Summary
Patients with gastroesophageal reflux disease (GERD) can present with typical or atypical symptoms. The aim of this study is to explore the underlying physiological and psychological mechanisms that lead to different symptomatic manifestations of GERD. A total of 238 patients diagnosed with GERD underwent gastroscopy, 24 h multichannel intraluminal impedance-pH (MII-pH) monitoring, and psychological assessment with questionnaires. Patient symptoms were used to classify GERD into phenotypes of typical reflux syndrome (TRS, n = 87), reflux chest pain syndrome (RCS, n = 98), and extraesophageal syndromes (EES, n = 53). 38 healthy volunteers served as controls. Reflux parameters and baseline impedance values (BIVs) were acquired from MII-pH monitoring results. A subset of subjects were biopsied from the lower esophagus; certain immune cells were stained with immunohistochemistry. BIVs in GERD patients (TRS, RCS, and EES) were significantly lower than in healthy controls and TRS patients exhibited the lowest BIVs (all P < 0.01). This indicated that the extent of mucosal injury differed across groups. TRS patients had higher acid exposure time (AET) compared to RCS, EES and controls (all P < 0.05). RCS patients had more intraepithelial T lymphocyte (IEL) and mast cell (MC) infiltration, and higher psychometric scores compared to TRS patients and controls (all P < 0.05), suggesting a possible stress-related esophageal hypersensitivity basis. TRS patients are characterized by acid reflux and correlated mucosal injury, which explains their typical reflux symptoms. RCS patients exhibit less acid-related injury but possible psychological stress-related esophageal hypersensitivity, which could be the main cause of their esophageal pain.
Keywords: acid reflux, baseline impedance, esophageal hypersensitivity, reflux chest pain
INTRODUCTION
Gastroesophageal reflux disease (GERD) is typically diagnosed by a combination of clinical symptoms, response to acid suppression, as well as objective tests (upper endoscopy, 24 h multichannel intraluminal impedance-pH (MII-pH) monitoring). The Montreal consensus classifies GERD-related symptoms as a set of syndromes: typical reflux syndrome (TRS), reflux chest pain syndromes (RCS), syndromes with esophageal injury (SEI), and extraesophageal syndromes (EES).1
Little research has discussed the underlying mechanisms from the perspective of these clinical syndromes. The widely used GERD classification is based on the aforementioned objective tests and includes reflux esophagitis (RE), nonerosive reflux disease (NERD), and Barrett esophagus. NERD is defined as troublesome reflux symptoms in the absence of esophageal mucosal breaks but with a pathological range of acid exposure. In contrast, reflux hypersensitivity (RH) is defined as having normal acid exposure but a positive relationship between symptoms and reflux events by the Rome IV committee.2,3 Functional heartburn (FH) is a burning retrosternal discomfort without any type of reflux underlying symptoms. However, only a small portion of GERD patients have undergone diagnostic tests to get a definitive diagnosis of one of the above-mentioned GERD phenotypes. This paper seeks to understand more about the different symptoms in GERD.
Over half of GERD patients are NERD patients. All NERD patients have no visible lesions during the endoscopy, but this does not necessarily mean their mucosa is intact; varying degrees of subtle mucosal injury can be found microscopically.4 MII-pH monitoring has traditionally been used to monitor gastroesophageal reflux. Research has increasingly demonstrated that the decreased esophageal intraluminal baseline impedance value (BIV) is associated with impaired mucosal integrity.4–7 Therefore, morphological and even functional integrity of esophageal mucosa can be evaluated by measuring intraluminal baseline impedance.
The proposed mechanisms for esophageal symptoms include direct reflux damage, motility disorder, esophageal hypersensitivity, altered central stimuli processing, and psychological comorbidity.8 The role of direct reflux damage in GERD has been extensively studied. Negative emotions have been increasingly recognized to influence the perception of visceral pain. It has been shown that patients who do not respond to proton pump inhibitor (PPI) therapy are more likely to have psychosocial comorbidity than those who were successfully treated with a PPI.9 We speculate that different symptom manifestations have their own dominant mechanisms.
We conducted this study (1) to investigate the underlying mechanisms responsible for different esophageal symptomatic syndromes, (2) to evaluate reflux severity and mucosal injury among patients with different syndromes, and (3) to explore possible factors responsible for esophageal hypersensitivity.
MATERIALS AND METHODS
Study subjects
Between October 2014 and April 2016, we enrolled patients with typical and/or atypical reflux symptoms who visited the outpatient gastroenterology clinic at Peking University Third Hospital. Typical reflux symptoms are defined as heartburn and/or regurgitation. Atypical symptoms are defined as chest pain, belching, nausea, dysphagia, odynophagia, globus sensation, with or without extraesophageal symptoms such as chronic dry cough/laryngitis/pharyngitis. The inclusion criteria for patients were as follows: (1) aged higher than 18 years; (2) complaining of typical or atypical GERD symptoms at least twice a week for 6 months; (3) undergoing gastroscopy, 24 h MII-pH monitoring, and filling out questionnaires within the same week; (4) off PPI therapy >1 month. The study excluded people with: (1) systemic diseases such as ischemic cardiac pain, chronic pulmonary disease, severe ENT disorders, and underlying psychiatric illness; (2) gastrointestinal (GI) disorders such as GI tumor, previous GI surgery, peptic ulcer, gallstones, severe esophageal motility disorders, esophageal stricture, and Barrett's esophagus. Healthy volunteers with no digestive or systemic symptoms were recruited as controls. The protocol for this study was approved by the Ethics Committee of the Peking University Health Science Center. All subjects gave informed consent.
Study protocol
All subjects who agreed to participate in this study underwent careful vetting, which included a medical history, clinical examination, routine biochemistry, ECG, upper abdominal ultrasound, and gastroscopy to exclude certain systemic and GI diseases. Then it was decided whether subjects were eligible for this study. Within the following week, subjects were asked to finish 24 h MII-pH monitoring and esophageal manometry, and to complete a Symptom Checklist 90-R (SCL90R) questionnaire. Subjects with severe esophageal motility disorders were excluded from participating, and the list of subjects was finalized. Qualified GERD patients were then grouped by their chief complaints according to the Montreal definition: (1) TRS group, patients who emphasized troublesome heartburn and/or regurgitation; (2) RCS group, patients who emphasized chest pain or epigastric pain without associated symptoms of typical reflux or with pain overshadowing typical reflux symptoms; (3) EES group, patients who complained of chronic dry cough, chronic laryngitis, chronic pharyngitis, along with typical reflux symptoms or atypical symptoms such as dysphagia, odynophagia, and/or globus sensation. We also summarized numbers of RE, NERD, and RH/FH patients in each symptomatic group as reference data. During the final four months of this project, a subset of subjects were consecutively biopsied during gastroscopy in the distal esophagus. Biopsy tissues were processed routinely for immunohistochemistry (IHC) staining. Gastroesophageal reflux parameters and intraluminal BIVs were obtained from MII-pH monitoring. SCL90R total and dimension scores were calculated based on questionnaires.
Study procedures
Gastroesophageal reflux parameters and baseline impedance acquirement
All subjects underwent 24 h MII-pH monitoring using an ambulatory monitoring system (MMS, Netherlands). Six impedance values (z1 to z6) were recorded at 17, 15, 9, 7, 5, and 3 cm above the lower esophageal sphincter. BIVs were selected from the impedance recording curve between the time frame of 10–11am (the average of three measurements); data readings were not taken during patient swallowing, reflux, or when pH value reached <6. Gastroesophageal reflux parameters such as acid exposure time (AET, the fraction of total recording time at pH < 4.2) and reflux episodes (acidic (pH ≤ 4), weakly acidic (4 < pH < 7), weakly alkaline (pH ≥ 7) and gas) were all calculated by the MMS software package.
Intraepithelial immune cell quantification
Subjects were biopsied in the distal esophagus (5 cm above the Z-line) during gastroscopy, avoiding any erosive lesion. Intraepithelial CD3-positive T lymphocytes (IELs) and tryptase-positive mast cells (MCs) were identified by IHC staining. Primary antibodies were rabbit polyclonal anti-CD3 (1:150; DakoCytomation, A0452) and mouse monoclonal antitryptase (1:500; Abcam, ab2378). Visualization of positive reactions was obtained by incubation of sections with secondary antibody (Dako, Rabbit/Mouse GK500705/10).
Cell quantification was performed on IHC staining sections. Immune cells were unevenly distributed. Therefore, we counted all positive-stained cells (under ×200 magnification) in each section; cell count was then divided by section area. Sectional areas were photographed at ×40 magnification and calculated with a Nikon E600 scale, using the Image-pro plus software (Media Cybernetics). Accordingly, cell density was expressed as number of cells per mm2.
Psychological profiles—SCL90R
Broad psychological profiles were assessed by SCL90R. Patients were asked to rate the severity of their experiences with 90 symptoms. The symptoms were assigned to nine dimensions: somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. Also, a category called “additional items” was designed to assess quality of daily life. The total SCL90R score and 10 subscale scores were calculated.
Statistical analysis
All data are expressed as mean ±SE or number (%). Continuous variables such as demographic information, BIVs, reflux parameters, cell counts, and psychometric scores were compared across groups using analysis of variance, followed by post-hoc testing to determine differences between groups. Categorical variables such as proportions of gender, diagnosis, or abnormal refluxes were compared using a chi-square test. Pearson's correlation statistics was used for correlation analysis of mast cell density and BIV. All test results with a P value <0.05 were considered statistically significant. Statistical analyses were completed using the SPSS 16.0 (SPSS Inc., USA).
RESULTS
A total of 238 GERD patients (87 TRS, 98 RCS, and 53 EES) and 38 healthy subjects were included in this study. The clinical characteristics of these patients are described in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| TRS | RCS | EES | Control | P value* | P value** | |
|---|---|---|---|---|---|---|
| Number | 87 | 98 | 53 | 38 | ||
| Age (years) | 49.2 ± 1.5 | 52.6 ± 1.3 | 51.4 ± 2.0 | 47.6 ± 3.2 | 0.142 | 0.221 |
| Male:female | 46:41 | 42:56 | 16:37 | 18:20 | 0.069 | 0.031 |
| BMI (kg/m2) | 24.4 ± 3.8 | 23.1 ± 2.8 | 24.5 ± 3.4 | 21.8 ± 3.2 | 0.017 | 0.11 |
| RE | 37 (43%) | 24 (25%) | 13 (24%) | – | – | 0.015 |
| Endoscopy negative and AET > 4.2% (NERD) | 27 (31%) | 21 (21%) | 11 (21%) | – | – | 0.237 |
| Endoscopy negative and AET < 4.2% (RH and FH) | 23 (26%) | 53 (54%) | 29 (55%) | – | – | <0.001 |
| AET > 4.2% | 55 (63%) | 38 (39%) | 20 (38%) | 5 (13%) | 0.001 | 0.001 |
BMI, body mass index; EES, extraesophageal syndrome; FH, functional heartburn; NERD, non-erosive reflux disease; RCS, reflux chest pain syndrome; RE, reflux esophagitis; RH, reflux hypersensitive; TRS, typical reflux syndrome
*P value, comparison of TRS, RCS, EES and controls by one-way ANOVA;
**P value, comparison of TRS, RCS, EES by one-way ANOVA. Data are expressed as mean ± SE and number (%).
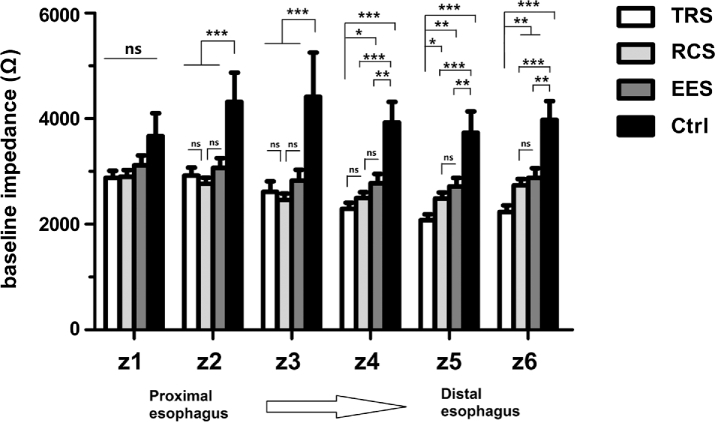
BIVs in GERD patients were lower than those in controls from z2 to z6 (all P ≤ 0.001) (Fig. 1). There were no significant differences of BIVs among TRS, RCP, and EES patients in z1, z2, and z3. However, BIVs in TRS patients were significantly lower than BIVs in RCS patients in z5 (P = 0.016) and z6 (P = 0.006). BIVs in RCS patients were significantly lower than BIVs in controls from z2 to z6 (all P < 0.001). BIVs in TRS patients were significantly lower than BIVs in EES patients in z4, z5, and z6 (P = 0.02, 0.002, and 0.004), but not in z1, z2, and z3. In summary, BIVs from the lower esophagus (z5, z6) consistently exhibited the following trend: TRS < RCS/EES < Controls.
Fig. 1.
Esophageal baseline impedance levels from 24 h MII-pH monitoring. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. ns, not statistically significant. Ctrl, controls; EES, extraesophageal syndromes; RCS, reflux chest pain syndrome; TRS, typical reflux syndrome.
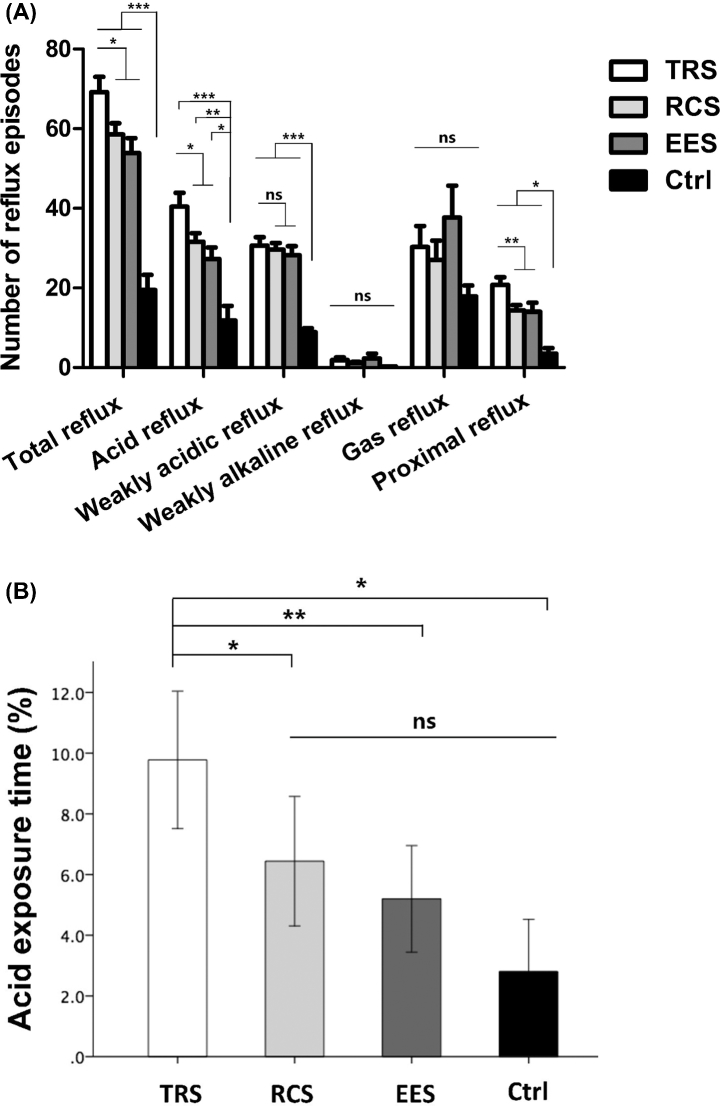
GERD patients exhibited higher numbers of total reflux, acid reflux, and weakly acidic reflux episodes compared to controls (all P < 0.001) (Fig. 2A). Within GERD groups, TRS patients showed higher numbers of total and acid reflux episodes compared with RCS (P = 0.016 and P = 0.02) and EES (P = 0.004 and P = 0.004) patients. AET in TRS patients (9.8 ± 1.1%) was significantly higher than AET in RCS (6.4 ± 1.0%), EES (5.2 ± 0.9%) patients, and controls (2.8 ± 0.8%) (P = 0.02, 0.008, and 0.012). No significant difference of AET was found between the RCS, EES, and control groups (Fig. 2B).
Fig. 2.
Gastroesophageal reflux parameters. (A) Numbers of various reflux episodes calculated by MMS software. (B) Acid exposure time calculated by MMS software. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. ns, not statistically significant.
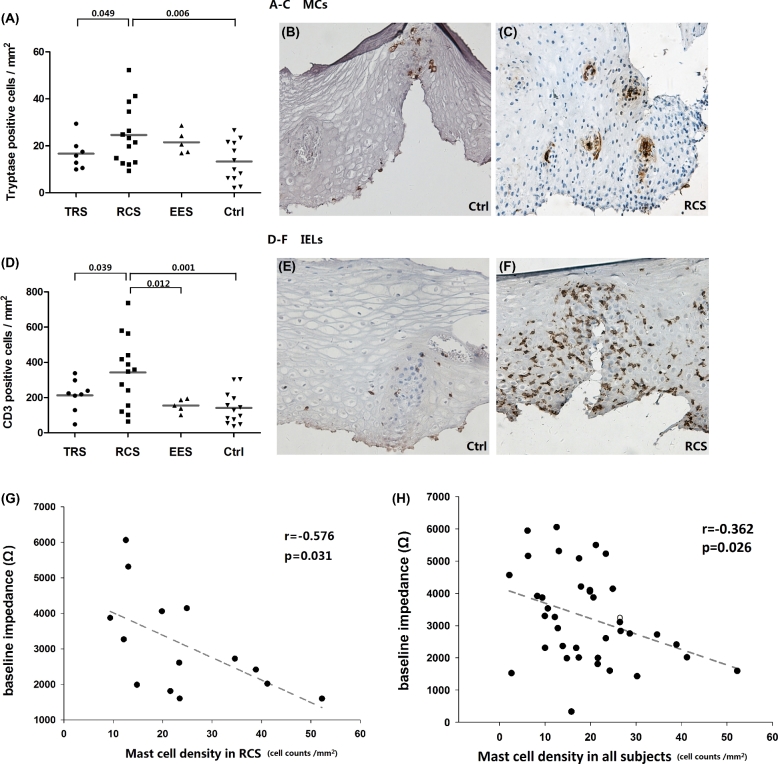
A total of 40 subjects (8 TRS, 14 RCS, 5 EES, and 13 controls) were biopsied. MCs in RCS patients were significantly higher than those in TRS patients and controls (24.7 ± 3.4 vs. 16.6 ± 2.6/mm2, P = 0.049; 24.7 ± 3.4 vs. 13.3 ± 2.5/mm2, P = 0.006) (Fig. 3A). Demonstrating a similar trend, IELs in RCS patients were also higher than those in TRS patients and controls (342.8 ± 53.0 vs. 213.2 ± 32.1/mm2, P = 0.039; 342.8 ± 53.0 vs. 141.6 ± 25.1/mm2, P = 0.001) (Fig. 3D). MCs were sparse in the esophagus, mostly around the papillary. Upon activation (Fig. 3C), MCs tended to cluster and cell shape became fuzzier, probably as a result of increased intracellular tryptase production or extracellular tryptase release. In healthy individuals, IELs were dispersed along the basal layer or peripapillary area (Fig. 3E). In RCS patients, IEL infiltrations were much more severe (Fig. 3F), and still typically distributed in peripapillary area.
Fig. 3.
Esophageal intraepithelial infiltration of mast cells and T lymphocytes. (A) Density of tryptase-positive MCs among groups. (B and C) IHC staining of MCs in a control subject and a RCS patient (×200). (D) Density of IELs among groups. (E and F) IHC staining of IELs in a control and a RCS patient (×200). (G) & (H) Correlation analyses between baseline impedance level (z6) and MC density in RCS group (n = 14) and in all subjects (n = 38). Each dot represents an individual, solid lines represent mean value.
A significant negative correlation was found between MC density and BIV (z6) in RCS group (r = −0.576, P = 0.031, n = 14) (Fig. 3G). The correlation analysis for MC and BIV in all subjects was also significant (r = −0.362, P = 0.026, n = 38) (Fig. 3H), but with a decreased R value. If we eliminated RCS patients to analyze this correlation in the remaining subjects (TRS, EES & controls), it became insignificant (r = −0.184, P = 0.39, n = 24, not shown in Fig. 3). Thus we assumed that the significant correlation was due to RCS patients.
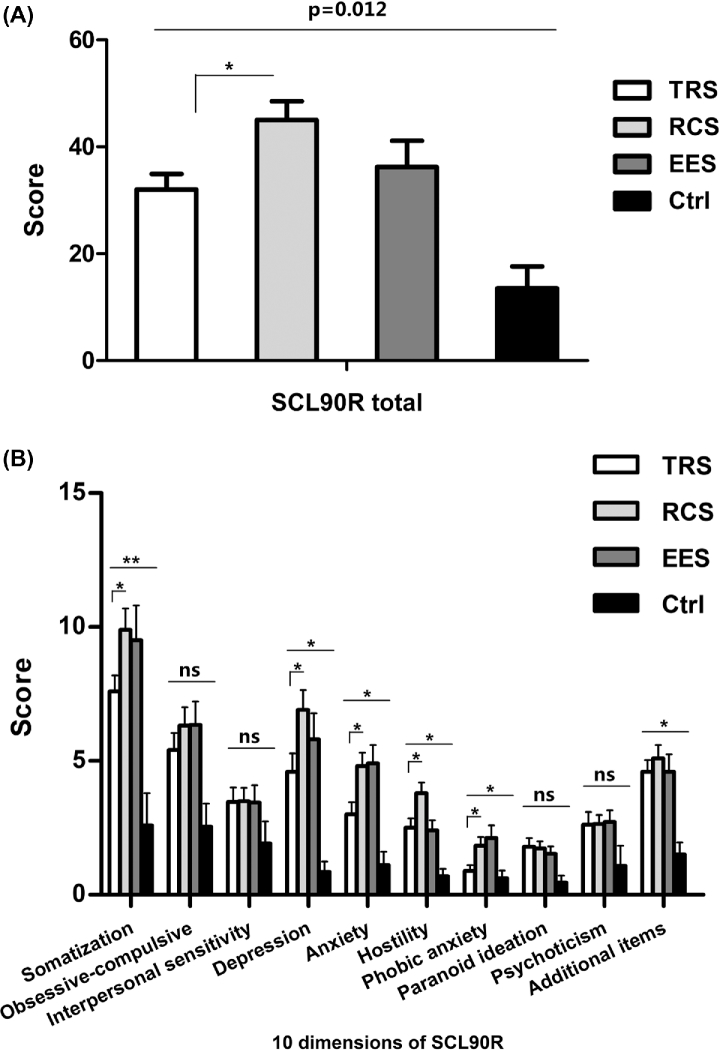
As summarized in Figure 4, GERD patients displayed higher psychometric scores than controls in terms of SCL90R total score (Fig. 4A) and several SCL90R subscale scores (Fig. 4B) (all P < 0.05). It is worth noting that the RCS patients displayed even higher SCL90R total scores than TRS patients; this suggested that psychological comorbidity tended to occur more often in RCS patients. When we looked into the 10 psychological dimensions in SCL90R, we found that RCS patients had significantly higher scores than TRS patients in somatization, depression, anxiety, hostility, and phobic anxiety.
Fig. 4.
Psychometric profiles among groups. (A) SCL-90R total score. (B) SCL-90R subscale scores. Data are presented as mean ± SEM. *P < 0.05. ns, not statistically significant.
DISCUSSION
In this study, we have provided the first evidence for the underlying pathogenesis of different symptomatic syndromes in GERD patients. We demonstrate that TRS patients have major gastroesophageal acid reflux and relevant mucosal injury; as a result they exhibit the lowest esophageal baseline impedance levels and the most troublesome reflux symptoms. RCS patients are characterized by less acid injury but more profound mucosal immune activation and psychological abnormalities, which may be related to esophageal hypersensitivity status leading to esophageal pain.
GERD impressions are heterogeneous reflux symptoms. For this reason, we classified patients by chief complaint and then determined physiological features in different symptomatic groups. As shown in Table 1, RE patients represented 43% of the TRS population and 25% of the RCS population, whereas RH and FH patients represented only 26% of the TRS population but 54% of the RCS population. This indicates the proportions of patients with mucosal injury differed across symptomatic groups. The typical reflux symptoms in TRS patients may largely be due to mucosal injury. The extent of mucosal injury in RE can easily be evaluated through endoscopy. Mucosal injuries in endoscopic negative NERD and RH/FH are usually evaluated by histological criteria such as basal cell hyperplasia, papillary elongation, inflammatory cell infiltration, and dilated intercellular spaces, whereas BIV is a new parameter to evaluate mucosal injury. GERD patients exhibit lower BIV than healthy controls, RE patients exhibit even lower BIV than NERD patients.5,6 Our previous study demonstrated that BIV correlates with AET and dilated intercellular spaces.7 Therefore BIV is a real-time measurable parameter to assess mucosal lesion. As seen in Figure 1, BIV decreased in GERD patients from the distal to proximal esophagus, which means that reflux injury is extensive and not just limited to the distal esophagus or localized erosive site. TRS patients showed even lower BIV than RCS and EES patients in distal (z5 & z6) but not proximal-mid (z1 to z4) esophagus, indicating that TRS patients have more severe mucosal injury in the distal esophagus. As the distal esophagus interacts most frequently with reflux components, we assume that TRS patients should have the severest reflux among the studied groups.
Reflux parameters are summarized in Figure 2. As we assumed, TRS patients exhibited higher AET and acid reflux episodes than RCS patients, which was in concordance with the differences in BIV, RE proportion, and symptom severity. Since BIV and RE are both confirmed to correlate with acid exposure severity, this is unsurprising. There was no significant difference of AET among RCS, EES, and controls, probably because more than half of the RCS and EES population were RH or FH patients who had normal AET (Table 1). The number of episodes of weakly acidic reflux and weakly alkaline reflux was similar among TRS, RCS, and EES patients, but higher than controls. Therefore weakly acidic and weakly alkaline reflux events do not seem to be responsible for the differences between TRS and RCS.
As shown in Figure 3, intraepithelial MCs and IELs were greater in RCS patients than in TRS patients and controls. The concordant increase of these immune cells caused us to assume that RCS patients might have a low-grade mucosal inflammation, and a tendency toward visceral hypersensitivity for esophageal pain. The GI mucosal inflammatory infiltrate is dominated by MCs, eosinophils, and intraepithelial lymphocytes.10 It is well established that MC activation generates epithelial barrier and neuromuscular dysfunction, promoting visceral hypersensitivity.11–14 Stress is a common activator of MCs. Several types of stress mediators induce MC activation and consequent release of vasoactive mediators, contributing to permeability alteration, barrier dysfunction, hyperalgesia, inflammation, and motility change.14–16 Yu et al. have demonstrated that the activation of esophageal MCs increases vagal nociceptive C fibers’ excitability and indicates that MCs play an important role in esophageal inflammatory reaction and nociception.17,18 Our previous study demonstrates that MCs are involved in stress-induced esophageal mucosal dysfunction in rodents and suggests that proteinase activated receptors 2 (PAR2) could be a potential intermediary factor.19 PAR2 is a target receptor of mast cell-derived tryptase. PAR2 is expressed on enterocytes, GI smooth muscle cells, and capsaicin-sensitive neurons in the esophagus, and regulates GI mucosa inflammation and barrier function.20–22 Kandulski et al. identify PAR2 expression in human esophageal epithelium and demonstrate the functional importance of PAR2-mediated pathways in the pathogenesis of GERD-associated mucosal alterations.23 In the human gut, MC activation has been suggested to alter pain perception in response to infections, stress and emotions in functional GI disorders (FGIDs). In summary, broad evidence supports a potential correlation of MCs with esophageal barrier integrity and hypersensitivity.
In the RCS group, we observed a significant negative correlation between MCs and BIV, indicating that immune activation affects mucosal integrity. Our previous research demonstrates that BIV correlates with morphological alterations such as dilated intercellular spaces and decreases of tight junction protein.7 It is therefore reasonable to see that MCs correlate with BIV. However, as we described in this study's results, this correlation was not seen in the TRS, EES & controls. Our explanation is that the dominant pathogenesis in TRS patients is acid-induced inflammatory response not involving MCs. Acid-related BIV decrease and MC-related BIV decrease are different processes, yet both result in mucosal structural alterations. The IELs in chronic esophageal mucosal inflammation are typically T lymphocytes.24 Very little research has explained the mechanism of how lymphocytes function in the inflammatory process of GERD; however, many inflammatory diseases frequently made worse by stress involve MCs in communication with T cells.25–27 We believe that there is a certain internal correlation in the increase of IELs and MCs in RCS patients.
The above evidence supports the relationship between low-grade immune activation and esophageal hypersensitivity to pain. Since the patients with less acid exposure (RCS vs. TRS) exhibit more immune activation, certain stimuli other than gastroesophageal reflux are likely causing this immune activation. Psychological comorbidity is commonly seen in FGIDs and GERD.28,29 Anxiety and depression increase GERD-related symptoms in population-based studies.30 GERD patients who have a poor correlation of symptoms with acid reflux events display a higher level of anxiety compared to patients who have a close correlation of symptoms with acid reflux events.31 It is not known whether this comorbidity is the cause or the consequence of GI dysfunction, but it is generally accepted that psychological symptoms and GI symptoms amplify each other via the so-called brain-gut axis. In our research, GERD patients had higher psychological scores than healthy controls, which mirror the findings of previous research. Our new finding is that psychological scores in RCS patients were even higher than TRS patients in terms of somatization, depression, anxiety, hostility, and phobic anxiety, suggesting that disease-specific treatment plans (PPI, behavioral therapy, or pain modulators) should take psychological factors into account.
In this study, the trend of psychological scores was similar to the trend of immune cells across groups: the RCS group exhibited the highest level both in immune cells and in psychological scores, indicating a potential connection between psychological comorbidity and immune activation. Accumulating evidence links psychological disturbance to immune activation.32 Our research is unable to confirm the onset sequence of immune activation and psychological disorder in RCS patients. However, we tend to interpret immune activation and related GI hypersensitivity as the downstream effects of psychological factors because otherwise it is difficult to explain how a less irritated esophagus (RCS vs. TRS) generates persistent pain and a broad scale of psychological problems.
So far, we have not discussed the EES patients. As we defined, EES patients are relatively heterogeneous as they demonstrate various atypical extraesophageal symptoms. We only collected a small number of histology samples from EES patients and were therefore unable to make definitive conclusions about this group. Evidence substantiating a beneficial effect of reflux treatments on the EES is weak, so reflux is rarely the sole cause of extraesophageal symptoms.1 Similarly, the reflux parameters in this study also demonstrated that acid exposure in EES patients was not as severe as acid exposure in TRS patients. In addition, we found that the EES group resembled the RCS group in many aspects, including a high female sex ratio, baseline impedance level, reflux parameters, RE/NERD/RH/FH constituent ratio, and even some psychological profiles. We therefore speculate that EES symptoms are to some extent linked to visceral hypersensitivity.
Potential limitations in this study are as follows: (1) The grouping criteria are relatively subjective. Narration bias could have caused some patients to be classified wrongly, especially for patients exhibiting both typical and atypical symptoms. This would affect the generalizability of the conclusions. (2) Although the sample size of the entire study was fairly large, the number of biopsied samples was smaller. Especially for the control group, it is better to have all participants biopsied to ensure no underlying diseases such as eosinophilic esophagitis. Because of this, our hope is that further studies will corroborate our findings. (3) The data in our study are cross-sectional; as a result, we can only prove a correlative relationship and not a causal relationship. In future research, patients need to be subgrouped by onset sequence of GI or psychological symptoms, and interventions such as pain-modulators or mast cell stabilizers may be added.
Overall, we have determined that various types of esophageal symptomatic syndromes are correlated with different mechanisms. Typical reflux symptoms are the manifestation of excessive acid exposure and consequent mucosal injury, while atypical reflux symptoms or even extraesophageal symptoms have a basis in esophageal hypersensitivity, which may be triggered by psychological stress and correlated immune activation.
Acknowledgments
This work was supported by the National Key Technology Research and Design Program of China (2012BAI06B02).
Notes
Specific author contributions: Study concept and design: Chanjuan Zhong; Data collection and analysis: Chanjuan Zhong, Drafting of manuscript: Chanjuan Zhong; Subject recruitment: Kun Wang, Kuiliang Liu; Performance of clinical procedure: Kun Wang, Kuiliang Liu; Maintaining database: Hong Liu, Kuiliang Liu; Histological analysis: Hui Su; Interpretation of data and important intellectual content: Jing Wu; Project conceiving and initiation: Liping Duan; Critical revision of the manuscript and study supervision: Liping Duan.
References
- 1. Vakil N, van Zanten SV, Kahrilas P et al. . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900–20. [DOI] [PubMed] [Google Scholar]
- 2. Drossman D A, Hasler W L. Rome IV—functional GI disorders: disorders of gut-brain interaction. Gastroenterology 2016; 150: 1257–61. [DOI] [PubMed] [Google Scholar]
- 3. Aziz Q, Fass R, Gyawali C P et al. . Esophageal disorders. Gastroenterology 2016; 150: 1368–79. [DOI] [PubMed] [Google Scholar]
- 4. Woodland P, Sifrim D. Esophageal mucosal integrity in nonerosive reflux disease. J Clin Gastroenterol 2014; 48: 6–12. [DOI] [PubMed] [Google Scholar]
- 5. Farre R, Blondeau K, Clement D et al. . Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 2011; 60: 885–92. [DOI] [PubMed] [Google Scholar]
- 6. Kessing B F, Bredenoord A J, Weijenborg P W et al. . Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol 2011; 106: 2093–7. [DOI] [PubMed] [Google Scholar]
- 7. Zhong C, Duan L, Wang K et al. . Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol 2013; 48: 601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickman R, Maradey-Romero C, Fass R. The role of pain modulators in esophageal disorders—no pain no gain. Neurogastroenterol Motil 2014; 26: 603–10. [DOI] [PubMed] [Google Scholar]
- 9. Nojkov B, Rubenstein J H, Adlis S A et al. . The influence of co-morbid IBS and psychological distress on outcomes and quality of life following PPI therapy in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2008; 27: 473–82. [DOI] [PubMed] [Google Scholar]
- 10. Wouters M M, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 2016; 65: 155–68. [DOI] [PubMed] [Google Scholar]
- 11. Rodewald H R, Feyerabend T B. Widespread immunological functions of mast cells: fact or fiction? Immunity 2012; 37: 13–24. [DOI] [PubMed] [Google Scholar]
- 12. Klooker T K, Braak B, Koopman K E et al. . The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010; 59: 1213–21. [DOI] [PubMed] [Google Scholar]
- 13. van den Wijngaard R M, Klooker T K, Welting O et al. . Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil 2009; 21: 1107–94. [DOI] [PubMed] [Google Scholar]
- 14. Wallon C, Yang P C, Keita A V et al. . Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008; 57: 50–58. [DOI] [PubMed] [Google Scholar]
- 15. Barreau F, Cartier C, Ferrier L et al. . Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 2004; 127: 524–34. [DOI] [PubMed] [Google Scholar]
- 16. Zheng P Y, Feng B S, Oluwole C et al. . Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut 2009; 58: 1473–9. [DOI] [PubMed] [Google Scholar]
- 17. Yu S, Kollarik M, Ouyang A et al. . Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 2007; 293: G850–6. [DOI] [PubMed] [Google Scholar]
- 18. Yu S, Gao G, Peterson B Z et al. . TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 2009; 297: G34–42. [DOI] [PubMed] [Google Scholar]
- 19. Zhong CJ, Wang K, Zhang L et al. . Mast cell activation is involved in stress-induced epithelial barrier dysfunction in the esophagus. J Dig Dis 2015; 16: 186–96. [DOI] [PubMed] [Google Scholar]
- 20. Cenac N, Chin A C, Garcia-Villar R et al. . PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol 2004; 558: 913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu H, Miller D V, Lourenssen S et al. . Proteinase-activated receptor-2 activation evokes oesophageal longitudinal smooth muscle contraction via a capsaicin-sensitive and neurokinin-2 receptor-dependent pathway. Neurogastroenterol Motil 2010; 22: 210–6, e67. [DOI] [PubMed] [Google Scholar]
- 22. Itoh Y, Sendo T, Oishi R. Physiology and pathophysiology of proteinase-activated receptors (PARs): role of tryptase/PAR-2 in vascular endothelial barrier function. J Pharmacol Sci 2005; 97: 14–19. [DOI] [PubMed] [Google Scholar]
- 23. Kandulski A, Wex T, Monkemuller K et al. . Proteinase-activated receptor-2 in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol 2010; 105: 1934–43. [DOI] [PubMed] [Google Scholar]
- 24. Rubio CA, Sjodahl K, Lagergren J. Lymphocytic esophagitis. Am J Clin Pathol 2006; 125: 432–7. [PubMed] [Google Scholar]
- 25. Theoharides T C, Alysandratos K D, Angelidou A et al. . Mast cells and inflammation. Biochim Biophys Acta 2012; 1822: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suurmond J, van Heemst J, van Heiningen J et al. . Communication between human mast cells and CD4(+) T cells through antigen-dependent interactions. Eur J Immunol 2013; 43: 1758–68. [DOI] [PubMed] [Google Scholar]
- 27. Shefler I, Salamon P, Reshef T et al. . T cell-induced mast cell activation: a role for microparticles released from activated T cells. J Immunol 2010; 185: 4206–12. [DOI] [PubMed] [Google Scholar]
- 28. Pinto-Sanchez M I, Ford A C, Avila C A et al. . Anxiety and depression increase in a stepwise manner in parallel with multiple FGIDs and symptom severity and frequency. Am J Gastroenterol 2015; 110: 1038–48. [DOI] [PubMed] [Google Scholar]
- 29. Lackner J M, Gudleski G D, Thakur E R et al. . The impact of physical complaints, social environment and psychological functioning on IBS patients’ health perceptions: looking beyond GI symptom severity. Am J Gastroenterol 2014; 109: 224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizyed I, Fass S S, Fass R. Review article: gastro-oesophageal reflux disease and psychological comorbidity. Aliment Pharmacol Ther 2009; 29: 351–8. [DOI] [PubMed] [Google Scholar]
- 31. Rubenstein J H, Nojkov B, Korsnes S et al. . Oesophageal hypersensitivity is associated with features of psychiatric disorders and the irritable bowel syndrome. Aliment Pharmacol Ther 2007; 26: 443–52. [DOI] [PubMed] [Google Scholar]
- 32. Dantzer R, O’Connor J C, Freund G G et al. . From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]