Abstract
Oxytocin (OT) elicits weight loss in diet-induced obese (DIO) rodents, nonhuman primates, and humans, in part, by reducing food intake. Chronic OT administration produces more sustained weight loss in high fat diet (HFD)-fed DIO rodents relative to chow-fed controls, but the reasons for this effect remain unclear. We hypothesized that HFD-induced obesity is associated with elevated OT receptor (OXTR) binding in brain regions where OT is known to cause decreased food intake and that this sensitized neural system is one mechanism by which OT preferentially elicits weight loss in DIO rodents. We therefore determined the impact of diet (HFD vs chow) and drug treatment (chronic OT infusion vs vehicle) on 1) OXTR binding in hindbrain and forebrain sites where OT suppresses food intake relative to control sites that express OXTR and 2) forebrain vasopressin 1a receptor (AVPR1a) density to evaluate the specificity of any OT effects. Using quantitative receptor autoradiography, we found that 1) diet composition failed to alter OXTR or AVPR1a binding; 2) chronic OT treatment produced largely global reductions in forebrain OXTR and AVPR1a binding without significantly altering hindbrain OXTR binding. These findings suggest that forebrain OXTR and AVPR1a are down-regulated in response to chronic OT treatment. Given that chronic intranasal OT may be used as a therapeutic strategy to treat obesity, future studies should consider the potential downregulatory effect that chronic treatment can have across forebrain and hindbrain nonapeptide receptors and assess the potential contribution of both receptor subtypes to the outcome measures.
Keywords: Obesity, oxytocin, food intake, receptor autoradiography
Introduction
The neurohypophyseal hormone oxytocin (OT) is synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus and is well-recognized for its peripheral effects to stimulate uterine contraction during parturition and milk ejection during lactation. Existing studies have extended this knowledge by providing evidence that OT also plays an important role in the central nervous system (CNS) in promoting prosocial behaviors (Striepens et al., 2011, Yamasue et al., 2012) and in the regulation of energy balance (Blevins and Baskin, 2015, Lawson, 2017, Leng and Sabatier, 2017, Leslie et al., 2018, Plessow et al., 2018, Spetter et al., 2018). We and others have demonstrated that OT is effective in reducing weight gain and/or evoking weight loss in both diet-induced obese (DIO) and genetically obese rodent models with impaired (Deblon et al., 2011, Maejima et al., 2011, Zhang et al., 2011, Zhang and Cai, 2011, Morton et al., 2012, Maejima et al., 2017, Roberts et al., 2017) or defective leptin signaling (Morton et al., 2012, Altirriba et al., 2014, Plante et al., 2015). OT dose-dependently reduces food intake, whether administered systemically, directly into the CNS, or intranasally (Blevins and Baskin, 2015). This effect is observed in rodents fed a standard chow or low fat diet (Rinaman and Rothe, 2002, Maejima et al., 2011, Zhang et al., 2011, Morton et al., 2012, Ho et al., 2014, Noble et al., 2014, Ong et al., 2015, Blevins et al., 2016, Roberts et al., 2017, Klockars et al., 2018), sucrose (Mullis et al., 2013, Herisson et al., 2016), and high fat diet (HFD) (Deblon et al., 2011, Maejima et al., 2011, Zhang et al., 2011, Zhang and Cai, 2011, Morton et al., 2012, Blevins et al., 2016, Maejima et al., 2017). For these reasons, OT has attracted widespread interest as a therapeutic strategy to treat obesity, given that these effects have been recently translated to DIO nonhuman primates (Blevins et al., 2015) and obese humans (Zhang et al., 2013, Lawson et al., 2015, Thienel et al., 2016, Hsu et al., 2017).
Chronic OT administration (either infusions or repeated administration) elicits sustained weight loss in HFD-fed mice and rats (Deblon et al., 2011, Maejima et al., 2011, Zhang et al., 2011, Blevins et al., 2016, Maejima et al., 2017, Roberts et al., 2017), but it tends to be ineffective or produce only transient effects on body weight in chow-fed or low fat diet-fed rodents (Deblon et al., 2011, Maejima et al., 2011, Zhang et al., 2011, Blevins et al., 2016, Maejima et al., 2017, Roberts et al., 2017). These effects on weight loss are mainly attributed to reductions in food intake (Maejima et al., 2011, Blevins et al., 2016, Roberts et al., 2017). The presence of fat (Deblon et al., 2011, Maejima et al., 2011, Zhang et al., 2011, Blevins et al., 2016, Maejima et al., 2017, Roberts et al., 2017) and carbohydrates (i.e. sugar) (Miedlar et al., 2007, Sclafani et al., 2007, Olszewski et al., 2010, Mullis et al., 2013, Herisson et al., 2014, Blevins et al., 2015, Olszewski et al., 2015, Cherepanov et al., 2017) in the HFD may contribute to the effectiveness of chronic OT to elicit lasting suppression of food intake in HFD subjects, but differences in cholesterol levels between diets have also been hypothesized to play a role. Gimpl (Gimpl and Fahrenholz, 2001) has reported that cholesterol in the diet may enhance the affinity state of the OXTR, which may partly explain the increased effectiveness of chronic OT in HFD-fed rodents relative to chow-fed rodents in reducing food intake and eliciting weight loss. Based on these collective findings, we tested the hypothesis that HFD-induced obesity is associated with elevated OXTR binding in brain regions where OT reduces food intake: the arcuate nucleus (ARC (Maejima et al., 2014)), the ventromedial hypothalamus (VMH (Noble et al., 2014, Klockars et al., 2017)), and the nucleus of the solitary tract (NTS (Ong et al., 2015)). This sensitized neural OT system could be one mechanism by which OT could have enhanced effectiveness to elicit weight loss in DIO rodents.
Existing studies indicate that OT may interact with the vasopressin 1a receptors (AVPR1a) as OT is reported to have only a 10-fold selectivity for OXTR relative to vasopressin 1a receptor (AVPR1a) (Kimura et al., 1994, Postina et al., 1996, Gimpl and Fahrenholz, 2001). This is likely due to the structural similarity between OT and AVP, which differ by only two amino acids (Gimpl and Fahrenholz, 2001), and between the mammalian OXTR and AVPR1a, which share approximately 50% sequence homology (Gimpl and Fahrenholz, 2001). Previous studies from rodent models (including mice, rats, hamsters and prairie voles) suggest that acute or chronic OT administration can act through AVPR1a to elicit measured effects on neural activation, behavior and physiology (Yamamoto et al., 2004, Bales et al., 2007, Schorscher-Petcu et al., 2010, Sala et al., 2011, Song et al., 2014, Flicks et al., 2016, Everett et al., 2018, Guoynes et al., 2018). Thus, an important question is whether chronic infusions of OT (at doses that elicit weight loss) may also impact AVPR1a density in brain areas that contribute to the effects of OT to reduce food intake and whether these effects occur in a diet-specific manner. We therefore determined the impact of HFD and chronic OT treatment on OXTR binding in areas where OT reduces food intake relative to control areas that express OXTR, as well as AVPR1a binding in several forebrain areas to evaluate the specificity of action of OT.
Experimental Procedures
Materials and Methods
Animals
Adult male outbred Sprague-Dawley (CD® IGS) (≈ 6–7 months/490–887 g) were obtained from Charles River Laboratories International (Wilmington, MA). All rats in this study were maintained on a HFD or chow diet and received a 21-day chronic third ventricular (3V) infusions of OT (16 nmol/day) or saline vehicle. These rats are a subset of those used in a previously published metabolic study (Blevins et al., 2016). All rats were housed individually in Plexiglas cages in a temperature controlled room (22±2°C) under a 12:12-h light-dark cycle (lights off at 1 p.m.). Rats had ad libitum access to water and either a HFD containing 60% kcal from fat (Research Diets, D12492, New Brunswick, NJ) or a low fat chow diet containing 13% kcal from fat (LabDiet, St. Louis, MO). The current research protocols were approved both by the Institutional Animal Care and Use Committee of the Veterans Affairs Puget Sound Health Care System (VAPSHCS) and the University of Washington in accordance with NIH Guidelines for the Care and Use of Animals.
Brain removal and slicing
HFD-fed or chow-fed control rats were euthanized with an overdose of ketamine hydrochloride (214.3 mg/kg), xylazine (10.71 mg/kg) and acepromazine (2.2 mg/kg) (IP) following a 21-day infusion of OT or saline vehicle as previously described (Blevins et al., 2016). Brains were immediately removed, flash frozen in isopentane and stored frozen at −80°C. They were then sliced on a cryostat at 20 μm and mounted onto Superfrost slides (Fisherbrand, Fisher Scientific, PA). All slides were stored at −80°C in sealed slide boxes with desiccants until the time of assay.
Receptor autoradiography
Receptor autoradiography for OXTR and AVPR1a receptor was performed as described previously (Perkeybile et al., 2015). Slides were removed from −80°C and allowed to thaw at room temperature. Next, they were lightly fixed in 0.1% paraformaldehyde (pH 7.4), washed twice in 50 mM Tris base (pH 7.4; to remove endogenous hormones), and incubated for 1 hr in tracer buffer (pH 7.4) containing 50 pM 125l- ornithine vasotocin analog (125l-OVTA) or 125l- linear vasopressin antagonist (125I-LVA) to target OXTR and AVPR1a, respectively (radioligands from PerkinElmer, Waltham, MA). Finally, unbound radioligand was removed after slides were rinsed in several washes of 50 mM Tris base with 10 mM MgCl2 (pH 7.4) and dipped in deionized water before being allowed to air dry. Once dried, the slides were exposed to Carestream BioMax MR film (Kodak, Rochester, NY) for 3 days and then developed and analyzed.
Quantification
A rat brain atlas (Paxinos and Watson, 2007) was used to determine the anatomical boundaries of the following regions of interest (ROI): ARC, VMH, central nucleus of the amygdala (CeA), lateral septum (LS), subiculum (Sub), area postrema (AP), dorsal motor nucleus of the vagus (DMV), and NTS. Our analysis focused on ROIs where OT is known to act to affect food intake: ARC, VMH, and NTS. In order to evaluate the specificity of any measurable effects, we also included in our analysis a few forebrain ROIs which have dense receptor binding in rats but have not been linked to OT’s effects on food intake: the CeA and Sub (for OXTR) and the CeA and LS (for AVPR1a). In the hindbrain, these control regions included the AP and DMV.
The 125l-OVTA and 125I-LVA binding was quantified in the following manner, which has been described previously (Freeman et al., 2017). For the forebrain study, the optical binding density (OBD) was quantified directly from the film using a light box, a top mounted camera, and the MCID Core Digital Densitometry system (Cambridge, UK). For the hindbrain study, which was performed prior to our acquisition of the MCID system, we used ImageJ (NIH; Bethesda, MD, USA) to measure OBD from digitized images of the films. These films were scanned and digitized using an Epson Perfection V500 Photo scanner, and the resulting autoradiogram image files were imported into ImageJ 64 for analysis. In both studies, quantification was carried out in the same way: OBD values from a set of 125l autoradiography standards (American Radiolabeled Chemicals, Inc., St. Louis, MO, USA) were loaded into the software and used to generate a standard curve, from which OBD values for each ROI were extrapolated. For each specimen, average OBD values were calculated for each ROI, as well as for one background area where no binding was detected, which served as a measure of non-specific binding. This average background/non-specific binding was subtracted from the ROI measurements to yield normalized OBDs across specimens and correct for individual variation in non-specific binding across individuals of this outbred strain of rats.
Data Analysis
In order to control for the multiple brain regions examined, we divided our analyses into three groups: OXTR forebrain binding, OXTR hindbrain binding, and AVPR1a forebrain binding. We then performed multivariate analysis of variance (MANOVA) on each group. Analysis was performed in SAS 9.4 (SAS Institute, Cary, NC), using proc glm. Assumptions of analysis of variance were tested. A significant result in the multivariate analysis was followed by separate ANOVAs for each brain area in that analysis. Alpha was set at p < 0.05 and all tests were two-tailed.
Results
Effects on OXTR density in the hindbrain
We conducted this study to examine the extent to which diet (HFD vs chow) and chronic drug treatment (OT vs vehicle) may impact OXTR binding in a hindbrain area (NTS) known to express OTR (Loup et al., 1989, Verbalis et al., 1995, Gould and Zingg, 2003, Yoshida et al., 2009) and that contributes to the effects of OT on food intake relative to control sites (AP, DMV) that express OXTR (Vaccari et al., 1998, Yoshida et al., 2009) but have not been directly linked to the central effects of OT to reduce food intake (AP, DMV). OT treatment did not significantly predict OXTR binding in the hindbrain (Wilks’ lambda = 0.547, F3,9 = 2.49, p = 0.127; Figure 1). There was no effect of diet (Wilks’ lambda = 0.604, F3,9 =1.97, p = 0.190), and no diet by drug interaction (Wilks’ lambda = 0.833, F3,9 = 0.60, p = 0.631). Due to the overall lack of drug effect, we do not claim significance for individual hindbrain areas. However, it is worth noting that had they been examined alone, OT would have reduced OXTR binding in both the DMV (F1 =8.11, p = 0.016) and the NTS (F1 = 5.52, p = 0.039).
Figure 1. Optical binding density of OXTR from the hindbrain following chronic 3V OT and vehicle infusions in rats that were fed a high fat diet (HFD) or chow.
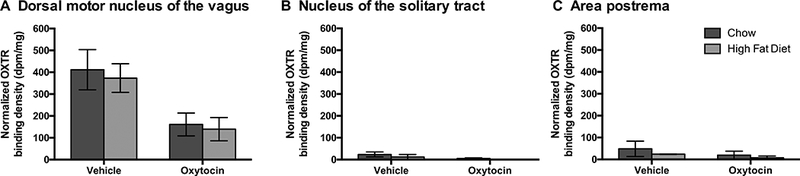
Data are expressed as mean ± SEM (n=2–6 rats per group). *P<0.05 HFD vs. chow.
Effects on OXTR density in the forebrain
We conducted this study to examine the extent to which diet (HFD vs chow) and drug treatment (OT vs vehicle) may impact OXTR binding in forebrain areas that express OXTR (Vaccari et al., 1998, Yoshida et al., 2009, Hidema et al., 2016), are linked to the control of food intake (ARC, VMH, CeA) and potentially contribute to the metabolic effects of OT in HFD-fed rats as has been reported previously in chow-fed rats and mice (Maejima et al., 2014, Noble et al., 2014, Klockars et al., 2017, Klockars et al., 2018). To determine whether the neural effects were specific only to brain areas that are known to contribute to the effects of OT on food intake, we also included the Sub, given that this site expresses OXTR but has not been linked to the central effects of OT to reduce food intake. We found that OT treatment significantly down-regulated OXTR binding in the forebrain (Wilks’ lambda = 0.340, F4,20 = 9.69, p < 0.001; Figures 2–3). There was no effect of diet (Wilks’ lambda = 0.839, F4,20 = 0.96, p = 0.453), and no diet by drug interaction (Wilks’ lambda = 0.792, F4,20 = 1.31, p = 0.299). OXTR binding was significantly affected by treatment in the ARC (r2 = 0.566, F1 = 29.00, p < 0.0001), the CeA (r2 = 0.501, F1 = 18.74, p < 0.001), the VMH (r2 = 0.593, F1 = 32.34, p < 0.0001), and Sub (r2 = 0.587, F1 = 26.93, p < 0.0001).
Figure 2. Optical binding density of OXTR binding from the forebrain following chronic 3V OT and vehicle infusions in rats that were fed a high fat diet (HFD) or chow.
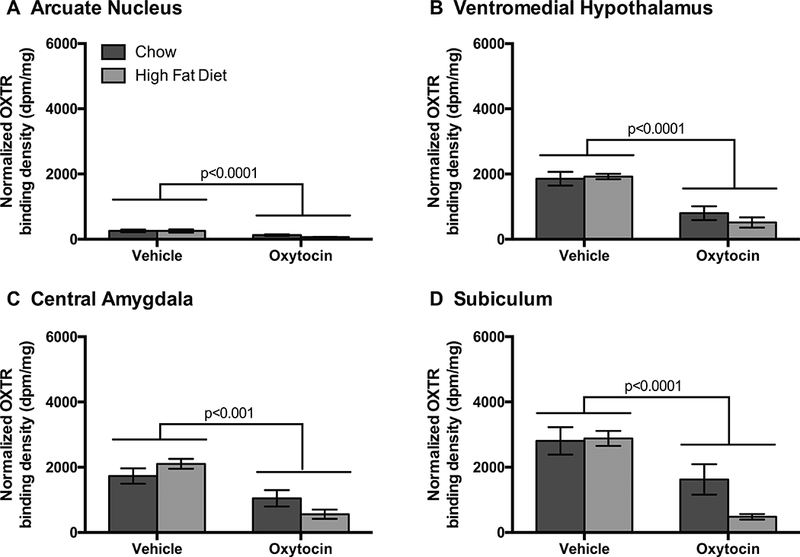
Data are expressed as mean ± SEM (n=6–9 rats per group). *P<0.05 HFD vs. chow.
Figure 3. Representative autoradiograms of forebrain OXTR binding in saline (A,C,E) and OT (B,D,F) treated rats.
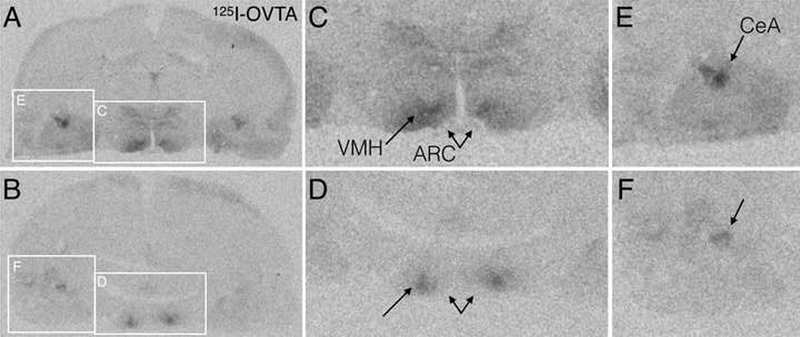
A,B. Full coronal sections at the level of the ventromedial hypothalamus (VMH), arcuate nucleus (ARC), and central amygdala (CeA), with white boxes indicating the zoomed in regions in panels C-F. C,D. Digitally zoomed images of the hypothalamus showing reduced OXTR binding after OT treatment (D) compared to saline (C). E,F. Digitally zoomed images of the amygdala showing reduced OXTR binding after OT treatment (F) compared to saline (D).
Effects on AVPR1a density in the forebrain
In addition, we examined the structurally similar AVPR1a in forebrain sites with previously reported AVP binding (Dorsa et al., 1984, Szot et al., 1990) and AVP1a mRNA expression (Ostrowski et al., 1992, Ostrowski et al., 1994), in order to evaluate the specificity of any OT effects, given the precedent for cross-talk between these two systems (Yamamoto et al., 2004, Bales et al., 2007, Gupta et al., 2008, Schorscher-Petcu et al., 2010, Sala et al., 2011, Song et al., 2014, Guoynes et al., 2018). To determine whether the neural effects were specific to forebrain areas that contribute to the central effects of OT on food intake (ARC, VMH, CeA) (Maejima et al., 2014, Noble et al., 2014, Klockars et al., 2017, Klockars et al., 2018), we also included the lateral septum (LS) because it expresses AVPR binding sites (Baskin et al., 1983) [including AVPR1a (Grundwald et al., 2016)] in rats but has not been linked to the central effects of OT to reduce food intake. OT treatment also significantly down-regulated AVPR1a receptor binding in the forebrain (Wilks’ lambda = 0.516, F4,25 = 5.87, p = 0.002; Figures 4–5). There was no effect of diet (Wilks’ lambda = 0.889, F4,25 = 0.78, p = 0.548), and no diet by drug interaction (Wilks’ lambda = 0.943, F4,25 = 0.38, p = 0.0823). AVPR1a was significantly affected by treatment in the ARC (r2 = 0.293, F1 = 10.44, p = 0.003), the VMFI (r2 = 0.279, F1 = 10.50, p = 0.003), and the LS (r2 = 0.326, F1 = 11.24, p = 0.002). However, OT treatment did not affect AVPR1a binding in the CeA (r2 = 0.072, F1 = 0.75, p = 0.393).
Figure 4. Optical binding density of AVPR1a binding from the forebrain following chronic 3V OT infusions in rats that were fed a high fat diet (HFD) or chow.
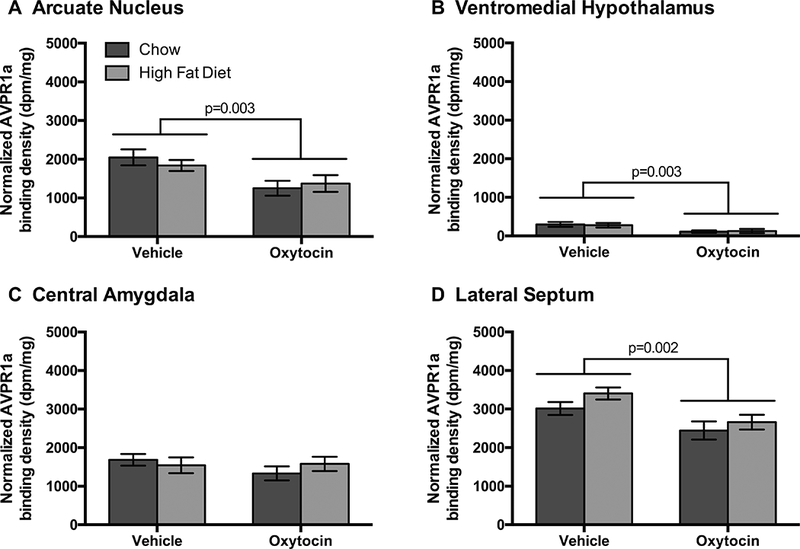
Data are expressed as mean ± SEM (n=7–9 rats per group). *P<0.05 HFD vs. chow.
Figure 5. Representative autoradiograms of forebrain AVPR1a binding in saline (A,C,E) and OT (B,D,F) treated rats.
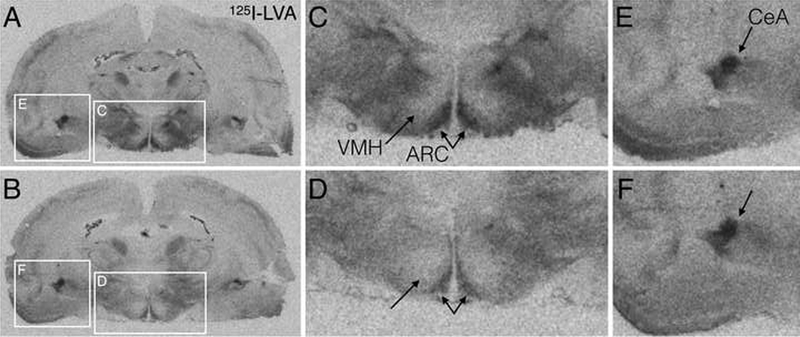
A,B. Full coronal sections at the level of the ventromedial hypothalamus (VMH), arcuate nucleus (ARC), and central amygdala (CeA), with white boxes indicating the zoomed in regions in panels C-F. C,D. Digitally zoomed images of the hypothalamus showing reduced AVPR1a binding after OT treatment (D) compared to saline (C). E,F. Digitally zoomed images of the amygdala showing no change in AVPR1a binding between saline treated (E) and OT treated (F) rats.
Discussion
Chronic OT administration produces more sustained effects to evoke weight loss in HFD-fed DIO rodents relative to chow-fed controls (Deblon et al., 2011, Maejima et al., 2011, Zhang et al., 2011, Blevins et al., 2016, Maejima et al., 2017, Roberts et al., 2017), but the reasons for this effect remain unclear. We hypothesized that HFD-induced obesity is associated with elevated OXTR binding in brain regions where OT has been shown to be effective in reducing food intake (ARC, VMH, CeA, NTS) and that this sensitized neural OT system is one mechanism by which OT could have enhanced effectiveness to elicit weight loss in DIO rodents. To test this hypothesis, we determined the impact of HFD diet and chronic OT treatment on OXTR binding in hindbrain (NTS) and forebrain (ARC, CeA, VMH) sites where OT suppresses food intake, relative to control sites that express OXTR (AP, DMV, Sub) but have not been linked to the central effects of OT on food intake. Additionally, we also measured forebrain vasopressin 1a receptor (AVPR1a) density in the ARC, CeA and VMH relative to the LS to evaluate the specificity of any OT effects.
We found that diet composition failed to alter OXTR or AVPR1a density and that chronic OT treatment down-regulated OXTR density in forebrain. In addition, chronic OT treatment down-regulated forebrain AVPR1a density with the exception of the CeA. Collectively, these findings suggest that both forebrain OXTR and AVPR1a are down-regulated in response to chronic OT treatment. Given that chronic intranasal OT may be used as a therapeutic strategy, future studies should assess the extent to which both receptor subtypes may contribute to outcome measures. Future plans for the use of chronic OT treatment in humans or animal studies also be aware of the the potential downregulatory effect that this course of treatment can have across forebrain nonapeptide receptors.
Our findings indicate that chronic OT treatment was not associated with a significant downregulation of hindbrain OXTR in both HFD-fed or chow-fed rats, although it is worth noting that OT would have reduced OXTR binding in DMV and the NTS had the ROIs been examined alone. Moreover, there was also no significant impact of diet on OXTR binding within the hindbrain. We acknowledge the possibility that there may have been a floor effect that prevented the detection of OXTR downregulation. It will be important in future studies to address this possibility with a larger sample size and to assess the temporal effects of chronic OT on hindbrain OXTR binding and whether OXTR downregulation coincides with OT’s waning effectiveness to reduce food intake in HFD-fed rats.
OXTRs in NTS are anatomically positioned to control energy balance by reducing food intake and stimulating energy expenditure by increasing brown adipose tissue (BAT) thermogenesis. The NTS is a CNS site with sympathetic outflow to BAT and is linked to the control of BAT thermogenesis (Bamshad et al., 1999, Li et al., 2007, Morrison and Nakamura, 2011). Recent studies have identified an anatomical circuit comprised of projections from the parvocellular PVN (pPVN) to the NTS (Kong et al., 2012) that then project to the raphe pallidus (RPa), a brain area known to regulate sympathetic control of BAT thermogenesis via projections to spinal cord sympathetic pre-ganglionic neurons (Morrison and Nakamura, 2011, Morrison, 2016). Existing studies have extended these anatomical findings to provide more direct support for a role of endogenous OT action at hindbrain NTS OXTRs in the control of food intake (Blouet et al., 2009, Baskin et al., 2010, Matarazzo et al., 2012, Ong et al., 2015) and thermogenesis (Ong et al., 2017) in rats and mice. Furthermore, acute 4V (Ho et al., 2014, Ong et al., 2015) or NTS (Ong et al., 2015) administration of OT reduces food intake and stimulates BAT temperature (Roberts et al., 2017) as well as core temperature (Ong et al., 2017). In addition, our recent studies show chronic fourth ventricular (4V) OT is associated with prolonged weight loss that is mediated, in part, by reductions in food intake (Roberts et al., 2017). OXTRs in both the NTS and DMV may contribute to the effects of OT to reduce food intake, in part, by inhibiting gastric motility or gastric emptying (Rogers and Hermann, 1987, McCann and Rogers, 1990, Flanagan et al., 1992, Cruz et al., 2007, Holmes et al., 2013). Future studies that assess the effects of OT to reduce food intake in rats with gastric cannulas that remain open (sham feeding; no gastric distension) or closed (real feeding) would help identify whether decreases in gastric emptying are required for OT to reduce food intake.
Two key questions remain to better understand the role of specific populations of OXTR within the brain in contributing to the prolonged effects of chronic CNS administration of OT on weight loss:1) at what point does the OXTR downregulation occur following continuous OT infusion and 2) does this coincide with the inability of chronic CNS OT to produce sustained reductions in food intake that extend beyond 2–3 weeks? The extent to which downregulation of OXTRs in forebrain, midbrain and hindbrain areas contribute to the inability of chronic CNS OT to elicit long-term reductions of food intake await further investigation (Leslie et al., 2018).
Another mechanism that may contribute to the more prolonged effectiveness of OT to evoke weight loss in DIO animals maintained on a HFD includes an enhanced affinity state of OXTR, due to cholesterol in the HFD. OXTRs are found in either a high or low affinity state, and cholesterol is required for high-affinity binding of OT (Gimpl and Fahrenholz, 2001). The presence of cholesterol in media that contains solubilized OXTRs can potentially enhance high-affinity OT binding and stabilize the OXTR in a high affinity state (Gimpl et al., 1995). Gimpl indicated that “cholesterol acts as an allosteric modulator and stabilizes the receptor in a high-affinity state for agonists and antagonists”. Given that the HFDs used across many studies (60% kcal from fat, D12492) may contain up to 279.6 to 300.8 mg/kg cholesterol (D12492 from 2006 or 2011 formulations) relative to 200 mg/kg in chow diet (13% kcal from fat; 5001) the potential role of cholesterol levels is a possible complicating factor in interpreting the results of studies on the impact of OT on HFD-fed animals. However, with the exception of the CeA, we did not find evidence of enhanced binding in the CNS in vehicle-treated DIO rats relative to chow-fed controls. Future studies that examine the role of cholesterol on both OXTR binding and OXTR mRNA and protein expression in DIO rodents maintained on a HFD relative to chow-fed controls will be helpful in identifying whether enhanced OXTR binding may contribute to the more prolonged effectiveness of OT in DIO animals.
Although the majority of existing studies provide data to support that cholesterol may certainly contribute to the enhanced effectiveness of OT in DIO rodent models it does not appear to be required as others have demonstrated a more robust effect of OT to elicit weight loss following chronic subcutaneous administration in mice on a cholesterol-free HFD relative to a low fat diet (Maejima et al., 2011, Maejima et al., 2017). Maejima and colleagues further demonstrated that OT-elicited weight loss was negatively correlated with initial body weight in mice maintained on the HFD such that OT was more effective in those mice on the HFD that were more obese. In contrast, there was no correlation between OT-elicited weight loss and initial body weight in mice maintained on the low fat diet. While we did not directly measure changes in CNS OXTR mRNA or OXTR protein expression in this study, others have found that 7-week exposure to a HFD in a genetic model of obesity is associated with increased OXTR mRNA in peripheral tissues, including adipose tissue (Gajdosechova et al., 2014, Yi et al., 2015) and skeletal muscle (Gajdosechova et al., 2014). This may be a response to the reduction of circulating OT that is observed in certain genetic models of obesity (Gajdosechova et al., 2014, Plante et al., 2015). Given that others have demonstrated a direct action of OT on adipocytes (Deblon et al., 2011, Yi et al., 2015), this may also explain, in part, the more robust effectiveness of chronic systemic OT to evoke weight loss in DIO mice. These findings are also consistent with those that have found reductions in serum OT in DIO mice (Zhang et al., 2011, Zhang and Cai, 2011) although this finding is not always evident in DIO mice (Maejima et al., 2017) and rats (Morton et al., 2012) for reasons that may be related to length of exposure to HFD or time when measurements occurred. It will be important to examine the extent to which OXTRs on adipocytes are necessary for systemic OT to elicit weight loss in DIO rodents in future studies.
Our findings that show a largely global pattern of downregulation of CNS OXTRs in response to chronic OT infusion are largely consistent with what others have shown in the CNS following chronic intracerebroventricular (ICV) infusions in rats (Insel et al., 1992) and mice (Peters et al., 2014) fed a standard low fat or chow diet. Insel and colleagues reported that lateral ventricular administration of OT over 10 days, at a dose that enhanced social behavior (≈ 2.38 nmol/day) (Witt et al., 1992), produced a global reduction of OXTR binding in the VMH, anterior olfactory nucleus (AOP), bed nucleus of the stria terminalis (BNST), CeA, and the ventral septum (VS) in rats (Insel et al., 1992). Similarly, Peters and colleagues demonstrated that lateral ventricular infusion of OT over 15 days, at a dose that produced anxiogenic behavior ≈ 0.0238 or 0.238 nmol/day), produced a global downregulation of OXTR in the dorsolateral septum (DLS), ventrolateral septum (VLS), basolateral amygdala, medial amygdala, CeA, and median raphe nucleus in mice (Peters et al., 2014). In contrast, Insel and colleagues demonstrated that repeated administration (2x daily over 4 days) of OT (1000 ng) did not produce any obvious alterations in OXTR binding in the AOP, BNST, amygdala, VMH, and VS at 24 h following the last injection although there appeared to be a slight reduction in OXTR binding in the AOP at 72 h post-treatment (Insel et al., 1992).
We and others have found that 2–4x daily administration of OT is also effective in reducing body weight in obese nonhuman primates and humans (Zhang et al., 2013, Blevins et al., 2015) suggesting that repeated intermittent administration of OT might be an effective strategy to promote weight loss. Moreover, others have shown that long-term intermittent delivery of anorexigenic peptides (2–4x daily 3-h infusions) [e.g. PYY(3–36), exendin-4] into the periphery is an effective strategy to produce sustained reductions in food intake and weight loss in DIO rodents (Reidelberger et al., 2011, Henry et al., 2015). Now that OT is currently being tested as a weight-loss therapy in pre-clinical trials in humans, it will be important to consider delivery approach (long-term intermittent delivery vs continuous delivery) and the potential impact on OXTR downregulation when optimizing a treatment regimen to elicit weight loss in obese humans.
The finding that chronic OT infusion downregulates AVPR1a binding is also consistent with what others have suggested in regards to specificity of OT action. The effects of in vitro bath application of OT to increase contractions in rabbit ejaculatory tissues was blocked by the V1a antagonist, SR49059 (Gupta et al., 2008). In addition, ICV administration of OT appears to control certain forms of social behavior in hamsters and mice through the AVPR1a (Sala et al., 2011, Song et al., 2014). Recent reports also indicate that chronic intranasal OT reduces vasopressin-immunoreactive cells in the paraventricular nucleus of male prairie voles (Guoynes et al., 2018). This does not appear to be limited to only chronic administration of OT as a single acute injection of OT on postnatal day 1 produces changes in both OT (Yamamoto et al., 2004) and AVP (Yamamoto et al., 2004, Bales et al., 2007) immunoreactive cells in the hypothalamus of prairie voles. Furthermore, acute intraperitoneal administration of OT is reported to elicit 1) c-Fos in various CNS sites (Hicks et al., 2016), locomotor activity (Hicks et al., 2016), analgesia and scratching (Schorscher-Petcu et al., 2010), as well as 2) inhibit methamphetamine-elicited behaviors (Everett et al., 2018), in part, through AVPR1a in rodent models. However, in contrast to the adult-onset obesity phenotype observed with OT or OXTR null mice (Takayanagi et al., 2008, Camerino, 2009), AVPR1a null mice are hypermetabolic (Hiroyama et al., 2007) and existing data from acute studies in our lab and others suggest that the effects of CNS OT to reduce food intake (Rinaman and Rothe, 2002, Ho et al., 2014, Ong et al., 2015, Klockars et al., 2017), sucrose consumption (Mullis et al., 2013), and increase BAT temperature (Roberts et al., 2017) are mediated by OXTRs. However, further tests will need to rule out the possibility that effects of chronic 4V OT may be mediated, in part, by AVPR1a receptors in the NTS. It will also be important to examine when the OXTR and AVPR1a downregulation occurs and whether this coincides with the waning of OT-elicited changes in food intake. If so, this may explain, in part, why the effects of chronic OT to reduce food intake do not typically extend beyond a 2–3 week period.
Conclusions
Collectively, these findings indicate that chronic OT, at a dose that elicits weight loss and reductions in fat mass (Blevins et al., 2015), produces global reductions in OXTR binding in forebrain sites where OT has been shown to be effective in reducing food intake (ARC, CeA, VMH) in addition to control sites that are also linked to the control of food intake and express OXTRs (Sub). Furthermore, OT also produced largely similar reductions in AVPR1a binding in the ARC and VMH as well as a control site that is linked to the control of food intake that expresses AVPR1a (LS), but it failed to produce significant reductions in AVPR1a binding in the CeA. Our findings indicate that sustained OT treatment was not associated with a significant downregulation of hindbrain OXTR in both HFD-fed or chow-fed rats nor was there a significant impact of diet on OXTR binding within the hindbrain. It will be important to assess whether OXTR and AVPR1a downregulation may be causally related to OT’s waning effectiveness to reduce food intake in HFD-fed animals or whether these effects occur afterwards. Given the excitement over the use of chronic intranasal OT as potential therapeutic to treat obesity (Ott et al., 2013, Zhang et al., 2013, Blevins et al., 2015, Lawson et al., 2015, Kuppens et al., 2016, Striepens et al., 2016, Thienel et al., 2016, Hsu et al., 2017, Lawson, 2017, Plessow et al., 2018, Spetter et al., 2018), autism spectrum disorder (Striepens et al., 2011, Yamasue et al., 2012, Lacivita et al., 2017), and schizophrenia (Striepens et al., 2011, Montag et al., 2013, Ota et al., 2018) and the precedent for cross-talk between these OT and AVP, future studies should also assess the extent to which both receptor subtypes may contribute to the effects of OT when it is administered chronically.
Highlights.
Diet composition failed to alter oxytocin (OXTR) or vasopressin 1a receptor (AVPR1a) binding in forebrain and hindbrain.
Chronic oxytocin (OT) treatment was associated with largely global reductions in forebrain OXTR and AVPR1a binding.
It will be important to consider the effect that chronic OT treatment can have across forebrain nonapeptide receptors.
ACKNOWLEDGMENTS
The authors thank the technical support of Vishu Anekonda, Benjamin Thompson, Zachary Roberts, James Graham and Dr. Peter Havel.
This material was based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (VA). This work was also supported by the VA Merit Review Awards 1l01BX001213–01A1 and BX004102–01, NIH R01DK115976, and from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service to James Blevins. This work was also supported by grants to Karen Bales (NIH HD060117 and NIH HD071998). This work was also supported by the Energy Balance and Glucose Metabolism Core of the Nutrition Obesity Research Center at the University of Washington and supported by National Institutes of Health (NIH) grant P30 DK035816.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altirriba J, Poher AL, Caillon A, Arsenijevic D, Veyrat-Durebex C, Lyautey J, Dulloo A, Rohner-Jeanrenaud F (2014) Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology 155:4189–4201. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS (2007) Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience 144:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ (1999) CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol 276:R1569–1578. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE (2010) A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology 151:4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, Petracca F, Dorsa DM (1983) Autoradiographic localization of specific binding sites for [3H][Arg8]vasopressin in the septum of the rat brain with tritium-sensitive film. Eur J Pharmacol 90:155–157. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Baskin DG (2015) Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiol Behav 152:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, Havel PJ (2015) Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 308:R431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Thompson BW, Anekonda VT, Ho JM, Graham JL, Roberts ZS, Hwang BH, Ogimoto K, Wolden-hanson TH, Nelson JO, Kaiyala KJ, Havel PJ, Bales KL, Morton GJ, Schwartz MW, Baskin DG (2016) Chronic CNS oxytocin signaling preferentially induces fat loss in high fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol 308:R431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Jo YH, Li X, Schwartz GJ (2009) Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 29:8302–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino C (2009) Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 17:980–984. [DOI] [PubMed] [Google Scholar]
- Cherepanov SM, Akther S, Nishimura T, Shabalova AA, Mizuno A, Ichinose W, Shuto S, Yamamoto Y, Yokoyama S, Higashida H (2017) Effects of three lipidated oxytocin analogs on behavioral deficits in CD38 knockout mice. Brain Sci. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA (2007) A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol 292:R291–307. [DOI] [PubMed] [Google Scholar]
- Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, Piscitelli F, Legros JJ, Geenen V, Foti M, Wahli W, Di Marzo V, Rohner-Jeanrenaud F (2011) Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One 6:e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsa DM, Petracca FM, Baskin DG, Cornett LE (1984) Localization and characterization of vasopressinbinding sites in the amygdala of the rat brain. J Neurosci 4:1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett NA, McGregor IS, Baracz SJ, Cornish JL (2018) The role of the vasopressin V1A receptor in oxytocin modulation of methamphetamine primed reinstatement. Neuropharmacology 133:1–11. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM (1992) Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 578:256–260. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, Bales KL (2017) Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Soc Neurosci. 12:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdosechova L, Krskova K, Segarra AB, Spolcova A, Suski M, Olszanecki R, Zorad S (2014) Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. The J Endocrinol. 220:333–343. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 81:629–683. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Klein U, Reilander H, Fahrenholz F (1995) Expression of the human oxytocin receptor in baculovirus-infected insect cells: high-affinity binding is induced by a cholesterol-cyclodextrin complex. Biochemistry 34:13794–13801. [DOI] [PubMed] [Google Scholar]
- Gould BR, Zingg HH (2003) Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience 122:155–167. [DOI] [PubMed] [Google Scholar]
- Grundwald NJ, Benitez DP, Brunton PJ (2016) Sex-dependent effects of prenatal stress on social memory in rats: a role for differential expression of central vasopressin-1a receptors. J Neuroendocrinol. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guoynes CD, Simmons TC, Downing GM, Jacob S, Solomon M, Bales KL (2018) Chronic intranasal oxytocin has dose-dependent effects on central oxytocin and vasopressin systems in prairie voles (Microtus ochrogaster). Neuroscience 369:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J, Russell R, Wayman C, Hurley D, Jackson V (2008) Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br J Pharmacol. 155:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KE, Elfers CT, Burke RM, Chepurny OG, Holz GG, Blevins JE, Roth CL, Doyle RP (2015) Vitamin B12 conjugation of peptide-YY(3–36) decreases food intake compared to native peptide-YY(3–36) upon subcutaneous administration in male rats. Endocrinology 156:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herisson FM, Brooks LL, Waas JR, Levine AS, Olszewski PK (2014) Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. Neuroreport 25:909–914. [DOI] [PubMed] [Google Scholar]
- Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK (2016) Oxytocin acting in the nucleus accumbens core decreases food intake. J Neuroendocrinol. 28. [DOI] [PubMed] [Google Scholar]
- Hicks C, Ramos L, Dampney B, Baracz SJ, McGregor IS, Hunt GE (2016) Regional c-Fos expression induced by peripheral oxytocin administration is prevented by the vasopressin 1A receptor antagonist SR49059. Brain Res Bull 127:208–218. [DOI] [PubMed] [Google Scholar]
- Hidema S, Fukuda T, Hiraoka Y, Mizukami H, Hayashi R, Otsuka A, Suzuki S, Miyazaki S, Nishimori K (2016) Generation of Oxtr cDNA(HA) -Ires-Cre Mice for Gene Expression in an Oxytocin Receptor Specific Manner. J Cell Biochem 117:1099–1111. [DOI] [PubMed] [Google Scholar]
- Hiroyama M, Aoyagi T, Fujiwara Y, Birumachi J, Shigematsu Y, Kiwaki K, Tasaki R, Endo F, Tanoue A (2007) Hypermetabolism of fat in V1a vasopressin receptor knockout mice. Mol Endocrinol 21:247–258. [DOI] [PubMed] [Google Scholar]
- Ho JM, Anekonda VT, Thompson BW, Zhu M, Curry RW, Hwang BH, Morton GJ, Schwartz MW, Baskin DG, Appleyard SM, Blevins JE (2014) Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology 155:2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA (2013) Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol 591:3081–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu EA, Miller JL, Perez FA, Roth CL (2018) Oxytocin and Naltrexone successfully treat hypothalamic obesity in a boy post-craniopharyngioma resection. J Clin Endocrinol Metab. 103:370–375. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Witt DM (1992) Homologous regulation of brain oxytocin receptors. Endocrinology 130:2602–2608. [DOI] [PubMed] [Google Scholar]
- Kimura T, Makino Y, Saji F, Takemura M, Inoue T, Kikuchi T, Kubota Y, Azuma C, Nobunaga T, Tokugawa Y, et al. (1994) Molecular characterization of a cloned human oxytocin receptor. Eur J Endocrinol 131:385–390. [DOI] [PubMed] [Google Scholar]
- Klockars OA, Klockars A, Levine AS, Olszewski PK (2018) Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake. Neuroreport. 29:504–510. [DOI] [PubMed] [Google Scholar]
- Klockars OA, Waas JR, Klockars A, Levine AS, Olszewski PK (2017) Neural basis of ventromedial hypothalamic oxytocin-driven decrease in appetite. Neuroscience 366:54–61. [DOI] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB (2012) GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens RJ, Donze SH, Hokken-Koelega AC (2016) Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin Endocrinol. 85:979–987. [DOI] [PubMed] [Google Scholar]
- Lacivita E, Perrone R, Margari L, Leopoldo M (2017) Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. J Med Chem 60:9114–9141. [DOI] [PubMed] [Google Scholar]
- Lawson EA (2017) The effects of oxytocin on eating behaviour and metabolism in humans. Nature reviews Endocrinology 13:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ (2015) Oxytocin reduces caloric intake in men. Obesity. 23:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Sabatier N (2017) Oxytocin - The Sweet Hormone? Trends Endocrinol Metab 28:365–376. [DOI] [PubMed] [Google Scholar]
- Leslie M, Silva P, Paloyelis Y, Blevins J, Treasure J (2018) A systematic review and quantitative meta-analysis of oxytocin’s effects on feeding. J Neuroendocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, Scarpace PJ (2007) Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab 293:E252–258. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ (1989) Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res 500:223–230. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Aoyama M, Sakamoto K, Jojima T, Aso Y, Takasu K, Takenosihita S, Shimomura K (2017) Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci Rep 7:8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T (2011) Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 3:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Sakuma K, Santoso P, Gantulga D, Katsurada K, Ueta Y, Hiraoka Y, Nishimori K, Tanaka S, Shimomura K, Yada T (2014) Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS letters 588:4404–4412. [DOI] [PubMed] [Google Scholar]
- Matarazzo V, Schaller F, Nedelec E, Benani A, Penicaud L, Muscatelli F, Moyse E, Bauer S (2012) Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J Neurosci 32:17097–17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC (1990) Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol 428:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlar JA, Rinaman L, Vollmer RR, Amico JA (2007) Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol 293:R1063–1068. [DOI] [PubMed] [Google Scholar]
- Montag C, Brockmann EM, Bayerl M, Rujescu D, Muller DJ, Gallinat J (2013) Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: a case-control study. World J Biol Psychiatry 14:500–508. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2016) Central control of body temperature F1000Res 5:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K (2011) Central neural pathways for thermoregulation. Front Biosci 16:74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE (2012) Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 302:E134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K, Kay K, Williams DL (2013) Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res 1513:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Billington CJ, Kotz CM, Wang C (2014) Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol 307:R737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Allen K, Levine AS (2015) Effect of oxytocin receptor blockade on appetite for sugar is modified by social context. Appetite 86:81–87. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schioth HB, Levine AS (2010) Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology 151:4736–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ZY, Alhadeff AL, Grill HJ (2015) Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol 308:R800–R806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ZY, Bongiorno DM, Hernando MA, Grill HJ (2017) Effects of endogenous oxytocin receptor signaling in nucleus tractus solitarius on satiation-mediated feeding and thermogenic control in male rats. Endocrinology 158:2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Bradley DJ, O’Carroll AM, Brownstein MJ, Young WS (1992) Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology 131:533–535. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS 3rd (1994) Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology 135:1511–1528. [DOI] [PubMed] [Google Scholar]
- Ota M, Yoshida S, Nakata M, Yada T, Kunugi H (2018) The effects of adjunctive intranasal oxytocin in patients with schizophrenia. Postgrad Med 130:122–128. [DOI] [PubMed] [Google Scholar]
- Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M (2013) Oxytocin reduces reward-driven food intake in humans. Diabetes 62:3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (eds.) (2007) The rat brain in stereotaxic coordinates Burlington: Academic Press. [Google Scholar]
- Perkeybile AM, Delaney-Busch N, Hartman S, Grimm KJ, Bales KL (2015) Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Front Behav Neurosci 9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID (2014) Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 42:225–236. [DOI] [PubMed] [Google Scholar]
- Plante E, Menaouar A, Danalache BA, Yip D, Broderick TL, Chiasson JL, Jankowski M, Gutkowska J (2015) Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 156:1416–1428. [DOI] [PubMed] [Google Scholar]
- Plessow F, Marengi DA, Perry SK, Felicione JM, Franklin R, Holmes TM, Holsen LM, Makris N, Deckersbach T, Lawson EA (2018) Effects of intranasal oxytocin on the blood oxygenation level-dependent signal in food motivation and cognitive control pathways in overweight and obese men. Neuropsychopharmacology 43:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R, Kojro E, Fahrenholz F (1996) Separate agonist and peptide antagonist binding sites of the oxytocin receptor defined by their transfer into the V2 vasopressin receptor. J Biol Chem 271:31593–31601. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Haver AC, Apenteng BA, Anders KL, Steenson SM (2011) Effects of exendin-4 alone and with peptide YY(3–36) on food intake and body weight in diet-induced obese rats. Obesity 19:121–127. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Rothe EE (2002) GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 283:R99–106. [DOI] [PubMed] [Google Scholar]
- Roberts ZS, Wolden-Hanson TH, Matsen ME, Ryu V, Vaughan CH, Graham JL, Havel PJ, Chukri DW, Schwartz MW, Morton GJ, Blevins JE (2017) Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 313:R357–R371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE (1987) Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides 8:505–513. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B (2011) Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 69:875–882. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS (2010) Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci 30:8274–8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA (2007) Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol 292:R1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JKt, Larkin TE 2nd, Huhman KL, Albers HE (2014) Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetter MS, Feld GB, Thienel M, Preissl H, Hege MA, Hallschmid M (2018) Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci Rep 8:2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R (2011) Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 32:426–450. [DOI] [PubMed] [Google Scholar]
- Striepens N, Schroter F, Stoffel-Wagner B, Maier W, Hurlemann R, Scheele D (2016) Oxytocin enhances cognitive control of food craving in women. Hum Brain Mapp 37:4276–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Ferris CF, Dorsa DM (1990) [3H]arginine-vasopressin binding sites in the CNS of the golden hamster. Neurosci Lett 119:215–218. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K (2008) Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19:951–955. [DOI] [PubMed] [Google Scholar]
- Thienel M, Fritsche A, Heinrichs M, Peter A, Ewers M, Lehnert H, Born J, Hallschmid M (2016) Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int J Obes (Lond) 40:1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL (1998) Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139:5015–5033. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM (1995) Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv Exp Med Biol 395:209–225. [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR (1992) Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav 43:855–861. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS (2004) Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 125:947–955. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Yee JR, Hurlemann R, Rilling JK, Chen FS, Meyer-Lindenberg A, Tost H (2012) Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction. J Neurosci 32:14109–14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KJ, So KH, Hata Y, Suzuki Y, Kato D, Watanabe K, Aso H, Kasahara Y, Nishimori K, Chen C, Katoh K, Roh SG (2015) The regulation of oxytocin receptor gene expression during adipogenesis. J Neuroendocrinol 27:335–342. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K (2009) Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci 29:2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D (2011) Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cai D (2011) Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab 301:E1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, Cai D (2013) Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 8:e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]


