five new enediynes 5, 6, 12, 13, and 20 were tested for cytotoxicity against selected human cancer cell lines, including central nervous system (SF-295), melanoma (SKMEL-5), breast (MDA-MB-231 and SKBR-3), and nonsmall cell lung (NCI-H226), with 1 as a control.4,17 They exhibited a broad range of IC50’s, with 1 as the most potent, followed by 6, 13, 12, 5, and 20 sequentially (Table 1). Since 1 differs from 6 only by the appended side chain, the slight decrease in potency suggests that the side chain plays a minor role in the cytotoxicity. In contrast, the increasing level of oxidation from 12, 13, to 6 correlated with an increase in potency, while a free hydroxyl group at C-7 appeared to be detrimental to the observed cytotoxicity, as exemplified by 5 and 20.
Table 1.
Cytotoxicity (IC50s in nM) of the Five New Enediynes (5, 6, 12, 13, 20), in comparison with TNM A (1), against Five Selected Human Cancer Cell Lines
| cell line | SF-295 | SKMEL-5 | MDA-MB-231 | SKBR-3 | NCI-H226 |
|---|---|---|---|---|---|
| 1 | 0.83 ± 0.07 | 0.56 ± 0.12 | 1.0 ± 0.4 | 0.90 ± 0.19 | 17 ± 1 |
| 5 | 51 ± 9 | 22 ± 1 | 29 ± 1 | 22 ± 1 | >100 |
| 6 | 2.2 ± 0.1 | 2.5 ± 0.1 | 2.1 ± 0.3 | 1.7 ± 0.1 | 31 ± 6 |
| 12 | 16 ± 2 | 13 ± 3 | 17 ± 3 | 14 ± 1 | >100 |
| 13 | 8.0 ± 0.8 | 9.1 ± 1.6 | 8.4 ± 1.9 | 6.8 ± 0.2 | >100 |
| 20 | 52 ± 2 | 24 ± 6 | 35 ± 4 | 16 ± 3 | >100 |
In summary, comparative analysis of the dyn, tnm, ucm, and ypm biosynthetic gene clusters led to the identification of four genes unique to the tnm cluster (Figure 1). Inactivation of the four genes in S. sp. CB03234 yielded mutants that accumulated 20 new TNM congeners, including five enediynes (Figure 3). The isolated TNM congeners provided new insights into the tailoring steps of 1 biosynthesis and enabled us to formulate a unified pathway for the four anthraquinone-fused enediynes, featuring 26 as a key common intermediate (Figure 4). These findings set the stage to investigate the mechanisms of how a common intermediate is channeled into the biosynthesis of each anthraquinone-fused enediyne. Engineering of the anthraquinone-fused enediyne biosynthetic machineries therefore promises to further expand the structural diversity, thereby defining the structure−activity relationship of the anthraqui-none-fused enediynes, for anticancer drug discovery and development.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported in part by National Institutes of Health Grants GM115575 (B.S.) and CA204484 (B.S. and C.R.). J.C. and I.C. were supported in part by postdoctoral fellowships from the State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, and the Chinese Scholarship Council(201606185009), and the German Research Foundation, respectively. This is Manuscript No. 29708 from The Scripps Research Institute.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b02584.
General experimental procedures; Tables S1−S8, Figures S1−S156 (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Galm U; Hager MH; Van Lanen SG; Ju J; Thorson JS; Shen B Chem. Rev 2005, 105, 739–758. [DOI] [PubMed] [Google Scholar]
- (2).Van Lanen SG; Shen B Curr. Top. Med. Chem 2008, 8, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Liang Z-X Nat. Prod. Rep 2010, 27, 499–528. [DOI] [PubMed] [Google Scholar]
- (4).Yan X; Ge H; Huang T; Hindra; Yang D; Teng Q; Crnovcic I; Li X; Rudolf JD; Lohman JR; Gansemans Y; Zhu X; Huang Y; Zhao L-X; Jiang Y; Nieuwerburgh FV; Rader C; Duan Y; Shen B mBio 2016, 7, e02104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Davies J; Wang H; Taylor T; Warabi K; Huang X-H; Andersen RJ Org. Lett 2005, 7, 5233–5236. [DOI] [PubMed] [Google Scholar]
- (6).Yan X; Chen JJ; Adhikari A; Yang D; Crnovcic I; Wang N; Chang C-Y; Rader C; Shen B Org. Lett 2017, 19, 6192–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Konishi M; Ohkuma H; Matsumoto K; Tsuno T; Kamei H; Miyaki T; Oki T; Kawaguchi H; VanDuyne GD; Clardy JJ Antibiot 1989, 42, 1449–1452. [DOI] [PubMed] [Google Scholar]
- (8).Liu W; Christenson SD; Standage S; Shen B Science 2002, 297, 1170–1173. [DOI] [PubMed] [Google Scholar]
- (9) (a).Kennedy DR; Gawron LS; Ju JH; Liu W; Shen B; Beerman TA Cancer Res 2007, 67, 773–781. [DOI] [PubMed] [Google Scholar]; (b) Kennedy DR; Ju J; Shen B; Beerman TA Proc. Natl. Acad. Sci. U. S. A 2007, 104, 17632–17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Liu W; Nonaka K; Nie L; Zhang J; Christenson SD; Bae J; Van Lanen SG; Zazopoulos E; Farnet CM; Yang CF; Shen B Chem. Biol 2005, 12, 293–302. [DOI] [PubMed] [Google Scholar]
- (11).Ahlert J; Shepard E; Lomovskaya N; Zazopoulos E; Staffa A; Bachmann BO; Huang K; Fonstein L; Czisny A; Whitwam RE; Farnet CM; Thorson JS Science 2002, 297, 1173–1176. [DOI] [PubMed] [Google Scholar]
- (12).Gao Q; Thorson JS FEMS Microbiol. Lett 2008, 282, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tokiwa Y; Miyoshi-Saitoh M; Kobayashi H; Sunaga R; Konishi M; Oki T; Iwasaki SJ Am. Chem. Soc 1992, 114, 4107–4110. [Google Scholar]
- (14).Cohen DR; Townsend CA Nat. Chem 2017, 10, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cohen DR; Townsend CA Angew. Chem., Int. Ed 2018, 57, 5650–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Liu L; Pan J; Wang Z; Yan X; Yang D; Zhu X; Shen B; Duan Y; Huang YJ Ind. Microbiol. Biotechnol 2018, 45, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nicolaou KC; Chen JS; Zhang H; Montero A Angew. Chem., Int. Ed 2008, 47, 185–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.