Abstract
The functional significance of the selective enrichment of the omega-3 essential fatty acid docosahexaenoic acid (DHA; 22C and 6 double bonds) in cellular membrane phospholipids of the nervous system is being clarified by defining its specific roles on membrane protein function and by the uncovering of the bioactive mediators, docosanoids and elovanoids (ELVs). Here, we describe the preferential uptake and DHA metabolism in photoreceptors and brain as well as the significance of the Adiponectin receptor 1 in DHA retention and photoreceptor cell (PRC) survival. We now know that this integral membrane protein is engaged in DHA retention as a necessary event for the function of PRCs and retinal pigment epithelial (RPE) cells. We present an overview of how a) NPD1 selectively mediates preconditioning rescue of RPE and PR cells; b) NPD1 restores aberrant neuronal networks in experimental epileptogenesis; c) the decreased ability to biosynthesize NPD1 in memory hippocampal areas of early stages of Alzheimer’s disease takes place; d) NPD1 protection of dopaminergic circuits in an in vitro model using neurotoxins; and e) bioactivity elicited by DHA and NPD1 activate a neuroprotective gene-expression program that includes the expression of Bcl-2 family members affected by Aβ42, DHA, or NPD1. In addition, we highlight ELOVL4 (ELOngation of Very Long chain fatty acids-4), specifically the neurological and ophthalmological consequences of its mutations, and their role in providing precursors for the biosynthesis of ELVs. Then we outline evidence of ELVs ability to protect RPE cells, which sustain PRC integrity. In the last section, we present a summary of the protective bioactivity of docosanoids and ELVs in experimental ischemic stroke. The identification of early mechanisms of neural cell survival mediated by DHA-synthesized ELVs and docosanoids contributes to the understanding of cell function, prohomeostatic cellular modulation, inflammatory responses, and innate immunity, opening avenues for prevention and therapeutic applications in neurotrauma, stroke and neurodegenerative diseases.
Keywords: ischemic stroke, retina degenerations, ELOVL4, epileptogenesis, Alzheimer’s disease, Parkinson’s disease
1. Introduction
Disease onset and progression prompts complex reactions that unsettle homeostasis (Becher et al., 2017; Korn and Kallies, 2017) including inflammation and innate immune responses aiming to restore cell integrity (Bazan, 2006; Calder et al., 2017; Christ et al., 2018). Pro-homeostatic mechanisms originate in specific cells according to the context where the event takes place, such as macrophages or microglia encompassing the formation of autocrine and/or paracrine chemokines, cytokines, and protective lipid mediators (Bazan, 2009, 2007; Calder, 2018, 2017; Hishikawa et al., 2017; Innes and Calder, 2018; Serhan, 2017; Serhan et al., 2015; Serhan and Petasis, 2011).
Several cells, including immune cells, blood vessels, neurons, astrocytes, retinal pigment epithelial (RPE) cells and others, are engaged with an overall collective goal of sustaining homeostasis by removing triggering factors , cell debris, and setting in motion cellular and tissue restoration. This review provides an overview of pro-homeostatic signaling elicited by docosanoids and elovanoids (ELVs), which results in inflammatory modulation and neuroprotection. It includes cellular and molecular events activated in the central nervous system (CNS) at the onset of ischemic stroke, epileptogenesis, Alzheimer’s disease (AD), Parkinson’s disease (PD) or retinal degenerations.
2. Preferential uptake and metabolism of DHA in rod and cone photoreceptor cells
DHA is enriched in the CNS, which includes the retina (Bazan, 2009, 2007, 2006; Gordon and Bazan, 1990), and serves as the precursor for neuroprotective and prohomeostatic 22-carbon docosanoids (Bazan, 2009, 2007; Bazan et al., 2010; Mukherjee et al., 2004) and of 32- or 34-carbon ELVs (Bhattacharjee et al., 2017; Jun et al., 2017). Conversely, DHA can be the target of excessive oxidative damage in the brain and in the retina, evolving into pathology (Hollyfield et al., 2008).
Photoreceptor cells (PRCs) selectively take up DHA, which esterifies to phospholipids used for PRC disc membrane biogenesis (Rodriguez de Turco et al., 1990). By participating in the recycling of DHA, from phagocytized disc membranes back to the retina, RPE cells play a central role in the conservation and delivery of rod outer segment (ROS)-derived DHA back to PRCs through the interphotoreceptor matrix following daily phagocytosis of ROS tips (Gordon et al., 1992).
The supply of DHA to the frog retina and its subsequent use by retinal cells (studied by autoradiography and biochemical methods) has begun defining its utilization during rod PRC renewal. Predominant uptake by the neural retina, mainly in ganglion cell axons, outer synaptic layer, and Müller cells, was observed after supplying radiolabeled DHA. Preferentially labeled Müller cells suggest their involvement as a transient storage site (Rice et al., 2015). After administration of 3H-22:6, most of the retinal label was seen in rod PRCs. Two different labeling patterns were found: diffuse pattern and a dense-label area at the base of the outer segments. This dense-label area expanded and reached the outer segment apex after 30 d, having conserved 3H-22:6 (even until after day 46), demonstrating that the lipid 3H-22:6 acylated to phospholipids in PRC membranes migrated apically and was then phagocytosed by the RPE. 3H-leucine in parallel displayed narrow protein bands and, because the edge of the 3H-leucine-labeled band (rhodopsin) and the dense-label region of 3H-22:6, migrated together, reaching the PRC tips at the same time. We suggested that the 3H-22:6-labeled phospholipids are noncovalently associated with rhodopsin (Gordon and Bazan, 1990).
3. AdipoR1 conserves DHA and promotes PRC survival
Adiponectin receptor 1 (AdipoR1) is a receptor for the hormone adiponectin, which promotes insulin sensitivity, displays anti-inflammatory properties, and is a pro-cell survival factor (Yamauchi et al., 2003; Yamauchi and Kadowaki, 2013). Adiponectin occupancy of AdipoR1 stimulates adenosine monophosphate-activated kinase (AMPK) (Iwabu et al., 2010; Mao et al., 2006) and induces AdipoR1-dependent ceramidase activity to regulate ceramide abundance (Holland et al., 2011). Adiponectin receptors are involved in metabolic syndrome, mainly due to the adiponectin’s ability to restore insulin sensitivity in obese, diabetic preclinical models via activation of AMPK and peroxisome proliferator-activated receptor alpha (PPAR-α) pathways in an AdipoR1/R2-dependent manner (Yamauchi and Kadowaki, 2013). AdipoR1 is a seven transmembrane domain protein with an inverse topology of G-protein coupled receptors, featuring an intracellular N-terminus and an extracellular C-terminus (Yamauchi et al., 2003). We found that AdipoR1 has an additional function independent of its cognate ligand adiponectin: it is a key regulator of DHA uptake/retention in the retina (Rice et al., 2015), where it is critical to maintain the normal structure and function. We have demonstrated that DHA uptake in PRCs depends on AdipoR1. Young postnatal mice deficient in AdipoR1 exhibit low DHA levels in the retina, and lipidomic analysis revealed that long-chain polyunsaturated fatty acid (26 to 38 C)-containing phosphatidylcholines (PC), were severely depleted in AdipoR1 knockout (KO) retinas. These biochemical alterations depress retinoid levels, which functionally impairs retinal physiology in otherwise structurally intact retinas. Subsequently, PRC degeneration occurs.
After DHA uptake by RPE cells from choriocapillaris through the interphotoreceptor matrix, the fatty acid reaches the PRC inner segments, where it is taken up and acylated to phospholipids then used for membrane biogenesis of OS and other parts of the PRC (Bazan et al., 2011a; Fliesler and Anderson, 1983). DHA then ends up amounting to more than 50% of OS phospholipid fatty acyl chains and therefore DHA-containing phospholipids in the retina and other cells are reservoirs for docosanoids (Bazan et al., 1984; Mukherjee et al., 2004). NPD1 is made on-demand when homeostatic disruptions, such as protein misfolding, Aβ peptide challenge, and/or uncompensated oxidative stress (UOS), emerge (Bazan et al., 2011a), prompting responses to counteract neuroinflammation consequences and neurodegeneration (Bazan, 2006). The significance of DHA for PRC function and retinal degenerations has been expanded by the finding that the fatty acid-selective retention/conservation is driven, at least in part by AdipoR1. Upon ablation of this receptor, DHA uptake is impaired and PRC-specific very-long-chain polyunsaturated fatty acids (VLC-PUFA)-containing PC molecular species are reduced; and PRC function and survival are compromised (Rice et al., 2015).
KOs of AdipoR1 and AdipoR2 mediate adiponectin-induced signaling in skeletal muscle and liver (Lin et al., 2013; Yamauchi et al., 2003). The CNS exhibits AdipoR1 expression abundantly, particularly in the RPE and neural retina (gene expression portal, BioGPS; Wu et al., 2009; Thundyil, 2012).
DHA is transported from the liver to the RPE cells (Scott and Bazan, 1989), from which it is taken up by PRCs, esterified into phospholipids and used for the biogenesis of PRC membranes as a major acyl chain of OS disk membranes. PC in PRCs comprises half of the retinal phospholipids and is richly endowed with DHA, as well as with VLC-PUFAs (Aveldaño, 1988). ELOVL4 (ELOngation of Very Long chain fatty acids-4) elongates DHA to 32-38 carbons (VLC-PUFAs) in PRCs. After activation to acyl-Co A, these fatty acids are selectively acylated at sn-1 of PCMS, whereas DHA is esterified at sn-2 (Fliesler and Anderson, 1983). Mutations in the ELOVL4 are causative of Stargardt’s disease (STGD3). Whether the SNP in the human AdipoR1 locus associated with AMD in a Finnish population (Thundyil et al., 2012) is related to an impairment in DHA uptake/retention and/or VLC-PUFAs remains to be determined. The precise mechanisms of the biosynthesis and molecular principles engaged in retaining and making these fatty acids available in PRCs are not fully understood. Our findings that PC-VLC-PUFAs are reduced when AdipoR1 is ablated provides the first mechanistic explanation of the requirements to sustain this key pathway for PRC survival. Moreover, our data indicate that VLC-PUFAs, after shedding and phagocytosis of PRC apical disk membranes, appear transiently in RPE cells, from where they are recycled back to the inner segments of PRCs. This recycling is similar to the short loop of DHA (Bazan et al., 2011a). Therefore, RPE cells do not have the ability to biosynthesize VLC-PUFAs.
Under conditions of UOS, DHA is released from membrane-associated phospholipids and converted to NPD1, a promoter RPE and PRC survival (Bazan et al., 2011a). The function, if any, of VLC-PUFAs after shedding and phagocytosis is not known. However, it is tempting to speculate that they may also serve a role as protective mediators by themselves or as precursors of bioactive mediators of RPE cell survival. Recently, an essential role for Mfsd2a, a member of the major facilitator superfamily, was shown to mediate DHA transport across the blood brain barrier (Nguyen et al., 2014; Wong et al., 2016). However, the ablation of Mfsd2a does not lead to retinal degeneration. The ablation of AdipoR1 led to the discovery that this receptor regulates DHA retention in the retina and is necessary for PRC structure and function. Moreover, the DHA changes are selective for the essential omega-3 fatty acid family since total levels of arachidonic acid (AA) (from the essential omega-6 fatty acid family) remained unchanged in AdipoR1−/− retinas. Lack of AdipoR1 causes an early-impaired visual cycle and a reduced electroretinogram (ERG), indicators of PRC degeneration onset. AdipoR1 promotes DHA uptake that enables its conversion to VLC-PUFA. The PRC-specific PCMS containing VLC-PUFAs are greatly reduced in AdipoR1−/−. Thus, AdipoR1 is necessary for the synthesis of VLC-PUFAs and their distribution of phospholipids in normal healthy retinas. Deficiencies in VLC-PUFAs in aging human retinas may trigger changes that are ultimately associated with AMD. Thus, our work identifies AdipoR1 as a key regulator of DHA, of VLC-PUFAs, and of the functional integrity of PRCs.
4. NPD1 selectively mediates preconditioning rescue of RPE and PR cells
Conditioned protection, or preconditioning, is an acquired protection or resilience by a cell, tissue, or organ to a lethal stimulus facilitated by a previous sub-lethal stressor or pharmacological stimulus. Preconditioning is categorized by the stimulus that elicits the protection such as ischemic, light preconditioning or hyperthermia. Preconditioning has been linked to trophic factor bioactivity (Ueki et al., 2009), phosphorylation of ERK 1 and 2, modulation of the pre-mitochondrial protein Bcl-xL (Káldi et al., 2003; Li et al., 2003), and activation of protein kinase C (PKC) in various cells (Dreixler et al., 2008). Protective preconditioning also correlates with non-esterified AA, linoleic acid, and lipoxygenase activity in rat myocardium (Murphy et al., 1995; Starkopf et al., 1998), suggesting that AA lipoxygenase products are involved in ischemic preconditioning via a PLA2 activation. DHA is also released during ischemic myocardium preconditioning (Murphy et al., 1995).
DHA is a product of PLA2 and a substrate of lipoxygenase activity, suggesting that DHA and docosanoids are involved in preconditioning. In fact, the free-pool size of DHA is increased and then form docosanoids, which play a critical role in survival signaling mechanisms during the protective actions of both in vitro and in vivo retinal preconditioning. Human RPE cells, this resilience is mitigated through 15-LOX-1 via DHA and involves the neurotrophin pigment epithelium-derived factor (PEDF), which also stimulates docosanoid production. Moreover, NPD1 prevents the loss of protection bestowed by 15-LOX-1 inhibition in vitro and protects PRCs from light damage, further suggesting that DHA and NPD1 are pivotal for the protective actions of cell survival during preconditioning.
Preconditioning guards retinal cells against oxidative stress and light damage. DHA and NPD1 enable cell survival in both in vitro and in vivo models of retinal preconditioning in a mechanism facilitated by 15-LOX-1, which synthesizes NPD1. This enabling also is enhanced by the PEDF, which stimulates synthesis of 17-hydroperoxy docosahexaenoic acid (17-HpDHA) and NPD1. Furthermore, a specific 15-LOX-1 inhibitor blocks this effect. The protective signaling demonstrated by preconditioning is specific to docosanoid signaling, despite the concomitant release of the omega-6 AA and eicosanoid synthesis (Knott et al., 2018).
5. Aberrant neuronal networks are restored by NPD1 in experimental epileptogenesis
Epileptogenesis is the latent period between an insult (such as traumatic brain injury, stroke, or infection) and the onset of clinical manifestations that often include generalized tonic-clonic seizures and interictal spikes (Dichter, 2009; Dudek and Staley, 2011). In addition, non-convulsive seizures and microseizures can also be present after brain injury in acquired epilepsies. Hippocampal electrical activity reflects the functionality of neuronal assembly (Buzsáki, 2010; Mizuseki et al., 2011; Sullivan et al., 2011). Thus, pathological brain oscillations during epileptogenesis reflect aberrant neuronal network activities that in turn lead to spontaneous recurrent seizures. Using multi-microelectrode arrays in freely moving mice and Golgi staining after status epilepticus (SE) induced by pilocarpine, we found that NPD1 reduces microseizures, pathological high-frequency oscillations (pHFO) and hippocampal dendritic spine loss.
Moreover, NPD1 restricted spontaneous recurrent seizures, the hallmark of epilepsy. Also, NPD1’s limited interneuronal cell loss, microgliosis, and in vivo evoked dentate gyrus (DG) electrical hyper-excitability. Thus, NPD1 rescues neuronal networks disruptions and this bioactivity may help contribute to identifying critical events in the onset of pathological circuit impairments including epileptogenesis (Bazan et al., 2011b; Musto et al., 2015, 2016). The progressive neuronal network alterations that lead to recurrent spontaneous seizures are the hallmark of epilepsy (Rakhade and Jensen, 2009) and exhibit electrical manifestations preceding seizures (Litt and Lehnertz, 2002), that includes pathological high-frequency oscillations (pHFO: >250 Hz) (Bähner et al., 2011; Bragin et al., 2010, 2000, 1999; Fisher et al., 1992; Jacobs et al., 2009; Litt et al., 2001; Litt and Lehnertz, 2002; Staba et al., 2002; Traub et al., 2001), propagation with neuronal networks recruitment (Dudek and Staley, 2011) and disruptions of synaptic homeostasis (Ramocki and Zoghbi, 2008).
High DHA consumption induces synaptic protection in Alzheimer’s disease models (Calon et al., 2004). NPD1 is decreased in CA1 area of hippocampus from Alzheimer’s disease (Bazan et al., 2011a), increases after seizures (Musto et al., 2011) and attenuates seizure severity progression and hippocampal hyper-excitability in a kindling model of temporal lobe epilepsy (Musto et al., 2011). Therefore, NPD1 restores neuronal network homeostasis during epileptogenesis. Using freely moving mice with implanted multiple microelectrode arrays (silicon probes) in hippocampal layers spontaneous microseizures and pHFO were attenuated by NPD1 administration during epileptogenesis. Also, hippocampal dendritic spine loss was limited by NPD1 and a striking NPD1-mediated attenuation of epileptic hyper-excitability and interneuronal loss in DG takes place. The responses in cells in different layers of the hippocampus to pathophysiological insults that lead to aberrant networks is crucial for understanding brain dysfunctions (Palop and Mucke, 2010; Roberson et al., 2011). Thus, cell-type-specific events underlying NPD1 bioactivity will contribute to identifying potential biomarker/s and therapeutic targets for epileptogenesis and other neurodegenerative diseases.
Physiological hippocampal field activity (Buzsáki et al., 2003) recorded through chronically implanted multi-microelectrode arrays after SE was disrupted showing fast ripples, interictal spikes, and bursts of spikes after three weeks post-SE (Shibley and Smith, 2002). Microseizures present in different hippocampal layers during epileptogenesis were characterized by a) simultaneous traces of high amplitude waves separated by a burst of spikes from stratum oriens associated with high amplitude spikes from the pyramidal layer; b) simultaneous traces from stratum radiatum-moleculare; c) spiking activity in the DG; and d) sinusoidal-like burst of spikes in DG or a group of high amplitude spikes associated with low amplitude background from the hilus. Thus,, these activity patterns resemble those of human epilepsy (Schevon et al., 2008; Stead et al., 2010). The microseizures showed a trend with theta oscillation and HFO (> 150 Hz) were detected in all hippocampal fields. However, only DG displayed a significant increase, specifically >200-300 Hz. pHFO (>200 Hz) reflects a synchronized burst-firing of aberrant neuron clusters mainly in DG (Bragin et al., 2011, 2000). Moreover, single unit activity from DG during the interictal period or free of microseizure activity after SE disclosed a bursting-like profile interrupted by a period of inactivity in epileptogenic mice compared with control mice. This bursting-like profile incrementally progressed and was interrupted by a silent period in a stepwise fashion.
Systemic administration of NPD1 modulates aberrant neuronal networks during epileptogenesis. NPD1 prevented disruption of ripple morphology and attenuated pHFO in DG. LFP represents excitatory post-synaptic potential activity from synchronized neurons (Buzsáki et al., 2004), and is thought to be a morphological signature of excitatory post-synaptic sites and damage in epilepsy (Kurz et al., 2008; Yuste, 2011). NPD1 remarkably limited dendritic spine loss in hippocampal layers. The effect of NPD1 on epileptogenesis led to SRS reduction at 3 weeks after SE onset. Moreover, NPD1 limited the number of hippocampal microseizures during epileptogenesis. This reduction in microseizures and pHFO was correlated with lower number of SRS. Also, NPD1 reduced the amplitudes of microseizures in hippocampal layers and limits the development of hippocampal hyper-excitability in a rapid kindling model of temporal lobe epilepsy (Musto et al., 2011) that displays a subtle alteration in hippocampal neuronal plasticity and damage (Morimoto et al., 2004). Loss of structural plasticity and cellular inhibition of granular cells takes place in DG in epilepsy (Covolan and Mello, 2000; Danzer et al., 2010; Thind et al., 2010). We stimulated the dorsal DG with sub-convulsive electrical impulses after SE in freely moving rats and observed sustained severe seizures and electrical responses in 93% of the animals after 14 days of SE. We then stimulated the DG and the evoked responses were recorded and found that NPD1 reduced after-discharge duration and the number of evoked spikes recorded after each stimulation from DG. Moreover, NPD1 treated animals preserved the integrity of interneuronal cell bodies in the DG hilus and showed attenuated gliosis and microgliosis.
The experimental approach followed here sets in motion injury-initiated cellular and molecular cascades that induce the generation of abnormal, synchronous and recurring epileptiform discharges (Sloviter and Bumanglag, 2013). NPD1 administration for 5 days beginning 24 h after SE was able to reduce SRS. Therefore, our study indicates that there is a therapeutic window for a down regulating of epileptogenesis during the first week after SE under the present conditions.
The aberrant neuronal network activity observed here from LFP recordings reflects the linearly summed postsynaptic potential from small populations of principal cells in epileptogenesis (Buzsáki et al., 2004; Kraskov et al., 2007; Lindén et al., 2011). The aberrant morphology of the electrical activity may be a marker of epileptogenesis (Bragin et al., 2010, 2000, 1999; Dichter, 2009; Jiruska and Bragin, 2011). Excitatory synaptic activity in the hippocampus takes place in dendritc spines (Matsuzaki et al., 2004; Takahashi et al., 2012; Yuste, 2011), and dendritic spine reorganization in DG occurs during seizure susceptibility development and SRS (Danzer et al., 2008; Isokawa, 2000, 1998). Micro-electrical activity during initiation of epileptic discharges observed in cytoarchitectonic layer IV of the visual cortex and layer V/VI of somatosensory cortex (Chatt and Ebersole, 1982) with robust dendrite plasticity and connections to and from other circuits, suggests high post-synaptic activities (Galvez et al., 2006). Moreover, dendritic spine rearrangement could result from early dendritic spine loss due to seizures (Guo et al., 2012; Kurz et al., 2008; Swann et al., 2000; Zeng et al., 2007). Our results suggest that NPD1 rescues vulnerable dendritic spines overactivated after SE (Mattson and Duan, 1999; Tao and Rolls, 2011). NPD1 reduces oxidative stress-induced apoptosis (Mukherjee et al., 2004) by modulation of Bcl-2 family of proteins (Anthony et al., 2011; Lukiw et al., 2005). We therefore hypothesized that NPD1 upregulates anti-apoptotic signaling in dendrites, and, as a consequence, counteracts the formation of aberrant neuronal network activity.
Since BDNF, NGF, NT3 and other neurotrophins are NPD1 synthesis agonists, this survival mechanism of NPD1 might potentiate neurotrophin activity (Mukherjee et al., 2007). Likewise, it could downregulate cyclooxgenase-2 activity during epileptogenesis (Serrano et al., 2011; Tu and Bazan, 2003) and limit neuroinflammatory signaling (Marcheselli et al., 2003). However, the precise molecular mechanism of NPD1 bioactivity in aberrant plasticity and dendritic injury in epileptogenesis should be defined.
pHFO one week after SE have similar profiles from those observed in the neocortex of chronic epileptic patients (Jacobs et al., 2009; Schevon et al., 2008; Stead et al., 2010) or from DG several weeks after intra-hippocampal injection of kainic acid (Bragin et al., 2010). pHFO may arise from localized small aberrant clusters of neurons (Jiruska and Bragin, 2011; Worrell et al., 2008). pHFO represent a periodic field of hyper-synchronized action potentials of principal cells; bursting neurons at low frequencies but in anti-phase (Foffani et al., 2007; Staley, 2007) or desynchronization of busting cells firing at lower frequencies (Ibarz et al., 2010). These neuronal activities could be enhanced by excitatory discharges to granule cells (Scimemi et al., 2006) and a lack of discharge for interneuron basket cells (Bragin et al., 2000), suggesting an imbalance of excitatory and inhibitory synaptic neurotransmission in DG (Stacey et al., 2011). Pyramidal cells can evoke epileptiform activity independent of neuronal connectivity (Miles and Wong, 1984). HFO can be observed when CA3 input to CA1 is interrupted, suggesting that the local network is engaged in the genesis of HFO (Maier et al., 2011). The loss of inhibitory modulation from sub-populations of interneurons after SE may facilitate pHFO mechanisms (Bragin et al., 2010). Although pHFO is postulated as a biomarker for epileptogenesis, it is not clear if pHFO is a cause or a consequence of epileptogenesis. However, such inhibitory activities are overwhelmed and subsequently fail (Trevelyan et al., 2006). Our results show that attenuation of pHFO during epileptogenesis by NPD1 limits SRS. One possible mechanism is that NPD1 contributes to improving the synchronicity of the basket cell network on pyramidal cells (Trevelyan, 2009) and thereby attenuates pHFO by modulating excitatory-inhibitory balance during epileptogenesis (Stark and Bazan, 2011).
DG contributes to spread seizures severity due to impairment of gamma-aminobutyric acid (GABA) inhibition (André et al., 2001; Buckmaster et al., 2009; Doherty and Dingledine, 2001; Lévesque et al., 2011; McAuliffe et al., 2011). NPD1 reduced the DG hyper-excitability after SE. Proper modulation of DG granule cell firing by GABAergic interneurons is decreased due to loss of specific interneuron populations or proper synaptic connectivity in that region (Bragin et al., 2011). This study demonstrated that reduced DG hyper-excitability by NDP1 was associated with protection of somatostatin and calretinin interneuronal cells in DG. Also, our observations support the evidence that DHA reduces those vulnerable interneuronal cell populations and NPD1 could be the mediator of that neuroprotection (Ferrari et al., 2008).
The observed concomitant microseizures and pHFO reflect aberrant network activities in hippocampal layers and the pHFO may act as an endogenous marker of a kindlinglike mechanism that recruits and synchronizes other aberrant neuronal networks, triggering SRS. Future work on NPD1 bioactivity will further explore its potential for the development of new therapeutic approaches for circuitry impairment as in epileptogenesis, Alzheimer’s and other neurodegenerative diseases.
6. Parkinson’s disease (PD)
Often PD clinical presentation takes place when 80% of striatal dopamine is reduced implying that sizable numbers of neurons are damaged early in the disease pathogenesis. Aiming to understand potential early events we have study the effects of NPD1 in experimental models using 1-methyl-4-phenylpyridinium ion (MPP+) or its precursor 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity. We have used differentiated primary neuronal culture from mesencephalic embryonic mice that displays 17–20 % of TH-positive neurons and shows potently retracted dendritic arbor distal segments by Sholl analysis, thus decreasing the maximum branch order reached. Under these conditions, we uncovered that NPD1 (100 nM) rescue dopaminergic neurons from apoptosis and sustains dendritic arbor of surviving neurons (Calandria et al., 2015). These findings suggest that mesencephalic TH-positive neurons cell damage and death is not a direct consequence of mitochondrial dysfunction alone (rotenone does not elicited a similar effect). The NPD1 bioactivity is currently being explored to prevent early loss of dopamine neurons and neurodegeneration in PD.
7. Alzheimer’s disease: Neuroprotection by NPD1
The first clear involvement of lipid mediators, or of any other brain-generated molecule, potentially beneficial in AD came from studies using brain samples from donors that judging from their plaque and tangle assessment were from moderate AD disease development (Zhao et al., 2011). Moreover, because fatty acids are generated rapidly postmortem, the sampling time was monitored by RNA spectral quality. Unesterified DHA levels were found to be higher in AD hippocampal CA1, whereas NPD1 levels in AD were one-twentieth of those in age-matched controls (Lukiw et al., 2005). Thus, a drop out of 11–41% of neurons is insufficient to account for the 20-fold reduction in NPD1 pool size in AD hippocampus when compared with age-matched controls. Therefore, despite some decreased availability of unesterified DHA, NPD1 levels were dramatically reduced, perhaps as the result of excessive oxidative stress and other events, and NPD1 beneficial bioactivity during neural cell degeneration may be lost. In these same human CA1 hippocampal samples, we also examined the levels of expression of a cytosolic changes in gene-expression patterns in Aβ42-, DHA-, or NPD1-stressed HN cells Gene Main Function Apoptotic Inflammatory Aβ42 + DHA + NPD1 Pro- Anti- HN cells were treated with Aβ42, DHA, or NPD1. Increases for Aβ42-, DHA-, or NPD1-treated HN cells are expressed as fold increases over untreated, age-matched controls. CEX-1 (GenBank U64197) is a marker for inflammatory and oxidative stress responses (Colangelo et al., 2002; McGeer and McGeer, 2003), and B94 (GenBank M92357) is a TNF-α-inducible proinflammatory element (Colangelo et al., 2002; Liao et al., 2004). Genes are classified according to major functions although many have multiple cellular roles. Analytical criteria were gene changes of at least 2-fold over control and P less than 0.05 (ANOVA) in Aβ42- stressed PLA2 (cPLA2) and 15-LOX, 2 key enzymes in the mobilization of DHA and NPD1 biosynthesis. In AD brain, when compared with age-matched controls, cPLA2 abundance was increased 4.6-fold and 15-LOX decreased almost 2-fold (P < 0.05). Decreased abundance of NPD1 in AD brain may be explained, at least in part, by a disruption in the expression and regulation of the PLA2 and/or 15-LOX-like enzymes essential for NPD1 biosynthesis.
These noxious stimuli further orchestrate pathogenic gene-expression programs in stressed brain cells, thereby linking a cascade of caspase-mediated cell death pathways with apoptosis and neuronal demise (Bazan and Lukiw, 2002; Lahiri et al., 2003). Neural mechanisms leading toward NPD1 generation from DHA thereby appear to redirect cellular fate toward successful brain cell aging. The Bcl-2 pro- and antiapoptotic gene families, sAPPα, and NPD1 lie along a cell fate–regulatory pathway whose component members are highly interactive, and have potential to function cooperatively in brain cell survival, acting through modulation of Aβ42-directed pathogenic events. Taken together, these data suggest that NPD1 induces an antiapoptotic, neuroprotective gene-expression program that regulates the secretion of Aβ peptides, resulting in the modulation of inflammatory signaling, neuronal survival, and the preservation of brain cell function. Agonists of NPD1 biosynthesis or NPD1 analogs may be useful for exploring new therapeutic strategies for AD and related neurodegenerative disease. HN cells, a primary coculture of human neurons and glia, are useful in vitro test system to study stress mechanisms during human brain cell development, aging, and AD (Bazan and Lukiw, 2002). DHA downregulates secretion of Aβ peptides from aging HN cells. Amyloidogenic Aβ peptides were progressively secreted from HN cells into the incubation medium throughout 8 weeks of culture. The amyloid plaque of AD shows Aβ40/Aβ42 enriched ratio (Guo et al., 1998; Selkoe and Kopan, 2003) and was about 10:1 throughout the 8-week period of cell incubation. After the addition of IL-1β, a potent inducer of reactive oxygen species and a promoter of oxidative stress a time-dependent release of both Aβ40 and Aβ42 was found. DHA attenuated Aβ peptide release. During oxidative stress in human RPE and ischemia/reperfusion in the brain, NPD1 elicits neuroprotection, suggesting that in aging HN cells, attenuation of the Aβ peptide neurotoxicity by DHA could be mediated, at least in part, by NPD1. Furthermore, NPD1 biosynthesis is enhanced by the neurotrophic peptide sAPPα, a 612-amino acid fragment derived from α-secretase-mediated cleavage of βAPP (Guo et al., 1998; Selkoe and Kopan, 2003). sAPPα promotes neuritogenesis and long-term survival of neurons in culture and protects brain cells against the toxicity of Aβ40 and Aβ42 peptides and excitotoxic and ischemic injury both in cell cultures and in vivo (Guo et al., 1998; Stein and Johnson, 2003). sAPPα generated via the α-secretase pathway does not give rise to the shorter amyloidogenic Aβ peptides; hence, the shunting of βAPP into the α-secretase pathway may be beneficial by the relative lowering of Aβ peptide abundance (Bahr et al., 1998; Bazan, 2003; Selkoe and Kopan, 2003; Stein and Johnson, 2003). Some of the neurotrophic activity of sAPPα may be elicited, at least in part, by an upregulation in the biosynthesis of NPD1 as a complementary cell-survival mechanism activated early in AD pathogenesis. sAPPα may activate NPD1 biosynthetic enzymes PLA2 and/or a 15-LOX– like enzyme integral to NPD1 biosynthesis (Calandria et al., 2009; Marcheselli et al., 2003). It is interesting that muscarine, a positive regulator of PLA2, is also a potent inducer of sAPPα in human neuroblastoma SH-SY5Y cells (Webster et al., 2002; Wood et al., 2000); therefore, the enzymatic pathways involving PLA2-mediated DHA and NPD1 biosynthesis may exhibit positive feedback regulation through sAPPα. sAPPα also protect neural cells against the proapoptotic thapsigargin, an inhibitor of the endoplasmic reticulum Ca2+-ATPase, and the adverse effect of thapsigargin can be abolished in cells overexpressing antiapoptotic Bcl-2 (Guo et al., 1998). Aβ42 elevated NPD1 pool size in the presence of added DHA. This action of Aβ42 peptide may represent a cytoprotective response of brain cells when confronted with a peptide that triggers oxidative stress. The unesterified DHA pool size assessed by liquid chromatography-photodiode array-electrospray ionization-tandem mass spectrometrybased (LC-PDA-ESI-MS-MS-based) lipidomic analysis (Marcheselli et al., 2003; Mukherjee et al., 2004) showed that neither Aβ42 nor sAPPα was able to promote unesterified DHA pool-size changes, indicating that a tight regulation of unesterified DHA may take place when sAPPα (100 μM) activates NPD1 production in the absence of added exogenous DHA.
The Aβ42 peptide-mediated apoptosis in both neurons and glia were shown to be counteracted by NPD1 to protect HN cells against Aβ42-induced cytotoxicity. Thus, 3-week-old HN cells were incubated for an additional 3.5 days in serum-free HN cell maintenance medium (HNMM) made 8 μM in Aβ42 peptide. Because selective cell loss may take place in older HN cell cultures (when neuronal cells drop out), the use of HN cells at a fixed age (and 50:50 neuronal/glial populations) was selected to minimize this possibility. Apoptosis was found to occur in both neurons and glia. When NPD1 (50 nM) was added to this test system, NPD1 protected both neurons and glia from Aβ42-directed apoptosis, as evidenced by quantification of Hoechst 33258 staining of compacted nuclei in control, Aβ42-treated, and Aβ42+NPD1-treated cell fields. Unlike control HN cells, Aβ-treated HN cells also exhibited retracted neurites; however, when treated with NPD1, cells assumed extended neurites and an overall morphology resembling that of control cell.
8. DHA and NPD1 induce pro-homeostatic gene-expression
Proinflammatory and apoptosis-related gene-expression in 4-week-old HN cells are enhanced after exposure to Aβ42 (Colangelo et al., 2002; Liao et al., 2004), whose RNA levels are upregulated in AD-patient brains (Colangelo et al., 2002), as well as Bcl-2 antiapoptotic (Bcl-xl, Bcl-2, and Bfl-1 (A1)) and proapoptotic (Bax and Bik; (Akhtar et al., 2004; Guo et al., 1998)). Aβ42 markedly upregulated a complex proapoptotic and proinflammatory gene-expression program. These analyses indicated significant Aβ42-mediated upregulation in the expression of a family of genes encoding the cytokines IL-1β, CEX-1, and TNF-α, COX-2, B94, and the proapoptotic Bax and Bik proteins (Guo et al., 1998; Metcalfe et al., 2004; Tarte et al., 2004). DHA and NPD1 each showed upregulation of Bcl-xl, Bcl-2, and Bfl-1 (A1), neuroprotective members of the Bcl-2 gene family, and relative down-regulation of Bax and Bik, proapoptotic members of the Bcl-2 gene family. Bax and Bik were upregulated, respectively, in Aβ42-treated cells over age-matched control cells, and these enhanced RNA levels were driven to the status of “no significant change” after treatment with DHA or NPD1. The antiapoptotic Bcl-2 family member Bfl-1 (A1) was upregulated by DHA and NPD1 to about 4- and 6-fold. In HN cells, DHA was induced a 5-fold increase in NPD1 at 4 weeks of culture; DHA decreased Aβ40 and Aβ42 peptide secretion from aging brain cells with concomitant NPD1 biosynthesis. In turn, NPD1 inhibited Aβ42-induced apoptosis in both glial and neuronal cell populations in a stressed HN cell model are in concordance with the findings of others on apoptosis in glial and neuronal cells in AD brain (Kitamura et al., 1999; Kobayashi et al., 2004; Paradisi et al., 2004). The brain samples used for lipidomic analysis, while the senile plaque densities in the CA1 region for control tissues used averaged 0.25 lesions/mm2, the senile plaque densities in the CA1 for all AD tissues used in the lipidomic study be achieved by decreasing γ-secretase activation, by decreasing anti-oxidative defenses, or both (Hashimoto et al., 2005; Lim et al., 2005). Along those lines, recent studies indicate that DHA attenuates neuronal degeneration and rescues learning ability in rodent models of AD (Calon et al., 2004; Hashimoto et al., 2005; Lim et al., 2005). Moreover, NPD1 (Bazan, 2005; Marcheselli et al., 2003; Mukherjee et al., 2004) is formed, while DHA downregulates Aβ release in aging HN cells in culture. Since NPD1 inhibits Aβ42-induced apoptosis in HN cells, DHA protection in cells in culture and in in vivo models may involve NPD1 synthesis.
Importantly, no significant change in neuronal and glial cell morphology in these cultures after 18 h of Aβ42 (or NPD1) treatment took place, and there was no sizable cell death at the concentrations of Aβ42 used over the time course investigated suggesting that Aβ42 is setting in motion potential cell damaging signals accompanied by the onset of apoptosis, and changes in gene-expression that in part emulate neurodegeneration as in AD. Secreted Aβ40 and Aβ42 during HN cell aging has implications in the development of Aβ-related neuropathology and resembles Aβ deposition during brain aging and in AD (Crapper et al., 1975; Pelvig et al., 2003; Terry, 1983). Intracellular processing of the transmembrane glycoprotein βAPP through a sequential β- and γ- secretase–catalyzed proteolysis generates Aβ peptides that are subsequently shuttled to the plasma membrane and secreted (Bazan, 2003; Stein and Johnson, 2003).
Interestingly, exposure of HN cells to the glial cell-derived, pro-inflammatory cytokine IL-1β significantly stimulated both Aβ40 and Aβ42 secretion as a function of HN cell aging. IL-1β directly stimulates γ-secretase-mediated cleavage of βAPP into Aβ peptides through a JNK-dependent MAPK pathway (Liao et al., 2004). Conversely, DHA suppressed both Aβ40 and Aβ42 peptide release. DHA attenuates lipid peroxides and reactive oxygen species in the cerebral cortex and the hippocampus of Aβ-infused rats, suggesting that DHA elicits neuroprotection by blocking Aβ40/Aβ42 neurotoxicity (Calon et al., 2004; Hashimoto et al., 2005) (Calon et al., 2004; Hashimoto et al., 2005).
Pro- and anti-apoptotic proteins are modulators proximal to mitochondria and irreversible cell damage. Pro-apoptotic proteins Bik and Bax were enhanced by Aβ42, but not by DHA or NPD1, whereas Bcl-2, Bcl-xl, and Bfl-1(A1) were enhanced by DHA. NPD1, on the other hand, promoted a much larger increase in anti-apoptotic Bcl-2 proteins. Bfl-1(A1) increased almost 6-fold. Anti-apoptotic Bcl-2 family members such as Bfl-1(A1) play critical roles in the survival of aged and terminally differentiated cells and break the mechanistic link between inflammatory signaling and apoptosis (Tarte et al., 2004). In fact, NPD1 also induces the anti-apoptotic Bcl-2 family of proteins Bcl-2 and Bcl-xl in oxidatively challenged human RPE cells (Mukherjee et al., 2004) and promotes cytoprotection. A further suggestion of the significance of NPD1 in AD is the observation that hippocampal CA1 from AD patients shows a dramatic reduction in NPD1. Whether decreased NPD1 levels in AD-brain hippocampal CA1 are the result or the cause of the AD process remains to be clarified. Since these tissues were sampled within 3 h postmortem, the NPD1 pool size may reflect the capacity of the CA1 hippocampal region to activate synthesis of the mediator.
In mouse brain undergoing ischemia/reperfusion, NPD1 increases during the initial 8 hours after 1 h of ischemia (Marcheselli et al., 2003). In the post-mortem brain, the differences found between age-matched controls and AD brains point to the relative inability of the AD CA1 region to accumulate NPD1.
Therefore, the interplay of DHA-derived neuroprotective signaling aims to counteract proinflammatory, cell-damaging events triggered by multiple, converging cytokine and amyloid peptide factors in AD. Amyloid peptide-mediated oxidative stress, the activation of microglia associated with Aβ peptide deposition, and excessive production of microglial-derived cytokines such as IL-1β and TNF-α support progressive inflammatory episodes in AD (McGeer and McGeer, 2003; Zhang et al., 2002; Kim et al., 2003; Hong et al., 2003). These noxious stimuli further orchestrate pathogenic gene- expression programs in stressed brain cells, thereby linking a cascade of caspase-mediated cell death pathways with apoptosis and neuronal demise (Bazan and Lukiw, 2002; Colangelo, 2002; Lahiri et al., 2003). NPD1 biosynthesis from DHA seems to redirect brain cell fate toward counteracting cell-aging events. The Bcl-2 pro- and antiapoptotic gene families, sAPPα, and NPD1 lie along a cell fate-regulatory pathway highly interactive, and that have potential to function cooperatively in brain cell survival, through modulation of Aβ42-directed pathogenic events and other disruptors of homeostasis. Taken together, these data suggest that NPD1 induces an anti-apoptotic, neuroprotective gene-expression program that includes modulation of uncompensated oxidative stress and regulation of the secretion of Aβ peptides, resulting in the modulation of inflammatory signaling, neuronal survival, and the preservation of brain cell function. Agonists of NPD1 biosynthesis or NPD1 analogs may be useful for exploring new therapeutic strategies for AD and related neurodegenerative disease.
9. ELOVL4
Neurons and PRCs express the elongase enzyme ELOVL4, which catalyzes the biosynthesis of (≥C28) omega-3 (n-3) very long chain polyunsaturated fatty acids (VLC-PUFAs). These fatty acids become acyl chains of PC and sphingolipids and are enriched in the inner segment of PRCs. ELOVL4 synthesizes VLC-PUFAs in the retina (Agbaga et al., 2010, 2008; Aveldaño, 1987) and testes (Oresti et al., 2010), and it synthesizes very-long-chain saturated fatty acids (VLC-SFAs) in the skin and brain (Cameron et al., 2007; Monroig et al., 2010). The ELOVL4 protein is targeted, via its C-terminal di-lysine motif KXKXX, to the ER for elongation via a four-step cyclical process of condensation, reduction, dehydration and reduction, yielding a fatty acid elongated by two C. The initial condensation reaction and rate-limiting step is catalyzed by an elongase and mediated by iron-coordinating histidines in the active site, which condenses malonyl CoA (the two-C donor) and a fatty acyl-CoA to yield a 3-keto-acyl-CoA intermediate. The 3-keto compound is then reduced to the 3-hydroxy product, dehydrated to a trans-2,3-enoyl fatty acyl-CoA, which is further reduced to form a fatty acid two C longer than the precursor. The initial and final reduction steps are catalyzed by 3-keto-acyl-CoA reductase (KAR), trans-2,3-enoyl-CoA reductase (TER) enzymes, respectively, both of which require NADPH as a cofactor. The dehydration step is carried out by one of four different 3-hydroxyacyl-CoA dehydratases (HACD1, HACD2, HACD3, and HACD4), and the chain length of the final product is determined by the particular elongase that catalyzes the reaction.
10. ELOVL4 mutations
ELOVL4 mutations cause spinocerebellar ataxia (Bourassa et al., 2015; Cadieux-Dion et al., 2014; Ozaki et al., 2015) impaired neural development, neuronal dysfunction, hyper-excitability, seizures (Agbaga, 2016; Aldahmesh et al., 2011) juvenile macular degeneration in autosomal dominant STGD3, with loss of central vision, progressive degeneration of the macula and peripheral retina (Agbaga et al., 2008, 2010; Agbaga, 2016; Aveldaño, 1987; Bernstein et al., 2001; Cameron et al., 2007; Edwards et al., 2001; Maugeri et al., 2004; Monroig et al., 2010; Zhang et al., 2001), and early functional defects in RPE and PRCs (Kuny et al., 2015).
Mutations in ELOVL4 have been proposed to cause dominant STGD3 due to loss of its C-terminal endoplasmic reticulum (ER) retention signal, leading to mislocalization (Agbaga, 2016; Agbaga et al., 2010, 2008; Ambasudhan et al., 2004; Karan et al., 2005; Vasireddy et al., 2005) of the truncated ELOVL4 protein that in turn causes uncompensated cellular stress and PRC death. Alternatively, mislocalization of an enzymatically active truncated ELOVL4 protein from the ER leads to accumulation of toxic products (i.e., 3-keto intermediates) because the truncated protein still contains the putative active site. Production and accumulation of these toxic keto intermediates by the truncated ELOVL4 could be an additive insult to the overall reduction in the ELOVL4-derived products, i.e. VLC-PUFAs. Furthermore, ELOVL4 KO mice display VLC-PUFA-deficient PRC terminals with reduced rod terminal vesicles and a disorganized outer plexiform layer (Agbaga et al., 2014; Bennett et al., 2014).
After VLC-PUFAs are generated via ELOVL4, they are incorporated into phospholipids in the PRC inner segment, where they become part of the PRC outer membrane biogenesis (Aveldaño, 1987) and tightly interact with rhodopsin (Aveldaño, 1988), and are assumed to be important in PRC longevity, PRC synaptic function, and neuronal connectivity. However, the mechanisms by which VLC-PUFAs exert this important protective role remain unknown. Herein, we have explored an alternative mechanistic rationale for the significance of ELOVL4 in PRC survival. The genetic ablation of AdipoR1 leads to depletion of the PCMS that contains C32:6n3 or C34:6n3, and DHA (C22:6n3), which in turn leads to photoreceptor degeneration that resembles various human forms of retinal degenerative diseases (Rice et al., 2015). Thus, shortage in key protective mediators derived from VLC-PUFAs may be a key factor in these diseases.
DHA recycles daily during PRC outer-segment renewal through the RPE. Upon sensing the need for pro-homeostatic signaling, DHA also serves as the precursor for docosanoids (e.g., NPD1). DHA also can be the target of excessive oxidative damage (leading to the formation of protein adducts) that evolves into retinal pathology. Most of the known lipids mediators, including prostaglandins, resolvins and NPD1, are derived from 20-22 C length fatty acid precursors. We have found a family of bioactive lipid mediators in retina that we call elovanoids (ELVs). ELV-N32 and ELV-N34 are di-hydroxylated derivatives of 32:6n-3 or 34:6n-3 length fatty acid precursors, respectively. The precursors of ELVs are made by the elongase ELOVL4 that converts C26-derived fatty acids from eicosapentaenoic acid (EPA) or DHA to very long chain polyunsaturated fatty acids (≥C28). PRCs express this evolutionarily conserved enzyme. Mutant ELOVL4 causes juvenile macular degeneration in autosomal dominant STGD3 and early functional defects in RPE cells and PRCs.
11. Elovanoids
ELVs are a new class of endogenous pro-homeostatic lipid mediators that counteract UOS, oxygen glucose deprivation (OGD), N-methyl-d-aspartate (NMDA)-induced excitotoxicity or MCAo-induced ischemic stroke. ELVs are made rapidly when disruptors of homeostasis evolve and when brain or retinal cells need to counteract neuroinflammatory responses to protect their integrity and to sustain survival and brain/retina functions.
Bifurcation pathways from PC by phospholipase A1 (PLA1) (blue, to ELVs) or PLA2 (yellow, to NPD1 or other docosanoids). DHA through ELOVL4 leads to the synthesis of 32:6n-3, 34n-3 and other very-long-chain polyunsaturated fatty acids containing omega-3s (VLC-PUFAs,n-3). These fatty acids are then esterified at the C1 (sn-1) position of PC, which has DHA in the C2 (sn-2) position. PLA1 or/and PLA2 release the fatty acids that lead to the formation of either ELVs, NPD1, or other docosanoids. Then, free 32:6n- 3 and 34n-3 result in the synthesis of ELV-N32 or ELV-N34 respectively. A hydroperoxyde intermediate and a putative GPCR for ELVs and NPD1 are depicted.
ELVs are di-hydroxylated derivatives of 32:6n-3 or 34:6n-3 biosynthesized in the brain by ELOVL4, an enzyme essential for the synthesis of very long chain-fatty acids with chain lengths ≥ 28 carbons that is mainly expressed in neurons and enriched in the hippocampus (Pastore et al., 2017). These ≥ 28 carbons VLC fatty acids are critical to brain function (Bhattacharjee et al., 2017; Demaerschalk et al., 2016; Jun et al., 2017; Poulin et al., 2016; Rau et al., 2012; Tasca et al., 2015; Tasic et al., 2016; Zeisel et al., 2015; Zhang et al., 2014). Moreover, mutations in ELOVL4 are causative of developmental or neurodegenerative disorders. Heterozygous inheritance of ELOVL4 mutations causes Stargardt-like Macular Dystrophy or Spinocerebellar Ataxia type 34. Homozygous inheritance of ELOVL4 mutations causes seizures, intellectual disability, ichthyosis, and premature death. However, biologically active derivatives and mechanisms for the function of ELOVL4 and of its products has remind elusive. Thus, 32:6n-3 or 34:6n-3 yield ELV-N32 and ELV-N34, respectively. ELVs are unlike any known lipid messenger since most of them are made from 20-22 C length precursors. The specific mechanisms that need to be defined are how the biosynthesis of the novel neuroprotective ELVs takes place and is regulated, and which cell/s are targeted. The first mechanism is the reservoir of the ELV precursors: that is, a PC molecular species that contains VLC-PUFAs,n-3 (precursors of ELVs) at the C1 and DHA at the C2 position. Whether or not cellular sensing of ischemic-reperfusion in ischemic stroke activates the release of one of these ELVs or of both, and the timing of this release, are unknown. Therefore, our goal is to test whether the ELVs might be part of a heretofore-unexpected signal bifurcation mechanism that aims to sustain neural cell integrity.
The complete structures and stereochemistry of elovanoids ELV-N32 and ELV-N34 from neuronal cultures were established through a direct comparison with compounds prepared via stereocontrolled total organic synthesis by adapting our previous method (Petasis et al., 2012). ELV-N32 and ELV-N34 were prepared by stereo-controlled total chemical synthesis. These synthetic ELVs with fully defined structures and stereochemistry allowed us to determine the complete R/S configuration as well as the Z/E geometry of the double bonds in these mixed neuronal cell culture-derived ELVs. Further validation of these structural assignments was established by synthesizing stereochemically pure deuterium-labeled ELVs, and by matching them with endogenously produced molecules in neuronal cultures by liquid chromatography tandem mass spectrometry (LC-MS/MS), we further confirmed their structure and stereochemistry. ELVs and their precursors were detected in neuronal cultures under OGD stress (Jun et al., 2017). We used m/z 499 -> 93 and 499 -> 401 MRM transitions for ELV-N32 detection, and m/z 527 -> 93 and 527 -> 429 transitions for ELV-N34 detection. For their corresponding mono-hydroxy precursors, we used m/z 483 -> 385 for 27-hydroxy-C32:6n3, and m/z 511 -> 413 for 29-hydroxyl-C34:6n3. For further identification, we performed full fragmentation on ELVs and found good matches to the synthetically produced standards. Both ELVs had UV maxima at 275 nm that are consistent with a conjugated triene structure. Following matching of synthetic ELVs with ELVs derived from mixed neuronal cells in culture, the complete structure and stereochemistry of ELV-N32 and ELV-N34 were established. The structures of ELV-N32 and ELV-N34, derived from a 34, were determined to be: ELV-N32: (14Z,17Z,20R,21E,23E,25Z,27S,29Z)-20,27-dihydroxydo-triaconta-14,17,21,23,25,29- hexaenoic acid; and ELV-N34: (16Z,19Z,22R,23E,25E,27Z, 29S,31Z)-22,29-dihydroxytetra-triaconta-16,19,23,25,27,31-hexaenoic acid.
12. Docosanoids and ELVs in experimental ischemic stroke
Stroke is the foremost cause of long-term disability in the United States (Demaerschalk et al., 2016), and it is also a burden to the healthcare system, with direct medical stroke-related costs expected to nearly triple from $71.6 to $184.1 billion between 2012 and 2030 (Demaerschalk et al., 2016). Hospitalizations for ischemic stroke increased among adolescents and young adults (aged 5-44 years) between 1995 and 2008 (Demaerschalk et al., 2016; Lloyd-Jones et al., 2010), underscoring the importance and urgency of stroke-related research. Focal ischemic stroke leads to impaired sensorimotor and cognitive functions with 70-80% of patients displaying hemiparesis soon after stroke. Stroke affects men and women equally up to the age of menopause; thereafter females are more susceptible to stroke.
Reperfusion after experimental ischemic stroke triggers lipid peroxidation and cellular damage including neuronal injury. DHA from membrane phospholipids during brain ischemia is a source of lipid peroxides. Leukocyte infiltration and enhanced pro-inflammatory gene expression also contribute to stroke damage. We have identified stereospecific messengers from docosahexaenoate-oxygenation pathways in a mouse stroke model. Widely used to prevent cerebrovascular disease, aspirin activates an additional pathway, which includes the 17R-resolvins. The newly discovered brain messenger 10,17S-docosatriene potently inhibited leukocyte infiltration, NF-κB, and cyclooxygenase-2 induction in experimental stroke and elicited neuroprotection. Further, in neural cells in culture, this lipid messenger also inhibited both interleukin 1-β-induced NF-κB activation and cyclooxygenase-2 expression. Thus, the bioactive docosanoids generated in vivo counteract leukocyte-mediated injury as well as pro-inflammatory gene induction. These results not only contest the notion that DHA participates solely in brain damage but also demonstrate that this fatty acid is the endogenous precursor to a neuroprotective signaling response mediator to ischemia-reperfusion.
Now, we have demonstrated in ischemia-reperfusion brain the formation of stereospecific DHA-oxygenation pathways that lead to the synthesis of novel messengers. We uncovered two DHA-oxygenation pathways: the first pathway forms the messenger 10,17S-docosatriene, and the second pathway, active in the presence of aspirin, formation a resolvin-type messenger (17R-DHA) as we called it then. The pathways have the potential for exerting counter-regulatory cellular and molecular signaling in brain injury. The data at that time demonstrated that the novel 10,17S-docosatriene is a potent inhibitor of ischemia-reperfusion-induced PMN infiltration and pro-inflammatory gene induction and that it inhibits cytokine-mediated pro-inflammatory gene activation in neural cells in culture. Overall, 10,17S-docosatriene potently elicited neuro-protection in vivo by reducing the stroke infarct volume 48 h after MCAo (middle cerebral artery occlusion).
Aspirin enhanced formation in brain of 17R-series resolvins that were found at that time to be cytoprotective and are counter-regulators of inflammation outside the nervous system (Serhan et al., 2000). Still, now a days it could be argued that these compounds enhance the actions of docosatrienes (NPD1 mainly), which are the counter-regulatory mediators generated from DHA to decrease leukocyte recruitment and to limit leukocyte-mediated inflammation and brain damage. The implied switch, from endogenous to aspirin-triggered DHA-derived lipid mediators that enhance this protective action, continuous to be of interest given the wide use of aspirin.
The synthesis of 10,17S-docosatriene after 1 h of MCAo coincides with free DHA availability that results from phospholipase A2 activation (Bazán, 1970; Bazan and Allan, 1998; Yoshida et al., 1984) as well as with reperfusion reoxygenation. The endogenous brain synthesis of 10,17S-docosatriene that peaks at8hof reperfusion may be a response of such insufficient magnitude as to counteract leukocyte infiltration and pro-inflammatory gene induction under the present experimental conditions. Thus, the relatively large ischemic insult produced by 1 h of MCAo followed by several h of reperfusion may overcome the ability of the endogenously generated docosanoids to elicit neuroprotection. Therefore, exogenous administration of 10,17S-docosatriene directly into the cerebroventricular system through continuous infusion during the initial 2 days of reperfusion did indeed exert neuroprotection. During 24–72 h of reperfusion is when most brain leukocyte infiltration occurs (Chatzipanteli et al., 2000; Chopp et al., 1996; Matsuo et al., 1994; Royo et al., 1999). Leukocytes accumulate in an area surrounding the infarct and are thought to possess a multifactorial ability to promote injury in brain ischemia-reperfusion (Hallenbeck and Kochanek, 1998). Moreover, PMN in-filtration as well as amoeboid microglia along the edges of the stroke infarct may be responsible for expansion of the penumbra region, which occurs from 16 h onward after MCAo followed by reperfusion (Mabuchi et al., 2000). Here we demonstrate that 10,17S-docosatriene administered continuously by intracerebroventricular perfusion inhibited such infiltration and subsequently reduced the stroke volume by 50% after 48 h. The amount of docosanoid infused was 1 g over a 48-h period at a rate of 250 nl/h. This observation implies that 10,17S-docosatriene is neuroprotective. Once these leukocytes infiltrate the brain, they release IL-1, tumor necrosis factor, and other cytokines, as well as myeloperoxidase, which in turn catalyzes the formation of additional reactive oxygen species. Myeloperoxidase in inter-cellular spaces of the brain is a highly effective enzymatic catalyst for the initiation of lipid peroxidation (Zhang et al., 2002), and brain cells are richly endowed with a major target of that process, polyunsaturated fatty acyl phospholipids (Bazan, 1990; Bazan and Allan, 1998).
The bioactivity of the novel docosanoid 10,17S-docosatriene (named as such and then confirmed to be NPD1) was further studied at the cellular level. We chose IL-1 as the trigger, because this cytokine increases during brain ischemia-reperfusion as a result of PMN infiltration as well as activation of microglia and macrophages (Mabuchi et al., 2000). Whether 10,17S-docosatriene bioactive inhibition of PMN infiltration and its blocking of pro-inflammatory gene expression are independent events or part of the same signaling still remains to be defined. What is clear is that the outcome of infusing 10,17S-docosatriene (NPD1) is neuroprotective against ischemia-reperfusion damage. DHA and NPD1 display potent neuroprotective bioactivity when given 1 hour after 2 hours of MCAo in rats (Bazan et al., 2012; Belayev et al., 2018, 2017, 2011; Calandria et al., 2015; Eady et al., 2012; Hong et al., 2015, 2014; Serhan et al., 2011).
ELV treatment 1 h after 2 h of MCAo protected both neurons, astrocytes and the SMI- 71–positive blood vessel cells in the cortex (Fig. 2). Vascular integrity facilitates neurogenesis and synaptogenesis that in turn contribute to improved functional recovery. Neurogenesis can be reactivated as a response to injury (Font et al., 2010) and new neurons from progenitors occurs in the subgranular zone of the DG, the subventricular zone of some cortical areas, the substantia nigra, and the periinfarcted areas (Arvidsson et al., 2002). Neural stem cells persist in the forebrain subventricular zone in a niche containing endothelial cells that might stimulate neural stem cells expansion and neurogenesis. Ischemic stroke augments neurogenesis and angiogenesis, but how endothelial cells influence stroke-induced neurogenesis is unknown. After cerebral ischemia, the integrity of the neurovascular unit (NVU) is compromised, allowing uncontrolled entry of molecules into the brain parenchyma that worsens damage caused by ischemia (Haley and Lawrence, 2017). Here, we measured ischemic disruption of the NVU by infiltration of endogenous IgG into the brain parenchyma. Treatment with ELV-N34 and ELVN34 attenuated NVU disruption induced by MCA-o. We showed here that the newly identified ELVs protected neurons undergoing OGD or NMDA receptor-mediated excitotoxicity. Moreover, ELVs attenuated infarcts, rescued ischemic core and penumbra, diminished NVU damage, and promoted cell survival along with neurological/behavioral recovery (Fig. 3 and 4).
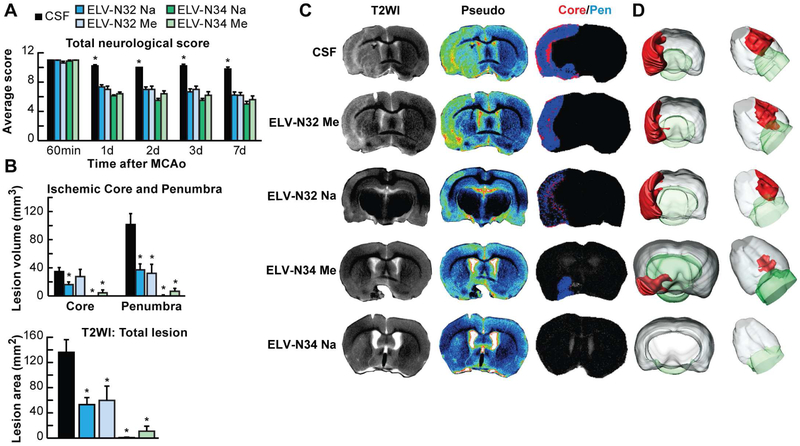
Fig. 2. ELV-N32 and ELV-N34 improve neurological/behavioral score, protect the penumbra, and reduce MRI lesion volumes after ischemic stroke.
(A) Total neurological score (normal score, 0; maximal score, 12) during MCAo (60 min) and at various times after treatment. At 60 min of MCAo, all animals had a score of 11 (of a possible 12). ELV-treated rats had significantly improved neurological scores on days 1, 3, and 7 compared to the vehicle (CSF)–treated group. (B) Ischemic core, penumbra, and total lesion volumes, computed from T2WI on day 7, were significantly reduced by ELV treatment compared to the vehicle group. (C) Representative T2WI, pseudo images, core/penumbra, and (D) 3D infarct volumes computed from T2WI on day 7. Core and penumbra were extracted from the entire brain. Core (red) and penumbral (blue) tissues were automatically identified in vehicle- and ELV-treated animals using the computational MRI method Hierarchical Region Splitting for penumbra identification. T2 hyperintensities were observed in the ischemic core and penumbra of vehicle-treated rats, consistent with edema formation. In contrast, ELV-treated animals had smaller lesion sizes. 3D reconstructions are from the same animal in each group on day 7. Values shown are means ± SD (n = 5 to 6 per group) (*P < 0.05, versus CSF group; repeated-measures ANOVA, followed by Bonferroni tests). From: (Bhattacharjee et al., 2017).
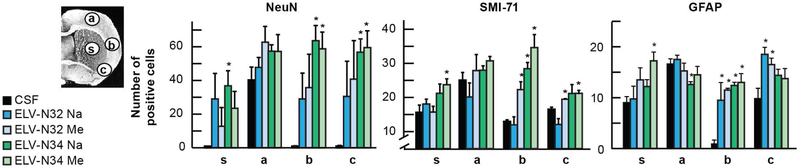
Fig. 3. ELV-N32 and ELV-N34 attenuate experimental ischemic stroke-induced neuronal and astrocyte cellular damage.
Coronal brain diagram (bregma, +1.2 mm) showing locations of regions for NeuN-, SMI-71-, and GFAP-positive cell counts in the cortex and striatum (s). Numbers of NeuN-positive neurons, SMI-71-positive vessels, and GFAP-positive astrocytes, increased by ELV treatment in the ischemic core (s) and different penumbral areas, are shown. Values shown are means ± SD (*P < 0.05, significantly different from vehicle; repeated-measures ANOVA, followed by Bonferroni tests; n = 5 to 6 per group). From: (Bhattacharjee et al., 2017).
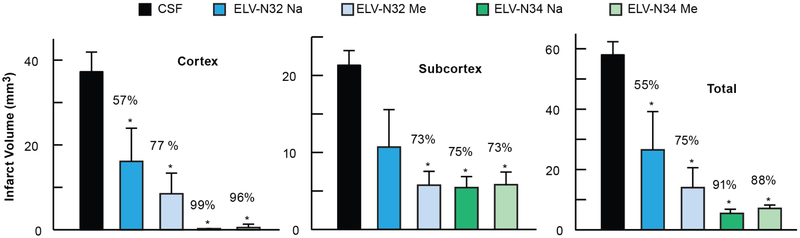
Fig. 4. ELV-N32 and ELV-N34 diminish Neurovascular Unit disruption and reduce brain infarction after ischemic stroke.
Cortical, subcortical, and total corrected infarct volumes. All ELV treatments markedly reduced cortical, subcortical, and total infarct volumes compared to the vehicle-treated group. Values shown are means ± SD (*P < 05, significantly different from vehicle; repeated-measures ANOVA, followed by Bonferroni tests; n = 5 to 6 per group). From: (Bhattacharjee et al., 2017).
13. Concluding Remarks
The identification of specific bioactive mediators from essential omega-3 fatty acids opens new modes of thinking and exploration concerning the significance of nutrition in healthcare and medicine. Clearly, there are many forms of neural cell damage and death, apoptosis and various forms of necrosis, most of them interrelated (Galluzzi et al., 2018, 2015). Critical to sustaining CNS integrity, particularly when confronted with trauma and/or chronic diseases, and ultimately sight and cognition, is understanding how to enable diet and other physiological measures. For example, evolving data is uncovering defined consequences of the western diet on immune memory, inflammation and other consequences (Christ et al., 2018). The nascent data indicates that DHA biology broadly impacts cell homeostasis and failures in its supply or metabolism leads to pathology. One of the emerging lessons is that preventive strategies should be rigorously considered to mend onset and early progression of chronic diseases, chiefly those that are neurodegenerative. NPD1 is highlighted here as a neuroprotective mediator in ischemic stroke, as defined by cellular and molecular targets, and demonstrated that the pathway that leads to its formation was enhanced in its neuroprotective bioactivity by the presence of aspirin (Marcheselli et al., 2003) by aspirin-triggered NPD1 (Bazan et al., 2012; Serhan et al., 2015; Serhan and Petasis, 2011).
The bioactivity of docosanoids and ELVs provides mechanistic rationale to understand cellular homeostatic regulation. ELVs are the first identified bioactive mediators from VLC-PUFA,n-3, which are the biosynthetic products of elongase ELOVL4. The structure and stereochemistry of ELVs with 32 and 34 carbons (ELV-N32, ELV-N34) was established (Bhattacharjee et al., 2017; Jun et al., 2017). ELV availability is abolished in the retinas of mice with genetically ablated AdipoR1 (Jun et al., 2017). The liver supplies dietary DHA (or derived from dietary 18:3n3) to tissues (Scott and Bazan, 1989) and captured by AdipoR1, followed by elongation in the inner segment of PRCs by ELOVL4 to VLC-PUFA,n-3 and incorporation into PCMS, which are also endowed with DHA (Fig. 1). Despite the low-abundance of EPA in the retina compared to DHA, ELOVL4 uses EPA as a preferred substrate (Yu et al., 2012). Retroconversion of DHA to EPA takes place in peroxisomes, and gives rise to the 26 C PUFA, the substrate for ELOVL4. During daily PRC OS renewal, PCMS interact with rhodopsin and end up in the RPE after shedding and phagocytosis. In the RPE, UOS or other disruptors of homeostasis triggers the release of 32:6n3 and 34:6n3 that in turn generate a hydroperoxyl and subsequently an ELV-N32 or ELV-N34, respectively. This newly discovered pathway that 32:6n-3 or 34:6n-3 yield novel mediators of 32 C or 34 C in length, contrasts to most known lipid mediators derived from 20 C or 22 C length precursors.
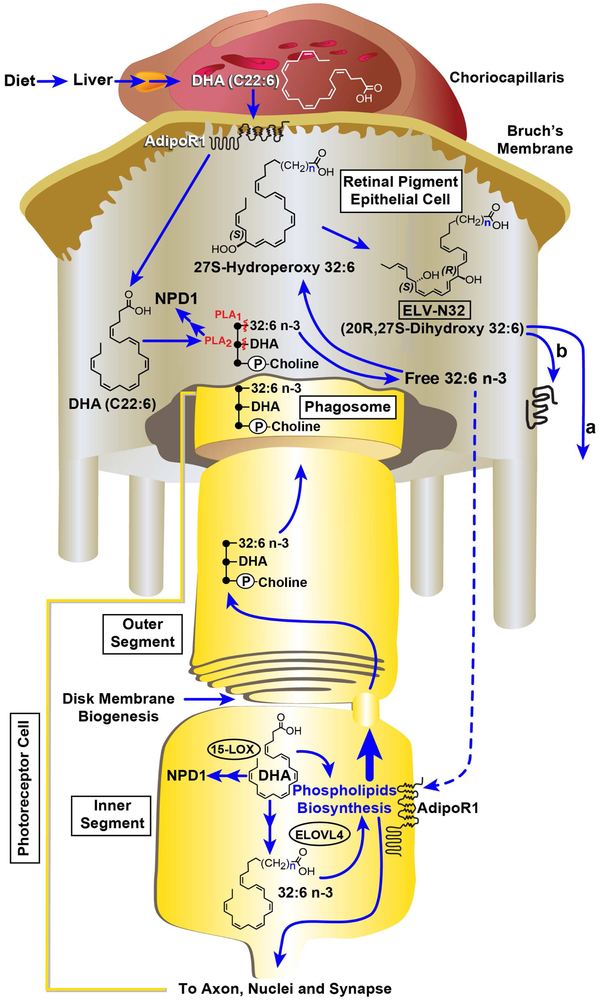
Fig. 1. Cell-specific ELV-synthesis and its targets.
The liver supplies DHA or 18:3 (not shown) from the diet. AdipoR1 captures DHA in the RPE as well as in the inner segments of PRCs. ELOVL4 generates 32:6 n-3 or 34:6 n-3 from EPA/DHA and other VLC-PUFAs,n-3. In this cartoon, for simplicity, only 32:6 n-3 synthesis to ELV-32 is highlighted, although 34:6 n3 follows a similar route toward the ELV 34 synthesis. In the inner segment the VLC-PUFAs,n-3 became acylated into C1 (sn-1) of phosphatidylcholine (PC, it also contains at sn-2 DHA). This phospholipid molecular species (PCMS) is used in the biogenesis of outer segment disc membranes, where the phospholipid tightly interacts with rhodopsin (Aveldaño, 1988). After shedding and phagocytosis by the RPE cells, the PC, upon impending disruptors of homeostasis, releases free 32:6 n-3 or 34:6 n-3 (by a Pl A1) and/or DHA (by a Pl A2). The 32:6 n-3 or 34:6 n-3 are then converted into ELVs following VLC-PUFAs,n-3 hydroperoxide intermediates. In 32:6 n-3 and ELV-N32, (CH2) n represents 11 carbons. Autocrine (A) / paracrine (B) pro-homeostatic signaling by ELVs sustain integrity of RPE and of PRC.
ELVs biosynthesis in human RPE cells is activated during UOS (Jun et al., 2017) targeting pro-homeostatic functions to preserve PRC integrity. The VLC-PUFA,n-3 are incorporated at position sn-1 of PC while DHA is located at position sn-2 (Fig.1). Therefore, ELV and docosanoid biosynthesis alternative regulation by specific phospholipases A1 and A2, suggest a novel pro-homeostatic mechanism. When cells are confronted with UOS, paracrine or autocrine bioactivity of NPD1 and /or ELVs takes place. Moreover, ELVs target and enhance the expression of pro-survival and pro-homeostatic proteins in the RPE cells undergoing UOS.
RPE cells sustain PRC functional integrity, and their demise is involved in the onset of most forms of retinal degenerations. One of the functions of the RPE cell is to retrieve DHA during PRC renewal and return it through the interphotoreceptor matrix to the PRC inner segment for new OS disc membrane biogenesis. Recently, we found that AdipoR1 is necessary for DHA availability to PRCs (Rice et al., 2015), and others have found that a single amino acid mutation in this receptor is causative of autosomal dominant retinitis pigmentosa (Zhang et al., 2016). Our work has disclosed that genetic ablation of this receptor leads to PRC degeneration and to shutting off VLC-PUFA,n-3 synthesis in the retina. Thus, the pool size of free 32:6n3 and of 34:6n3 in retinas of AdipoR1 KO mice is drastically decreased as compared with that in WT.
PUFA elongation in the inner segment of PRC by ELOVL4 leads to the biosynthesis of VLC-PUFAs,n-3 and their insertion at the sn-1 position of PC for biogenesis of PRC disk membranes. However, under conditions of stress, these VLC-PUFAs are cleaved by phospholipase A1 (PLA1) for the synthesis of mono- and di-hydroxy VLC-PUFAs (ELVs). Light-induced oxidative stress in mouse retinas triggers the production of free 32:6n3 and 34:6n3, as well as their mono- and di-hydroxy derivatives. In AdipoR1 KO mice, no detectable amounts of these molecules were found. Therefore, the lack of the VLC-PUFA,n-3 precursor DHA results in retinal degeneration, preceded by a remarkable downregulation of the free VLC-PUFA,n-3 molecular species and ELV biosynthesis. These observations support the concept that VLC-PUFA,n-3 are precursors of novel bioactive mediators that elicit pro-homeostatic protective PRC bioactivity.
Several neuroprotective strategies for stroke have shown promise; however, no treatment has demonstrated efficacy. The mechanistic cellular and molecular principles unraveled thus far should be instructive for stroke treatment translation because the failure of brain cells to survive ischemia-reperfusion damage may not be due to biology gone awry, but rather due to specific signals and mechanisms intended to be protective in the face of homeostasis disruptions that lead to neural circuits dysfunctions (Bazan, 2014).
ELVs exerted neuroprotection as well in a preclinical model of ischemic stroke and have helped us begin deciphering the thus-far elusive molecular principles of DHA-mediated neuroprotection. The complex network of neuroinflammation responses, which is beneficially targeted by ELVs at the onset of reperfusion after an ischemic stroke, is also uncovering the transcriptional activation of genes; thus, ELVs and docosanoids, including NPD1 drive the process by which a potential network of new pathways and synergies for neuroprotection might operate. The findings of ELVs open a different mechanistic perspective as compared with other endogenous pro-homeostatic and neuroprotective signaling because they stem from a phospholipid molecular species that is endowed with acyl chains with two different PUFA precursors of bioactive lipids, VLC-PUFAs and DHA (Fig 1). DHA is located at the sn-2 position of the phospholipid, the VLC-PUFAs,n-3 are located at the sn-1 position of the phospholipid and are subject to alternatively or concomitantly regulated pathways. Therefore, these findings revealed a different signal bifurcation pro-homeostatic and neuroprotective mechanism to sustain neural cell integrity. We anticipate that other ELVs longer as well, might also be endogenously made to regulate cell function. ELVs as a therapeutic agent have the potential for focal ischemic stroke and other conditions that engage inflammatory/homeostatic disruptions. Since inflammation is determined to be a common early event in many chronic diseases, especially neurodegenerative diseases, understanding endogenous protective mechanisms is important to identifying potential therapeutics for these diseases. Preconditioning is an endogenous phenomenon that can contribute to elucidate these protective mechanisms. Therefore, the molecular protective preconditioning signaling of DHA and of NPD1 are the bases for acquired protection or resilience when the retina, an integral part of the CNS, is confronted with a lethal stimulus.
The bioactivities of docosanoids and ELVs have implications related to both the understanding of cell function and how the brain, retina and immune cells modulate inflammatory/injury responses. These mechanisms are central to the design of new therapeutics for neurologic diseases. Novel DHA-signaling pathways are leading to answer the clinically important questions regarding undiscovered non-redundant targets in stroke, traumatic head injury, spinal cord injury, and other diseases that involve neuroinflammatory/immune components. The potent bioactivity of docosanoids and ELVs demonstrates the existence of endogenous critical defenses and targets for preventive (dietary/life style/behavioral modification strategies) and for potential therapeutic neuroprotective interventions in these diseases.
Vitae.
Founder/Director, LSU Neuroscience Center of Excellence; Professor of Neurology; Yvette C./Ernest C. Villere Chair for Retinal Degenerations and Boyd Professor; Founder/Editor-in-Chief, Molecular Neurobiology; member of Founding Senate, DZNE (2009–2016, Germany); Board of Governors’ Chairman Emeritus; ARVO Foundation; Foreign Adjunct Professor, Karolinska Institutet, Stockholm.
Awards include: Javits Neuroscience Investigator NINDS, NIH; Citation Classic, “Neural Stimulation or Onset of Cerebral Ischemia Activates Phospholipase A2” Current Contents,1991; Elected, Royal Academy of Sciences, and of Medicine, Spain; Fellow, Royal College of Physicians ,Ireland; Doctor Honoris Causa, Universidad Tucuman, Argentina; Endre Balazs Prize, ISER; Proctor Medal, ARVO; Chevreul Medal, France; Alkmeon International Prize; Miroslaw M. Mossakowski Medal, Polish Academy of Sciences.
Acknowledgements:
To my laboratory members and collaborators during the years. This work was supported by the National Eye Institute grant EY005121 (NGB), the National Institute of Neurological Disorders and Stroke grants NS046741 (NGB) and NS2302, the National Institute of General Medical Sciences grant P30 GM103340 (NGB), and the Eye, Ear, Nose and Throat Foundation.
Abbreviations
- AA
arachidonic acid
- AD
Alzheimer’s disease
- AdipoR1
adiponectin receptor 1
- AMD
age-related macular degeneration
- AMPK
adenosine monophosphate-activated kinase
- CNS
central nervous system
- DG
dentate gyrus
- DHA
docosahexaenoic acid
- ELOVL4
elongation of very long chain fatty acids protein 4
- ELV
elovanoid
- EPA
eicosapentaenoic acid
- ER
endoplasmic reticulum
- ERG
electroretinogram
- HFO
high-frequency oscillation
- KO
knockout
- MCAo
middle cerebral artery occlusion
- NMDA
N-methyl-d-aspartate
- NPD1
neuroprotectin D1
- NVU
neurovascular unit
- OGD
oxygen glucose deprivation
- OS
outer segment
- PC
phosphatidylcholine
- PCMS
phosphatidylcholine molecular species
- PEDF
pigment epithelium-derived factor
- pHFO
pathological high-frequency oscillations
- PKC
protein kinase C
- PD
Parkinson’s disease
- PPAR
peroxisome proliferator-activated receptor
- PRC
photoreceptor cell
- ROS
rod outer segment
- RPE
retinal pigment epithelium
- SE
status epilepticus
- SRS
subretinal space
- STGD3
Stargardt’s disease-3
- UOS
uncompensated oxidative stress
- VLC-PUFA
very long-chain polyunsaturated fatty acid
- VLC-SFA
very long-chain saturated fatty acid
Footnotes
Declaration of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agbaga M-P, 2016. Different Mutations in ELOVL4 Affect Very Long Chain Fatty Acid Biosynthesis to Cause Variable Neurological Disorders in Humans. Adv. Exp. Med. Biol 854, 129–135. 10.1007/978-3-319-17121-0_18 [DOI] [PubMed] [Google Scholar]
- Agbaga M-P, Brush RS, Mandal MNA, Henry K, Elliott MH, Anderson RE, 2008. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci 105, 12843–12848. 10.1073/pnas.0802607105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaga M-P, Mandal MNA, Anderson RE, 2010. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J. Lipid Res 51, 1624–1642. 10.1194/jlr.R005025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaga M-P, Tam BM, Wong JS, Yang LL, Anderson RE, Moritz OL, 2014. Mutant ELOVL4 that causes autosomal dominant stargardt-3 macular dystrophy is misrouted to rod outer segment disks. Invest. Ophthalmol. Vis. Sci 55, 3669–3680. 10.1167/iovs.13-13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RS, Ness JM, Roth KA, 2004. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim. Biophys. Acta 1644, 189–203. 10.1016/j.bbamcr.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, Rizzo WB, Alkuraya FS, 2011. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am. J. Hum. Genet 89, 745–750. https://doi.org/10.1016yj.ajhg.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambasudhan R, Wang X, Jablonski MM, Thompson DA, Lagali PS, Wong PW, Sieving PA, Ayyagari R, 2004. Atrophic macular degeneration mutations in ELOVL4 result in the intracellular misrouting of the protein. Genomics 83, 615–625. https://doi.org/10.1016Zj.ygeno.2003.10.004 [DOI] [PubMed] [Google Scholar]
- André V, Marescaux C, Nehlig A, Fritschy JM, 2001. Alterations of hippocampal GAbaergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus 11, 452–468. 10.1002/hipo.1060 [DOI] [PubMed] [Google Scholar]
- Anthony DF, Sin YY, Vadrevu S, Advant N, Day JP, Byrne AM, Lynch MJ, Milligan G, Houslay MD, Baillie GS, 2011. β-Arrestin 1 inhibits the GTPase- activating protein function of ARHGAP21, promoting activation of RhoA following angiotensin II type 1A receptor stimulation. Mol. Cell. Biol 31, 1066–1075. 10.1128/MCB.00883-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O, 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med 8, 963–970. 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- Aveldaño MI, 1988. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry 27, 1229–1239. [DOI] [PubMed] [Google Scholar]
- Aveldaño MI, 1987. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J. Biol. Chem 262, 1172–1179. [PubMed] [Google Scholar]
- Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M, Draguhn A, 2011. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc. Natl. Acad. Sci. U. S. A. 108, E607–616. 10.1073/pnas.1103546108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G, 1998. Amyloid beta protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J. Comp. Neurol 397, 139–147. [PubMed] [Google Scholar]
- Bazán NG, 2014. Is there a molecular logic that sustains neuronal functional integrity and survival? Lipid signaling is necessary for neuroprotective neuronal transcriptional programs. Mol. Neurobiol 50, 1–5. 10.1007/s12035-014-8897-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, 2009. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res 50 Suppl, S400–405. 10.1194/jlr.R800068-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, 2007. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci 48, 4866–4881; biography 4864–4865. 10.1167/iovs.07-0918 [DOI] [PubMed] [Google Scholar]
- Bazan NG, 2006. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 29, 263–271. 10.1016/j.tins.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Bazan NG, 2005. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. Zurich Switz 15, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, 2003. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res 44, 2221–2233. 10.1194/jlr.R300013-JLR200 [DOI] [PubMed] [Google Scholar]
- Bazan NG, 1990. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system, in: Wurtman R, Wurtman J (Eds.), Nutrition and the Brain. Raven Press, New York, pp. 1–24. [Google Scholar]
- Bazan NG, 1970. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta 218, 1–10. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Allan G, 1998. Platelet-activating factor and other bioactive lipids, in: Ginsberg MD, Bogousslavsky (Eds.), Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management. Blackwell Science Publishers, Malden, MA, pp. 532–555. [Google Scholar]
- Bazan NG, Birkle DL, Reddy TS, 1984. Docosahexaenoic acid (22:6, n-3) is metabolized to lipoxygenase reaction products in the retina. Biochem. Biophys. Res. Commun 125, 741–747. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN, 2010. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res 51, 2018–2031. 10.1194/jlr.R001131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, Zhang C, Jun B, Obenaus A, Fredman G, Zhu M, Winkler JW, Petasis NA, Serhan CN, Belayev L, 2012. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp. Neurol 236, 122–130. 10.1016/j.expneurol.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Lukiw WJ, 2002. Cyclooxygenase-2 and presenilin-1 gene expression induced by interleukin-1beta and amyloid beta 42 peptide is potentiated by hypoxia in primary human neural cells. J. Biol. Chem 277, 30359–30367. 10.1074/jbc.M203201200 [DOI] [PubMed] [Google Scholar]
- Bazan NG, Molina MF, Gordon WC, 2011a. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr 31, 321–351. 10.1146/annurev.nutr.012809.104635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Musto AE, Knott EJ, 2011b. Endogenous Signaling by Omega-3 Docosahexaenoic Acid-derived Mediators Sustains Homeostatic Synaptic and Circuitry Integrity. Mol. Neurobiol 44, 216–222. 10.1007/s12035-011-8200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Spath S, Goverman J, 2017. Cytokine networks in neuroinflammation. Nat. Rev. Immunol 17, 49–59. 10.1038/nri.2016.123 [DOI] [PubMed] [Google Scholar]
- Belayev L, Hong S-H, Menghani H, Marcell SJ, Obenaus A, Freitas RS, Khoutorova L, Balaszczuk V, Jun B, Oriá RB, Bazan NG, 2018. Docosanoids Promote Neurogenesis and Angiogenesis, Blood-Brain Barrier Integrity, Penumbra Protection, and Neurobehavioral Recovery After Experimental Ischemic Stroke. Mol. Neurobiol 10.1007/s12035-018-1136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG, 2011. Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl. Stroke Res 2, 33–41. 10.1007/s12975-010-0046-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Mukherjee PK, Balaszczuk V, Calandria JM, Obenaus A, Khoutorova L, Hong S-H, Bazan NG, 2017. Neuroprotectin D1 upregulates Iduna expression and provides protection in cellular uncompensated oxidative stress and in experimental ischemic stroke. Cell Death Differ. 24, 1091–1099. 10.1038/cdd.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett LD, Hopiavuori BR, Brush RS, Chan M, Van Hook MJ, Thoreson WB, Anderson RE, 2014. Examination of VLC-PUFA-deficient photoreceptor terminals. Invest. Ophthalmol. Vis. Sci 55, 4063–4072. 10.1167/iovs.14-13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Tammur J, Singh N, Hutchinson A, Dixon M, Pappas CM, Zabriskie NA, Zhang K, Petrukhin K, Leppert M, Allikmets R, 2001. Diverse macular dystrophy phenotype caused by a novel complex mutation in the ELOVL4 gene. Invest. Ophthalmol. Vis. Sci 42, 3331–3336. [PubMed] [Google Scholar]
- Bhattacharjee S, Jun B, Belayev L, Heap J, Kautzmann M-A, Obenaus A, Menghani H, Marcell SJ, Khoutorova L, Yang R, Petasis NA, Bazan NG, 2017. Elovanoids are a novel class of homeostatic lipid mediators that protect neural cell integrity upon injury. Sci. Adv 3, e1700735 10.1126/sciadv.1700735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa CV, Raskin S, Serafini S, Teive HAG, Dion PA, Rouleau GA, 2015. A New ELOVL4 Mutation in a Case of Spinocerebellar Ataxia With Erythrokeratodermia. JAMA Neurol. 72, 942–943. https://doi.Org/10.1001/jamaneurol.2015.0888 [DOI] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J, 2011. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia 52, 45–52. 10.1111/j.1528-1167.2010.02896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Staba RJ, 2010. High-frequency oscillations in epileptic brain. Curr. Opin. Neurol 23, 151–156. 10.1097/WCO.0b013e3283373ac8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wilson CL, Fried I, Buzsaki G, 1999. High-frequency oscillations in human brain. Hippocampus 9, 137–142. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J, 2000. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia 41 Suppl 6, S144–152. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X, 2009. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J. Neurosci. Off. J. Soc. Neurosci 29, 8259–8269. 10.1523/JNEUR0SCI.4179-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, 2010. Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68, 362–385. 10.1016/j.neuron.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A, 2003. Hippocampal network patterns of activity in the mouse. Neuroscience 116, 201–211. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Geisler C, Henze DA, Wang X-J, 2004. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 27, 186–193. 10.1016/j.tins.2004.02.007 [DOI] [PubMed] [Google Scholar]
- Cadieux-Dion M, Turcotte-Gauthier M, Noreau A, Martin C, Meloche C, Gravel M, Drouin CA, Rouleau GA, Nguyen DK, Cossette P, 2014. Expanding the clinical phenotype associated with EL0VL4 mutation: study of a large French-Canadian family with autosomal dominant spinocerebellar ataxia and erythrokeratodermia. JAMA Neurol. 71, 470–475. 10.1001/jamaneurol.2013.6337 [DOI] [PubMed] [Google Scholar]
- Calandria JM, Asatryan A, Balaszczuk V, Knott EJ, Jun BK, Mukherjee PK, Belayev L, Bazan NG, 2015. NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death Differ. 22, 1363–1377. 10.1038/cdd.2014.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG, 2009. Selective Survival Rescue in 15-Lipoxygenase-1- deficient Retinal Pigment Epithelial Cells by the Novel Docosahexaenoic Acid- derived Mediator, Neuroprotectin D1. J. Biol. Chem 284, 17877–17882. 10.1074/jbc.M109.003988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, 2018. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc. Nutr. Soc 77, 52–72. 10.1017/S0029665117003950 [DOI] [PubMed] [Google Scholar]
- Calder PC, 2017. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans 45, 1105–1115. 10.1042/BST20160474 [DOI] [PubMed] [Google Scholar]
- Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, Visioli F, 2017. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev 40, 95–119. 10.1016/j.arr.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Ashe KH, Frautschy SA, Cole GM, 2004. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron 43, 633–645. 10.1016/j.neuron.2004.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DJ, Tong Z, Yang Z, Kaminoh J, Kamiyah S, Chen H, Zeng J, Chen Y, Luo L, Zhang K, 2007. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int. J. Biol. Sci 3, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatt AB, Ebersole JS, 1982. The laminar sensitivity of cat striate cortex to penicillin induced epileptogenesis. Brain Res. 241, 382–387. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD, 2000. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 20, 531–542. 10.1097/00004647-200003000-00012 [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Jiang N, Zhang RL, Prostak J, 1996. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 16, 578–584. 10.1097/00004647-199607000-00007 [DOI] [PubMed] [Google Scholar]
- Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag SJCFM, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E, 2018. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 172, 162–175.e14. 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ, 2002. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res 70, 462–473. 10.1002/jnr.10351 [DOI] [PubMed] [Google Scholar]
- Covolan L, Mello LE, 2000. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 39, 133–152. [DOI] [PubMed] [Google Scholar]
- Crapper DR, Dalton AJ, Skoptiz M, Scott JW, Hachinski VC, 1975. Alzheimer degeneration in Down syndrome. Electrophysiologic alterations and histopathologic findings. Arch. Neurol 32, 618–623. [DOI] [PubMed] [Google Scholar]
- Danzer SC, He X, Loepke AW, McNamara JO, 2010. Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy. Hippocampus 20, 113–124. 10.1002/hipo.20589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, Kotloski RJ, Walter C, Hughes M, McNamara JO, 2008. Altered morphology of hippocampal dentate granule cell presynaptic and postsynaptic terminals following conditional deletion of TrkB. Hippocampus 18, 668–678. 10.1002/hipo.20426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE, American Heart Association Stroke Council and Council on Epidemiology and Prevention, 2016. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 47, 581–641. 10.1161/STR.0000000000000086 [DOI] [PubMed] [Google Scholar]
- Dichter MA, 2009. Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch. Neurol 66, 443–447. 10.1001/archneurol.2009.10 [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R, 2001. Reduced excitatory drive onto interneurons in the dentate gyrus after status epilepticus. J. Neurosci. Off. J. Soc. Neurosci 21, 2048–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler JC, Shaikh AR, Shenoy SK, Shen Y, Roth S, 2008. Protein kinase C subtypes and retinal ischemic preconditioning. Exp. Eye Res 87, 300–311. 10.1016/j.exer.2008.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE Staley KJ, 2011. Seizure probability in animal models of acquired epilepsy: A perspective on the concept of the preictal state. Epilepsy Res., Special Issue on Epilepsy Research UK Workshop 2010 on “Preictal Phenomena” 97, 324–331. 10.1016/j.eplepsyres.2011.10.017 [DOI] [PubMed] [Google Scholar]
- Eady TN, Belayev L, Khoutorova L, Atkins KD, Zhang C, Bazan NG, 2012. Docosahexaenoic acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PloS One 7, e46151 10.1371/journal.pone.0046151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Donoso LA, Ritter R, 2001. A novel gene for autosomal dominant Stargardt-like macular dystrophy with homology to the SUR4 protein family. Invest. Ophthalmol. Vis. Sci 42, 2652–2663. [PubMed] [Google Scholar]
- Ferrari D, Cysneiros RM, Scorza CA, Arida RM, Cavalheiro EA, de Almeida A-CG, Scorza FA, 2008. Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: Quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav. EB 13, 36–42. 10.1016/j.yebeh.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S, 1992. High- frequency EEG activity at the start of seizures. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 9, 441–448. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE, 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res 22, 79–131. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L, 2007. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55, 930–941. https://doi.Org/10.1016/j.neuron.2007.07.040 [DOI] [PubMed] [Google Scholar]
- Font MA, Arboix A, Krupinski J, 2010. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr. Cardiol. Rev 6, 238–244. 10.2174/157340310791658802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, Baehrecke EH, Bazan NG, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Campanella M, Candi E, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin K-M, Di Daniele N, Dixit VM, Dynlacht BD, El-Deiry WS, Fimia GM, Flavell RA, Fulda S, Garrido C, Gougeon M-L, Green DR, Gronemeyer H, Hajnoczky G, Hardwick JM, Hengartner MO, Ichijo H, Joseph B, Jost PJ, Kaufmann T, Kepp O, Klionsky DJ, Knight RA, Kumar S, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lugli E, Madeo F, Malorni W, Marine J-C, Martin SJ, Martinou J-C, Medema JP, Meier P, Melino S, Mizushima N, Moll U, Muñoz-Pinedo C, Nuñez G, Oberst A, Panaretakis T, Penninger JM, Peter ME, Piacentini M, Pinton P, Prehn JH, Puthalakath H, Rabinovich GA, Ravichandran KS, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Shi Y, Simon H-U, Stockwell BR, Szabadkai G, Tait SW, Tang HL, Tavernarakis N, Tsujimoto Y, Vanden Berghe T, Vandenabeele P, Villunger A, Wagner EF, Walczak H, White E, Wood WG, Yuan J, Zakeri Z, Zhivotovsky B, Melino G, Kroemer G, 2015. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 22, 58–73. 10.1038/cdd.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona- Gutierrez D, Cecconi F, Chan FK-M, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin K-M, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine J-C, Martin SJ, Martinou J-C, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon H-U, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G, 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF, 2006. Vibrissa-signaled eyeblink conditioning induces somatosensory cortical plasticity. J. Neurosci. Off. J. Soc. Neurosci 26, 6062–6068. 10.1523/JNEUROSCI.5582-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WC, Bazan NG, 1990. Docosahexaenoic acid utilization during rod photoreceptor cell renewal. J. Neurosci 10, 2190–2202. 10.1523/JNEUROSCI.10-07-02190.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WC, Rodriguez de Turco EB, Bazan NG, 1992. Retinal pigment epithelial cells play a central role in the conservation of docosahexaenoic acid by photoreceptor cells after shedding and phagocytosis. Curr. Eye Res 11, 73–83. [DOI] [PubMed] [Google Scholar]
- Guo D, Arnspiger S, Rensing NR, Wong M, 2012. Brief seizures cause dendritic injury. Neurobiol. Dis 45, 348–355. 10.1016/j.nbd.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Robinson N, Mattson MP, 1998. Secreted beta-amyloid precursor protein counteracts the proapoptotic action of mutant presenilin-1 by activation of NF- kappaB and stabilization of calcium homeostasis. J. Biol. Chem 273, 12341–12351. [DOI] [PubMed] [Google Scholar]
- Haley MJ, Lawrence CB, 2017. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 37, 456–470. 10.1177/0271678X16629976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck J, Kochanek P, 1998. Inflammatory responses in cerebral Ischemia. Role of Leukocytes, in: Ginsberg MD, Bogousslavsky J (Eds.), Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management. Blackwell Science Publishers, Malden, MA, pp. 489–506. [Google Scholar]
- Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O, 2005. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr 135, 549–555. 10.1093/jn/135.3.549 [DOI] [PubMed] [Google Scholar]
- Hishikawa D, Valentine WJ, Iizuka-Hishikawa Y, Shindou H, Shimizu T, 2017. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 591, 2730–2744. 10.1002/1873-3468.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE, 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17, 55–63. 10.1038/nm.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL, 2008. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med 14, 194–198. 10.1038/nm1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-H, Belayev L, Khoutorova L, Obenaus A, Bazan NG, 2014. Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J. Neurol. Sci 338, 135–141. 10.1016/j.jns.2013.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-H, Khoutorova L, Bazan NG, Belayev L, 2015. Docosahexaenoic acid improves behavior and attenuates blood-brain barrier injury induced by focal cerebral ischemia in rats. Exp. Transl. Stroke Med 7, 3 10.1186/s13231-014-0012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L, 2010. Emergent dynamics of fast ripples in the epileptic hippocampus. J. Neurosci. Off. J. Soc. Neurosci 30, 16249–16261. 10.1523/JNEUROSCI.3357-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes JK, Calder PC, 2018. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 132, 41–48. 10.1016/j.plefa.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Isokawa M, 2000. Remodeling dendritic spines of dentate granule cells in temporal lobe epilepsy patients and the rat pilocarpine model. Epilepsia 41 Suppl 6, S14–17. [DOI] [PubMed] [Google Scholar]
- Isokawa M, 1998. Remodeling dendritic spines in the rat pilocarpine model of temporal lobe epilepsy. Neurosci. Lett 258, 73–76. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T, 2010. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464, 1313–1319. 10.1038/nature08991 [DOI] [PubMed] [Google Scholar]
- Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau C-ECF, Gotman J, 2009. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia 50, 1780–1792. 10.1111/j.1528-1167.2009.02067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Bragin A, 2011. High-frequency activity in experimental and clinical epileptic foci. Epilepsy Res. 97, 300–307. 10.1016/j.eplepsyres.2011.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun B, Mukherjee PK, Asatryan A, Kautzmann M-A, Heap J, Gordon WC, Bhattacharjee S, Yang R, Petasis NA, Bazan NG, 2017. Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity. Sci. Rep 7, 5279 10.1038/s41598-017-05433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káldi I, Martin RE, Huang H, Brush RS, Morrison KA, Anderson RE, 2003. Bright cyclic rearing protects albino mouse retina against acute light-induced apoptosis. Mol. Vis 9, 337–344. [PubMed] [Google Scholar]
- Karan G, Yang Z, Howes K, Zhao Y, Chen Y, Cameron DJ, Lin Y, Pearson E, Zhang K, 2005. Loss of ER retention and sequestration of the wild-type ELOVL4 by Stargardt disease dominant negative mutants. Mol. Vis 11, 657–664. [PubMed] [Google Scholar]
- Kitamura Y, Taniguchi T, Shimohama S, 1999. Apoptotic cell death in neurons and glial cells: implications for Alzheimer’s disease. Jpn. J. Pharmacol 79, 1–5. [DOI] [PubMed] [Google Scholar]
- Knott EJ, Gordon WC, Jun B, Do K, Bazan NG, 2018. Retinal Pigment Epithelium and Photoreceptor Preconditioning Protection Requires Docosanoid Signaling. Cell. Mol. Neurobiol 38, 901–917. 10.1007/s10571-017-0565-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi M, Nakano H, Shimazaki M, Sugimori K, Koshino Y, 2004. Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer’s disease. J. Alzheimers Dis JAD 6, 623–632; discussion 673–681. [DOI] [PubMed] [Google Scholar]
- Korn T, Kallies A, 2017. T cell responses in the central nervous system | Nature Reviews Immunology. Nat. Rev. Immunol 17, 179–194. 10.1038/nri.2016.144 [DOI] [PubMed] [Google Scholar]
- Kraskov A, Quiroga RQ, Reddy L, Fried I, Koch C, 2007. Local field potentials and spikes in the human medial temporal lobe are selective to image category. J. Cogn. Neurosci 19, 479–492. 10.1162/jocn.2007.19.3.479 [DOI] [PubMed] [Google Scholar]
- Kuny S, Cho WJ, Dimopoulos IS, Sauvé Y, 2015. Early Onset Ultrastructural and Functional Defects in RPE and Photoreceptors of a Stargardt-Like Macular Dystrophy (STGD3) Transgenic Mouse Model. Invest. Ophthalmol. Vis. Sci 56, 7109–7121. 10.1167/iovs.15-17567 [DOI] [PubMed] [Google Scholar]
- Kurz JE, Moore BJ, Henderson SC, Campbell JN, Churn SB, 2008. A cellular mechanism for dendritic spine loss in the pilocarpine model of status epilepticus. Epilepsia 49, 1696–1710. 10.1111/j.1528-1167.2008.01616.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Chen D, Vivien D, Ge Y-W, Greig NH, Rogers JT, 2003. Role of cytokines in the gene expression of amyloid beta-protein precursor: identification of a 5’-UTR-binding nuclear factor and its implications in Alzheimer’s disease. J. Alzheimers Dis. JAD 5, 81–90. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bortel A, Gotman J, Avoli M, 2011. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol. Dis 42, 231–241. 10.1016/j.nbd.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Cao W, Anderson RE, 2003. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Invest. Ophthalmol. Vis. Sci 44, 4968–4975. [DOI] [PubMed] [Google Scholar]
- Liao Y-F, Wang B-J, Cheng H-T, Kuo L-H, Wolfe MS, 2004. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma- secretase-mediated cleavage of amyloid precursor protein through a JNK- dependent MAPK pathway. J. Biol. Chem 279, 49523–49532. 10.1074/jbc.M402034200 [DOI] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Frautschy SA, Cole GM, 2005. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. Off. J. Soc. Neurosci 25, 3032–3040. 10.1523/JNEUROSCI.4225-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Qiu Y, Liu Y, Mohan R, Li Q, Lei B, 2013. Expression of adiponectin and its receptors in type 1 diabetes mellitus in human and mouse retinas. Mol. Vis 19, 1769–1778. [PMC free article] [PubMed] [Google Scholar]
- Lindén H, Tetzlaff T, Potjans TC, Pettersen KH, Grün S, Diesmann M, Einevoll GT, 2011. Modeling the spatial reach of the LFP. Neuron 72, 859–872. 10.1016/j.neuron.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Litt B, Esteller R, Echauz J, D’Alessandro M, Shor R, Henry T, Pennell P, Epstein C, Bakay R, Dichter M, Vachtsevanos G, 2001. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron 30, 51–64. [DOI] [PubMed] [Google Scholar]
- Litt B, Lehnertz K, 2002. Seizure prediction and the preseizure period. Curr. Opin. Neurol 15, 173–177. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, 2010. Executive summary: heart disease and stroke statistics−−2010 update: a report from the American Heart Association. Circulation 121, 948–954. 10.1161/CIRCULATIONAHA.109.192666 [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Pappolla M, Pelaez RP, Bazan NG, 2005. Alzheimer’s disease--a dysfunction in cholesterol and lipid metabolism. Cell. Mol. Neurobiol 25, 475–483. 10.1007/s10571-005-4010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M, 2000. Contribution of Microglia/Macrophages to Expansion of Infarction and Response of Oligodendrocytes After Focal Cerebral Ischemia in Rats. Stroke 31, 1735–1743. 10.1161/01.STR.317.1735 [DOI] [PubMed] [Google Scholar]
- Maier N, Tejero-Cantero A, Dorrn AL, Winterer J, Beed PS, Morris G, Kempter R, Poulet JFA, Leibold C, Schmitz D, 2011. Coherent phasic excitation during hippocampal ripples. Neuron 72, 137–152. 10.1016/j.neuron.2011.08.016 [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim R-Y, Liu F, Dong LQ, 2006. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol 8, 516–523. 10.1038/ncb1404 [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG, 2003. Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-mediated Leukocyte Infiltration and Pro-inflammatory Gene Expression. J. Biol. Chem 278, 43807–43817. 10.1074/jbc.M305841200 [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Onodera H, Shiga Y, Nakamura M, Ninomiya M, Kihara T, Kogure K, 1994. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke 25, 1469–1475. 10.1161/01.STR.25.7.1469 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H, 2004. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, 1999. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J. Neurosci. Res 58, 152–166. [PubMed] [Google Scholar]
- Maugeri A, Meire F, Hoyng CB, Vink C, Van Regemorter N, Karan G, Yang Z, Cremers FPM, Zhang K, 2004. A novel mutation in the ELOVL4 gene causes autosomal dominant Stargardt-like macular dystrophy. Invest. Ophthalmol. Vis. Sci 45, 4263–4267. 10.1167/iovs.04-0078 [DOI] [PubMed] [Google Scholar]
- McAuliffe JJ, Bronson SL, Hester MS, Murphy BL, Dahlquist-Topalá R, Richards DA, Danzer SC, 2011. Altered patterning of dentate granule cell mossy fiber inputs onto CA3 pyramidal cells in limbic epilepsy. Hippocampus 21, 93–107. 10.1002/hipo.20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL, 2003. Inflammatory processes in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 741–749. 10.1016/S0278-5846(03)00124-6 [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, Hunter HR, Bloor DJ, Lieberman BA, Picton HM, Leese HJ, Kimber SJ, Brison DR, 2004. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol. Reprod. Dev 68, 35–50. 10.1002/mrd.20055 [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK, 1984. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J. Physiol 356, 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsáki G, 2011. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat. Neurosci 14, 1174–1181. 10.1038/nn.2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroig Ó, Rotllant J, Cerdá-Reverter JM, Dick JR, Figueras A, Tocher DR, 2010. Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1801, 1145–1154. 10.1016/j.bbalip.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ, 2004. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol 73, 1–60. 10.1016/j.pneurobio.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG, 2007. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. U. S. A 104, 13152–13157. 10.1073/pnas.0705949104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG, 2004. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. U. S. A 101, 8491–8496. 10.1073/pnas.0402531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Glasgow W, Fralix T, Steenbergen C, 1995. Role of lipoxygenase metabolites in ischemic preconditioning. Circ. Res 76, 457–467. [DOI] [PubMed] [Google Scholar]
- Musto AE, Gjorstrup P, Bazan NG, 2011. The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia 52, 1601–1608. 10.1111/j.1528-1167.2011.03081.x [DOI] [PubMed] [Google Scholar]
- Musto AE, Rosencrans RF, Walker CP, Bhattacharjee S, Raulji CM, Belayev L, Fang Z, Gordon WC, Bazan NG, 2016. Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci. Rep 6 10.1038/srep30298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musto AE, Walker CP, Petasis NA, Bazan NG, 2015. Hippocampal Neuro-Networks and Dendritic Spine Perturbations in Epileptogenesis Are Attenuated by Neuroprotectin D1. PLoS ONE 10 10.1371/journal.pone.0116543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL, 2014. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506. 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- Oresti GM, Ayuza Aresti PL, Gigola G, Reyes LE, Aveldano MI, 2010. Sequential depletion of rat testicular lipids with long-chain and very long-chain polyenoic fatty acids after X-ray-induced interruption of spermatogenesis. J. Lipid Res 51, 2600–2610. 10.1194/jlr.M006395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Doi H, Mitsui J, Sato N, likuni Y, Majima T, Yamane K, Irioka T, Ishiura H, Doi K, Morishita S, Higashi M, Sekiguchi T, Koyama K, Ueda N, Miura Y, Miyatake S, Matsumoto N, Yokota T, Tanaka F, Tsuji S, Mizusawa H, Ishikawa K, 2015. A Novel Mutation in ELOVL4 Leading to Spinocerebellar Ataxia (SCA) With the Hot Cross Bun Sign but Lacking Erythrokeratodermia: A Broadened Spectrum of SCA34. JAMA Neurol. 72, 797–805. 10.1001/jamaneurol.2015.0610 [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2010. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci 13, 812–818. 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F, 2004. Astrocyte modulation of in vitro beta-amyloid neurotoxicity. Glia 46, 252–260. 10.1002/glia.20005 [DOI] [PubMed] [Google Scholar]
- Pastore D, Pacifici F, Capuani B, Palmirotta R, Dong C, Coppola A, Abete P, Roselli M, Sbraccia P, Guadagni F, Lauro D, Rundek T, Della-Morte D, 2017. Sex-Genetic Interaction in the Risk for Cerebrovascular Disease. Curr. Med. Chem 24, 2687–2699. 10.2174/0929867324666170417100318 [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Regeur L, Oster S, Pakkenberg B, 2003. Neocortical glial cell numbers in Alzheimer’s disease. A stereological study. Dement. Geriatr. Cogn. Disord 16, 212–219. 10.1159/000072805 [DOI] [PubMed] [Google Scholar]
- Petasis NA, Yang R, Winkler JW, Zhu M, Uddin J, Bazan NG, Serhan CN, 2012. Stereocontrolled total synthesis of neuroprotectin D1 / protectin D1 and its aspirin-triggered stereoisomer. Tetrahedron Lett. 53, 1695–1698. 10.1016/j.tetlet.2012.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J-F, Tasic B, Hjerling-Leffler J, Trimarchi JM, Awatramani R, 2016. Disentangling neural cell diversity using single-cell transcriptomics. Nat. Neurosci 19, 1131–1141. 10.1038/nn.4366 [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE, 2009. Epileptogenesis in the immature brain: emerging mechanisms. Nat. Rev. Neurol 5, 380–391. 10.1038/nrneurol.2009.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY, 2008. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 455, 912–918. 10.1038/nature07457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau TF, Lu Q, Sharma S, Sun X, Leary G, Beckman ML, Hou Y, Wainwright MS, Kavanaugh M, Poulsen DJ, Black SM, 2012. Oxygen glucose deprivation in rat hippocampal slice cultures results in alterations in carnitine homeostasis and mitochondrial dysfunction. PloS One 7, e40881 10.1371/journal.pone.0040881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Calandria JM, Gordon WC, Jun B, Zhou Y, Gelfman CM, Li S, Jin M, Knott EJ, Chang B, Abuin A, Issa T, Potter D, Platt KA, Bazan NG, 2015. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun 6, 6228 10.1038/ncomms7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu G-Q, Palop JJ, Noebels JL, Mucke L, 2011. Amyloid- β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci 31, 700–711. 10.1523/JNEUROSCI.4152-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Gordon WC, Peyman GA, Bazan NG, 1990. Preferential uptake and metabolism of docosahexaenoic acid in membrane phospholipids from rod and cone photoreceptor cells of human and monkey retinas. J. Neurosci. Res 27, 522–532. 10.1002/jnr.490270413 [DOI] [PubMed] [Google Scholar]
- Royo NC, Wahl F, Stutzmann JM, 1999. Kinetics of polymorphonuclear neutrophil infiltration after a traumatic brain injury in rat. Neuroreport 10, 1363–1367. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Waziri A, Branner A, Sosunov A, Schroeder CE, Emerson RG, 2008. Microphysiology of epileptiform activity in human neocortex. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 25, 321–330. 10.1097/WNP.0b013e31818e8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Schorge S, Kullmann DM, Walker MC, 2006. Epileptogenesis is associated with enhanced glutamatergic transmission in the perforant path. J. Neurophysiol 95, 1213–1220. 10.1152/jn.00680.2005 [DOI] [PubMed] [Google Scholar]
- Scott BL, Bazan NG, 1989. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci 86, 2903–2907. 10.1073/pnas.86.8.2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D, Kopan R, 2003. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci 26, 565–597. 10.1146/annurev.neuro.26.041002.131334 [DOI] [PubMed] [Google Scholar]
- Serhan CN, 2017. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 31, 1273–1288. 10.1096/fj.201601222R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K, 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med 192, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N, 2015. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 1851, 397–413. 10.1016/j.bbalip.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA, 2011. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol 18, 976–987. 10.1016/j.chembiol.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA, 2011. Resolvins and protectins in inflammation resolution. Chem. Rev 111, 5922–5943. 10.1021/cr100396c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R, 2011. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J. Neurosci. Off. J. Soc. Neurosci 31, 14850–14860. 10.1523/JNEUROSCI.3922-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley H, Smith BN, 2002. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 49, 109–120. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Bumanglag AV, 2013. Defining “epileptogenesis” and identifying “antiepileptogenic targets” in animal models of acquired temporal lobe epilepsy is not as simple as it might seem. Neuropharmacology 69, 3–15. 10.1016/j.neuropharm.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J, 2002. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J. Neurophysiol 88, 1743–1752. 10.1152/jn.2002.88.4.1743 [DOI] [PubMed] [Google Scholar]
- Stacey WC, Krieger A, Litt B, 2011. Network recruitment to coherent oscillations in a hippocampal computer model. J. Neurophysiol 105, 1464–1481. 10.1152/jn.00643.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, 2007. Neurons skip a beat during fast ripples. Neuron 55, 828–830. https://doi.org/10.10167j.neuron.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Stark DT, Bazan NG, 2011. Synaptic and extrasynaptic NMDA receptors differentially modulate neuronal cyclooxygenase-2 function, lipid peroxidation, and neuroprotection. J. Neurosci. Off. J. Soc. Neurosci 31, 13710–13721. 10.1523/JNEUROSCI.3544-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkopf J, Andreasen TV, Bugge E, Ytrehus K, 1998. Lipid peroxidation, arachidonic acid and products of the lipoxygenase pathway in ischaemic preconditioning of rat heart. Cardiovasc. Res 37, 66–75. [DOI] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, Worrell GA, 2010. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain J. Neurol 133, 2789–2797. 10.1093/brain/awq190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Johnson JA, 2003. Genetic programming by the proteolytic fragments of the amyloid precursor protein: somewhere between confusion and clarity. Rev. Neurosci 14, 317–341. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsaki G, 2011. Relationships between Hippocampal Sharp Waves, Ripples, and Fast Gamma Oscillation: Influence of Dentate and Entorhinal Cortical Activity. J. Neurosci 31, 8605–8616. 10.1523/JNEUROSCI.0294-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Al-Noori S, Jiang M, Lee CL, 2000. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus 10, 617–625. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y, 2012. Locally synchronized synaptic inputs. Science 335, 353–356. 10.1126/science.1210362 [DOI] [PubMed] [Google Scholar]
- Tao J, Rolls MM, 2011. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J. Neurosci. Off. J. Soc. Neurosci 31, 5398–5405. 10.1523/JNEUROSCI.3826-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarte K, Jourdan M, Veyrune JL, Berberich I, Fiol G, Redal N, Shaughnessy J, Klein B, 2004. The Bcl-2 family member Bfl-1/A1 is strongly repressed in normal and malignant plasma cells but is a potent anti-apoptotic factor for myeloma cells. Br. J. Haematol 125, 373–382. 10.1111/j.1365-2141.2004.04908.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca CI, Dal-Cim T, Cimarosti H, 2015. In vitro oxygen-glucose deprivation to study ischemic cell death. Methods Mol. Biol. Clifton NJ 1254, 197–210. 10.1007/978-1-4939-2152-2_15 [DOI] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, Bertagnolli D, Goldy J, Shapovalova N, Parry S, Lee C, Smith K, Bernard A, Madisen L, Sunkin SM, Hawrylycz M, Koch C, Zeng H, 2016. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci 19, 335–346. 10.1038/nn.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R, 1983. Cortical morphometry in Alzheimer’s disease, in: Katzman E (Ed.), Biological Aspects of Alzheimer’s Disease, Bradbury Report. Cold Spring Harbor Laboratory Press, Spring Harbor, NY, pp. 95–98. [Google Scholar]
- Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS, 2010. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J. Comp. Neurol 518, 647–667. https://doi.Org/10.1002/cne.22235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thundyil J, Pavlovski D, Sobey CG, Arumugam TV, 2012. Adiponectin receptor signalling in the brain. Br. J. Pharmacol 165, 313–327. 10.1111/j.1476-5381.2011.01560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA, 2001. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J. Neurosci. Off. J. Soc. Neurosci 21, 9478–9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, 2009. The direct relationship between inhibitory currents and local field potentials. J. Neurosci. Off. J. Soc. Neurosci 29, 15299–15307. 10.1523/JNEUROSCI.2019-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R, 2006. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J. Neurosci. Off. J. Soc. Neurosci 26, 12447–12455. 10.1523/JNEUROSCI.2787-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B, Bazan NG, 2003. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp. Neurol 179, 167–175. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Le Y-Z, Chollangi S, Muller W, Ash JD, 2009. Preconditioning-induced protection of photoreceptors requires activation of the signal-transducing receptor gp130 in photoreceptors. Proc. Natl. Acad. Sci. U. S. A. 106, 21389–21394. 10.1073/pnas.0906156106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasireddy V, Vijayasarathy C, Huang J, Wang XF, Jablonski MM, Petty HR, Sieving PA, Ayyagari R, 2005. Stargardt-like macular dystrophy protein ELOVL4 exerts a dominant negative effect by recruiting wild-type protein into aggresomes. Mol. Vis 11, 665–676. [PubMed] [Google Scholar]
- Webster NJ, Green KN, Peers C, Vaughan PFT, 2002. Altered processing of amyloid precursor protein in the human neuroblastoma SH-SY5Y by chronic hypoxia. J. Neurochem 83, 1262–1271. [DOI] [PubMed] [Google Scholar]
- Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DLA, Ghosh S, Nguyen LN, Barathi VA, Yeo SW, Luu CD, Wenk MR, Silver DL, 2016. Mfsd2a Is a Transporter for the Essential ω−3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem 291, 10501–10514. 10.1074/jbc.M116.721340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MW, Segal JA, Mark RJ, Ogden AM, Felder CC, 2000. Inflammatory cytokines enhance muscarinic-mediated arachidonic acid release through p38 mitogen-activated protein kinase in A2058 cells. J. Neurochem 74, 2033–2040. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B, 2008. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain J. Neurol 131, 928–937. 10.1093/brain/awn006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T, 2013. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 17, 185–196. 10.1016/j.cmet.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T, 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769. 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Harik SI, Busto R, Santiso M, Martinez E, Ginsberg MD, 1984. Free fatty acids and energy metabolites in ischemic cerebral cortex with noradrenaline depletion. J. Neurochem 42, 711–717. [DOI] [PubMed] [Google Scholar]
- Yu M, Benham A, Logan S, Brush RS, Mandal MNA, Anderson RE, Agbaga M-P, 2012. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. J. Lipid Res 53, 494–504. 10.1194/jlr.M021386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, 2011. Dendritic spines and distributed circuits. Neuron 71, 772–781. 10.1016/j.neuron.2011.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo- Branco G, Hjerling-Leffler J, Linnarsson S, 2015. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142. 10.1126/science.aaa1934 [DOI] [PubMed] [Google Scholar]
- Zeng L-H, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M, 2007. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J. Neurosci. Off. J. Soc. Neurosci 27, 11604–11613. 10.1523/JNEUROSCI.0983-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang C, Shen Y, Chen N, Wang L, Liang L, Guo T, Yin X, Ma Z, Zhang B, Yang L, 2016. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa. Hum. Genet 135, 1375–1387. 10.1007/s00439-016-1730-2 [DOI] [PubMed] [Google Scholar]
- Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, Kakuk LE, Lagali PS, Wong PW, MacDonald IM, Sieving PA, Figueroa DJ, Austin CP, Gould RJ, Ayyagari R, Petrukhin K, 2001. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet 27, 89–93. 10.1038/83817 [DOI] [PubMed] [Google Scholar]
- Zhang R, Brennan M-L, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL, 2002. Myeloperoxidase Functions as a Major Enzymatic Catalyst for Initiation of Lipid Peroxidation at Sites of Inflammation. J. Biol. Chem 277, 46116–46122. 10.1074/jbc.M209124200 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci 34, 11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG, 2011. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARY-mediated mechanisms in Alzheimer’s disease models. PloS One 6, e15816 10.1371/journal.pone.0015816 [DOI] [PMC free article] [PubMed] [Google Scholar]