Abstract
Cancers of the gastrointestinal (GI) track are a serious global health problem. The human GI tract is home to trillions of microorganisms that known as gut microbiota and have established a symbiotic relationship with the host. The human intestinal microbiota plays an important role in the development of the gut immune system, metabolism, nutrition absorption, production of short-chain fatty acids and essential vitamins, resistance to pathogenic microorganisms, and modulates a normal immunological response. Microbiota imbalance has been involved in many disorders including inflammatory bowel disease, obesity, asthma, psychiatric illnesses, and cancers. Oral administration of probiotics seems to play a protective role against cancer development as a kind of functional foods. Moreover, clinical application of probiotics has shown that some probiotic strains can reduce the incidence of post-operative inflammation in cancer patients. In the present narrative review, we carried out update knowledge on probiotic effects and underlying mechanism to GI cancers. Currently, it is accept that most commercial probiotic products are generally safe and can used as a supplement for cancer prevention and treatment. Nevertheless, well-designed, randomized, double blind, placebo-controlled human studies are required to gain the acceptance of the potential probiotics as an alternative therapy for cancer control..
Key Words: Probiotic, Prebiotics, Gastrointestinal cancer, Gut microbiota
Introduction
Nowadays, besides of introducing the new technology and methodology for diagnostic and management of GI cancers, some additional aspects are becoming increasingly important, including the maintenance of health a counteracting cancers by health benefits of using probiotics and prebiotics in human nutrition. Probiotics are defined as living bacteria that, when consumed in sufficient quantities, carry the health benefits of the host (1). The main benefit of using probiotic is to help the host maintain the microbial balance of the intestine, reduce pathogenic gastrointestinal microorganisms, the improvement of bowel regularity, and the restoration of intestinal microbiota homeostasisin antibiotic-associated diarrhea (2). Furthermore, several studies have shown potential of probiotics in cancer prevention and treatment through microbiota modulation, immune modulation, reduced bacterial translocation, enhanced gut barrier function, anti-inflammatory and anti-pathogenic activity, with effects on reducing tumor formation and metastasis (3, 4). The possible association between probiotics and GI neoplasm has mainly been evaluated in relation to colorectal cancer (CRC) and gastric-cancer-related Helicobacter pylori (H. pylori) (5-9). These results indicate the probiotics are potential dietary supplements against neoplastic predisposition through extensive effects on the immune system of host (25–29). The current narrative review summarizes the update knowledge on probiotic effects and underlying mechanism to GI cancers. Moreover, we presented the comprehensive review of the evidence from clinical studies using probiotics in the prevention or/ and treatment of GI cancers.
Probiotics
Most of the probiotic products currently available contain lactic acid bacteria (LAB) which belong to the Lactobacillus and Bifidobacterium (10). Some of the most important probiotic microorganisms that used in human nutrition are listed in Table 1. Most microorganisms recognized to date as probiotics are Gram-positive, with Lactobacillus and Bifidobacterium being the main species used as treatments of gastrointestinal disorders (11). However, some Gram-negatives are also used as probiotics. The best example of this group is Escherichia coli Nissle 1917 (EcN) (12), also known as “Mutaflor,” which has been used in recent years to treat chronic constipation and colitis in Germany (13, 14). Two of the most commercially important LAB that playing an important role in the dairy products are Streptococcus thermophilus and Lactococcus lactis (15).
Table 1.
Probiotic microorganisms used in human nutrition
| Type Lactobacillus | Type Bifidobacterium | Lactic Acid Bacteria | Other Microorganisms |
|---|---|---|---|
| L. acidophilus (a) | B. adolescentis (a) | Enterococcus faecium (a) | Bacillus clausii (a) |
| L. amylovorus (b) | B. animalis (a) | Lactococcus lactis (b) | Escherichia coli Nissle 1917(a) |
| L. casei (a), (b) | B. bifidum (a) | Streptococcus thermophiles (a) | Saccharomyces cerevisiae (boulardi) (a) |
| L. gasseri (a) | B. breve (b) | ||
| L. helveticus (a) | B. infantis (a) | ||
| L. johnsonii (b) | B. longum (a) | ||
| L. pentosus (b) | |||
| L. plantarum (b) | |||
| L. reuteri (a) | |||
| L. rhamnosus (a), (b) |
Mostly as pharmaceutical products;
mostly as food additives
Selection criteria for probiotic strains
According to the World Health Organization (WHO), Food and Agriculture Organization (FAO), and European Food Safety Authority (EFSA), probiotic strains must meet both safety and functionality for human and animal health, as well as to their technological usefulness (Table 2). Microorganism that used as probiotics should meet the terms of GRAS (Generally Regarded as Safe) and QPS (Qualified Presumption of Safety) status and the safety of a strain is defined as the absence of association with pathogenic cultures, and the antibiotic resistance profile. Functional aspects define their survival in the gastrointestinal tract and its safety effects (16, 17). Due to the rapid growth of the probiotic market, the probiotic survival and maintenance of their properties throughout the storage and distribution process is very important (18, 19). Finally, Suitable probiotic strains should have a positive effect on the host, transfer through the intestinal tract, adhere to the epithelial cells of the intestine, produce antimicrobial agents against the pathogen and stabilize the intestinal microflora (20).
Table 2.
Selection criteria for probiotic strains
| Criteria | Required properties |
|---|---|
| Safety | Human or animal origin Isolated from the gastrointestinal tract of healthy individuals History of safe use Precise diagnostic identification (phenotype and genotype traits) Absence of data regarding an association with infective disease Absence of the ability to cleave bile acid salts No adverse effects Absence of genes responsible for antibiotic resistance localized in non-stable elements |
| Functionality | Competitiveness with respect to the microbiota inhabiting the intestinal ecosystem Ability to survive and maintain the metabolic activity, and to grow in the target site Resistance to bile salts and enzymes Resistance to low pH in the stomach Competitiveness with respect to microbial species inhabiting the intestinal ecosystem Antagonistic activity towards pathogens Resistance to bacteriocins and acids produced by the endogenic intestinal microbiota Adherence and ability to colonize some particular sites within the host organism An appropriate survival rate in the gastrointestinal system |
| Technological usability |
Easy production of high biomass amounts and high productivity of cultures Viability and stability of the desired properties of probiotic bacteria during the fixing process High storage survival rate in finished products Guarantee of desired sensory properties of finished products Genetic stability Resistance to bacteriophages |
Probiotics and GI cancers
Gastrointestinal cancer refers to malignant conditions of the gastrointestinal (GI) tract and other organs involved in digestive system which includes cancers of the esophagus, gallbladder, liver, pancreas, gastric, small intestine, colon and rectum (21). GI cancers are a major health problem, accounting for 25% of all cancers and 9% of all causes of cancer death in the world (22). Colorectal, gastric and esophageal cancers are the third, fourth and eighth most common cancers with 1.4, 1 and 0.45 million new cases in 2012, respectively (23). GI cancers are considered as multifactorial diseases, complex relationships between genetics, epigenetics, immunity, environmental factors, diet and lifestyle that can interact with the gut microbiota and functions during the tumor genesis and growth (24, 25).
There has been an increased interest in the scientific community on the use of probiotic therapy for prevention and treatment of a number of GI disorders, including irritable bowel syndrome (IBS), inflammatory bowel diseases (IBD), pathogenic bacterial or viral infection, and antibiotic associated diarrhea (26, 27). There is also epidemiological evidence that supports a protective role of probiotics against cancer (28). Substantial research has demonstrated that probiotics possess anti-proliferative or pro-apoptotic activities in GI cancers, among which colonic cancer cells and gastric cancer cells were most commonly studied (29, 30). Several studies were performed on the health benefits of milk fermented with Lactobacillus casei and L. acidophilus and the results indicate the positive effects of these probiotics on increase of tumor cell apoptosis (31, 32). Previous studies indicated the anti-proliferative role of L. rhamnosus GG strain in both human gastric cancer cells and colonic cancer cells (33-35), while another probiotic product named Bifidobacterium adolescentis SPM0212 inhibited the proliferation of three human colon cancer cell lines including HT-29, SW 480, and Caco-2 (36). Other probiotic products or strains that exerted antitumor activities against human colon cancer cells included Bacillus polyfermenticus (37), L. acidophilus 606 (38), LGG/Bb12 (39), and LGG/Bifidobacterium animalis subsp. lactis (40). In addition, Cousin et al. reported that fermented milk containing Propionibacterium freudenreichii enhanced the cytotoxicity of camptothecin that was used as a chemotherapeutic agent for gastric cancer (41).
Colorectal cancer
Colorectal cancer (CRC) is the third most common cancer worldwide with more than 1 million new cases annually and is responsible for death of more than 500,000 people (42). Evidence has shown that taking probiotics is a protective approach for proper maintaining of healthy gut microbiota and also reducing the risk of colon cancer risk (43). Contrary to many in-vitro and in-vivo studies in animal models and cancer cell lines of human, few randomized placebo-controlled trials (RCTs) studies have reported the effect of probiotics on prevention and inhibition of intestinal carcinogenesis (44-46). The benefits of probiotics are not only limited to the prevention of intestinal cancers, but they can also include the prevention of symptoms and complications in patients undergoing colorectal surgery for cancer and who receiving intestinal cancer treatment (8, 47-51). In table 3 we summarized the results of clinical trials studies regarding the effect of probiotics intervention for prevention or/and treatment of colorectal cancers.
Table 3.
Clinical trials of probiotics interventions for prevention, post-operative complications and treatment of CRC
| Type of intervention | Patients | Probiotic strain | Length of treatment | Outcome | Ref |
|---|---|---|---|---|---|
| Prevention | 38 healthy patients | L. rhamnosus | 4 weeks | Reduce of b-glycosidase activity by 10% and urease activity by 13% | (44) |
| 17 healthy patients | RS vs. BF lactis | 4 weeks | Induced unique changes in fecal microflora but did not significantly alter any other fecal, serum, or epithelial variables. | (45) | |
| 10 CRC and 20 healthy patients | L. gasseri (LG21) | 12 weeks | A deterioration of the intestinal environment was observed in the colorectal cancer patients in comparison to the healthy controls, and the intestinal environment improved when probiotics was taken. | (46) | |
| Prevention of post-operative complication | 100 CRC patients undergoing surgery (placebo group/ probiotics group n=50/50) |
L. plantarum
L. acidophilus B. longum |
16 days | Improvement in the integrity of gut mucosal barrier and decrease in infections complications |
(8) |
| 124 CRC patients undergoing surgery (placebo group/ probiotics group n=80/84) |
L. acidophilus
L. plantarum B. lactis BB S. boulardii |
15 days | Decreased the rate of all postoperative major complication, gene expression of TNF and circulating concentrations of IL-6 were under the control of SOCS3 in the probiotics group |
(47) | |
| 156 CRC patients undergoing surgery (placebo group/ probiotics group n=81/75) |
E. faecalis
C. butyricum B. mesentericus |
15 days | Probiotic treatment reduce superficial incisional surgical site infections (SSIs) in patients undergoing CRC surgery | (48) | |
| 60 CRC patients undergoing surgery (placebo group/ probiotics group n=30/30) |
B. longum
L. acidophilus E. faecalis |
12 days | Faster recovery of bowel function, lower incidences of diarrhea, and slightly lower rate of bacteremia.in probiotic group | (49) | |
| Chemotherapy and radiation therapy related toxicity | 150 CRC patients undertreated | L. rhamnosus GG | 24 weeks | Patients had less diarrhea, less abdominal pain, less hospital care, and had fewer chemo dose reductions due to bowel toxicity |
(50) |
| 490 gynecological cancer and CRC patients |
VSL#3 (a mixture of 8 probiotics) |
From the 1st day of radiation therapy |
Significant decrease of diarrhea (31.6 vs. 51.8%) and Severe diarrhea (1.4 vs. 55.4%) |
(51) |
The results of few clinical trial studies showed the effect of probiotics on manipulate the composition of gut microbiota, thus positively affect the host by improving intestinal barrier integrity, inhibiting growth of pathogens, reducing metabolism of pro-carcinogenic substances (44-46). Therefore, probiotics are effective in preventing and inhibiting the growth of intestinal cancer. In addition, several RCTs studies demonstrate that the use of probiotics in patients undergoing abdominal surgery is a promising approach to the prevention of post-operative superficial incisional surgical site infections (SSIs) and improvement in the integrity of gut mucosal barrier (47-49). Furthermore, the patients’ quality of life was also improved, shortening the duration of post-operative hospital stay and the period needed for antibiotics administration. Chemotherapy and radiotherapy as the conventional therapies for cancers can changes in the composition of the gut microbiota; these disruptions could also participate in the development of mucositis, particularly diarrhea and bacteraemia (52, 53). The prevention of cancer therapy-induced mucositis by probiotics has been investigated in randomized clinical trials with some promising results. Two trial studies on CRC patients who were undergoing chemotherapy and radiotherapy indicated a significantly decreased incidence of diarrhea by administration of L. rhamnosus GG and VSL#3 (a mixture of 8 probiotics) (50, 51).
Gastric cancer
Gastric cancer (GC) represents the second cause of cancer-related death worldwide, accounting for approximately 10% of newly diagnosed cancers (23). Although GC incidence rate declined in recent last years, the 5 year survival rate of this neoplasm is under 25% with regional variations (54). Studies on probiotics and gastric cancer are mainly focused on eliminating Helicobacter pylori (H. pylori) infection as the major risk factors of gastric cancer (GC) (55). H. pylori is a Gram-negative bacterium which can disrupt the acid mucus barrier and colonize the gastric epithelium, is found in patients who are suffering from chronic gastritis, peptic ulcer and gastric cancer (56, 57). Inhibitory effects of probiotics on H. pylori infection have been observed in several animal models containing B. bifidum, L. acidophilus, L. rhamnosus, L. salivarius and several other probiotic strains (58).
In recent years, the success of eradication therapies of H. pylori by combination therapy of proton pump inhibitor (PPI) and two antibiotics therapy (clarithromycin plus amoxicillin or metronidazole) has been declined, due to the development of H. pylori resistant strains. According to recently meta-analysis, using probiotics as a supplementation with antibiotic therapy is very useful to the H. pylori eradication (59-61). In table 4 we summarized the results of clinical trials studies regarding the effect of probiotics in association with antibiotics treatment in eradication of H. pylori colonization. The results of these studies suggest that probiotic supplementation during antibiotic therapy to H. Pylori eradication, decreases adverse side effects, resulting in better compliance and, in some cases, improved rates of eradication. In addition, gastric tumor promoting proliferation of lymphoid tissue disappeared after successful eradication (62, 63). One of the proposed mechanisms for probiotic treatment is that these microbes can be present in the stomach and even live in it temporarily, increase the immune response and reduce the effect of H. pylori inflammation on the host gastric mucosa (64).
Table 4.
Clinical trials using probiotics in association with combination therapy of H. pylori eradication
| Patients | Probiotic strain | Length of treatment | Outcome | Ref |
|---|---|---|---|---|
| 120 dyspeptic adults | L. acidophilus LB | 10 days | Eradication rate ↑, side effects ↔ | (93) |
| 60 asymptomatic adults | L. rhamnosus GG | 14 days | Eradication rate ↔, side effects ↓ | (94) |
| 120 asymptomatic adults | L. rhamnosus GG | 14 days | Eradication rate ↔, adverse effects ↑ | (95) |
| 160 dyspeptic adults |
L. acidophilus La5 B. lactis Bb12 |
4 weeks | Eradication rate ↑, adverse effects ↓ | (96) |
| 85 asymptomatic adults |
L. rhamnosus GG S. boulardii L. acidophilus La5 B. lactis Bb12 |
2 weeks | Eradication rate ↔, adverse effects ↓ | (97) |
| 70 dyspeptic adults with resistant H. pylori |
L. casei ssp L. casei DG |
10 days | Eradication rate ↔, adverse effects ↓ | (98) |
| 86 dyspeptic children | L. casei | 2 weeks | Eradication rate ↑, adverse effects ↔ | (99) |
| 40 dyspeptic children | L. reuteri | 20 days | Eradication rate ↔, adverse effects ↓ | (100) |
| 138 dyspeptic adults with resistant H. pylori |
L. acidophilus La5 B. lactis Bb12 |
4 weeks | Urease activity↓ during pretreatment, eradication rate ↑, side effects ↓ | (101) |
| 65 children |
B. animalis
L. casei |
unclear | Eradication rate ↑ | (102) |
| 118 individuals |
L. rhamnosus LC
P. freudenreichii B. breve |
4 weeks | Eradication ↔, urease activity ↓, gastritis and H. pylori colonization ↓ | (103) |
| 90 individuals | L. reuteri | 6 weeks | Eradication rate ↑ | (104) |
Increase,
decrease,
no effect
Other GI cancers
Unlike many studies on CRC and GC, there are few studies that suggest probiotic role in the prevention and treatment of other GI cancers such as pancreas and liver cancer. Pancreatic cancer is the 12th most common cancer in the world with 338,000 new cases and 7th most frequent cause of death worldwide with 331,000 deaths per year, but the etiology is still unknown (23, 65). Some previous studies supports a multifaceted role of probiotics in preventing pancreatic cancer by modulating pancreatitis and other risk factors such as diabetes, pancreatic necrosis, inflammation and obesity (66-68). Table 5 shows the results of clinical trials studies regarding the effect of probiotics on severe acute pancreatitis (SAP). The results of meta-analysis on six clinical trial studies indicate that probiotics did not significantly effects on the clinical outcomes of patients with SAP (69). Therefore, the available data are not sufficient to draw conclusions about the effects of probiotics in pancreatic cancer because of the limited number of trials and their heterogeneity. The types of probiotics and treatment strategies are very important in the heterogeneity of clinical outcomes reported in different RCTs.
Table 5.
Clinical trials of probiotics interventions in patients with severe acute pancreatitis (SAP)
| Patients | Probiotic strain | Length of treatment | Outcome | Ref |
|---|---|---|---|---|
| 45 SAP patients | L. plantarum | 7 days | reducing pancreatic sepsis and the number of surgical interventions | (67) |
| 25 SAP patients |
B. longum
L. bulgaricus S. thermophilus |
7 days | The time of abdominal pain alleviation, serum amylase restoration, the incidence rate of complications and mean of hospitalization were significantly decreased in group treated with probiotics | (105) |
| 62 SAP patients |
P. pentosaceus
L. mesenteroides L. paracasei L. plantarum with bioactive fibers |
7 days | symbiotic may prevent organ dysfunctions in the late phase of severe acute pancreatitis | (66) |
| 298 SAP patients |
L. acidophilus
L. casei L. salivarius L. lactis B. bifidum B. lactis |
28 days | Probiotic prophylaxis with this combination of probiotic strains did not reduce the risk of infectious complications and was associated with an increased risk of mortality. | (68) |
| 90 SAP patients |
P. pentosaceus
L. mesenteroides L. paracasei L. plantarum with bioactive fibers |
unclear | Synbiotic supplements was associated with lower infection rate lower rate of surgical interventions, shorter ICU and hospital stay and reduced mortality rate | (106) |
| 70 SAP patients |
B. longum
L. bulgaricus E. faecalis |
14 days | Early enteral nutrition (EN) with addition of probiotics resulted in significant lowering of the level of pro-inflammatory cytokines, earlier restoration of gastrointestinal function, decrease of complications and shortening of hospitalization | (107) |
Liver cancer is the fifth most common cancer in men and the ninth in women and is the second most common cause of death from cancer worldwide, estimated to be responsible for nearly 746,000 deaths in 2012 (23). The gut microbiome has been related to the development of liver disorders such as liver fibrosis (70), non-alcoholic fatty liver disease (71) and more recently, liver cancer (72). In the previous year, it was reported that probiotics inhibit hepatocellular carcinoma (HCC) progression in mice (73). Feeding a probiotics mixture to tumor-injected mice could shift the composition of gut microbiota and reduce the size of liver tumors. In addition to the reduction of tumor size, angiogenic factors were down regulated by probiotics administration.
Anti-carcinogenic mechanisms of probiotics on GI cancers
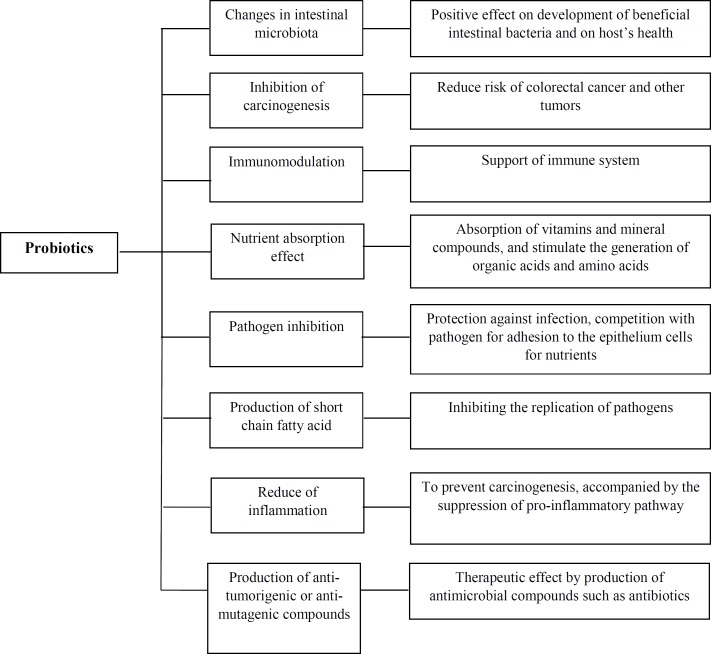
Theoretically, probiotics are able to reduce cancer risk by several mechanisms. Oral administration of probiotics has multiple effects such as normalization of gut microbiota, improvement of the gastrointestinal barrier, inhibition of potential pathogens, anti-inflammatory activities and suppression of tumor formation and growth. Figure 1 presents a scheme of the possible anti-carcinogenic mechanisms of probiotics.
Figure 1.
Anti-carcinogenic mechanisms of probiotics
Probiotics have abundant anticancer benefits and have a major impact on the quantitative and/or qualitative changes of the intestinal microbiota. The intestinal microbiota has been linked to GI cancer development also by production of toxic and genotoxic bacterial metabolites that can lead to mutations by binding specific cell surface receptors and affecting intracellular signal transduction. Specific strains of bacteria are involved in the pathogenesis of cancer, including Streptococcus bovis, Bacteroides, clostridia, and H. pylori (74-76). On the contrary, some bacterial strains, including L. acidophilus and B. longum, inhibit carcinogenic tumor growth in the colon (77, 78). Thus, a balance between “detrimental” and “beneficial” bacteria has implications in setting the stage for cancer. Shifting the proportion of microbes has been reported to influence carcinogen bioactivation and thus cancer risk. It is increasingly apparent that dietary components can significantly modify this balance. In addition, probiotics also affect the intestinal microbiological compositions, thus positively affect the host by improving intestinal barrier integrity, inhibiting growth of pathogens, reducing metabolism of pro-carcinogenic substances.
The benefits of probiotics are not only limited to the prevention and inhibition of carcinogenic agents, but they can also include the therapeutic effect and the prevention of cancer treatment complications. The therapeutic effect of probiotics can be due to the production of antimicrobial compounds such as bacteriocins and antibiotics. Bacteriocins produced by LAB are peptides or small proteins that are frequently inhibitory towards many undesirable bacteria, including food-borne pathogens (79). It has also been suggested that LAB or a soluble compound produced by the bacteria may interact directly with tumor cells in culture and inhibit their growth (36). The competitive behavior of probiotics with pathogens is related to adhesion to epithelial cells (80). Several studies that characterized LAB from different origins has shown that the ability to adhere to epithelial cells is strain dependent (81-83). The suppressive effect of probiotics was also associated with production of short chain fatty acids (SCFAs), which could be reflected, by the enrichment of SCFAs-related pathway (84, 85).
Chronic inflammation has been recognized as a risk factor of cancer (86). For example; inflammatory bowel disease (IBD) is a risk factor of colon cancer and the risk of HCC can be increased by inflammatory conditions, such as hepatitis B, C virus infection (87). Inflammation not only plays a role in colitis-associated colon cancer, but may also happen in sporadic colon cancer and affect the progression of cancer (88, 89). L. rhamnosus GG was reported to prevent colon carcinogenesis, accompanied by the suppression of NFkB pathway (90), a pro-inflammatory pathway that links IBD and colon cancer (91, 92). Li et al. (73) showed a reduction of pro-inflammatory cytokine IL-17 by probiotics in HCC model, revealing the possible relationship between immunomodulatory effect and anticancer effect of probiotics.
Conclusion
Probiotics have become very important in medicine because of their useful effects on the host health. Numerous in vitro studies and animal models show positive effects of probiotics on gastrointestinal cancers by various mechanisms, including anti-carcinogenic effects, anti-mutagenic properties, modification of differentiation process in tumor cells, production of short chain fatty acids, alteration of tumor gene-expressions, activation of the host’s immune system, inhibition of the bacteria that convert pro-carcinogens to carcinogens, alteration of colonic motility and transit time, as well as reduction of intestinal pH to reduce microbial activity. Different mechanisms can be involved in these beneficial effects, mainly via modulation of gut microbiota, which thereby influences host metabolism and immunity. Nevertheless, human clinical trials of the application of probiotics as bio therapeutics against cancer with adequate follow-up results are still lacking. Therefore, extensive clinical trials studies on human are required to identify the potential strains, dosages and administration regimes for specific types and stages of cancer as an alternative therapy for cancer treatment
Acknowledgment
This study is related to the project NO 1396/65554 From the Student research Committee, Shahid Beheshti University for Medical Sciences, Tehran, Iran. We also appreciate the ‘Student Research Committee’ and ’Research and Technology Chancellor’ in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Indian Council of Medical Research Task Force; Co-ordinating Unit ICMR; Co-ordinating Unit DBT. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res. 2011;134:22–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Floch MH, Walker WA, Madsen K, Sanders ME, Macfarlane GT, Flint HJ, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011;45:S168–71. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]
- 3.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–40. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–88. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 5.Russo F, Linsalata M, Orlando A. Probiotics against neoplastic transformation of gastric mucosa: effects on cell proliferation and polyamine metabolism. World J Gastroenterol. 2014;20:13258–72. doi: 10.3748/wjg.v20.i37.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasouli BS, Ghadimi-Darsajini A, Nekouian R, Iragian GR. In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. J Cancer Res Ther. 2017;13:246–51. doi: 10.4103/0973-1482.204897. [DOI] [PubMed] [Google Scholar]
- 7.Khoder G, Al-Menhali AA, Al-Yassir F, Karam SM. Potential role of probiotics in the management of gastric ulcer. Exp Ther Med. 2016;12:3–17. doi: 10.3892/etm.2016.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taremi M, Khoshbaten M, Gachkar L, EhsaniArdakani M, Zali M. Hepatitis E virus infection in hemodialysis patients: a seroepidemiological survey in Iran. BMC Infect Dis. 2005;17(5):36. doi: 10.1186/1471-2334-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–96. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. 2001;73:S365–73. doi: 10.1093/ajcn/73.2.365s. [DOI] [PubMed] [Google Scholar]
- 11.Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol. 2006;17:204–10. doi: 10.1016/j.copbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Nissle A. [Explanations of the significance of colonic dysbacteria & the mechanism of action of E coli therapy (mutaflor)] Medizinische. 1959;4:1017–22. [PubMed] [Google Scholar]
- 13.Mollenbrink M, Bruckschen E. [Treatment of chronic constipation with physiologic Escherichia coli bacteria Results of a clinical study of the effectiveness and tolerance of microbiological therapy with the E coli Nissle 1917 strain (Mutaflor)] Med Klin (Munich) 1994;89:587–93. [PubMed] [Google Scholar]
- 14.Schutz E. The treatment of intestinal diseases with Mutaflor A multicenter retrospective study. Fortschr Med. 1989;107:599–602. [PubMed] [Google Scholar]
- 15.Felis GE, Dellaglio F. Taxonomy of Lactobacilli and Bifidobacteria. Curr. Issues Intestinal Microbiol. 2007;8:44–61. [PubMed] [Google Scholar]
- 16.Anadon A, Martinez-Larranaga MR, Aranzazu Martinez M. Probiotics for animal nutrition in the European Union Regulation and safety assessment. Regul Toxicol Pharmacol. 2006;45:91–5. doi: 10.1016/j.yrtph.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Gaggia F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141:S15–28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Markowiak P, Slizewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017:9. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Cerbo A, Palmieri B. Review: The market of probiotics. Pak J Pharm Sci. 2015;28:2199–206. [PubMed] [Google Scholar]
- 20.Orlando A, Russo F. Intestinal microbiota, probiotics and human gastrointestinal cancers. J Gastrointest Cancer. 2013;44:121–31. doi: 10.1007/s12029-012-9459-1. [DOI] [PubMed] [Google Scholar]
- 21.Pourhoseingholi MA, Vahedi M, Baghestani AR. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol Hepatol Bed Bench. 2015;8:19–27. [PMC free article] [PubMed] [Google Scholar]
- 22.Ghoncheh M, Salehiniya H. Inequality in the Incidence and Mortality of All Cancers in the World. Iran J Public Health. 2016;45:1675–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Beyond gastric adenocarcinoma: Multimodality assessment of common and uncommon gastric neoplasms. Abdom Radiol (NY) . 2017;42:124–40. doi: 10.1007/s00261-016-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serban DE. Gastrointestinal cancers: influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014;345:258–70. doi: 10.1016/j.canlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang MM, Cheng JQ, Xia L, Lu YR, Wu XT. Monitoring intestinal microbiota profile: a promising method for the ultraearly detection of colorectal cancer. Medical hypotheses. 2011;76:670–2. doi: 10.1016/j.mehy.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Zuccotti GV, Meneghin F, Raimondi C, Dilillo D, Agostoni C, Riva E, et al. Probiotics in clinical practice: an overview. J Int Med Res. 2008;36:1a–53. doi: 10.1177/14732300080360S101. [DOI] [PubMed] [Google Scholar]
- 27.De Preter V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health? Mol Nutr Food Res. 2011;55:46–57. doi: 10.1002/mnfr.201000451. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, et al. Cancer-preventing attributes of probiotics: an update. Int J Food Sci Nutr. 2010;61:473–96. doi: 10.3109/09637480903455971. [DOI] [PubMed] [Google Scholar]
- 29.Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci. 2008;9:854–63. doi: 10.3390/ijms9050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafter J. The effects of probiotics on colon cancer development. Nutr Res Rev. 2004;17:277–84. doi: 10.1079/NRR200484. [DOI] [PubMed] [Google Scholar]
- 31.Lee JW, Shin JG, Kim EH, Kang HE, Yim IB, Kim JY, et al. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. J Vet Sci . 2004;5:41–8. [PubMed] [Google Scholar]
- 32.Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus and L casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer. 2010;62:371–8. doi: 10.1080/01635580903407197. [DOI] [PubMed] [Google Scholar]
- 33.Russo F, Orlando A, Linsalata M, Cavallini A, Messa C. Effects of Lactobacillus rhamnosus GG on the cell growth and polyamine metabolism in HGC-27 human gastric cancer cells. Nutr Cancer. 2007;59:106–14. doi: 10.1080/01635580701365084. [DOI] [PubMed] [Google Scholar]
- 34.Orlando A, Messa C, Linsalata M, Cavallini A, Russo F. Effects of Lactobacillus rhamnosus GG on proliferation and polyamine metabolism in HGC-27 human gastric and DLD-1 colonic cancer cell lines. Immunopharmacol Immunotoxicol. 2009;31:108–16. doi: 10.1080/08923970802443631. [DOI] [PubMed] [Google Scholar]
- 35.Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V, et al. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC21 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer. 2012;64:1103–11. doi: 10.1080/01635581.2012.717676. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y, Lee D, Kim D, Cho J, Yang J, Chung M, et al. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch Pharm Res. 2008;31:468–73. doi: 10.1007/s12272-001-1180-y. [DOI] [PubMed] [Google Scholar]
- 37.Ma EL, Choi YJ, Choi J, Pothoulakis C, Rhee SH, Im E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int J Cancer. 2010;127:780–90. doi: 10.1002/ijc.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y, Oh S, Yun HS, Oh S, Kim SH. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett Appl Microbio. 2010;51:123–30. doi: 10.1111/j.1472-765X.2010.02859.x. [DOI] [PubMed] [Google Scholar]
- 39.Borowicki A, Michelmann A, Stein K, Scharlau D, Scheu K, Obst U, et al. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr Cancer. 2011;63:151–60. doi: 10.1080/01635581.2010.516874. [DOI] [PubMed] [Google Scholar]
- 40.Stein K, Borowicki A, Scharlau D, Schettler A, Scheu K, Obst U, et al. Effects of synbiotic fermentation products on primary chemoprevention in human colon cells. J Nutr Biochem. 2012;23:777–84. doi: 10.1016/j.jnutbio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Cousin FJ, Jouan-Lanhouet S, Dimanche-Boitrel MT, Corcos L, Jan G. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PloS One. 2012;7:e31892. doi: 10.1371/journal.pone.0031892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017;65:311–5. doi: 10.1136/jim-2016-000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So SS, Wan ML, El-Nezami H. Probiotics-mediated suppression of cancer. Curr Opin Oncol. 2017;29:62–72. doi: 10.1097/CCO.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 44.Hatakka K, Holma R, El-Nezami H, Suomalainen T, Kuisma M, Saxelin M, et al. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp shermanii JS on potentially carcinogenic bacterial activity in human colon. Int J Food Microbiol . 2008;128:406–10. doi: 10.1016/j.ijfoodmicro.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Worthley DL, Le Leu RK, Whitehall VL, Conlon M, Christophersen C, Belobrajdic D, et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am J Clin Nutr. 2009;90:578–86. doi: 10.3945/ajcn.2009.28106. [DOI] [PubMed] [Google Scholar]
- 46.Ohara T, Yoshino K, Kitajima M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology . 2010;57:1411–5. [PubMed] [Google Scholar]
- 47.Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J Surg. 2015;39:2776–83. doi: 10.1007/s00268-015-3071-z. [DOI] [PubMed] [Google Scholar]
- 48.Aisu N, Tanimura S, Yamashita Y, Yamashita K, Maki K, Yoshida Y, et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp Ther Med. 2015;10:966–72. doi: 10.3892/etm.2015.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Xia Y, Chen H, Hong L, Feng J, Yang J, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget. 2016;7:8432–40. doi: 10.18632/oncotarget.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97:1028–34. doi: 10.1038/sj.bjc.6603990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroentero. 2007;13:912–5. doi: 10.3748/wjg.v13.i6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409–21. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 53.Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PloS One. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer . 2009;125:666–73. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 55.Bhandari A, Crowe SE. Helicobacter pylori in gastric malignancies. Current gastroenterology reports. 2012;14:489–96. doi: 10.1007/s11894-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 56.Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385–99. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashtari S, Pourhoseingholi MA, Molaei M, Taslimi H, Zali MR. The prevalence of Helicobacter pylori is decreasing in Iranian patients. Gastroenterol Hepatol Bed Bench. 2015;8:S23–9. [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu XY, Liu F. Probiotics as an adjuvant treatment in Helicobacter pylori eradication therapy. J Dig Dis. 2017;18:195–202. doi: 10.1111/1751-2980.12466. [DOI] [PubMed] [Google Scholar]
- 59.Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–68. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 60.Losurdo G, Cubisino R, Barone M, Principi M, Leandro G, Ierardi E, et al. Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis. World J Gastroenterol. 2018;24:139–49. doi: 10.3748/wjg.v24.i1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu R, Chen K, Zheng YY, Zhang HW, Wang JS, Xia YJ, et al. Meta-analysis of the efficacy of probiotics in Helicobacter pylori eradication therapy. World J Gastroenterol. 2014;20:18013–21. doi: 10.3748/wjg.v20.i47.18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gisbert JP, Calvet X. Review article: common misconceptions in the management of Helicobacter pylori-associated gastric MALT-lymphoma. Aliment Pharmacol Ther. 2011;34:1047–62. doi: 10.1111/j.1365-2036.2011.04839.x. [DOI] [PubMed] [Google Scholar]
- 63.Kokkola A, Valle J, Haapiainen R, Sipponen P, Kivilaakso E, Puolakkainen P. Helicobacter pylori infection in young patients with gastric carcinoma. Scand J gastroenterol. 1996;31:643–7. doi: 10.3109/00365529609009143. [DOI] [PubMed] [Google Scholar]
- 64.Du YQ, Su T, Fan JG, Lu YX, Zheng P, Li XH, et al. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18:6302–7. doi: 10.3748/wjg.v18.i43.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pourhoseingholi MA, Ashtari S, Hajizadeh N, Fazeli Z, Zali MR. Systematic review of pancreatic cancer epidemiology in Asia-Pacific Region: major patterns in GLOBACON 2012. Gastroenterol Hepatol Bed Bbench. 2017;10:245–57. [PMC free article] [PubMed] [Google Scholar]
- 66.Olah A, Belagyi T, Poto L, Romics L Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepato-gastroenterology. 2007;54:590–4. [PubMed] [Google Scholar]
- 67.Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–7. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 68.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 69.Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18:R57. doi: 10.1186/cc13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–49. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 71.Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PloS One. 2013;8:e62885. doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Sung CY, Lee N, Ni Y, Pihlajamaki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306–15. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasmi G, Andoni R, Mano V, Kraja D, Muco E, Kasmi I. Streptococcus bovis isolated in haemoculture a signal of malignant lesion of the colon. Clin Lab. 2011;57:1007–9. [PubMed] [Google Scholar]
- 75.Nakamura J, Kubota Y, Miyaoka M, Saitoh T, Mizuno F, Benno Y. Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol Immunol. 2002;46:487–90. doi: 10.1111/j.1348-0421.2002.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 76.Strofilas A, Lagoudianakis EE, Seretis C, Pappas A, Koronakis N, Keramidaris D, et al. Association of helicobacter pylori infection and colon cancer. J Clin Med Res. 2012;4:172–6. doi: 10.4021/jocmr880w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang JH, Shim YY, Cha SK, Reaney MJ, Chee KM. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J Med Microbiol. 2012;61:361–8. doi: 10.1099/jmm.0.035154-0. [DOI] [PubMed] [Google Scholar]
- 78.Foo NP, Ou Yang H, Chiu HH, Chan HY, Liao CC, Yu CK, et al. Probiotics prevent the development of 1,2-dimethylhydrazine (DMH)-induced colonic tumorigenesis through suppressed colonic mucosa cellular proliferation and increased stimulation of macrophages. J Agric Food Chem. 2011;59:13337–45. doi: 10.1021/jf203444d. [DOI] [PubMed] [Google Scholar]
- 79.De Vuyst L, Leroy F. Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Agric Food Chem. 2007;13:194–9. doi: 10.1159/000104752. [DOI] [PubMed] [Google Scholar]
- 80.Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–37. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, et al. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbio . 2008;125:286–92. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Collado MC, Isolauri E, Salminen S. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol Lett. 2008;285:58–64. doi: 10.1111/j.1574-6968.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 83.Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilan CG, Salminen S. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J Food Prot. 2006;69:2011–5. doi: 10.4315/0362-028x-69.8.2011. [DOI] [PubMed] [Google Scholar]
- 84.Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 87.Ashtari S, Pourhoseingholi MA, Sharifian A, Zali MR. Hepatocellular carcinoma in Asia: Prevention strategy and planning. World Hepatol. 2015;7:1708–17. doi: 10.4254/wjh.v7.i12.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–29. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 89.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–8. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacouton E, Chain F, Sokol H, Langella P, Bermudez-Humaran LG. Probiotic Strain Lactobacillus casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front Immunol. 2017;8:1553. doi: 10.3389/fimmu.2017.01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 93.Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, et al. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14:1625–9. doi: 10.1046/j.1365-2036.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 94.Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–9. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 95.Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, et al. Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion. 2001;63:1–7. doi: 10.1159/000051865. [DOI] [PubMed] [Google Scholar]
- 96.Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin YS, et al. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:1669–75. doi: 10.1046/j.1365-2036.2002.01335.x. [DOI] [PubMed] [Google Scholar]
- 97.Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, et al. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–9. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- 98.Tursi A, Brandimarte G, Giorgetti GM, Modeo ME. Effect of Lactobacillus casei supplementation on the effectiveness and tolerability of a new second-line 10-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med Sci Monit. 2004;10:Cr662–6. [PubMed] [Google Scholar]
- 99.Sykora J, Valeckova K, Amlerova J, Siala K, Dedek P, Watkins S, et al. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol. 2005;39:692–8. doi: 10.1097/01.mcg.0000173855.77191.44. [DOI] [PubMed] [Google Scholar]
- 100.Lionetti E, Miniello VL, Castellaneta SP, Magista AM, de Canio A, Maurogiovanni G, et al. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24:1461–8. doi: 10.1111/j.1365-2036.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- 101.Sheu BS, Cheng HC, Kao AW, Wang ST, Yang YJ, Yang HB, et al. Pretreatment with Lactobacillus- and Bifidobacterium-containing yogurt can improve the efficacy of quadruple therapy in eradicating residual Helicobacter pylori infection after failed triple therapy. Am J Clin Nutr. 2006;83:864–9. doi: 10.1093/ajcn/83.4.864. [DOI] [PubMed] [Google Scholar]
- 102.Goldman CG, Barrado DA, Balcarce N, Rua EC, Oshiro M, Calcagno ML, et al. Effect of a probiotic food as an adjuvant to triple therapy for eradication of Helicobacter pylori infection in children. Nutrition. 2006;22:984–8. doi: 10.1016/j.nut.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Myllyluoma E, Veijola L, Ahlroos T, Tynkkynen S, Kankuri E, Vapaatalo H, et al. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy--a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol Ther. 2005;21:1263–72. doi: 10.1111/j.1365-2036.2005.02448.x. [DOI] [PubMed] [Google Scholar]
- 104.Ojetti V, Bruno G, Ainora ME, Gigante G, Rizzo G, Roccarina D, et al. Impact of Lactobacillus reuteri Supplementation on Anti-Helicobacter pylori Levofloxacin-Based Second-Line Therapy. Gastroenterol Res Pract. 2012;2012:740381. doi: 10.1155/2012/740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y. Adjuvant therapy for probiotics in patients with severe acute pancreatitis: an analysis of 14 cases [in Chinese] Shijie Huaren Xiaohua Zazhi. 2007;15:302–4. [Google Scholar]
- 106.Plaudis H, Pupelis G, Zeiza K, Boka V. Early low volume oral synbiotic/prebiotic supplemented enteral stimulation of the gut in patients with severe acute pancreatitis: a prospective feasibility study. Acta Chir Belg. 2012;112:131–8. doi: 10.1080/00015458.2012.11680811. [DOI] [PubMed] [Google Scholar]
- 107.Cui LH, Wang XH, Peng LH, Yu L, Yang YS. [The effects of early enteral nutrition with addition of probiotics on the prognosis of patients suffering from severe acute pancreatitis] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:224–8. doi: 10.3760/cma.j.issn.2095-4352.2013.04.011. [DOI] [PubMed] [Google Scholar]