Abstract
Aim:
The aim of this systematic review was to determine if the human colon, through the lower gut-liver axis, drives PSC activity by assessing the progression of the disease in patients with and without colectomy for colonic disease.
Background:
The gut-liver axis is involved in the pathogenesis of liver disease. Abnormal immune-mediated responses to intestinal microbiome are implicated in primary sclerosing cholangitis (PSC) however the mechanisms remain poorly understood. Currently, no single animal model recapitulates all attributes of PSC in humans and this limits further studies of gut-liver interactions.
Methods:
A systematic search of PubMed, Medline, and Scopus was performed for articles that contained the terms “colectomy” or “bowel resection” AND “primary sclerosing cholangitis” up to 15th April 2018. Articles were reviewed by 2 reviewers and raw data collated. A Forest plot was used to illustrate the effect of colectomy on subsequent liver transplantation for PSC. Linear regression was used to estimate mortality risk.
Results:
Colectomy appeared to have no effect on PSC progression, although high-quality studies were lacking. Rates of liver transplantation or transjugular intrahepatic portosystemic shunt for PSC were not affected by colectomy (OR 0.59, 95% CI 0.14 - 2.53, p=0.48). Mortality risk following colectomy in patients with PSC is 2.11% per year (95% CI 0.03% - 4.18%, p=0.032, R2 = 0.722).
Conclusion:
Current evidence is limited but suggests colectomy does not affect the progression of PSC in patients with colonic disease. Pathogenic micro-organisms or antigens that drive PSC may not be limited to the lower gut.
Key Words: Primary sclerosing cholangitis, Inflammatory bowel disease, Colectomy, Procto-colectomy
Introduction
The gut-liver axis, a concept thought to be first introduced in 1987 by Volta et al (1-2), describes the complex interactions between the gut microbiome, the small and large bowel, the immune system and the liver. Recent advances in gastroenterology have implicated the gut-liver axis in alcoholic liver disease, non-alcoholic fatty liver disease, primary biliary cholangitis, and primary sclerosing cholangitis (PSC) (3). In PSC, immune-mediated processes lead to chronic inflammation of both intra-hepatic and extra-hepatic bile ducts, eventually causing liver cirrhosis. Approximately 47-76% of patients with PSC also suffer from inflammatory bowel disease (IBD) (4), however, a causal link has not been established. Dysbiosis in the gut microbiome, increased intestinal permeability, translocation of circulating pro-inflammatory cytokines in the portal vein, and circulating auto-antibodies are hallmark characteristics in the pathophysiology of PSC (3), however little else is known about the triggering microorganism, relevant antigens, or its location within the gastrointestinal tract.
The role of the colon in the pathogenesis of PSC has long served as a controversial point of debate. Early small sample sized case series (5-7) have previously reported impressive rates of improvement in PSC for patients who had undergone colectomy for concomitant ulcerative colitis (UC). However, such results were irreproducible in larger cohort studies of UC patients (8-9). In support of gut involvement in PSC, basic science research has elucidated the recruitment and preferential binding of chemokine (C-C motif) receptor 9 positive - integrin α4β7 positive (CCR9+ α4β7+) T-lymphocytes to abnormally upregulated mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), vascular adhesion protein-1 (VAP-1) and gut homing chemokine (C-C motif) ligand (CCL25) on hepatic endothelial cells, and the up-regulation of toll-like receptors on biliary epithelial cells and T-Helper type 17 (TH17) cells as responses of the gut-liver axis specific to PSC (10-13). Clinically, a retrospective study from a transplant centre has also demonstrated that colectomy conferred a protective effect to liver grafts against recurrent PSC (14). Such conflicting reports in literature preclude any strong conclusions from being made.
In looking at animal models to understand the role of the colon in the pathogenesis of PSC, current disease models of PSC remain sub-optimal and not a single animal model to date is able to fully recapitulate all attributes of the disease seen in humans (15). As a result, further studies of gut-liver interactions are restricted and this impacts on our understanding of the disease and our ability to develop potential treatments. In order to determine if the lower gut-liver axis (colon-microbiome-immune interactions) drives the disease activity in PSC, the aim of this systematic review was to determine if total colectomy had any effect on the progression of PSC.
Methods
Literature search
The systematic review was performed following standard PRISMA guidelines (16). A search of Medline, Cochrane, and Scopus databases was performed for articles containing the terms "colectomy" AND "primary sclerosing cholangitis" or “bowel resection” AND "primary sclerosing cholangitis" that were published up to April 2018. Unpublished literature was identified through the OpenGrey database. Additional studies that were not included in the database search were identified through searching the reference lists of retained articles.
Inclusion and exclusion criteria
All observational studies, except for case reports and small case series (less than five patients), were included for further evaluation when available. All patients with a confirmed diagnosis of PSC were included. Patients who had PSC but not undergone colectomy were used as a control group, and total colectomy or procto-colectomy were considered as an identical intervention, where applicable. The exclusion criteria for meta-analyses were: (i) studies that did not report sufficient primary data, (ii) studies with irrelevant content and (iii) studies that were not accessible by the UK Access Management Federation.
Data extraction
Two authors (JO and MFB) independently reviewed all titles and abstracts then assessed articles against the inclusion criteria for analysis. A third reviewer (YAN) resolved any differences. The primary outcome measure was the progression of PSC as determined by serological or histological evidence, defined pragmatically on the specific criteria used within each study. Secondary outcome measures were the rate of liver transplantation (including re-transplantation) or transjugular intrahepatic portosystemic shunts (TIPSS) when liver transplantation was unsuitable, and mortality rates. Data were extracted independently by authors JO and MFB using a standardized form. Data extracted included serological or histological progression of PSC, transplantation rates, and mortality rates. Quality of the studies included was assessed using a modified Newcastle–Ottawa Scale.
Data synthesis and analysis
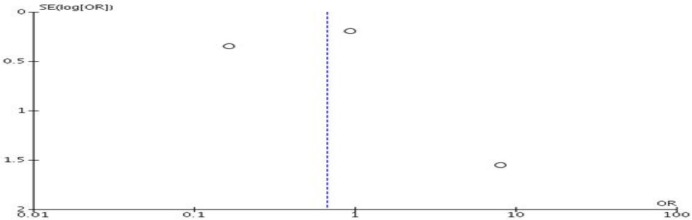
Forest plots were used to illustrate effect size between studies, where possible. Each study included had an odds ratio (OR) with respective 95 percent confidence interval (CI) calculated. A Mantel-Haenszel (M-H) statistical method was performed, calculating an overall OR for respective outcomes: an OR less than 1.00 inferred a worse survival for the colectomy group versus the control group, whilst an OR greater than 1.00 inferred a better survival. The significance level was set to 5% for all tests and alternative hypotheses were two-sided. High heterogeneity between studies was presumed and a random-effects model was used. A funnel plot was employed to assess for potential publication bias (Appendix 1).
Appendix 1.
Funnel plot for studies induced in liver transplantation and TIPSS rate
All-cause mortality following colectomy was plotted for each study against respective mean study follow up. A mean-weighted linear regression was performed to estimate the change in all-cause mortality per year.
Statistical analysis was performed using Review Manager (RevMan) Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and GraphPad Prism 5.0 (GraphPad Software, La Jolla California USA).
Results
675 studies were identified from the initial literature search (Figure 1). Following removal of duplicates and abstract screening, twenty-four full-text articles were reviewed. After excluding articles with insufficient data (n=2), irrelevant content (n=5) and inaccessibility (n=2), a total of fifteen articles were included in the final review (8-28).
Figure 1.
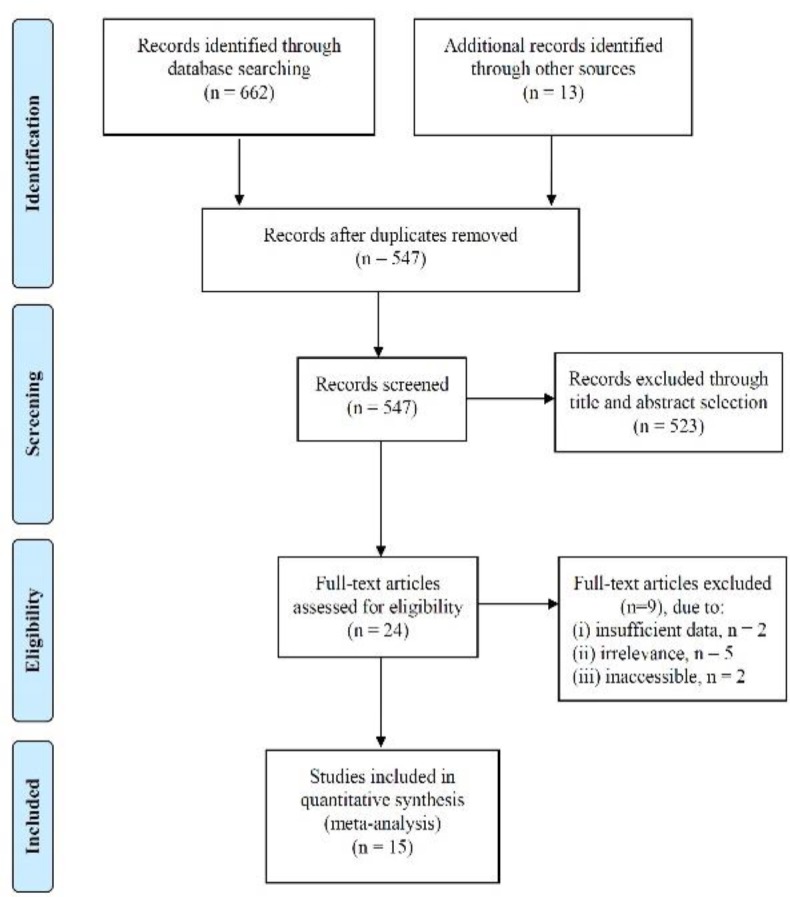
Systematic review flow diagram
Rate of PSC Progression
Overall, colectomy did not appear to have an effect on PSC progression. Two studies directly comparing patients with PSC following colectomy versus no colectomy were identified (Table 1) but there was no significant difference between both groups of patients. No randomized control trials were identified; the limited number of studies identified precluded additional quantitative analysis.
Table 1.
PSC progression rates in patients with colonic disease, with and without colectomy
| Study, Year | Type of Resection | Follow-up (years) | Sample size in study |
No change to PSC activity or PSC progression |
||
|---|---|---|---|---|---|---|
| Colectomy, n |
No Colectomy, n | Colectomy, n (%) |
No Colectomy, n (%) |
|||
| Cangemi et al, 1989 (8) | PC | 3 | 13 | 17 | 13 (100%) | 17 (100%) |
| Alabraba et al,2009 (14) | PC or CO | 6.9 | 46 | 169 | 15 (32.6%) | 39 (23.1%) |
| Aitola et al, 1994 (17) | PC or CO | 4.8 | 7 | 5 (71.4%) | ||
| Mikkola et al, 1995 (18) | PC or Co | 9 | 13 | 4 (30.8%) | ||
| Goudet et al, 2000 (9) | PC or CIA | 10 | 36 | 18 (50.0%) | ||
| Cho et al, 2008 (19) | IPA | 4.3 | 22 | 2 (9.1%) | ||
| Lepisto et al, 2009 (20) | PC, IPA | 11 | 30 | 15 (50%) | ||
PC = Proctocolectomy, Co = Colectomy, IPA = Ileal pouch-anal canal anastomosis, CIA = Colectomy with ileorectal anastomosis,
= no control group in study
One study (8) followed up 30 patients in total over 3 years follow-up, reporting disease progression in all patients, both colectomy and non-colectomy. Another study (14) reported data on 46 patients who had undergone a colectomy and 169 patients as the control, for an average follow-up of 6.9 years, and noted PSC progression rates as 32.6% and 23.1% respectively (p=0.19).
Five further observational studies were identified with a follow-up period ranging between 4.3 to 11 years (9, 17-20). However, these studies did not have control groups so statistical comparisons were not possible. The reported PSC progression rates ranged between 9.1% and 50%. Due to heterogeneity in the definitions of PSC progression used in the studies, linear regression analysis was not attempted.
Rates of Liver Transplantation or TIPSS
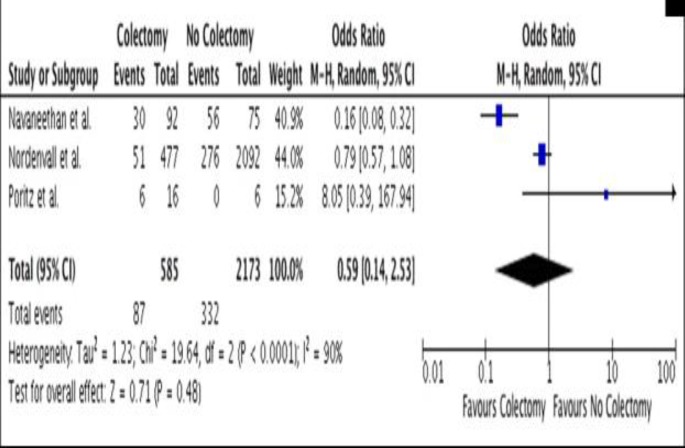
Six studies (20-25) reporting liver transplantation rates (or TIPSS in cases where liver transplantation was unsuitable) were included (Table 2). Follow-up ranged from 5.9 to 13.4 years, with rates reported between 9% and 50%. Three studies (21-23) directly compared colectomy versus no colectomy in this patient cohort. Following forest plot analysis, no significant effect of colectomy on either the rates of liver transplantation or TIPSS was demonstrated (OR 0.59, 95% CI 0.14-2.53, p=0.48) (Figure 2).
Table 2.
Liver transplantation or TIPSS rates in patients with colonic disease, with and without colectomy
| Study, Year | Type of Resection | Follow-up (years) | Sample size in study |
Transplantation or TIPSS |
||
|---|---|---|---|---|---|---|
| Colectomy, n | No Colectomy, n | Colectomy, n (%) | No Colectomy, n (%) |
|||
| Poritz et al, 2003 (21) | PC or Co | 7 | 16 | 6 | 6 (37.5%) | * |
| Navaneethan et al, 2011 (22) | PC, PBI, or IPA | 13.4 | 92 | 75 | 30 (32.6%) | 56 (74.7%) |
| Nordenvall et al, 2018 (23) | Co or SR | 5.9 | 477 | 2092 | 51 (10.7%) | 276 (13.2%) |
| Lepisto et al, 2009 (20) | PC | 11 | 30 | 15 (50%) | ||
| Mathis et al, 2012 (24) | PC | 5.9 | 100 | 9 (9%) | ||
| Lian et al, 2012 (25) | Co | 15 | 23 | 9 (39.1%) | ||
OLTx = Liver Transplant, PC = Proctocolectomy, Co = Colectomy, SR = Segmental large bowel resection, IPA = Ileal pouch-anal canal anastomosis, PBI = proctocolectomy with Brooke’s ileostomy,
= no control group in study
Figure 2.
Forest Plot-Effect of colectomy on liver transplantation of TIPSS in PSC patients
All-Cause Mortality Rates
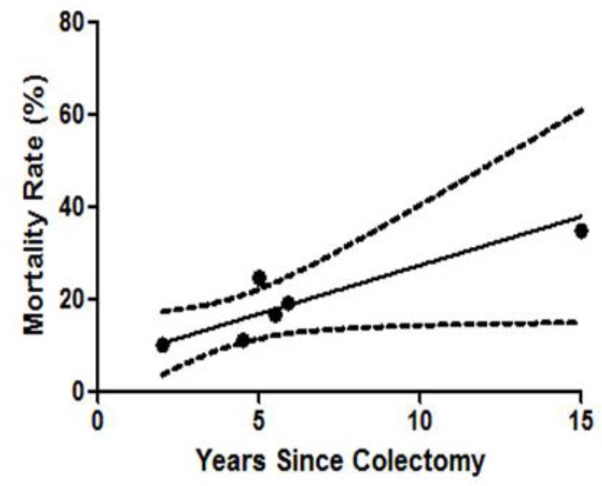
Seven studies were identified that reported all-cause mortality rates following colectomy (8, 23, 25-29) (Table 3). Mean-weighted linear regression analysis (Figure 3) demonstrated a 2.11% per year mortality risk (CI 0.03% to 4.18%, p=0.032 R2 = 0.722) for patients with PSC who have undergone colectomy.
Table 3.
Studies reporting mortality rates in PSC patients with colonic disease, with and without colectomy
| Study, Year | Type of Resection | Follow-up (years) | Sample size in study |
Mortality Rates |
||
|---|---|---|---|---|---|---|
| Colectomy, n |
No Colectomy, n | Colectomy, n (%) | No Colectomy, n (%) |
|||
| Cangemi et al, 1989 [8] | PC | 3 | 13 | 17 | 2 (15.3%) | 2 (11.8%) |
| Nordenvall et al, 2018 [23] | Co | 5.9 | 477 | 2092 | 83 (17.4%) | 426 (20.4%) |
| Post et al, 1994 [26] | PC, Co | 0.1 | 24 | 3 (12.5%) | ||
| Penna et al, 1996 [27] | IPA | 4.5 | 54 | 6 (11.1%) | ||
| Gorgun et al, 2005 [28] | IPA | 5 | 65 | 16 (24.6%) | ||
| Lian et al, 2012 [25] | Co | 15 | 23 | 8 (34.8%) | ||
| Treeprasertsuk et al, 2013 29] | Co | 5.5 | 78 | 13 (16.7%) | ||
PC = Proctocolectomy, Co = Colectomy, IPA = Ileal pouch-anal canal anastomosis,
= no control group in study
Figure 3.
Linear regression of mortality following colectomy; gradient 2.11, p= 0.032 R2=0.722
Two studies (8, 23) directly compared colectomy versus no colectomy group in PSC patients. One study (8) followed up 30 patients for 3 years and demonstrated similar mortality between the two groups, with rates of 15.3% and 11.8% respectively. The other study (23) identified, followed 2569 patients for a median time of 5.9 years, and also showed no difference in mortality rates for all time points of colectomy, reporting 17.4% versus 20.4% respectively.
Discussion
Our results suggest that cumulatively there is limited evidence in the current literature to demonstrate any beneficial effect of colectomy on the disease activity of PSC. Nordenvall et al (23) reported that colectomy before the diagnosis of PSC was associated with lower liver transplantation and death rates in IBD-PSC patients and no effect was observed if colectomy was performed after PSC was diagnosed. Though interesting, these findings contradict a study by Alabraba et al (14) which reported that colectomy before or during liver transplantation for PSC significantly reduced PSC recurrence and liver re-transplantation rates. However, both the numbers in these specific subgroups were sub-optimal. Taking into consideration that there is a lack of an established scientific mechanism through which these effects are achieved, an argument that these observations are due to chance alone could also be made.
We were able to calculate an estimated mortality rate, based on the included studies, for patients with PSC following colectomy at 2.11% per year. Putting this into context, mortality rates for all PSC patients reported in the literature ranges between 3.3-5.8% (35-39), and whilst our data may therefore suggest a lower mortality rate post-colectomy, the heterogeneity and low quality in the studies included precludes any definitive conclusions to be drawn. In considering the above, together with the lack of robust evidence, we believe colectomy should not be offered as a treatment option for severe PSC until better patient studies or scientific advances are able to demonstrate otherwise.
The main limitation of this systematic review was the lack of high-quality studies for meta-analyses in current literature. No randomized control trials were identified and only a small proportion of studies had a control group so that an effect size could be estimated. Another limitation was the high heterogeneity between studies, which was in part due to varying criteria of how disease progression was measured and defined within these studies. In this regard, it precluded meaningful quantitative analyses in this systematic review. Lastly, though the statistical analyses performed herein were robust, effects from small sample sizes in the meta-analyses and publication bias cannot be excluded completely. Nonetheless, the main aim of this systematic review was to study PSC activity after the interference of the lower gut-liver axis is achieved through a "surgical knock-out" of the colon in humans.
As animal models remain sub-optimal, observational studies such as case-control and association studies, although do not demonstrate causality, still provide important information and have contributed too much of our understanding of PSC (3). However, even in reviewing clinical studies of PSC in recent literature, it seems the role of the colon in the pathogenesis of PSC remains poorly understood and the chasm between basic sciences and clinical observations remain wide and poorly bridged. Invariably, this is reflected in the limited treatment options in clinical practice. It is also noteworthy that although well designed longitudinal studies could offer alternative means to identify causative microorganisms, the lack of a reliable biomarker in early disease, low prevalence of the disease, and poor accessibility of the biliary tree restrict the conduct of these studies (15).
Moving away from animal models and clinical observational studies, in vitro longitudinal and high throughput screens of the microbiome in the lower GI tract offers the possibility of identifying the pathogenic microorganisms in PSC as the cost of interrogating the microbiome becomes more affordable. Unfortunately, even if a pathogen is identified, the transition from mechanistic studies to identify a target for drug action and then to drug safety studies preclude the use of any pharmacological treatments in the near future. In considering novel and potential treatments for PSC that are on the horizon, faecal transplantation is perhaps the closest at being introduced into clinical practice. Fecal transplantation could potentially reverse gut dysbiosis in PSC and provide a means of controlling PSC progression where current drug treatments have failed. Interestingly, a clinical trial assessing the effects of faecal transplantation in patients with PSC is currently in progress (ClinicalTrials.gov, Identifier: NCT02424175) and the results of this study are eagerly awaited. Alternatively, advances in regenerative medicine (40,41) have made considerable progress in our understanding of cholangiocyte biology. In vivo studies and transplantation of lab-grown bile ducts are being undertaken in porcine models which could potentially be used in the treatment of large duct PSC (University of Cambridge, UK).
In summary, the colon as part of the lower gut-liver axis is likely to be involved in the pathogenesis of PSC but is unlikely to be the key factor driving the disease and higher-quality larger clinical trials are required. The pathogenic microbiome or antigens could potentially be identified in the midgut, since total colectomy has shown little effect on PSC activity. Though our understanding of the immune-mediated disease remains poor, rapid advances in regenerative medicine, high throughput screening of the gut microbiome, and research targeting the gut-liver axis are likely to make headway in developing new treatments for the disease.
Acknowledgment
JO is supported by the W D Armstrong fellowship at the University of Cambridge.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Volta U, Bonazzi C, Bianchi FB, Baldoni AM, Zoli M, Pisi E. IgA antibodies to dietary antigens in liver cirrhosis. Ric Clin Lab. 1987;17:235–42. doi: 10.1007/BF02912537. [DOI] [PubMed] [Google Scholar]
- 2.Vajro P, Paolella G, Fasano A. Microbiota And Gut-Liver Axis: A Mini-Review On Their Influences On Obesity And Obesity Related Liver Disease. J Pediatr Gastroenterol Nutr. 2013;56:461–8. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabi , et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J Hepatol. 2012;56:1181–8. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Olsson R, Hulten L. Concurrence of ulcerative colitis and chronic active hepatitis: clinical courses and results of colectomy. Stand J Gastroenterol. 1975;10:331–5. [PubMed] [Google Scholar]
- 6.Stauffer MH, Sauer WG, Dearing WH, Baggenstoss AH. The spectrum of cholestatic hepatic disease. JAMA. 1965;191:829–37. doi: 10.1001/jama.1965.03080100047011. [DOI] [PubMed] [Google Scholar]
- 7.Wood RA, Cuschieri A. Is sclerosing cholangitis complicating ulcerative colitis a reversible condition? Lancet. 1980;2:716–8. doi: 10.1016/s0140-6736(80)91935-2. [DOI] [PubMed] [Google Scholar]
- 8.Cangemi JR, Weisner RH, Beaver SJ, Ludwig J, MacCarty RL, Dozois RR, et al. Effect of proctocolectomy for chronic ulcerative colitis on the natural history of primary sclerosing cholangitis. Gastroenterology. 1989;96:790–4. [PubMed] [Google Scholar]
- 9.Goudet P, Dozois RR, Kelly KA, Ilstrup DM, Phillips SF. Characteristics and evolution of extraintestinal manifestations associated with ulcerative colitis after proctocolectomy. Dig Surg. 2001;18:51–5. doi: 10.1159/000050097. [DOI] [PubMed] [Google Scholar]
- 10.Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–72. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 11.Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hubscher SG, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9 gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–7. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karrar A, Broome U, Sodergren T, Jaksch M, Bergquist A, Bjornstedt M, et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504–14. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Katt J, Schwinge D, Schoknecht T, Quaas A, Sobotka I, Burandt E, et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 2013;58:1084–93. doi: 10.1002/hep.26447. [DOI] [PubMed] [Google Scholar]
- 14.Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–40. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 15.Fickert P, Pollheimer MJ, Beuers U, Lackner C, Hirschfield G, et al. Characterization of animal models for primary sclerosing cholangitis (PSC) J Hepatol. 2014;60:1290–303. doi: 10.1016/j.jhep.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Aitola PT, Mattila J, Halonen PJ, Matikainen MJ. The effect of colectomy on histological and biochemical liver changes associated with ulcerative colitis. Eur J Gastroenterol Hepatol. 1994;6:803. [Google Scholar]
- 18.Mikkola K, Kiviluoto T, Riihelä M, Taavitsainen M, Järvinen HJ. Liver involvement and its course in patients operated on for ulcerative colitis. Hepatogastroenterology. 1995;42:68–72. [PubMed] [Google Scholar]
- 19.Cho CS, Dayton MT, Thompson JS, Koltun WA, Heise CP, Harms BA. Proctocolectomy-ileal pouch-anal anastomosis for ulcerative colitis after liver transplantation for primary sclerosing cholangitis: a multi-institutional analysis. J. Gastrointest. Surg. 2008;12:1221–6. doi: 10.1007/s11605-008-0528-5. [DOI] [PubMed] [Google Scholar]
- 20.Lepistö A, Kivistö S, Kivisaari L, Arola J, Järvinen HJ. Primary sclerosing cholangitis: outcome of patients undergoing restorative proctocolecetomy for ulcerative colitis. Int J Colorectal Dis. 2009;24:1169–74. doi: 10.1007/s00384-009-0773-4. [DOI] [PubMed] [Google Scholar]
- 21.Poritz LS, Koltun WA. Surgical management of ulcerative colitis in the presence of primary sclerosing cholangitis. Dis Colon Rectum. 2003;46:173–8. doi: 10.1007/s10350-004-6520-6. [DOI] [PubMed] [Google Scholar]
- 22.Navaneethan U, Venkatesh PGK, Lashner BA, Shen B, Kiran RP. The impact of ulcerative colitis on the long-term outcome of patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;35:1045–53. doi: 10.1111/j.1365-2036.2012.05063.x. [DOI] [PubMed] [Google Scholar]
- 23.Nordenvall C, Olen O, Nilsson PJ, von Seth E, Ekbom A, Bottai M, et al. Colectomy prior to diagnosis of primary sclerosing cholangitis is associated with improved prognosis in a nationwide cohort study of 2594 PSC-IBD patients. Aliment Pharmacol Ther. 2018;4:238–45. doi: 10.1111/apt.14393. [DOI] [PubMed] [Google Scholar]
- 24.Mathis KL, Benavente-Chenhalls LA, Dozois EJ, Wolff BG, Larson DW. Short- and long-term surgical outcomes in patients undergoing proctocolectomy with ileal pouch-anal anastomosis in the setting of primary sclerosing cholangitis. Dis Colon Rectum. 2011;54:787–92. doi: 10.1007/DCR.0b013e318217eea7. [DOI] [PubMed] [Google Scholar]
- 25.Lian L, Menon , KVN , Shen B, Remzi F, Kiran RP. Inflammatory bowel disease complicated by primary sclerosing cholangitis and cirrhosis: is restorative proctocolectomy safe? Dis Colon Rectum. 2012;55:79–84. doi: 10.1097/DCR.0b013e3182315745. [DOI] [PubMed] [Google Scholar]
- 26.Post AB, Bozdech JM, Lavery I, Barnes DS. Colectomy in patients with inflammatory bowel disease and primary sclerosing cholangitis. Dis Colon Rectum. 1994;37:175–8. doi: 10.1007/BF02047543. [DOI] [PubMed] [Google Scholar]
- 27.Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schlek C, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38:234–9. doi: 10.1136/gut.38.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorgun E, Remzi FH, Manilich E, Preen M, Shen B, Fazio VW. Surgical outcome in patients with primary sclerosing cholangitis undergoing ileal pouch-anal anastomosis: a case-control study. Surgery. 2005;138:631–7. doi: 10.1016/j.surg.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Treeprasertsuk S, Björnsson E, Sinakos E, Weeding E, Lindor KD. Outcome of patients with primary sclerosing cholangitis and ulcerative colitis undergoing colectomy. World J Gastrointest Pharmacol Ther. 2013;4:61–8. doi: 10.4292/wjgpt.v4.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindberg J, Stenling R, Palmqvist R, Rutegård J. Early onset of ulcerative colitis: long-term follow-up with special reference to colorectal cancer and primary sclerosing cholangitis. J Pediatr Gastroenterol Nutr. 2008;46:534–8. doi: 10.1097/MPG.0b013e31815a98ef. [DOI] [PubMed] [Google Scholar]
- 31.Cooperman AM, Judd ES. The role of colectomy in hepatic disease accompanying ulcerative and granulomatous colitis Current status of a continuing problem. Mayo Clin Pro. 1972;47:36–8. [PubMed] [Google Scholar]
- 32.Eade MN, Cooke WT, Brooke BN. Liver disease in ulcerative colitis II The long-term effect of colectomy. Ann Intern Med. 1970;72:489–97. doi: 10.7326/0003-4819-72-4-489. [DOI] [PubMed] [Google Scholar]
- 33.Kartheuser AH, Dozois RR, LaRusso NF, Weisner RH, Ilstrup DM, Schlek CD. Comparison of surgical treatment of ulcerative colitis associated with primary sclerosing cholangitis: ileal pouch-anal anastomosis versus Brooke ileostomy. Mayo Clin Proc. 1996;71:74–756. doi: 10.4065/71.8.748. [DOI] [PubMed] [Google Scholar]
- 34.Dirks E, Singer MV. (Ulcerative colitis and primary sclerosing cholangitis-does proctocolectomy have a therapeutic effect?) Z Gastroenterol. 1990;28:222–4. [PubMed] [Google Scholar]
- 35.Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48:939–44. doi: 10.1016/j.jhep.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Broomé U, Olsson R, Loof L, Bodemar G, Hultcrantz R, Danielsson A, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–5. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingham JGC, Kochar N, Gravenor M B. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126:1929–30. doi: 10.1053/j.gastro.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 38.Ueda Y, Kaido T, Okajima H, Hata K, Anazawa T, Yoshizawa A, et al. Long-term Prognosis and Recurrence of Primary Sclerosing Cholangitis After Liver Transplantation: A Single-Center Experience. Transplant Direct. 2017;3:e334. doi: 10.1097/TXD.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tischendorf JJW, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107–14. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 40.Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845–52. doi: 10.1038/nbt.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23:954–63. doi: 10.1038/nm.4360. [DOI] [PubMed] [Google Scholar]