Abstract
There is accumulating evidence that both dehydroepiandrosterone (DHEA) and cortisol play an important role in regulating physical maturation and brain development. High DHEA levels tend to be associated with neuroprotective and indirect anabolic effects, while high cortisol levels tend to be associated with catabolic and neurotoxic properties. Previous literature has linked the ratio between DHEA and cortisol levels (DC ratio) to disorders of attention, emotional regulation and conduct, but little is known as to the relationship between this ratio and brain development. Due to the extensive links between the amygdala and the cortex as well as the known amygdalar involvement in emotional regulation, we examined associations between DC ratio, structural covariance of the amygdala with whole-brain cortical thickness, and validated report-based measures of attention, working memory, internalizing and externalizing symptoms, in a longitudinal sample of typically developing children and adolescents 6 to 22 years of age. We found that DC ratio predicted covariance between amygdalar volume and the medial anterior cingulate cortex, particularly in the right hemisphere. DC ratio had a significant indirect effect on working memory through its impact on prefrontal-amygdalar covariance, with higher DC ratios associated with a prefrontal-amygdalar covariance pattern predictive of higher scores on a measure of working memory. Taken together, these findings support the notion, as suggested by animal and in vitro studies, that there are opposing effects of DHEA and cortisol on brain development in humans, and that these effects may especially target prefrontal-amygdalar development and working memory, in a lateralized fashion.
Keywords: Adrenarche, Androgens, Adolescence, Puberty, Attention, Human Brain
1. INTRODUCTION
Cortisol and dehydroepiandrosterone (DHEA) are the most abundant adrenal hormones of the hypothalamic-pituitary-adrenal (HPA) axis and exert potent, pleiotropic effects on human physiology including regulation of immune, metabolic and cognitive function (Kamin and Kertes 2017). The role of cortisol in supporting acute stress responses by drawing upon the brain and the body’s energy reserves (i.e. increasing the body’s catabolic state) has been extensively investigated (Nicolaides et al. 2014). Notably, cortisol may lead to increased neurotoxicity (and other adverse physical effects) when it remains elevated for long periods of time, for example, in the context of prolonged and inescapable exposure to psychological stress (Kamin and Kertes, 2017).
In comparison to cortisol, the study of DHEA has been relatively neglected, but it is in fact the most abundantly produced steroid hormone across the lifespan, maintained at high levels from middle childhood until the third decade of life (Adams, 1985). Further, humans and the great apes are the only species demonstrated to exhibit adrenarche, a key developmental event leading to increased production of dehydroepiandrosterone (DHEA) (Remer, Boye, Hartmann and Wudy, 2005). Taken together, this evidence suggests that DHEA may have played an important evolutionary role in the development of the human brain. Interestingly, DHEA possesses anti-glucocorticoid actions that may be carried out through a variety of mechanisms, including but not limited to antagonists effects on the glucocorticoid receptor (Kalimi, Shafagoj, Loria, Padgett and Regelson, 1994) as well as an indirect enhancement of the body’s anabolic state (as opposed to a catabolic state) (Campbell, 2011). As such, cortisol and DHEA are thought to serve opposing functions, with DHEA mitigating the effects of cortisol (Chen et al 2015). Measurement of the ratio between the two hormones may therefore provide a more accurate assessment of HPA-axis function than either hormone measured alone (Sollberger and Ehlert 2015).
Cortisol modulates neuroglial structure and function through glucocorticoid and mineralocorticoid receptors that translocate from the cell cytoplasm to the nucleus to modulate gene transcription (Madalena et al 2017). Non-genomic signaling pathways have also been identified and may underlie rapid effects within seconds to minutes (Joels, Sarabdjitsingh and Karst, 2012). Direct exposure to glucocorticoids in vitro or stress models which trigger cortisol release (such as immobilization or social defeat) have been demonstrated to increase dendritic arborization of baso-lateral amygdala neurons (Vyas, Mitra, Shankaranarayana Rao and Chattarji, 2002). In glial cells, exposure to glucocorticoids inhibits proliferation and differentiation of oligodendrocytes, thus inhibiting myelination (Alonso, 2000). Glucocorticoid and in vivo stress paradigms enhance proliferation of microglial cells and may induce lasting dysregulation of their inflammatory function (Nair and Bonneau, 2006).
In contrast to cortisol, DHEA appears to play an important role in enhancing neuronal and glial plasticity, through a variety of peripheral and central mechanisms (Compagnone and Mellon, 2000; Li, Cui, Zhang, Zhou, Ge, Sokabe and Chen, 2008; Maninger, Wolkowitz, Reus, Epel and Mellon, 2009). Of these, the most relevant to human development may be the anti-glucocorticoid effects of DHEA, which play an important role in metabolically active brain regions. Through these anti-glucocorticoid effects, DHEA preserves anabolic potential by increasing reserves in mitochondrial energy (Campbell, 2011). Because release of neurotransmitters during neuronal firing is thought to rely directly on mitochondrial energy, these effects of DHEA represent a direct mechanism through which it could alter activity-dependent synaptogenesis (Vos, Lauwers and Verstreken, 2010).
Extending these cellular and molecular studies, studies of brain development implicate cortisol and DHEA in the regulation of large-scale brain networks involved in cognitive control. Cortisol appears to regulate structure and function of the hippocampus, amygdala and prefrontal cortex extending to the anterior cingulate (McEwen, Gray and Nasca, 2015). Although fewer studies have examined the effect of DHEA, it appears to be involved in regulating cortical plasticity in a comparable set of brain networks including the left dorsolateral prefrontal cortex, right temporo-parietal junction, right premotor and right entorhinal cortex (Herting, Gautam, Spielberg, Dahl and Sowell, 2015; Nguyen, McCracken, Ducharme, Cropp, Botteron, Evans and Karama, 2013b). DHEA also shows significant uptake in the amygdala (Regelson and Kalimi, 1994; Sripada, Marx, King, Rajaram, Garfinkel, Abelson and Liberzon, 2013).
Acute administration of exogenous cortisol appears to have varying effects on executive function, depending on the task and the time course of administration. Interestingly, cortisol administration appears to impair working memory tested shortly afterwards (Shields, Bonner and Moons, 2015). Induction of stress or direct administration of cortisol or other glucocorticoids reduces performance in tests of working memory and set-shifting (Plessow, Fischer, Kirschbaum and Goschke, 2011) though some studies have yielded opposing results (Henckens, van Wingen, Joels and Fernandez, 2011). In contrast – and in line with the notion that DHEA opposes the effects of cortisol – exogenous DHEA administration reduces activity in brain regions associated with generating negative emotion and enhances activity in regions linked to emotional regulation, reducing negative affect and impairing encoding of highly emotional stimuli (Sripada, Marx, King, Rajaram, Garfinkel, Abelson and Liberzon, 2013). Cortisol and DHEA have also been implicated in both internalizing and externalizing disorders (Kamin and Kertes, 2017). Elevated cortisol levels are consistently identified as biological correlates of depression and anxiety. Whether psychopathology is a cause or consequence of neuroendocrine dysregulation remains controversial. However, a number of studies have identified elevated cortisol or elevated cortisol/DHEA ratio as risk factors that predate the onset of depressive symptoms in children and adolescents (Goodyer, Herbert and Tamplin, 2003). In addition, higher DHEA and lower cortisol levels have been shown, in several, though not all studies, to correlate to lower severity of externalizing disorders such as oppositional defiant and conduct disorder as well as lower severity of attention deficit/hyperactivity symptoms (Golubchik, Mozes, Maayan and Weizman, 2009; Wang, Huang, Hsiao, Chiang, Wu, Shang and Chen, 2011).
It is important to note conversely that acute stress responses including cortisol elevation are thought to be important for maintaining arousal and thus for facilitating cognitive function (Kamin and Kertes, 2017). Studies support the notion of inadequate stress cortisol responses in conduct disordered children (Fairchild, van Goozen, Stollery, Brown, Gardiner, Herbert and Goodyer, 2008). Further, caution should be taken in interpreting studies of exogenous administration of DHEA or cortisol which produce acute supra-physiological surges in hormone levels and may have different effects from those of more chronic hormonal changes. The optimal situation is likely a dynamic balance of DHEA and cortisol that is appropriate to respond to the prevailing internal and external stressors.
Taken together, these studies provide compelling evidence for an anatomical and functional brain circuit that is regulated by DHEA and cortisol, and that may be relevant to developmental changes in cognition and behavior. A central component of this circuit is the amygdala, traditionally thought to be a key component of the affective brain. Interestingly, there is accumulating evidence to suggest that the amygdala is also crucially involved in the ‘bottom-up’ regulation of primary (i.e. perceptual salience) and secondary (i.e. goal-directed) attentional processes (Pessoa, 2008). The relationship between primary and secondary attentional processes may be facilitated by reciprocal connections between amygdala and cortex whereby activation of the amygdala alters the structure and function of cortical regions in a manner specific to each sensory modality (Phelps and LeDoux, 2005).
These structural effects of the amygdala are supported by previous findings of anatomical covariance between the amygdala and bilateral dorsolateral and dorsomedial prefrontal, inferior parietal, as well as bilateral orbital and ventromedial prefrontal cortices (Albaugh, Ducharme, Collins, Botteron, Althoff, Evans, Karama and Hudziak, 2013). Structural covariance refers to the examination of anatomic correlations between brain structures, has emerged recently as a compelling addition to existing modalities investigating brain function and structure (Alexander-Bloch, Giedd and Bullmore, 2013). Structural covariance has been shown to replicate and complement task-based and resting-state functional connectivity networks, diffusion tensor imaging-based tractography, and may represent both a measure of genotypic variance and environmental influences on the central nervous system (CNS) (Alexander-Bloch, Giedd and Bullmore, 2013). Although structural covariance associations do not necessarily prove anatomical connectivity, it has been demonstrated that there is longitudinal maturational coupling of cortical thickness changes in brains areas within anatomical/functional networks, such as the default mode network (Raznahan 2011). This process likely underlies the progressive integration of network-level covariance between 5 and 18 years of age (Zielinski 2010).
In sum, based on the available evidence, the ratio between DHEA and cortisol levels (DC ratio) may regulate the relationship, or covariance, between the amygdala and the cortex. This impact of DHEA and cortisol on anatomical circuits may in turn lead to functional alterations (Raznahan, Lerch, Lee, Greenstein, Wallace, Stockman, Clasen, Shaw and Giedd, 2011) in attentional processes as well as in the severity of internalizing and externalizing symptoms. We hypothesized that: (1) DC ratio may be associated with the development of prefrontal-amygdalar circuits from middle childhood to young adulthood; and (2) anatomical variation in prefrontal-amygdalar circuits related to higher DC ratios may be associated with higher levels of attention/working memory, lower severity of anxious-depressed symptoms, and higher severity of aggression/rule-breaking behaviors. To test these hypotheses, we examined associations between DC ratio, structural covariance of the amygdala with whole-brain cortical thickness, and tests of attention, working memory, anxious-depressed symptoms and aggression/rule-breaking behavior, in a longitudinal sample of typically developing children and adolescents 6 to 22 years of age.
2. METHODS AND MATERIALS
2.1. Sample Characteristics
The National Institutes of Health (NIH) MRI Study of Normal Brain Development is a multi-site project that aimed to provide a normative database to characterize healthy brain maturation. Subjects were recruited across the United States with a population-based sampling method seeking to achieve a representative sample in terms of income level, race and ethnicity (Evans, 2006). All experiments on human subjects were conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the adequate understanding and written parental consent, as well as assent of the subjects (or consent, if >=18 years old). The sample was limited to developmentally healthy children with rigorous exclusion criteria, described in detail elsewhere (Evans, 2006). In particular, any children with a current or past treatment for language disorder (simple articulation disorders not exclusionary); and a lifetime history of Axis I psychiatric disorder (except for simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency)’ were excluded from the study.
Subjects underwent repeated hormonal sampling, magnetic resonance brain imaging (MRI), and neurocognitive testing every 2 years, with a maximum of 3 visits over 4 years. After strict quality control of MRI data (see section 2.2) and the exclusion of scans without hormonal measurements, cognitive, or behavioral parameters, 224 subjects (F=128) were used for brain-hormone analyses (353 scans; F=207) and 200 subjects (F=114) for brain-cognitive or brain-behavioral analyses (282 scans; F=163) (see Table 1 for more details). Overall, participants were aged between 6 and 22 years old, with a mean age of 13 (SD = 3.56 years).
Table 1:
Sample Characteristics
| Sample used for DC ratio-Structural Covariance | Sample used for Structural Covariance-Cognition | ||
|---|---|---|---|
| # Scans per Visit # | 1 | n = 126 scans (F = 73) | n = 115 scans (F = 64) |
| 2 | n = 129 scans (F = 80) | n = 98 scans (F = 62) | |
| 3 | n = 98 scans (F = 54) Total = 353 scans (F = 207) |
n = 69 scans (F = 37) Total = 282 scans (F = 163) |
|
| # Participants per # Scans Completed |
1 scan | 121 (F = 68) | 130 (F = 73) |
| 2 scans | 77 (F = 41) | 58 (F = 33) | |
| 3 scans | 26 (F = 19) Total = 224 (F = 128) |
12 (F = 8) Total = 200 (F = 114) |
|
| Testosterone Time 1 (pg/mL) |
1 | n = 126, mean = 66.90, SD = 54.30 | n = 115, mean = 68.52, SD = 55.63 |
| 2 | n = 129, mean = 50.32, SD = 31.20 | n = 98, mean = 47.92, SD = 28.02 | |
| 3 | n = 98, mean = 68.99, SD = 74.59 | n = 69, mean = 63.06, SD = 80.04 | |
| Testosterone Time 2 (pg/mL) |
1 | n = 126, mean = 60.38, SD = 46.50 | n = 115, mean = 62.88, SD = 47.61 |
| 2 | n = 129, mean = 45.46, SD = 30.10 | n = 98, mean = 44.80, SD = 30.46 | |
| 3 | n = 98, mean = 67.68, SD = 61.92 | n = 69, mean = 61.75, SD = 64.45 | |
| Cortisol Time 1 (μg/dL) | 1 | n = 126, mean = 0.22, SD = 0.18 | n = 115, mean = 0.22, SD = 0.18 |
| 2 | n = 129, mean = 0.17, SD = 0.11 | n = 98, mean = 0.16, SD = 0.11 | |
| 3 | n = 98, mean = 0.21, SD = 0.19 | n = 69, mean = 0.21, SD = 0.20 | |
| Cortisol Time 2 (μg/dL) | 1 | n = 126, mean = 0.15, SD = 0.12 | n = 115, mean = 0.16, SD = 0.12 |
| 2 | n = 129, mean = 0.11, SD = 0.08 | n = 98, mean = 0.12, SD = 0.08 | |
| 3 | n = 98, mean = 0.15, SD = 0.13 | n = 69, mean = 0.16, SD = 0.13 | |
| Estradiol Time 1(pg/mL) | 1 | n = 126, mean = 7.49, SD = 4.17 | n = 115, mean = 7.49, SD = 4.22 |
| 2 | n = 129, mean = 8.00, SD = 4.31 | n = 98, mean = 7.85, SD = 4.19 | |
| 3 | n = 98, mean = 10.87, SD = 6.85 | n = 69, mean = 11.53, SD = 7.05 | |
| Estradiol Time 2 (pg/mL) | 1 | n = 126, mean = 7.17, SD = 4.42 | n = 115, mean = 7.21, SD = 4.38 |
| 2 | n = 129, mean = 8.05, SD = 4.71 | n = 98, mean = 8.05, SD = 4.81 | |
| 3 | n = 98, mean = 11.49, SD = 6.95 | n = 69, mean = 12.18, SD = 6.99 | |
| DHEA Time 1 (pg/mL) | 1 | n = 126, mean = 102.23, SD = 101.81 | n = 115, mean = 106.81, SD = 104.91 |
| 2 | n = 129, mean = 194.08, SD = 204.37 | n = 98, mean = 184.16, SD = 180.65 | |
| 3 | n = 98, mean = 176.31, SD = 159.13 | n = 69, mean = 154.57, SD = 140.08 | |
| DHEA Time 2 (pg/mL) | 1 | n = 126, mean = 96.05, SD = 111.34 | n = 115, mean = 100.94, SD = 114.95 |
| 2 | n = 129, mean = 172.77, SD = 178.86 | n = 98, mean = 173.33, SD = 175.91 | |
| 3 | n = 98, mean = 207.42, SD = 212.33 | n = 69, mean = 194.68, SD = 204.20 | |
| Season of sampling | 1 | Spring = 38 | Spring = 33 |
| Summer = 49 | Summer = 45 | ||
| Fall = 16 | Fall = 16 | ||
| Winter= 23 | Winter = 21 | ||
| Total = 126 | Total = 115 | ||
| 2 | Spring = 33 | Spring = 24 | |
| Summer = 49 | Summer = 39 | ||
| Fall = 25 | Fall = 20 | ||
| Winter = 22 | Winter = 15 | ||
| Total = 129 | Total = 98 | ||
| 3 | Spring = 32 | Spring = 20 | |
| Summer = 37 | Summer = 30 | ||
| Fall = 13 | Fall = 12 | ||
| Winter = 16 | Winter = 7 | ||
| Total = 98 | Total = 69 | ||
| Collection Time 1 (min after midnight) |
1 | n = 126, mean = 680.09, SD = 137.06 | n = 115, mean = 682.79, SD = 140.84 |
| 2 | n = 129, mean = 711.65, SD = 123.23 | n = 98, mean = 710.67, SD = 110.87 | |
| 3 | n = 98, mean = 721.29, SD = 106.24 | n = 69, mean = 721.23, SD = 99.75 | |
| Collection Time 2 (min after midnight) |
1 | n = 126, mean = 819.21, SD = 152.99 | n = 115, mean = 824.12, SD = 157.61 |
| 2 | n = 129, mean = 869.03, SD = 99.42 | n = 98, mean = 865.68, SD = 91.33 | |
| 3 | n = 98, mean = 863.96, SD = 106.92 | n = 69, mean = 858.78, SD = 102.15 | |
| Age (years) | 1 | n = 126, mean = 12.48, SD = 3.29 Range: 4.88 to 18.24 |
n = 115, mean = 12.63, SD = 3.19 Range: 6.09 to 18.24 |
| 2 | n = 129, mean = 13.25, SD = 3.60 Range: 6.79 to 20.17 |
n = 98, mean = 12.92, SD = 3.33 Range: 6.79 to 18.90 |
|
| 3 | n = 98, mean = 14.32, SD = 3.63 Range: 9.08 to 22.10 |
n = 69, mean = 13.11, SD = 2.77 Range: 9.08 to 18.84 |
|
| Gender F = female, M = male |
1 | F = 73, M = 53 Total = 126 | F = 64, M = 51 Total = 115 |
| 2 | F = 80, M = 49 Total = 129 | F = 62, M = 36 Total = 98 | |
| 3 | F = 54, M = 44 Total = 98 | F = 37, M = 32 Total = 69 | |
| Pubertal stage | 1 | n = 126, mean = 2.14, SD = 1.16 | n = 115, mean = 2.19, SD = 1.18 |
| 2 | n = 129, mean = 2.30, SD = 1.28 | n = 98, mean = 2.20, SD = 1.24 | |
| 3 | n = 98, mean = 2.64, SD = 1.39 | n = 69, mean = 2.23, SD = 1.23 | |
| Handedness | 1 | L = 11, R = 115 Total = 126 | L = 8, R = 107 Total = 115 |
| 2 | L = 11, R = 118 Total = 129 | L = 8, R = 90 Total = 98 | |
| 3 | L = 9, R = 89 Total = 98 | L = 7, R = 62 Total = 69 | |
| Total brain volume (cm3) | 1 | n = 126, mean = 486.93, SD = 63.97 | n = 115, mean = 490.36, SD = 63.27 |
| 2 | n = 129, mean = 496.84, SD = 64.81 | n = 98, mean = 491.89, SD = 63.80 | |
| 3 | n = 98, mean = 512.15, SD = 75.57 | n = 69, mean = 509.81, SD = 82.21 | |
| Left amygdala | 1 | n = 126, mean = 1053.53, SD = 129.02 | n = 115, mean = 1059.49, SD = 130.63 |
| 2 | n = 129, mean = 1071.57, SD = 129.99 | n = 98, mean = 1072.85, SD = 133.56 | |
| 3 | n = 98, mean = 1102.61, SD = 128.40 | n = 69, mean = 1103.99, SD = 132.50 | |
| Right amygdala | 1 | n = 126, mean = 1073.90, SD = 120.11 | n = 115, mean = 1078.54, SD = 120.51 |
| 2 | n = 129, mean = 1091.64, SD = 121.71 | n = 98, mean = 1097.24, SD = 123.49 | |
| 3 | n = 98, mean = 1121.53, SD = 124.09 | n = 69, mean = 1115.88, SD = 129.23 | |
| Amygdala mean volume | 1 | n = 126, mean = 1063.72, SD = 119.36 | n = 115, mean = 1069.02, SD = 120.20 |
| 2 | n = 129, mean = 1081.61, SD = 120.48 | n = 98, mean = 1085.04, SD = 123.19 | |
| 3 | n = 98, mean = 1112.07, SD = 120.74 | n = 69, mean = 1109.94, SD = 125.81 | |
| Scanner |
1
2 3 4 5 6 7 8 9 10 11 12 |
n = 48; 13.5% n = 44; 12.4% n = 50; 14.1% n = 21; 5.9% n =40; 11.3% n =2; 0.8% n = 19; 5.4% n = 27; 7.6% n = 64; 18.0% n = 18; 5.1% n = 15; 4.2% n = 5; 1.7% |
n = 41; 14.5% n = 36; 12.8% n = 42; 14.9% n = 20; 7.1% n = 33; 11.8% n = 3; 1% n = 16; 5.7% n = 20; 7.1% n = 48; 16.2% n = 9; 3.4% n = 10; 3.7% n = 4; 1.7% |
2.2. Neuroimaging Measures
A three-dimensional T1-weighted (T1W) Spoiled Gradient Recalled (SPGR) echo sequence from 1.5 Tesla scanners was obtained on each participant, with 1mm isotropic data acquired sagittal from the entire head for most scanners. In addition, T2-weighted (T2W) and proton density-weighted (PDW) images were acquired using a two-dimensional (2D) multi-slice (2mm) dual echo fast spin echo (FSE) sequence. Fully automated analysis of whole-brain cortical thickness was done through the CIVET pipeline, developed at the Montreal Neurological Institute (MNI). First, a multistage quality control process was implemented, as described previously (Nguyen, McCracken, Ducharme, Botteron, Mahabir, Johnson, Israel, Evans and Karama, 2013; Nguyen, McCracken, Ducharme, Cropp, Botteron, Evans and Karama, 2013b), excluding subjects with white or gray matter artifacts. All quality-controlled MR images were subsequently processed through the CIVET pipeline. These processing steps have been described at length in other publications (Nguyen, McCracken, Ducharme, Botteron, Mahabir, Johnson, Israel, Evans and Karama, 2013; Nguyen, McCracken, Ducharme, Cropp, Botteron, Evans and Karama, 2013b).
Volumetric measures of the amygdala were obtained from MRI data using a fully automated segmentation method validated in human subjects. This method utilizes a large, manually labeled MRI dataset (n = 80) of young healthy adults that serves as a template library (Collins and Pruessner, 2010). The manual segmentation was done by four different raters, and intra-class intra-rater and inter-rater reliability varied between r=0.83 for the right and r=0.95 for the left amygdala (Pruessner, Li, Serles, Pruessner, Collins, Kabani, Lupien and Evans, 2000). From this manual segmentation, a fully automated method was derived, characterized by label fusion techniques that combine segmentations from a subset of ‘n’ most similar templates. Specifically, each template is used to produce an independent segmentation of the subject using the ANIMAL pipeline (Collins and Evans, 1997), followed by a thresholding step to eliminate cerebrospinal fluid, which results in ‘n’ different segmentations. To fuse the segmentations at each voxel, a voting strategy is used; the label with the most votes from the ‘n’ templates is assigned to the voxel. Combining multiple segmentations minimizes errors and maximizes consistency between segmentations. When using n = 11 templates, the label fusion technique has been shown to yield an optimal median Dice Kappa of 0.826 and Jaccard similarity of 0.703 for the amygdala (Collins and Pruessner, 2010).
2.3. Hormonal and Pubertal Measures
Preceding each MRI visit, children provided two 1–3 cm3 samples of saliva, before and after the neurocognitive testing, respectively (total 4 samples). These samples were assayed by enzyme-linked immunosorbent assay (ELISA) methods, and the average results used as a measure of hormonal levels. The intra-assay and inter-assay coefficients of variation (COVs) were 6.5% and 16.2% for DHEA, 3.1% and 7.2% for cortisol, 6.1% and 13.5% for testosterone, and 4.1% and 9.1% for estradiol, respectively (Salimetrics ELISA, State College, PA; Salimetrics Salivary ELISA Kit, State College, PA). Each subject’s set of two samples for each visit were analyzed in duplicate together on the same plate. Each plate reflected the recruitment targets of each site, which were themselves counterbalanced across sites according to specific age and sex targets. An aliquoting procedure was followed when samples when first taken. At the next MRI, a similar procedure was followed, and the child again provided two separate saliva samples for hormonal measurement before and after neurocognitive testing (total 4 samples, preceding the actual MRI visit). Note that DC ratio collected at time 1 (before the neurocognitive testing) and DC ratio collected at time 2 (after the neurocognitive testing) were analyzed separately, as they potentially represent different measures of stress response (anticipatory increase vs. post-stress reactivity). Of note, caution was taken to collect the samples not immediately before or after another stressful procedure such as MRI, to avoid confounding effects. For example, ‘post-stress’ hormonal samples done after neurocognitive testing could be confounded by an anticipatory increase in stress related to the MRI scan, if taken immediately prior to the scan. To control for diurnal, seasonal and gender variation in hormonal measures, we have included collection time, sex and season as covariates in hormonal analyses (see section 2.5).
To measure pubertal maturation, the Pubertal Development Scale (PDS) was administered by a physician to all subjects included in this study (Petersen, Crockett, Richards and Boxer, 1988). This scale has been shown to have good reliability (coefficient alpha: 0.77) and validity (r2=0.61–0.67) compared to physical examination (Petersen, Crockett, Richards and Boxer, 1988). During an interview with the child/adolescent, questions were asked about physical development. We computed a puberty variable consisting of 5 stages, representing increasing levels of physical maturity similar to Tanner staging, previously described (Nguyen, McCracken, Ducharme, Botteron, Mahabir, Johnson, Israel, Evans and Karama, 2013). Pubertal stage was also included as a covariate in hormonal analyses (see section 2.5).
2.4. Cognitive Measures
To measure levels of inattentive, internalizing and externalizing symptoms, we selected the Attention, Anxious-Depressed, Aggression and Rule-Breaking subscales of the Child Behavior Checklist (CBCL) and Young Adult Self-Report (YASR), respectively. The CBCL and YASR are age-appropriate instruments extensively used for assessing psychopathology and competence worldwide. These instruments consist of reports from the parents or young adults themselves on specific behaviors exhibited within the previous 6 months (Achenbach and Rescorla, 2001). The YASR was derived from items on the CBCL and serves as a self-report extension of the CBCL for young adults (Achenbach, 1997). Both the CBCL and YASR are reliable measures with high stability over time, validated in multiple cultures, with high internal consistency (Achenbach and Rescorla, 2001).
To measure working memory, we selected the Working Memory subscale of the Behavior Rating Inventory of Executive Function (BRIEF). The BRIEF uses parent ratings of executive function in the context of everyday problem solving. It measures executive function (including working memory) in an integrated, relativistic way, outlining the complex, priority-based decision-making that is demanded in real-world situations (Gioia, Isquith, Retzlaff and Espy, 2002). The BRIEF has demonstrated high test-retest reliability (r ≈0.82 for parent ratings) and high internal consistency (Cronbach’s alphas ≈ .80 - .98) (Gioia, Isquith, Guy, Kenworthy and Baron, 2000). Here we are using the BRIEF Working Memory scale, which measures the ‘on-line representational memory’, i.e. the capacity to hold information in mind for the purpose of completing a task, encoding information, or generating goals, plans and sequential steps to achieving goals. In other words, this scale measures the ability to sustain working memory for appropriate lengths of time in order to sustain performance and attention.
2.5. Statistical Analyses
Statistical analyses were done using SurfStat (Matlab toolbox designed by Keith J. Worsley, http://www.math.mcgill.ca/keith/surfstat/) and SPSS 21.0 (SPSS, Inc., Chicago, Illinois). Please see Table 2 for more details on statistical models used in this section.
Table 2: Description of statistical models.
The specific statistical term of interest is underlined in each model; the rest of the terms represent control variables.
‘id’ refers to a specific participant’s identification number: this term is included in order to identify and link all longitudinal data from the same participant
‘I’ to the identity matrix of the mixed effects model
‘CTh’ in section 2.5.2 refers to average cortical thickness of the brain regions found to be significant in section 2.5.1
| Methods section | Statistical model |
|---|---|
| 2.5.1 | |
|
DC Ratio & Cortico-Amygdalar
Covariance |
(1) Whole-brain CTh = 1 + DHEA/Cortisol*Amygdala + DC +Amygdala + Collection Time + Age + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I (2) Whole-brain CTh = 1 + DHEA/Cortisol*Amygdala*Sex + DHEA/Cortisol*Amygdala + Amygdala*Sex + DHEA/Cortisol*Sex + DC +Amygdala + Sex + Collection Time + Age + Scanner + Handedness + Total Brain Volume + random (id) + I (3) Whole-brain CTh = 1 + DHEA/Cortisol*Amygdala*Age + DHEA/Cortisol*Amygdala + Amygdala*Age + DHEA/Cortisol*Age + DC +Amygdala + Age + Collection Time + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I (4) Note that in order to limit the number of control variables per model: models (1), (2), (3) and (4) were retested while adding testosterone, estradiol, pubertal stage or season of sampling as additional covariates (one at a time) |
| 2.5.2 | |
|
Cortico-Amygdalar Covariance & Cognitive/Behavioral Measures
|
(1) Following significant results with the ‘DC Ratio*Amygdala’ term in section 2.5.2: Cognitive/Behavioral Scores = 1 + CTh*Amygdala + CTh + Amygdala + Sex + Age + Scanner + Handedness + Total Brain Volume + random (id) + I |
| 2.5.3 | |
|
Indirect effects of DC ratio
Through Cortico-Amygdalar Covariance |
(1) Beta coefficients and p-values extracted from section 2.5.1
(2) Beta coefficients and p-values extracted from section 2.5.2 (3) Beta coefficients and p-values from (1) and (2) were extracted from existing analyses, and entered in the Sobel-Goodman test calculator to formally test indirect effects effects |
2.5.1. DC Ratio and Cortico-Amygdalar Structural Covariance
Mixed effects designs were used to model the relationship between DC ratio and covariance of the amygdala with whole-brain, native-space cortical thickness (CTh), taking into account the within- and between-individual variances in this longitudinal sample, and controlling for the effects of age, sex, total brain volume, scanner, handedness, time of salivary sampling (see Table 2 for more details). All continuous variables were centered using their respective means.
A correction for multiple comparisons across the whole brain, using random field theory (RFT, p<0.05), was applied to all analyses. Determining power and sample size in neuroimaging studies is a challenging task because of the massive multiple comparisons among tens of thousands of correlated voxels. Two metrics are commonly used to control for multiple comparisons in this setting, namely the family-wise error rate (FWER) based on random field theory (RFT), defined as the probability of obtaining at least one false positive in a family of tests, and the false discovery rate (FDR), defined as the proportion of false positives among all rejected tests (Hayasaka, Peiffer, Hugenschmidt and Laurienti, 2007; Lindquist and Mejia, 2015).
In practice, the application of RFT proceeds in two stages. First, one estimates the smoothness of the statistical map in terms of resels. Next, the resel counts are used to compute the expected Euler characteristic at different thresholds u. This allows us to determine the threshold at which we would expect only α × 100% of equivalent statistical maps arising under the null hypothesis to contain at least one cluster above the given threshold. RFT is a mathematically elegant approach toward correcting for multiple comparisons, like other methods that control the FWER, and gives more stringent and conservative results than FDR. Thus, RFT is more appropriate than FDR in the context of this study, because of the exploratory nature of the hypotheses tested in this study, itself due to the lack of prior evidence regarding the role of DC ratio in brain development.
To examine associations between DC Ratio and structural covariance of the amygdala, we examined the significance of the term “DC ratio*Amygdala”, while controlling for all the aforementioned control variables (see example below, with the terms of interest underlined). To examine any distinct effects of DHEA and cortisol above and beyond those related to estradiol, testosterone or season of collection, these variables were also included as control variables in additional models. Finally, to test for age and sex effects on the relationship between DC ratio and cortico-amygdalar networks, we tested for interactions with age, i.e. ‘DC ratio*Amygdala*Age’ and interactions with sex, i.e. ‘DC ratio*Amygdala*Sex’ on whole-brain cortical thickness (see Table 2 for more details).
2.5.2. Cortico-Amygdalar Structural Covariance and Cognitive/Behavioral Measures
To examine associations between the brain circuits impacted by DC ratio and cognitive/behavioral measures, we averaged the cortical thickness (CTh) of brain regions found to be significant in the previous section (see section 2.5.1) and examined the significance of the term “CTh*Amygdala” on cognitive/behavioral measures, while controlling for all the aforementioned control variables (see Table 2 for more details).
2.5.3. Indirect Effects of DC Ratio through Cortico-Amygdalar Structural Covariance
We formally tested whether DC ratio had a significant indirect effect on cognitive/behavioral measures through its impact on cortico-amygdalar structural covariance. To examine the relationship between DC ratio and covariance of the amygdala with the cortical region found to be significant in section 2.5.1, we extracted the coefficients and p-values of the significant interaction term ‘DC Ratio*Amygdala’. To examine the relationship between cortico-amygdalar covariance and cognitive/behavioral measures found to be significant in section 2.5.2, we extracted the coefficients and p-values of the significant interaction term ‘CTh*Amygdala’. Finally, coefficients and p-values were extracted from existing analyses and entered in the Sobel-Goodman test calculator to formally test indirect effects (http://quantpsy.org/sobel/sobel.htm). This more traditional approach to test indirect effects, using Baron-Kenney’s criteria and augmented by a formal Sobel’s test, was preferred by our group to more recent methods that include bootstrapping. This is because of the complexity of our longitudinal data (multiple scans per subjects, different number of scans per subject). The traditional method treats each relationship (between predictor and moderator, and then between moderator and outcome) separately, allowing us to model the longitudinal component of the data. Finally, note that the same set of control variables (including age), as listed in sections 2.5.1 and 2.5.2 was used for the indirect-effect analyses.
3. RESULTS
3.1. Sample Characteristics
Table 1 details sample characteristics, including number of longitudinal scans and covariates of interest. The sample used for DHEA-related analyses included 224 participants (F=128), and 353 scans. Participants were aged between 6 and 22 years old, with a mean age of 13 (SD = 3.56 years). There was a significant rise in DC ratio between timepoint 1 and 2 (raw DC ratio mean +/− SEM for timepoint 1: 1357.02 +/− 142.46; timepoint 2: 1559.67 +/− 108.6; log DC ratio mean +/− SEM for timepoint 1: 2.82 +/− 0.03; timepoint 2: 2.95 +/− 0.02; Wilcoxon Signed Rank Test for related samples: standardized test statistic 4.911, p<0.005).
3.2. DC Ratio and Cortico-Amygdalar Structural Covariance
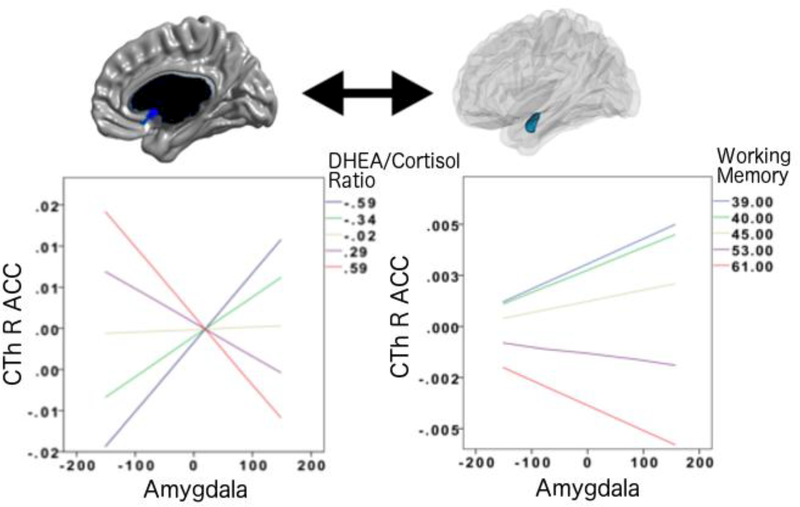
As shown in Figure 1, whole-brain analyses, controlling for the effects of age, sex, total brain volume, scanner, handedness and collection time of salivary samples, revealed that DC ratio (collected at time 2, after neurocognitive testing) is associated with the structural covariance between the right amygdala and CTh of the right medial (subgenual) anterior cingulate cortex (linear models, Brodmann area 25 (subgenual area), cluster-level p=0.047, 36 vertices, peak vertex id 41222 [x=2.16, y=11.37, z=−6.84], coefficient=0.20; standard error=0.05; p=1.45*10−4). Note that the relationship between this prefrontal region and the amygdala was present for both the left and right amygdala, but that it only met the RFT criteria for significance only on the right, while it remained a trend vertex-wise (p<=0.005) for the left amygdala. More specifically, lower DC ratios were associated with a more positive covariance between the amygdala and CTh of this prefrontal region, and higher DHEA levels were increasingly associated with more negative prefrontal-amygdalar covariance. Post-hoc analyses revealed that this net effect of DC ratio occurred as a result of the associations between increasing DHEA levels and more negative prefrontal-amygdalar covariance, and between increasing cortisol levels and more positive prefrontal-amygdalar covariance.
FIGURE 1. DC Ratio and Prefrontal-Amygdalar Structural Covariance.
This figure shows the associations between DC ratio, prefrontal-amygdalar structural covariance and working memory. The negative prefrontal-amygdalar covariance seen at higher DC ratios were associated with higher scores on higher scores on a ‘real-world’ parent rating of overall working memory (BRIEF). See Results for more detailed statistics.
All analyses consisted of mixed effects models that included all longitudinal data, as well as continuous measures of DC ratios and cognitive scores. Please note the Y axes of graphs list standardized residuals of cortical thickness (accounting for the effects of age, sex, handedness, scanner, and total brain volume in all analyses, as well as collection time for hormonal analyses).
Of note, there was no significant association between DC collected at time 1 (before neurocognitive testing) and cortico-amygdalar covariance. In addition, no other brain region met the threshold for significance (RFT, p<0.05) in the analysis involving DC collected at time 2 (after neurocognitive testing). Adding estradiol, testosterone, and season of sampling as control variables or performing log transformation of DC ratio to reduce skew did not result in any differences in the above findings. Finally, there were no significant age or sex interactions on the relationship between DC ratio and cortico-amygdalar structural covariance.
3.3. Cortico-Amygdalar Structural Covariance and Cognitive/Behavioral Measures
As shown in Figure 1, analyses controlling for the effects of age, sex, total brain volume, scanner and handedness, revealed that the prefrontal-amygdalar structural covariance (found in section 3.1 to be associated with DC ratio) was also associated with working memory scores as measured by the BRIEF (linear models, cluster-level coefficient=0.04; standard error=1.65*10−2; p=0.013). Lower scores on the BRIEF Working Memory test were associated with more positive prefrontal-amygdalar covariance (similar to the covariance seen with lower DC ratios), and higher working memory scores were associated with a more negative prefrontal-amygdalar covariance (similar to the covariance seen with higher DC ratios). Of note, no significant relationship emerged between prefrontal-amygdalar covariance and any other behavioral parameters, i.e. attention, anxious-depressed symptoms, aggression or rule-breaking behavior.
3.4. Indirect Effects of DC Ratio through Cortico-Amygdalar Structural Covariance
As shown in Table 3, formal tests of indirect effects (Sobel-Goodman) showed that DC ratio had a significant indirect effect on working memory through its impact on prefrontal-amygdalar structural covariance (Sobel test, test statistic=2.08, SE=0.004, p=0.04; Aroian test, test statistic=2.03, SE=0.004, p=0.04; Goodman test, test statistic=2.13, SE=0.004, p=0.03).
Table 3:
Indirect effects of DC Ratio on Working Memory through its Impact on Cortico-Amygdalar Structural Covariance
| Coefficient or test-statistic | Standard error | P-value | |
|---|---|---|---|
| DC ratio and mACC-AG covariance | b = 0.201 | SE = 0.0529 | p = 1.45*10−4 |
| mACC-AG and working memory | b = 0.041 | SE = 0.0165 | p = 0.013 |
| DC ratio and working memory | b = −1.489 | SE = 1.014 | p = 0.177 |
| Tests of Indirect effects* | Sobel test: Test-statistic = 2.080 |
Sobel test: SE = 0.004 |
Sobel test: p = 0.038 |
| Aroian test: Test-statistic = 2.031 |
Aroian test: SE = 0.004 |
Aroian test: p = 0.042 |
|
| Goodman test: Test-statistic = 2.132 |
Goodman test: SE = 0.004 |
Goodman test: p = 0.033 |
Note that there are three principal versions of the indirect effect ‘Sobel’ test - one that adds the third denominator term (Aroian, 1944/1947 - this is the version popularized by Baron & Kenny as the Sobel test), one that subtracts it (Goodman, 1960), and one that does not include it at all. In some situations, the results of these tests may be different –here, they are basically showing the same results in terms of test-statistics, standard errors, and significance.
4. DISCUSSION
The present study provides evidence that DHEA-Cortisol ratio may regulate working memory by modulating structural development of cortico-amygdalar networks. Specifically, we found significant associations between DC ratio and medial ACC-amygdala structural covariance, particularly in the right hemisphere. Higher DC ratio was also associated overall with a more negative prefrontal-amygdala covariance, with DHEA and cortisol associated with opposite covariance patterns (more negative prefrontal-amygdala covariance at higher DHEA levels, more positive prefrontal-amygdala covariance at higher cortisol levels). Further, we found that DC ratio had a significant indirect effect on working memory through its impact on prefrontal-amygdalar covariance, with higher DC ratios associated with a prefrontalamygdalar covariance pattern predictive of higher scores on an ecological test of working memory. Taken together, these findings support the notion, as suggested by animal and in vitro studies, that there may be opposing effects of DHEA and cortisol on brain development in humans, and that these effects may especially target prefrontal-amygdalar development and working memory in a lateralized fashion.
This work significantly contributes to our current understanding of the relationship between neuroendocrine function and brain development. Serial neuroimaging studies using task-based functional MRI measures suggest positive amygdala–prefrontal functional connectivity in early childhood (under 10 years of age) that switches to negative connectivity in early adolescence (Gee, Humphreys, Flannery, Goff, Telzer, Shapiro, Hare, Bookheimer and Tottenham, 2013). Prefrontal and cingulate cortex are theorized to exert a regulatory control on amygdala function; the switch in valence may therefore represent typical age-related improvement in anxiety (e.g. separation anxiety), emotional control and cognitive function (Beesdo, Lau, Guyer, McClure-Tone, Monk, Nelson, Fromm, Goldwin, Wittchen, Leibenluft, Ernst and Pine, 2009).
In line with this, stress and cortisol appear to dysregulate the same cortico-amygdalar circuit which extends primarily to prefrontal and cingulate cortices. Density of white matter connections between these areas is inversely correlated with severity of anxiety symptoms (Kim and Whalen, 2009). In adolescent females, early life stress predicted elevated cortisol levels which were associated with reduced functional connectivity between amygdala and ventro-medial prefrontal cortex over a decade later (Burghy, Stodola, Ruttle, Molloy, Armstrong, Oler, Fox, Hayes, Kalin, Essex, Davidson and Birn, 2012). These effects appear to be dose-dependent with higher daily cortisol levels associated with more dramatic reductions in functional connectivity. Further, recent studies from our group have demonstrated that DHEA may enhance working memory and performance in tests of visuo-motor attention by impacting the development of cortico-amygdalar and cortico-hippocampal networks, respectively (Nguyen, Haws, Fitzhugh, Torre, Hishaw and Alexander, 2016; Nguyen, Wu, Lew, Albaugh, Botteron, Hudziak, Fonov, Collins, Campbell, Booij, Herba, Monnier, Ducharme and McCracken, 2017). Taken together, these studies suggest that interactions between DHEA and cortisol may shape cortico-limbic development in a way that significantly alters cognitive and behavioral function.
The current study adds to this literature by highlighting the contribution of DC ratio to cortico-amygdalar circuit development and working-memory. Our finding of more significant effects for right vs. left amygdala (meeting RFT significance criteria for the right amygdala vs. vertex-wise trend p<=0.005 for the left amygdala) also hint at potential lateralization effects. Right lateralized abnormalities in prefrontal-amygdalar brain structure and function have also been described in studies of children with autism spectrum disorders and attention-deficit hyperactivity disorder (Sripada, Kessler, Fang, Welsh, Prem Kumar and Angstadt, 2014; Wei, Zhong, Nie and Gong, 2018). For example, a prior study has demonstrated that DHEA specifically modulates right ACC activity (Sripada, Marx, King, Rajaram, Garfinkel, Abelson and Liberzon, 2013). In that study, DHEA administration increased right ACC activity and decreased right amygdala activity (Sripada, Marx, King, Rajaram, Garfinkel, Abelson and Liberzon, 2013). Even though our study only targeted typically developing children, the present results can still serve to extend and support prior findings of lateralization effects in clinical populations, by hinting at the potentially preferential impact of DC ratio on the right prefrontal-amygdala circuit.
Interestingly, our findings only appeared in the hormonal samples collected after neurocognitive testing (Time 2), when DHEA levels were significantly higher than cortisol levels. These samples differ from those collected at Time 1 in that they were collected later during the day, when cortisol levels show a dip in their diurnal trajectories (while DHEA levels remain fairly stable throughout the day). Diurnal variation of the DC ratio is thus largely driven by the morning cortisol surge; testing earlier during the day (timepoint 1) may therefore be less reflective of the typically prevalent DC ratio than a late morning cortisol (timepoint 2). This generates several hypotheses: it is possible that (1) relatively high levels of DHEA are needed to exert counter-regulatory effects to those of cortisol, given the profound effect of cortisol on brain structure, function and development; and/or (2) the anti-glucocorticoid effects of DHEA may only emerge following a cortisol surge. Although not evident in our data set, DHEA has been found to be released at higher levels (along with cortisol) under stressful conditions (Dismukes, Meyer, Shirtcliff, Theall, Esteves and Drury, 2016), when it may play a particularly important role in counter-acting the effects of cortisol. As such, HPA axis reactivity would be very different in a context of anticipation (Time 1) vs. a context of post-stress reactivity (Time 2). This is consistent with reports that the effects of DHEA on cognition may predominate over those of cortisol in a situation of acute stress (Shields, Lam, Trainor and Yonelinas, 2016), as is potentially the case in our study. However, under conditions of chronic stress, DC ratio may rapidly drop, suggesting a potential impact for type and duration of stressor on any effects related to DC ratio. The relationships between DC ratio, brain structure and cognition may also be particularly significant during the key developmental period from middle childhood to young adulthood, when DHEA levels are at their peak. They may disappear in older adult samples who show declining DHEA levels. This is suggested by reports that in younger adult women 18–26 years old, DHEA levels may be associated with improved working memory (with the opposite relationship for cortisol), while in older postmenopausal women, DHEA replacement had no impact on cognitive outcomes (Davis, Panjari and Stanczyk, 2011; van Niekerk, Huppert and Herbert, 2001).
4.1. Strengths and Limitations
Strengths of our study include the large, longitudinal developmental dataset, including the repeated collection of hormonal, neuroimaging and measures of personality, behavior and cognition. The lack of association with tests measuring clinically significant behavioral parameters, i.e. any of the CBCL/YASR measures related to inattention, internalizing or externalizing symptoms, was not entirely unexpected, given this is a typically developing sample, with little variance in terms of psychopathology. Still, this suggests that, as would be expected, many other influences (environmental, hormonal and genetic) play a role in determining the clinical relevance of varying DC ratios on the development of an individual child.
One unexpected finding was the lack of any significant age interactions on the impact of DC ratio, in contrast to prior findings that DHEA-related effects on brain structure may be more prominent in pre-pubertal kids ages 4–13 years old (Nguyen, McCracken, Ducharme, Cropp, Botteron, Evans and Karama, 2013a). Yet, other reports suggest that the stress response (and buffering response) as measured by DC ratio may be relatively stable across the pubertal transition, supporting its potential role as a biomarker of mental health vulnerability (Ruttle, Shirtcliff, Armstrong, Klein and Essex, 2015).
Limitations of the present study include the absence of a formal path analysis to delineate the contribution of different components of covariance. In this complex biological system, the multiple bidirectional relationships and feedback loops (e.g. hormonal measures influence each other as well as brain structure; neurocognitive function may affect hormonal reactivity (Slattery, Grieve, Ames, Armstrong and Essex, 2013)) would contradict the fundamental assumptions of path analysis rendering it unreliable. Restricting the analysis to fewer factors might produce statistically satisfactory but biologically incomplete metrics. Reassuringly, our findings, though exploratory, are fairly consistent with previous studies employing diverse imaging and measurement strategies, as noted above.
The quality-control of some of our hormonal measures may represent a further study limitation. Although coefficients of variation (COVs) for our hormonal measures were generally under the usually accepted limits of 10 and 15% for inter and intra-assay variation respectively, the limit was marginally exceeded for the inter-assay COV of DHEA at 16.2% (see Methods section). We believe the large size of our dataset (224 subjects; 353 scans) renders it robust to this small degree of added noise.
Conclusions
In sum, our study reveals a significant relationship between DC ratio, the development of the right prefrontal-amygdalar structural network, and working memory. Together with previous investigations, our findings support the notion that DHEA and cortisol may play reciprocally antagonistic roles in brain development in humans, highlighting the importance of considering both simultaneously in neuroendocrine studies.
HIGHLIGHTS.
Prefrontal-amygdalar covariance varies as a function of DHEA-cortisol ratio
DHEA decreases prefrontal-amygdalar covariance, cortisol increases this covariance
DHEA-cortisol ratio impacts working memory through prefrontal-amygdalar covariance
Higher DHEA improves working memory, higher cortisol worsens working memory
DHEA and cortisol may play antagonistic roles during brain development in humans
ACKNOWLEDGEMENTS
This work was supported by Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02–3343, N01-MH9–0002, and N01-NS-9–2314, −2315, −2316, −2317, −2319 and −2320). Tuong-Vi Nguyen and Sherif Karama are both supported by salary awards by the FRQS Clinician Scientist Program and by CIHR operating grants.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Achenbach TM, 1997. Manual for the young adult self-report and young adult behavior checklist. University of Vermont, Department of Psychiatry, Burlington (VT). [Google Scholar]
- Achenbach TM, Rescorla LA, 2001. Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children,Youth, and Families, Burlington VT. [Google Scholar]
- Adams JB, 1985. Control of secretion and the function of C19-delta 5-steroids of the human adrenal gland. Molecular Cellular Endocrinology 41, 1–17. [DOI] [PubMed] [Google Scholar]
- Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ, 2013. Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: implications for the neural substrates of emotion regulation. Neuroimage 71, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E, 2013. Imaging structural co-variance between human brain regions. Nat Rev Neurosci 14, 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, 2000. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia 31, 219–231. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS, 2009. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of general psychiatry 66, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM, 2012. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature neuroscience 15, 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, 2011. Adrenarche and middle childhood. Hum Nat 22, 327–349. [DOI] [PubMed] [Google Scholar]
- Collins DL, Evans AC, 1997. Animal: Validation and Applications of Nonlinear Registration-Based Segmentation. International Journal of Pattern Recognition and Artificial Intelligence 11, 1271–1294. [Google Scholar]
- Collins DL, Pruessner JC, 2010. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage 52, 1355–1366. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH, 2000. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Front Neuroendocrin 21, 1–56. [DOI] [PubMed] [Google Scholar]
- Davis SR, Panjari M, Stanczyk FZ, 2011. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab 96, 1642–1653. [DOI] [PubMed] [Google Scholar]
- Dismukes AR, Meyer VJ, Shirtcliff EA, Theall KP, Esteves KC, Drury SS, 2016. Diurnal and stress-reactive dehydroepiandrosterone levels and telomere length in youth. Endocr Connect 5, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, 2006. The NIH MRI study of normal brain development. Neuroimage 30, 184–202. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM, 2008. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological psychiatry 64, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, 2013. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L, Baron IS, 2000. Test review: Behavior rating inventory of executive function. Child Neuropsychology 6, 235–238. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Retzlaff PD, Espy KA, 2002. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence 8, 249–257. [DOI] [PubMed] [Google Scholar]
- Golubchik P, Mozes T, Maayan R, Weizman A, 2009. Neurosteroid blood levels in delinquent adolescent boys with conduct disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 19, 49–52. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, 2003. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: a community-based longitudinal enquiry. Psychological medicine 33, 601–610. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Peiffer AM, Hugenschmidt CE, Laurienti PJ, 2007. Power and sample size calculation for neuroimaging studies by non-central random field theory. Neuroimage 37, 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joels M, Fernandez G, 2011. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proceedings of the National Academy of Sciences of the United States of America 108, 5801–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER, 2015. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. Plos One 10, e0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H, 2012. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacological reviews 64, 901–938. [DOI] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W, 1994. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem 131, 99–104. [DOI] [PubMed] [Google Scholar]
- Kamin HS, Kertes DA, 2017. Cortisol and DHEA in development and psychopathology. Hormones and behavior 89, 69–85. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ, 2009. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cui S, Zhang Z, Zhou R, Ge Y, Sokabe M, Chen L, 2008. DHEA-neuroprotection and -neurotoxicity after transient cerebral ischemia in rats. J Cereb Blood Flow Metab 29, 287–296. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Mejia A, 2015. Zen and the Art of Multiple Comparisons. Psychosomatic Medicine 77, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30, 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray JD, Nasca C, 2015. 60 YEARS OF NEUROENDOCRINOLOGY: Redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. The Journal of endocrinology 226, T67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH, 2006. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. Journal of neuroimmunology 171, 72–85. [DOI] [PubMed] [Google Scholar]
- Nguyen LA, Haws KA, Fitzhugh MC, Torre GA, Hishaw GA, Alexander GE, 2016. Interactive effects of subjective memory complaints and hypertension on learning and memory performance in the elderly. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 23, 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S, 2013. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex 23, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S, 2013a. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci 33, 10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S, 2013b. Interactive Effects of Dehydroepiandrosterone and Testosterone on Cortical Thickness during Early Brain Development. Journal of Neuroscience 33, 10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Wu M, Lew J, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins DL, Campbell BC, Booij L, Herba C, Monnier P, Ducharme S, McCracken JT, 2017. Dehydroepiandrosterone impacts working memory by shaping cortico-hippocampal structural covariance during development. Psychoneuroendocrino 86, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, 2008. On the relationship between emotion and cognition. Nature reviews. Neuroscience 9, 148–158. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A, 1988. A Self-Report Measure of Pubertal Status: Reliability, Validity, and Initial Norms. J Youth Adolescence 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE, 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187. [DOI] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Kirschbaum C, Goschke T, 2011. Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. Journal of cognitive neuroscience 23, 3218–3227. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC, 2000. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex 10, 433–442. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lerch Jason P., Lee N, Greenstein D, Wallace Gregory L., Stockman M, Clasen L, Shaw Phillip W., Giedd Jay N., 2011. Patterns of Coordinated Anatomical Change in Human Cortical Development: A Longitudinal Neuroimaging Study of Maturational Coupling. Neuron 72, 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regelson W, Kalimi M, 1994. Dehydroepiandrosterone (Dhea) - the Multifunctional Steroid .2. Effects on the Cns, Cell-Proliferation, Metabolic and Vascular, Clinical and Other Effects - Mechanism of Action. Ann Ny Acad Sci 719, 564–575. [DOI] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA, 2005. Urinary Markers of Adrenarche: Reference Values in Healthy Subjects, Aged 3–18 Years. Journal of Clinical Endocrinology & Metabolism 90, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ, 2015. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev Psychobiol 57, 688–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Bonner JC, Moons WG, 2015. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology 58, 91–103. [DOI] [PubMed] [Google Scholar]
- Shields GS, Lam JC, Trainor BC, Yonelinas AP, 2016. Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrino 67, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery MJ, Grieve AJ, Ames ME, Armstrong JM, Essex MJ, 2013. Neurocognitive function and state cognitive stress appraisal predict cortisol reactivity to an acute psychosocial stressor in adolescents. Psychoneuroendocrinology 38, 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, Angstadt M, 2014. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Human brain mapping 35, 4693–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, Liberzon I, 2013. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niekerk JK, Huppert FA, Herbert J, 2001. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrino 26, 591–612. [DOI] [PubMed] [Google Scholar]
- Vos M, Lauwers E, Verstreken P, 2010. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Frontiers in synaptic neuroscience 2, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S, 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Huang YS, Hsiao CC, Chiang YL, Wu CC, Shang ZY, Chen CK, 2011. Salivary dehydroepiandrosterone, but not cortisol, is associated with attention deficit hyperactivity disorder. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry 12, 99–109. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhong S, Nie S, Gong G, 2018. Aberrant development of the asymmetry between hemispheric brain white matter networks in autism spectrum disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 28, 48–62. [DOI] [PubMed] [Google Scholar]