Abstract
Aim:
To assess NT-proBNP as a biomarker for hyperdynamic circulation in decompensated cirrhosis.
Background:
Hyperdynamic circulation is common in decompensated cirrhosis. The previous studies reveal that N-terminal-proBNP (NT-proBNP) is elevated in cirrhosis.
Methods:
A prospective study involved 47 patients with decompensated cirrhosis. All of them underwent echocardiography with simultaneous measurement of blood pressure and heart rate. Cardiac output and systemic vascular resistance were calculated. The concentration of NT-proBNP in blood was measured with enzyme-linked immunosorbent assay.
Results:
In patients with decompensated cirrhosis, the concentration of NT-proBNP in blood directly correlated with end-diastolic volume (r=0.482; p<0.001), stroke volume (r= 0.566; p<0.001), cardiac output (r=0.556; p<0.001), volume of the left atrium (r=0.292; p=0.047), and inversely correlated with systemic vascular resistance (r=-0.538; p<0.001). There was no significant correlation between NT-proBNP and ejection fraction (p=0.083). Patients with hyperdynamic circulation have higher concentration of NT-proBNP (152÷476 pg/ml vs. 31÷133 pg/ml, p<0.001) regardless of the presence of diastolic dysfunction (p=0.222). According to ROC analysis, the best cut-off values for detection of hyperdynamic circulation in decompensated cirrhosis are considered to be 170.0 pg/ml of blood NT-proBNP, showing sensitivity and specificity of 72.0 and 86.4%, respectively. The positive and negative predictive value are 86.4% and 73.1%, AUC = 0.829 (0.709-0.949).
Conclusion:
NT-proBNP may serve as a non-invasive biomarker for hyperdynamic circulation in decompensated cirrhosis.
Key Words: Blood circulation, Liver cirrhosis, Biomarkers, Natriuretic peptide, Brain
Introduction
Hemodynamic changes in cirrhosis were first described more than 60 years ago (1,2) and include increased cardiac output, increased total blood volume, decreased blood pressure, and decreased systemic vascular resistance. Together, these changes constitute hyperdynamic circulation. Vasodilatation and hyperdynamic circulation are discussed to cause complications of cirrhosis including portal hypertension, hepatorenal and hepatopulmonary syndromes, and hepatic encephalopathy (3).
Non-selective beta-blockers remain the cornerstone of medical treatment of portal hypertension. However, the effect of non-selective beta-blockers depend on the severity of the hyperdynamic circulation and splanchnic vasodilation (4). No biomarker has been proposed for hyperdynamic circulation in cirrhosis, and hemodynamic parameters, such as cardiac output and systemic vascular resistance, are not usually determined before beta-blockers use. Hepatic venous pressure gradient was supposed for diagnosis of portal hypertension, but the measurement of it, is an expensive and invasive procedure and is not common (4). Thus, beta-blockers are usually used blindfold in cirrhosis. The use of a simple serum biomarker for hyperdynamic circulation may provide differential management with beta-blockers and control for their use, which may increase the effectiveness of therapy and reduce the incidence of side effects.
The endocrine function of the heart was first described in 1981, when the researchers injected the extract from the atria to rats and observed a significant increase in sodium and water excretion with urine (5). Myocardial hormone responsible for this effect was a 28 amino acid polypeptide (6), which was termed the atrial natriuretic peptide. Later, a similar peptide was isolated from the brain tissue and was called the brain natriuretic peptide (BNP) (7).
However, as it was revealed later that despite its name most of the BNP of blood is formed by the myocardium of the heart ventricles (8-9). After a significant increase in blood BNP in the patients with heart failure was described, this peptide began used as a biomarker of this disease (10). The physiological role of BNP is to reduce the volume of circulating blood to prevent cardiac overload. An increase in blood BNP in the patients with decompensated cirrhosis was established in 1992 (11). Most researchers consider BNP as a biomarker of cardiac dysfunction in cirrhosis (12-14).
It was found that the stretching of cardiomyocytes, which is observed in the dilatation of the left ventricle in heart failure, leads to an increase in the release of BNP into the blood (15). Since hyperdynamic circulation is also associated with the dilatation of the left ventricle (16), we assume that BNP in cirrhosis may serve as a biomarker for hyperdynamic circulation. It was shown that BNP is formed from proBNP after its cleavage into BNP and the N-terminal fragment (NT-proBNP) (17). NT-proBNP is more stable, therefore it may have better analytical characteristics.
The aim of this study is to assess NT-proBNP as a biomarker for hyperdynamic circulation in decompensated cirrhosis.
Methods
In this cross-sectional prospective study, 140 consecutive patients with cirrhosis admitted to the Department of Hepatology’s Clinic for Internal Diseases, Gastroenterology and Hepatology (the Clinic), at Sechenov University, Moscow, were screened for inclusion. Study procedures were explained to potential participants and written informed consent was obtained before enrollment. The study had been performed in accordance with the ethical standards laid down in an appropriate version of the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of Sechenov University.
Inclusion criteria were: diagnosis of decompensated cirrhosis verified by histology, clinical, biochemical, and ultrasound findings; age between 18 and 70 years. Severity of liver disease was determined using the Child–Turcotte–Pugh (CTP) scoring system, in which class A was defined as compensated cirrhosis and classes B and C were defined as decompensated cirrhosis.
Exclusion criteria included: cardiac disease, cancer or any other severe disease.
Of the original 140 patients screened for inclusion, 47 met criteria and were enrolled in the study. The other patients had compensated cirrhosis (n=61), cancer (n=4), other severe disease (n=9), and 19 patients declined to participate.
The concentration of NT-proBNP in blood was measured with enzyme-linked immunosorbent assay.
Echocardiography was performed at rest according to guidelines published by The American Society of Echocardiography (18). End-diastolic volume and end-systolic volume of the left ventricle were determined according to modified Simpson's disk method. Ejection fraction of the left ventricle was calculated as ((End-diastolic volume) - (End-systolic volume))/(End-diastolic volume). Stroke volume was calculated as (Doppler velocity time integral) × (cross-sectional aorta area). Systolic and diastolic blood pressure and heart rate were measured during stroke volume measurement using automatic oscillometric sphygmomanometer (AND, Japan). Mean arterial pressure was calculated as ((Systolic blood pressure) + 2 × (Diastolic blood pressure))/3. Cardiac output was calculated as (Stroke volume) × (Heart rate). Systemic vascular resistance was calculated as (Mean arterial pressure) / (Cardiac output). Diastolic dysfunction Grade I-IV was determined according to recent guideline (19).
Statistical analysis was performed with STATISTICA 10 software (StatSoft Inc., USA) and SPSS (IBM, USA). The differences between continuous variables were assessed with the Mann-Whitney test. Correlations between variables were computed using Spearman's rank correlation. Fisher's exact test was used to assess the difference between categorical variables. Data were represented as mean ± SD, except for NT-proBNP concentration, which was represented as interquartile range. P-value < 0.05 was considered statistically significant.
Results
The main parameters of participants are presented in Table 1. Blood NT-proBNP directly correlated with end-diastolic volume (r = 0.482; p <0.001), stroke volume (r = 0.566; p <0.001), cardiac output (r = 0.556; p <0.001), volume of the left atrium (r = 0.292; p = 0.047), and inversely correlates with systemic vascular resistance (r = -0.538; p <0.001). There were no significant correlations between NT-proBNP and ejection fraction (r = 0.226; p = 0.083), heart rate (p=0.087), and mean arterial pressure (p=0.151).
Table 1.
The main parameters of participants
| Cirrhosis class B CTP (n=27) | Cirrhosis class C CTP (n=20) | p-value | |
|---|---|---|---|
| CTP score | 7.86±0.86 | 10.90±1.29 | <0.001 |
| Age, year | 49.6±11.9 | 49.4±10.2 | 0.914 |
| Male/female | 12/15 | 16/4 | 0.015 |
| Etiology: alcohol | 16 | 14 | 0.328 |
| viral | 4 | 2 | 0.489 |
| autoimmune | 1 | 1 | 0.675 |
| mixed | 5 | 2 | 0.352 |
| cryptogenic | 1 | 1 | 0.675 |
| End-diastolic volume, ml | 115.1±19.6 | 126.0+31.4 | 0.302 |
| End-systolic volume, ml | 46.0±8.7 | 49.5±14.3 | 0.426 |
| Ejection fraction, % | 60.0±4.3 | 60.5±7.3 | 0.519 |
| Stroke volume, ml | 69.2±15.3 | 76.5±21.8 | 0.208 |
| Heart rate, bpm | 82.1±10.7 | 87.5±11.4 | 0.179 |
| Cardiac output, l/min | 5.71±1.45 | 6.77±2.38 | 0.168 |
| Systolic blood pressure, mm Hg | 111.3±9.8 | 110.5±9.4 | 0.739 |
| Diastolic blood pressure, mm Hg | 72.4±6.4 | 70.5±7.6 | 0.420 |
| Mean arterial pressure, mm Hg | 85.4±7.1 | 83.8±7.7 | 0.533 |
| Systemic vascular resistance, dyn х sec/cm5 | 1277±352 | 1125±443 | 0.126 |
| Left ventricular dilatation (present/absent) | 8/19 | 6/14 | 0.613 |
| Volume of the left atrium, ml | 30.0±8.5 | 38.3±12.9 | 0.005 |
| Left atrial dilatation (present/absent) | 5/22 | 10/10 | 0.024 |
| Hyperdynamic circulation (present/absent) | 14/13 | 11/9 | 0.533 |
| Е/А | 1.08±0.29 | 1.09±0.21 | 0.755 |
| Systolic heart dysfunction | 1 | 1 | 0.675 |
| Diastolic heart dysfunction Grade I | 6 | 2 | 0.242 |
| Diastolic heart dysfunction Grade II | 4 | 8 | 0.053 |
| Diastolic heart dysfunction Grade III-IV | 0 | 0 | 1.000 |
| NT-proBNP, pg/ml | 76.3÷311.9 | 56.8÷298.7 | 0.739 |
Systolic heart dysfunction (ejection fraction < 50% (10)) was revealed in 2 patients but they had a NT-proBNP level in the blood of less than 125 (cut-off for heart failure (10)). It was interesting that the decrease in the ejection fraction was not accompanied by an increase in the level of NT-proBNP in the blood, as it takes place in heart failure. In patients with elevated blood NT-proBNP (>125 pg/ml (10)), end-diastolic volume, stroke volume, cardiac output, the volume of the left atrium were higher, systemic vascular resistance was lower, ejection fraction, blood pressure, and heart rate were not significantly differ, hyperdynamic circulation, dilatation left atrium, and diastolic dysfunction Grade II were more often revealed (Table 2). In patients with left ventricular dilatation (end-diastolic volume > 150 ml in men and > 106 ml in women (18)), the level of NT-proBNP was higher, and this was also accompanied by an increase in stroke volume, cardiac output and a decrease in systemic vascular resistance without significant differences in ejection fraction, heart rate and blood pressure. Patients with dilation of the left atrium (volume > 34 ml (18)) had similar changes (Table 3). Among 15 patients with dilation of the left atrium, 3 patients had diastolic dysfunction Grade I and 12 ones had diastolic dysfunction Grade II. 80% of patients with dilation of the left atrium had hyperdynamic circulation. The volume of the left atrium correlated with cardiac output (r = 0.471; p = 0.001).
Table 2.
Main hemodynamic parameters according to blood NT-proBNP level
| Elevated NT-proBNP (n=26) | Normal NT-proBNP (n=21) | p-value | |
|---|---|---|---|
| End-diastolic volume, ml | 128.6±26.5 | 109.2±20.3 | 0 . 014 |
| End-systolic volume, ml | 49.2±11.8 | 45.3±10.8 | 0.369 |
| Ejection fraction, % | 61.6±4.2 | 58.5±6.7 | 0.131 |
| Stroke volume, ml | 79.1±17.5 | 63.9±14.3 | 0.003 |
| Heart rate, bpm | 85.6±11.5 | 82.9±10.9 | 0.380 |
| Systolic blood pressure, mm Hg | 110.8±9.8 | 111.2±9.5 | 0.940 |
| Diastolic blood pressure, mm Hg | 71.1±7.1 | 72.1±6.8 | 0.653 |
| Mean arterial pressure, mm Hg | 84.4±7.3 | 85.2±7.4 | 0.692 |
| Volume of the left atrium, ml | 36.8±13.7 | 28.1±4.8 | 0 . 049 |
| Left atrial dilatation (present/absent) | 14/12 | 1/20 | 0 . 006 |
| Cardiac output, l/min | 6.88±2.13 | 5.27±1.25 | 0. 007 |
| Hyperdynamic circulation (present/absent) | 19/7 | 6/15 | 0 . 003 |
| Systolic heart dysfunction | 0 | 2 | 0.194 |
| Diastolic heart dysfunction Grade I | 3 | 5 | 0.235 |
| Diastolic heart dysfunction Grade II | 12 | 0 | <0. 001 |
| Systemic vascular resistance, dyn х sec/cm5 | 1083±374 | 1368±373 | 0.014 |
Significant differences are marked in italics.
Table 3.
Blood NT-proBNP and the main hemodynamic parameters in decompensated cirrhosis depending on the presence of dilatation of the left ventricle and left atrium
| Left ventricular dilatation |
p | Left atrial dilatation |
p-value | |||
|---|---|---|---|---|---|---|
| Present (n=14) |
Absent (n=33) | Present (n=15) | Absent (n=32) | |||
| NT-proBNP, pg/ml | 115.3÷535.6 | 50.0÷196.6 | 0.042 | 196.6÷535.6 | 41.1÷168.9 | < 0.001 |
| Ejection fraction, % | 60.2±7.0 | 60.2±5.1 | 0.991 | 62.6±4.5 | 59.2±5.9 | 0.105 |
| Stroke volume, ml | 84.3±20.0 | 67.2±14.1 | 0.008 | 86.2±17.5 | 65.8±13.8 | < 0.001 |
| Heart rate, bpm | 87.2±10.7 | 83.2±11.1 | 0.301 | 87.9±11.5 | 82.8±10.8 | 0.189 |
| Mean arterial pressure, mm Hg | 81.4±8.9 | 86.1±6.1 | 0.085 | 82.0±5.9 | 86.0±7.6 | 0.085 |
| Cardiac output, l/min | 7.44±2.44 | 5.61±1.48 | 0.012 | 7.66±2.17 | 5.46±1.38 | 0.001 |
| Systemic vascular resistance, dyn х sec/cm5 | 960±309 | 1319±383 | 0.004 | 933±309 | 1342±367 | 0.001 |
| End-diastolic volume, ml | 139.3±26.4 | 111.5±20.5 | 0.002 | 137.9±27.5 | 111.3±19.9 | 0.003 |
| Hyperdynamic circulation (present/absent) | 11/3 | 14/19 | 0.024 | 12/3 | 13/19 | 0.012 |
Significant differences are marked in italics.
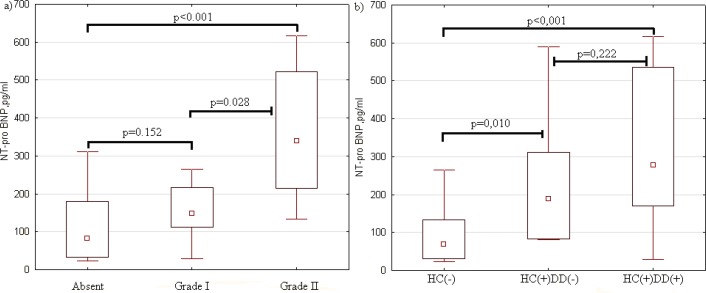
Diastolic dysfunction Grade I was not accompanied by a significant increase in blood NT-proBNP, in contrast to diastolic dysfunction Grade II (Figure 1a).
Figure 1.
Blood NT-proBNP (pg / ml) in decompensated cirrhosis depending on the presence of diastolic dysfunction (a) and hyperdynamic circulation (b). HC(-) - patients without hyperdynamic circulation (n = 22), HC(+)DD(-) - patients with hyperdynamic circulation without diastolic dysfunction (n = 10), HC(+)DD (+) - patients with hyperdynamic circulation and diastolic dysfunction (n = 15). The point in the middle of each box represents the mean. The length of the box represents the mean±SD. The error bars show the non-outlier range
Based on our previous study, we determined that for our population, the upper value of the normal range of cardiac output is 5.5 l/min (16). Cardiac output above this level was regarded as evidence of hyperdynamic circulation, which was detected in 25 (53.2%) patients. In these patients, the level of NT-proBNP was higher, and this was also accompanied by an increase in end-diastolic volume, stroke volume, heart rate, left atrial volume and a decrease in systemic vascular resistance without significant difference in blood pressure. In patients with hyperdynamic circulation, dilatation of the left atrium and diastolic dysfunction Grade II were more often revealed. Interestingly, an increase in blood NT-proBNP in patients with hyperdynamic circulation was accompanied by an increase in ejection fraction, while in heart failure, opposite changes were described (20) (Table 4).
Table 4.
Blood NT-proBNP and hemodynamic parameters in decompensated cirrhosis depending on the presence of hyperdynamic circulation
| Hyperdynamic circulation |
p | ||
|---|---|---|---|
| Present (n=25) | Absent (n=22) | ||
| NT-proBNP, pg/ml | 152÷476 | 31÷133 | <0.001 |
| End-diastolic volume, ml | 134.5±23.7 | 103.0±15.5 | <0.001 |
| End-systolic volume, ml | 50.4±11.2 | 44.2±11.0 | 0.072 |
| Ejection fraction, % | 62.6±3.6 | 57.6±7.4 | 0.001 |
| Stroke volume, ml | 84.1±14.9 | 58.9±8.7 | <0.001 |
| Heart rate, bpm | 89.7±10.7 | 78.4±8.5 | 0.001 |
| Systolic blood pressure, mm Hg | 110.0±9.1 | 112±10.0 | 0.388 |
| Diastolic blood pressure, mm Hg | 70.8±7.0 | 72.5±9.6 | 0.440 |
| Mean arterial pressure, mm Hg | 83.9±7.2 | 85.7±7.5 | 0.337 |
| Volume of the left atrium, ml | 36.5±13.3 | 28.9±7.4 | 0.043 |
| Left atrial dilatation (present/absent) | 12/13 | 3/19 | 0.012 |
| Systolic heart dysfunction | 0 | 2 | 0.214 |
| Diastolic heart dysfunction Grade I | 5 | 3 | 0.427 |
| Diastolic heart dysfunction Grade II | 10 | 2 | 0.016 |
| Systemic vascular resistance, dyn х sec/cm5 | 931±221 | 1532±301 | <0.001 |
Significant differences are marked in italics
In patients with hyperdynamic circulation, blood NT-proBNP was higher, regardless of the presence of diastolic dysfunction, but in ones with diastolic dysfunction there was a tendency for higher NT-proBNP values (Figure 1b).
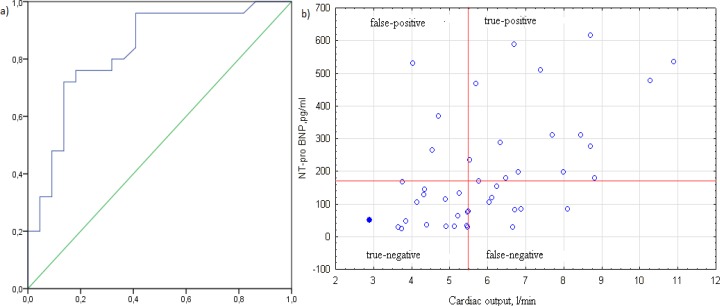
According to ROC analysis, the best cut-off values for detection of hyperdynamic circulation in decompensated cirrhosis are considered to be 170 pg/ml of blood NT-proBNP, showing sensitivity and specificity of 72.0 and 86.4%, respectively. The positive and negative predictive values were 86.4% and 73.1%. AUC = 0.829 (0.709-0.949), which correspond to a good biomarker characteristic (Figure 2).
Figure 2.
ROC analysis of blood NT-proBNP for detection of hyperdynamic circulation in decompensated cirrhosis (a) with a scattering diagram (b). Two truly positive results that significantly exceeded 700 pg/ml are not shown for the convenience of analyzing the scatter map
Discussion
An analysis of the results shows that an increase in blood NT-proBNP in decompensated cirrhosis is associated with an increase in the size of the left chambers of the heart as in heart failure. However, unlike the latter, in cirrhosis an increase in blood NT-proBNP are accompanied by an increase in stroke volume and cardiac output and a decrease in systemic vascular resistance without a significant change in ejection fraction. In decompensated cirrhosis, blood NT-proBNP directly correlates with end-diastolic volume, stroke volume, cardiac output, volume of the left atrium, inversely correlates with systemic vascular resistance, and does not correlate with ejection fraction, whereas in heart failure, a negative correlation with ejection fraction is shown (20). In contrast, patients with decompensated cirrhosis have a tendency to increase ejection fraction with an increase in blood NT-proBNP.
The findings allow suggesting that the pathogenesis of the increase in blood NT-proBNP in decompensated cirrhosis and systolic heart failure is different, but in both cases is associated with stretching of left ventricular cardiomyocytes.
In systolic heart failure, ejection fraction decreases which leads to decrease in stroke volume and, consequently, cardiac output. As a compensatory reaction in accordance with Starling's law, end-diastolic volume increases to increase stroke volume and cardiac output, but this is not always sufficient to normalize these. Therefore, an increase in NT-proBNP in systolic cardiac dysfunction is accompanied by a decrease in stroke volume and cardiac output and a compensatory increase in systemic vascular resistance to maintain blood pressure. In decompensated cirrhosis, reverse changes are observed. Endotoxemia leads to arterial vasodilation which leads to a decrease in systemic vascular resistance and blood pressure (21). In response to a decrease in blood pressure, barostatic mechanisms are activated, leading to fluid retention, which leads to increase in venous return and end-diastolic volume. These lead to an increase in stroke volume and cardiac output. Therefore, hyperdynamic circulation develops.
Thus, we showed that blood NT-proBNP in decompensated cirrhosis is a biomarker for hyperdynamic circulation, but not a biomarker for systolic cardiac dysfunction. As a result of the ROC analysis, we proposed a cut-off point for the value of this biomarker (170.0 pg/ml), which allows to identify patients with hyperdynamic circulation with high analytical reliability.
In one of the previous studies (12), there was no correlation between NT-proBNP and cardiac output in cirrhosis. The contradiction with our findings can be explained by the fact that in the cited work the authors did not divide patients into groups with compensated and decompensated cirrhosis, while hyperdynamic circulation was observed almost exclusively in patients with decompensated cirrhosis (16). In addition, if we analyze the regression curve of cardiac output to NT-proBNP, it can be seen that the correlation between these begins with a cardiac output level > 4.0 l/min, whereas cardiac output does not exceed this level in 42% of patients with the compensated cirrhosis (16).
In another study (13), it was shown that an increase in NT-proBNP is associated with diastolic dysfunction in cirrhosis. In our study, blood NT-proBNP was higher only in diastolic dysfunction Grade II when the left atrium volume increases, but not in diastolic dysfunction Grade I when, in most cases (62.5% in our work), dilation of left atrium is not observed. We suggest these due to the fact that blood NT-proBNP increases also as result of the extension of cardiomyocytes of left atrium, but not only as the result of the extension of cardiomyocytes of left ventricle. But the muscle mass of the left atrium less than muscle mass of the left ventricle. Therefore, the myocardium of the left atrium produces less NT-proBNP than myocardium of the left ventricle. At the same time, according to our findings, the increase in blood NT-proBNP in hyperdynamic circulation does not depend on the presence of diastolic dysfunction, but in patients with hyperdynamic circulation and diastolic dysfunction there is a tendency to higher blood NT-proBNP than in patients with hyperdynamic circulation but without diastolic dysfunction.
The use of NT-proBNP as a simple serum biomarker for hyperdynamic circulation may provide differential management with beta-blockers and control for their use, which may increase the effectiveness of therapy and reduce the incidence of side effects. Further searches are required to test this hypothesis.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025–33. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24:358–67. doi: 10.1016/0002-9343(58)90322-x. [DOI] [PubMed] [Google Scholar]
- 3.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–31. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 4.Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66:849–59. doi: 10.1016/j.jhep.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 6.Flynn TG, de Bold ML, de Bold AJ. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun. 1983;117:859–65. doi: 10.1016/0006-291x(83)91675-3. [DOI] [PubMed] [Google Scholar]
- 7.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y, Nakao K, Mukoyama M, Shirakami G, Itoh H, Hosoda K, et al. Rat brain natriuretic peptide--tissue distribution and molecular form. Endocrinology. 1990;126:2225–7. doi: 10.1210/endo-126-4-2225. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa Y, Nakao K, Mukoyama M, Hosoda K, Shirakami G, Arai H, et al. Natriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats The ventricle is a major site of synthesis and secretion of brain natriuretic peptide. Circ Res. 1991;69:491–500. doi: 10.1161/01.res.69.2.491. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 11.La Villa G, Romanelli RG, Casini Raggi V, Tosti-Guerra C, De Feo ML, Marra F, et al. Plasma levels of brain natriuretic peptide in patients with cirrhosis. Hepatology. 1992;16:156–61. doi: 10.1002/hep.1840160126. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen JH, Gøtze JP, Fuglsang S, Christensen E, Bendtsen F, Møller S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52:1511–7. doi: 10.1136/gut.52.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raedle-Hurst TM, Welsch C, Forestier N, Kronenberger B, Hess G, Herrmann E, et al. Validity of N-terminal propeptide of the brain natriuretic peptide in predicting left ventricular diastolic dysfunction diagnosed by tissue Doppler imaging in patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:865–73. doi: 10.1097/MEG.0b013e3282fb7cd0. [DOI] [PubMed] [Google Scholar]
- 14.Wong F, Siu S, Liu P, Blendis LM. Brain natriuretic peptide: is it a predictor of cardiomyopathy in cirrhosis? Clin Sci. 2001;101:621–8. [PubMed] [Google Scholar]
- 15.Hirose S, Hagiwara H, Takei Y. Comparative molecular biology of natriuretic peptide receptors. Can J Physiol Pharmacol. 2001;79:665–72. [PubMed] [Google Scholar]
- 16.Maslennikov RV, Driga AA, Ivashkin KV, Zharkova MS, Mayevskaya MV, Pavlov Ch F, et al. Small intestinal bacterial overgrowth syndrome and systemic inflammation in pathogenesis of hemodynamic changes at liver cirrhosis. Ross z gastroenterol gepatol koloproktol. 2017;27:45–56. [Google Scholar]
- 17.Vuolteenaho O, Ala-Kopsala M, Ruskoaho H. BNP as a biomarker in heart disease. Adv Clin Chem. 2005;40:1–36. [PubMed] [Google Scholar]
- 18.Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging . 2016;17:412. doi: 10.1093/ehjci/jew041. [DOI] [PubMed] [Google Scholar]
- 19.Jeong EM, Dudley SC. Diastolic dysfunction. Circ J. 2015;79:470–7. doi: 10.1253/circj.CJ-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prastaro M, Paolillo S, Savarese G, Dellegrottaglie S, Scala O, Ruggiero D, et al. N-terminal pro-b-type natriuretic peptide and left atrial function in patients with congestive heart failure and severely reduced ejection fraction. Eur J Echocardiogr. 2011;12:506–13. doi: 10.1093/ejechocard/jer070. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–84. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]