Abstract
Mitochondrial fatty acid β-oxidation (FAO) is a major catabolic process that degrades long-chain fatty acids. Recent reports reveal a broad role for FAO in cell fate control in endothelial cells, immune cells, and cancer cells. Concurrently, unique molecular pathways influenced by FAO have been identified that alter cell fate decisions.
Keywords: mitochondrion, metabolism, acetylation, endothelial cell, immune cell, cancer
Main text
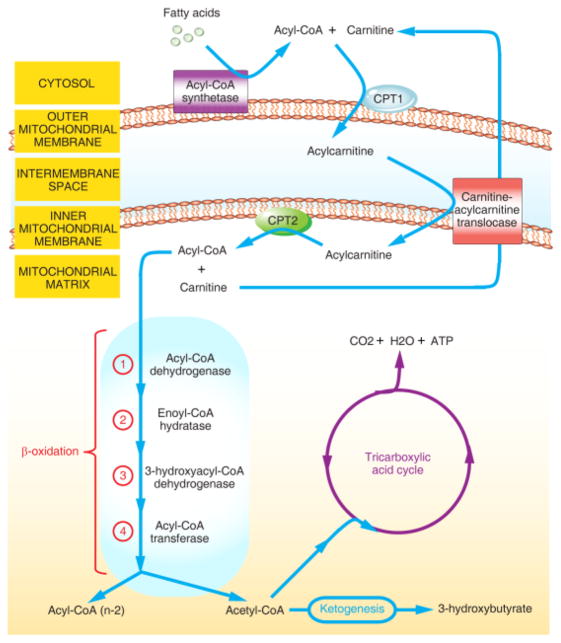
In 1904, the German chemist Georg Franz Knoop elucidated the key mechanisms that remove two carbon fragments from odd- and even-chain ω-phenyl fatty acids underlying fatty acid oxidation (FAO) [1]. Subsequently, many of the individual steps and at least 20 different enzymes or transporters in FAO have been further identified and successfully characterized. Notably, one crucial step in FAO is mitochondrial import of long-chain fatty acids from the cytosol into the mitochondrial matrix to generate ATP and acetyl-coenzyme A (acetyl-CoA). This process requires two successive enzymatic reactions that involve both the rate-limiting enzymes: carnitine palmitoyltransferase I (CPT1), located on the outer mitochondrial membrane, and CPT2, located on the inner mitochondrial membrane (Figure 1) [1].
Figure 1. Schematic Presentation of Fatty Acid Oxidation (FAO).
In the cytosol, long-chain fatty acids are first activated to form fatty acyl-coenzyme A (CoA) by acetyl-CoA synthetase enzymes. Second, carnitine palmitoyltransferase I (CPT1), located on the outer mitochondrial membrane, catalyzes the transesterification of the fatty acyl-CoA to acylcarnitine. Third, acylcarnitine transports into mitochondrial matrix via carnitine-acylcarnitine translocase. Forth, CPT2, located on the inner mitochondrial membrane, replaces carnitine with CoA. The above mentioned four steps are also known as carnitine shuttle. Fifth, acyl-CoA enters the β-oxidation spiral with acetyl-CoA as end products, which can be combusted in the tricarboxylic acid cycle or participate in ketogenesis. The stepwise degradation of acyl-CoA is catalyzed by four enzymes: (1) acetyl-CoA dehydrogenase, (2) enoyl-CoA hydratase, (3) 3-hydroxyacyl-CoA dehydrogenase and (4) acetyl-CoA transferase.
While FAO has long been regarded solely as an energy source, recent studies provide compelling evidence that establishes a link between FAO and cell fate determination. Although the regulation of cell determination has been studied in a variety of biological processes, its complexity and plasticity dictates further investigation to improve our understanding of its fundamental principles. In this ForumI article I summarize recent advances in metabolic FAO-related regulation of fate determination of representative cells including endothelial cells, immune cells, and cancer cells. Moreover, I speculate that maintaining a specific acetylation pattern of proteins by FAO, and hence by acetyl-CoA might represent one of the fundamental mechanisms underlying this regulation.
FAO in Endothelial Cell Fate Determination
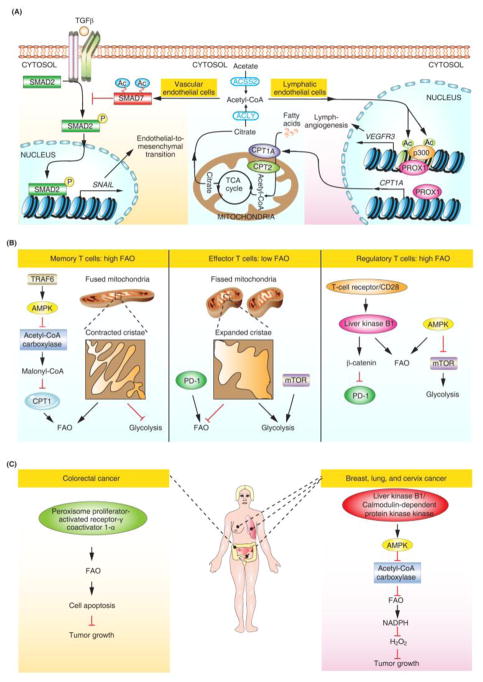
Endothelial cells form a single cell layer that lines the inside of blood vessels and lymphatics. Acquisition of mesenchymal characteristics by endothelial cells, also known as endothelial-to-mesenchymal-transition (EndoMT), occurs in normal cardiac development and pathologic conditions. During development, endocardial cells undergo EndoMT followed by migration into the endocardial cushion to form the heart valve mesenchyme [2]. Using mouse models containing an endothelium-specific deletion of CPT2, our group demonstrated that FAO is a pivotal regulator of EndoMT. FAO deficiency leads to augmented embryonic EndoMT, as evidenced by thickened cardiac valves. Furthermore, increased permeability resembling the pathological effects of EndoMT was observed in multiple vascular beds in these mice [2]. Importantly, our study, together with the work of Wong and colleagues, highlighted the role of acetyl-CoA as a central intermediate in FAO-associated protein acetylation [2, 3]. Mechanistically, FAO contributes to the acetylation and stability of SMAD7, a potent inhibitor of the transforming growth factor β (TGF-β)-induced EndoMT program (Figure 2A) [2]. Using conditional CPT1A knockout mice, Wong and colleagues showed a crucial role for FAO in lymphangiogenesis [3]. The regulatory axis involving transcription factor prospero homeobox 1 (PROX1), and the histone acetyltransferase p300 facilitates this process via upregulation of CPT1A expression and histone acetylation at lymphatic gene loci (Figure 2A) [3]. Together, these findings indicated a model in which FAO-regulated acetyl-CoA levels play an important role in protein acetylation in cell fate control in both vascular and lymphatic endothelium (Figure 2A).
Figure 2. Roles of FAO in Cell Fate Determination.
(A) FAO in endothelial cell fate determination. SNAIL is an EndoMT marker and VEGFR3 is a lymphatic gene. (B) FAO in immune cell fate determination. (C) FAO in cancer cell fate determination. Abbreviations: Ac, acetylation; Acetyl-CoA, acetyl coenzyme A; ACLY, ATP citrate lyase; ACSS2, acyl-coenzyme A synthetase short-chain family member 2; AMPK, AMP-activated kinase; CPT, carnitine palmitoyltransferase; FAO, fatty acid oxidation; mTOR, mechanistic target of rapamycin; P, phosphorylation; PD-1, programmed cell death 1; PROX1, prospero homeobox 1; TCA, tricarboxylic acid; TGF-β, transforming growth factor β; TRAF6, tumor necrosis factor receptor-associated factor 6.
FAO in Immune Cell Fate Determination
The recent interest of FAO in T cells was ignited by the discovery that FAO acts as a downstream mediator of the adaptor protein tumor necrosis factor receptor-associated factor 6 (TRAF6) for antigen stimulation [4]. Moreover, metformin-stimulated FAO promotes the generation of CD8+ memory T cells in the absence of TRAF6, and AMP-activated kinase (AMPK) activity underlies this TRAF6-mediated FAO (Figure 2B) [4]. Furthermore, a study of mitochondrial dynamics lends support to the idea that FAO is a pivotal regulator of memory T cell fate [5]. Mitochondrial cristae remodeling via fusion directs FAO adaptation in the establishment of memory T cells (Figure 2B) [5]. In contrast to memory T cells, effector T cell differentiation relies more on glycolysis, the metabolic pathway that converts glucose into pyruvate (Figure 2B) [6]. Indeed, cristae expansion via fission promotes glycolysis in activated effector T cells [5]. In addition to T memory cells and T effector cells, FAO-based metabolic programming occurs in regulatory T cells [7]. Independently of conventional AMPK signaling, liver kinase B1 (LKB1) programs the metabolic fitness of regulatory T cells by orchestrating FAO, and this metabolic fitness with appropriate FAO is required for preventing undesired immune responses in the control of immunological self-tolerance and homeostasis [7]. Intriguingly, LKB1 also contributes to the activation of β-catenin signaling for inhibition of the expression of the immune checkpoint molecule PD-1 (programmed cell death 1) (Figure 2B) [7]. PD-1 has also been implicated in the blockade of T-effector cell differentiation by enhancing FAO (Figure 2B) [8]. These two seminal studies suggest that manipulation of FAO in specific subsets of T cells may influence the efficacy of PD-1-based immunotherapy for cancer and autoimmune disease.
Similarly to the distinct metabolic phenotypes in regulatory and effector T cells [6], it seems that macrophage polarization into classically activated (M1) macrophages relies on glycolysis, while the alternatively activated (M2) macrophages require FAO [9]. Although these results have coupled immune cell metabolism with specific functional programs at a given point in space and time, much of evidence regarding FAO is based on biochemical and pharmaceutical approaches [9]. Our group and others have now begun to employ genetic models with conditional FAO deficiency to delineate the complexities of FAO in immune cell fate determination. Interestingly, genetic approaches using mice with myeloid linage-specific deletion of Cpt2 indicate that FAO is dispensable for the M2 program [9], suggesting that metabolic shift as a determining factor of cell fate seems to be more complex than was previously envisaged.
FAO in Cancer Cell Fate Determination
Opposing effects of FAO in cancer cell fate determination have been observed [10,11]. An increase in FAO was shown to contribute to peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α)-associated induction of colorectal cancer cell apoptosis and inhibition of tumorigenesis (Figure 2C) [10]. Contrary to its tumor-suppressive activity, increased FAO was found to promote cancer cell survival in conditions of energy stress [11]. This oncogenic FAO contributes to the LKB1/AMPK-regulated NADPH homeostasis in a set of cancer cell types derived from breast, lung, and cervix (Figure 2C) [11]. Collectively, these results emphasize the importance of the context-dependent nature of FAO in the fate control of cancer cells. However, there is no clear explanation for the opposing effects of FAO in different cancer cell types. Therefore, the potential beneficial effects of FAO on different types of cancer cells should be considered in appropriate conditions for future therapeutic application. For example, the term ‘triple-negative breast cancer’ was coined to describe breast cancer cells that lack expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2). Patients with triple-negative breast cancers generally have a high expression of the oncogene MYC and an increase in FAO [12]. Because common hormone therapy and drugs targeted to HER2 are ineffective for triple-negative breast cancers, pharmacologic inhibition of FAO holds great promise as a new therapeutic strategy for this subset of patients[12].
Concluding Remarks
This Forum article highlights recent genetic and functional data, which are beginning to provide evidence that FAO determines cell fate in a growing number of physiological and pathological conditions. Based on recent studies [2, 3], I propose a model detailing how FAO affects acetyl-CoA levels and protein post-translational modifications in cell fate decisions (Figure 2A). Current results also suggest that therapeutic manipulation of FAO holds great promise for the diagnosis and treatment of a wide range of human diseases in clinical settings.
Acknowledgments
The author sincerely apologizes to colleagues whose work could not be included due to space limitation. The author is grateful for the support from the National Institutes of Health (NIH) Intramural Program and the Leducq Foundation. The author thanks the NIH Fellows Editorial Board and Douglas Joubert, NIH Library Writing Center, for manuscript editing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Houten SM, et al. The Biochemistry and Physiology of Mitochondrial Fatty Acid beta-Oxidation and Its Genetic Disorders. Annu Rev Physiol. 2016;78:23–44. doi: 10.1146/annurev-physiol-021115-105045. [DOI] [PubMed] [Google Scholar]
- 2.Xiong J, et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol Cell. 2018;69(4):689–698. e7. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong BW, et al. The role of fatty acid beta-oxidation in lymphangiogenesis. Nature. 2017;542(7639):49–54. doi: 10.1038/nature21028. [DOI] [PubMed] [Google Scholar]
- 4.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck MD, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K, et al. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature. 2017;548(7669):602–606. doi: 10.1038/nature23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura M, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17(3):216–7. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Errico I, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci U S A. 2011;108(16):6603–8. doi: 10.1073/pnas.1016354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon SM, et al. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–5. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camarda R, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22(4):427–32. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]