Abstract
The optimal treatment of malignant pleural mesothelioma (MPM) has not yet been established and is still under investigation. Surgery is one of the pillars in the multimodality approach with the purpose of removing as much as visible tumor as possible and to relieve symptoms. To date, two major surgical procedures are available for removal or debulking of MPM that is considered to be resectable: [extended (e)] pleurectomy/decortication (P/D) and extrapleural pneumonectomy (EPP). Historically, EPP was regarded as the only way to achieve a macroscopic complete resection. However, in the last years, there is a shift in literature towards (e)P/D as the preferred surgical procedure whenever possible as several retrospective studies and meta-analyses showed a similar or lower long-term survival and higher perioperative mortality and postoperative morbidity in patients who been treated with EPP. On the other hand, no randomized-controlled trials regarding surgical treatment with (e)P/D or EPP exist and therefore level A evidence favoring one surgical procedure is lacking. In this review we provide a nuanced and well-considered answer to the question whether EPP is still indicated in the surgical treatment of MPM.
Keywords: Malignant pleural mesothelioma (MPM), surgery, extrapleural pneumonectomy (EPP), pleurectomy decortication (P/D)
Introduction
The optimal treatment of malignant pleural mesothelioma (MPM) has not yet been established and is still under investigation (1). Due to the ineffectiveness of single-modality therapy, treatments with curative intent tend to make use of a multimodality approach, combining (neo)adjuvant chemotherapy, surgery and radiotherapy (2,3). Surgery remains an important therapeutic option for MPM with the purpose to macroscopically remove visible tumor, to eliminate pleural effusion, to relieve symptoms such as dyspnea, to ease the pain, and to increase the efficacy of adjuvant therapy (4,5). However, surgery in MPM is rather cytoreductive than radical due to the intricate location and relation to surrounding normal tissue (6,7). Therefore, it is virtually impossible to obtain free resection margins and the aim of surgery is mainly to obtain a macroscopic resection by removing as much visible tumor as possible (6). Adjuvant chemotherapy and/or radiotherapy should complete the process by eliminating microscopic residual tumor at the surgical margins to prevent local recurrence or widespread hematogenous or lymphatic dissemination (2).
To date, two major surgical procedures are available for removal or debulking of MPM that is considered to be resectable: [extended (e)] pleurectomy/decortication (P/D) and extrapleural pneumonectomy (EPP), but this is subject to discussion, in particular regarding the most appropriate intervention (5,8-12).
P/D is defined as parietal and visceral pleurectomy with removal of all gross tumor. When the diaphragm and/or pericardium is resected as well, the procedure is defined as an extended P/D. Lung parenchyma is not resected (1,6,11).
EPP is defined as en bloc resection of the parietal and visceral pleura including the ipsilateral lung, pericardium and diaphragm, which are reconstructed with a soft tissue patch (Figure 1). In cases where the pericardium and/or diaphragm are not involved by tumor, these structures may be left intact (1,6,11).
Figure 1.
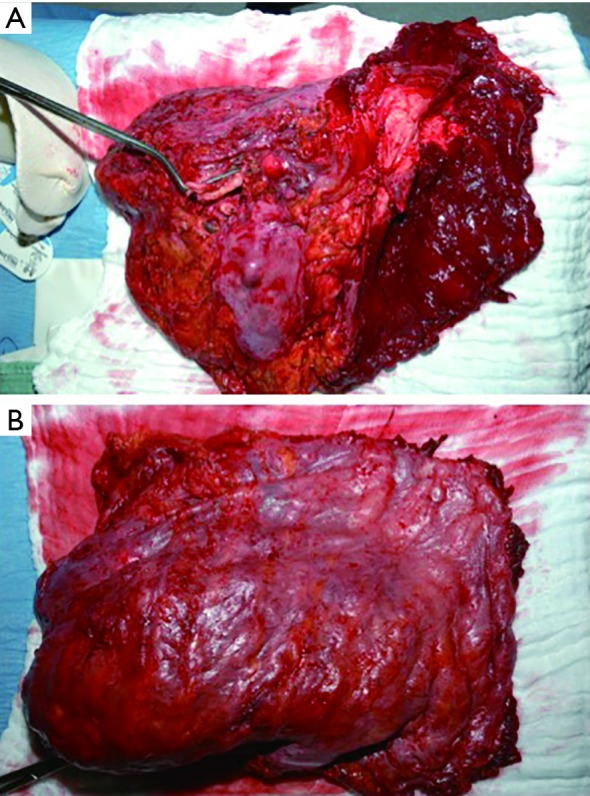
Resection specimen after EPP. (A) Hilar view with clamp on main bronchus; (B) costal view.
EPP vs. (e)P/D
The technique of tumor resection in MPM is one of the most debated topics in thoracic surgery, mainly due to the lack of evidence concerning this topic (9,11,13).
For many years, EPP was considered as the only surgical procedure which achieves a macroscopic complete resection and was applied in resectable patients independent of stage and histology (9,11). This was supported by the fact that EPP was able to extend the disease-free interval of TNM-stage I in the retrospective IASLC database (14).
As one would expect EPP should provide superior survival compared to P/D as it is the most extensive debulking surgery for MPM. Visceral pleurectomy at P/D is more likely to leave residual tumor cells compared to EPP because the connection between the visceral pleura and lung parenchyma is usually tighter than between the parietal pleura and chest wall (7). However, neither EPP nor (e)P/D will yield a complete R0 resection (13). Estimates are that even with EPP, positive margins on microscopy can be found in 70–100% of cases (15,16). To improve local control administering high-dose radiotherapy is most easily performed after EPP compared to (e)P/D, as there is an empty cavity without remaining lung parenchyma, and this is recommended by the National Comprehensive Cancer Network (NCCN) guidelines on MPM (17).
Despite these characteristics and advantages of EPP, in recent years there is a shift in literature towards (e)P/D as the preferred surgical procedure whenever possible.
In 2010 Cao et al. published a meta-analysis which demonstrated a significantly lower perioperative mortality and morbidity with a similar, if not superior, long-term survival for patients who underwent (e)P/D compared to EPP in a multimodality setting (18). Luckraz et al. reported that EPP without adjuvant therapy was an independent risk-factor for decreased survival (HR =9.2) in multivariate analysis with the inclusion of histological type, surgical procedure and type of adjuvant therapy (19). A paradigm shift in the surgical management of MPM was initiated.
Next, the Mesothelioma and Radical Surgery 1 (MARS 1) study was the first trial to randomize between EPP and no EPP after induction chemotherapy, as concerns arose on the role of extensive surgery for mesothelioma (20,21). This study could not demonstrate a significant advantage in survival after EPP versus no EPP and the authors even advised against the use of EPP because of its high morbidity and mortality rates (8). The results of this MARS 1 pilot trial gave rise to an intense debate in literature as mortality of EPP in the MARS was considerably higher compared to other centers and only a small number of patients underwent EPP (8,17). However, the idea that EPP being the most radical surgical procedure for MPM does not automatically result in superior survival—in contrast to other solid tumors—gained popularity.
Concerning postoperative quality of life (QoL) Rena et al. described EPP as a harmful procedure after their single-institution analysis of 77 patients. They concluded that patients submitted to EPP had a higher post-operative complication rate, a worse long-term QoL, a shorter residual life time after recurrent disease, despite a similar long-term survival when compared to P/D (22). Because MPM is an aggressive disease with a poor prognosis and limited life expectancy, assessment of the QoL after treatment should be included as an outcome measure as the remaining time should be as good as possible (5,9).
On the other hand, the technique of EPP has been refined over time and became a procedure with acceptable morbidity and mortality, especially in centers of experience where mortality rate became lower than 5% in the last decade (9,11,18). Centers with less than 5 EPP procedures per year have a mortality rate of 12.5% and as for morbidity, for instance, a significant higher incidence of postoperative acute respiratory distress syndrome (9,11). To optimize results EPP and (e)P/D should only be performed in high-volume thoracic surgical centers (17).
In 2014, the largest meta-analysis of survival after P/D vs. EPP to date by Taioli et al. demonstrated that perioperative 30-day mortality is significantly higher after EPP than after P/D (4.5% vs. 1.7%; P<0.05), and that EPP is associated with more postoperative complications than P/D. There was no statistically significant difference in survival between P/D and EPP at 2 years but only a modest difference in favor of P/D. However, given the increased risk associated with EPP, due to complexity and increased physiologic strain, EPP does not significantly improve survival to warrant the risk. Therefore, the authors suggested that P/D should be the preferred procedure when possible (23).
More recently, two further retrospective analyses have compared EPP and P/D with regards to morbidity, complications and overall survival. Kostron et al. investigated 167 patients who received multimodal treatment with induction chemotherapy combined with either EPP or P/D. Their main finding was that freedom of recurrence and postoperative morbidity were similar in both groups. However, the complication profile and the severity favored P/D (24). Similarly, Infante et al. performed a retrospective analysis of 163 patients who underwent EPP, P/D or palliative pleurectomy. They found that postoperative complications and mortality were similar in the EPP and P/D groups but that patients who underwent EPP had a six-fold higher risk of pleural sepsis when compared to P/D (25).
So why can EPP not live up to its expectations to significantly improve survival compared to (e)P/D? Several explanations for this contradiction have been provided:
❖ First, EPP is associated with higher perioperative mortality and morbidity due to disadvantages such as severe deterioration of postoperative cardiopulmonary function and QoL, and poorer tolerance to chemotherapy in case of recurrence (7,8,11,18-20,23,26). Moreover, due to the long lag time of 30–50 years after exposure to asbestos MPM generally affects patients in their late 50s to mid 60s and even older, causing them to be generally less fit for extensive surgery such as EPP (6,27);
❖ Second, patients who undergo (e)P/D have more opportunities for additional therapy (including EPP) after recurrence compared with patients who undergo EPP as first line treatment. Moreover, survival after recurrence was found to be longer in patients who underwent (e)P/D than in those who underwent EPP (21,28). However, the MARS 1 feasibility trial did not include enough patients in order to draw such conclusion (29);
❖ Third, because of better cardiopulmonary reserve, patients who undergo (e)P/D better tolerate postoperative non-oncological disorders such as pneumonia and cardiac failure compared to those who undergo EPP (7);
❖ Fourth, as the technique of EPP has refined over time (supra), the technique of (e)P/D has also improved with diaphragmatic and pericardial resection and acceptable macroscopic complete resection in the current era (11). Nowadays, in an increasing number of centers (e)P/D is even accepted as curative-intent surgery for MPM and most have abandoned EPP (7,30);
❖ Fifth, the recommendation of EPP for stage I MPM has not gained support in the current practice where centers perform (e)P/D in patients with limited tumor load, and reserve EPP for patients with advanced disease, especially fissure involvement (7,9,14).
So is EPP still indicated for MPM?
Current literature favors (e)P/D to EPP for several reasons:
As P/D is a procedure with a lower morbidity and mortality, it seems to be the most logical and preferred choice in the treatment of MPM (11,23). Others suggest that EPP should one day become of historical interest only due to repeated analyses of historical datasets that fail to show a benefit to EPP over (e)P/D (20). The current practice at the Hyogo College of Medicine in Japan is to perform the least invasive surgical procedure to achieve macroscopic tumor resection, being P/D. So P/D is indicated in most of cases, except those with extensive tumor invasion into the pulmonary parenchyma. Resection of the diaphragm, pericardium, and lung parenchyma is performed if required. Conversion to EPP is decided on the basis of intraoperative findings (7).
This concept is supported by Opitz and Weder who state that patients who are scheduled for P/D should be prepared to undergo EPP in case of extensive lung infiltration discovered during surgery as one surgical procedure does not fit for all patients with MPM. Lung preservation should be achieved whenever possible, but EPP may be a valuable solution in selected cases (9). This was already the conclusion of a systemic review of EPP for MPM carried out in 2010 (18). Filosso et al. have a similar view regarding this matter: they believe that due to inaccuracies of thoracic computed tomography (CT) and magnetic resonance imaging (MRI) scans in defining the extent of MPM, surgeons must be ready to perform both (e)P/D and EPP according to the tumor’s extent and the patient’s functional status (31).
The Clinical Practice Guidelines of European Society of Medical Oncology (ESMO) on MPM do not advocate a specific procedure to perform a macroscopic complete resection (6). The NCCN guidelines on MPM suggest P/D may be safer than EPP in early stage disease with favorable histology but does not conclude on which procedure is oncologically superior because of the lack of properly designed, well-performed randomized controlled trails. In more advanced disease, P/D reduces the risk for perioperative mortality and may be acceptable in terms of achieving complete macroscopic resection. However, the decision about whether to do P/D or EPP may not be made until surgical exploration (13).
Conclusions
There is still a lot of debate on which treatment modalities are optimal for the treatment of MPM. Surgery is one of the pillars in the multimodality approach with the purpose of removing as much as visible tumor as possible and to relieve symptoms. The two main surgical techniques that are used for this purpose are EPP and (e)P/D. Historically, EPP was regarded as the only way to achieve a macroscopic complete resection (9,11). In the last years, however, researchers have compared patients that have been treated with EPP or (e)P/D. They found that despite the more radical approach of EPP, long-term survival is similar or lower to that of (e)P/D (18,23). Moreover, a number of retrospective studies have demonstrated higher perioperative mortality and postoperative morbidity and a lower postoperative QoL in patients who have been treated with EPP (24,25). However, no randomized-controlled trials regarding surgical treatment with (e)P/D or EPP exist and therefore level A evidence favoring one surgical procedure is lacking. Currently, most authors propose a personalized view on the matter of deciding to treat with EPP or (e)P/D, namely by postponing the decision from pre-operatively to peroperative. They recommend starting with P/D as it is less invasive and to decide intraoperatively whether or not to switch to EPP if required to obtain a macroscopic complete resection. In this case, patients have to be informed that a more radical and invasive surgery is possible based on the peroperative findings (9,31).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.De Laet C, Domen A, Cheung KJ, et al. Malignant Pleural Mesothelioma: Rationale for a New TNM Classification. Acta Chir Belg 2014;114:245-9. 10.1080/00015458.2014.11681020 [DOI] [PubMed] [Google Scholar]
- 2.Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. 10.1183/09031936.00039510 [DOI] [PubMed] [Google Scholar]
- 3.van Thiel E, van Meerbeeck JP. European guidelines for the management of malignant pleural mesothelioma. Pol Arch Med Wewn 2010;120:503-10. [PubMed] [Google Scholar]
- 4.Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. 10.1053/j.semtcvs.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 5.Taioli E, van Gerwen M, Mihalopoulos M, et al. Review of malignant pleural mesothelioma survival after talc pleurodesis or surgery. J Thorac Dis 2017;9:5423-33. 10.21037/jtd.2017.11.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v31-9. 10.1093/annonc/mdv199 [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa S. Extrapleural pneumonectomy or pleurectomy/decortication for malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2014;62:516-21. 10.1007/s11748-014-0389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domen A, De Laet C, Vanderbruggen W, et al. Malignant pleural mesothelioma: single-institution experience of 101 patients over a 15-year period. Acta Chir Belg 2017;117:157-63. 10.1080/00015458.2016.1272253 [DOI] [PubMed] [Google Scholar]
- 9.Opitz I, Weder W. A nuanced view of extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Transl Med 2017;5:237. 10.21037/atm.2017.03.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki T, Yamasaki N, Tsuchiya T, et al. Is Pleurectomy/Decortication Superior to Extrapleural Pneumonectomy for Patients with Malignant Pleural Mesothelioma? A Single-Institutional Experience. Ann Thorac Cardiovasc Surg 2018;24:81-8. 10.5761/atcs.oa.17-00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batirel HF. Extrapleural pneumonectomy (EPP) vs. pleurectomy decortication (P/D). Ann Transl Med 2017;5:232. 10.21037/atm.2017.03.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherpereel A, Astoul P, Baas P, et al. [Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma]. Zhongguo Fei Ai Za Zhi 2010;13:C23-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. 10.6004/jnccn.2016.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. 10.1097/JTO.0b013e31826915f1 [DOI] [PubMed] [Google Scholar]
- 15.Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6. 10.1097/JTO.0b013e3181ae25bf [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. 10.1016/S0022-5223(99)70469-1 [DOI] [PubMed] [Google Scholar]
- 17.Van Schil PE, Opitz I, Weder W, et al. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J 2014;44:754-64. 10.1183/09031936.00207213 [DOI] [PubMed] [Google Scholar]
- 18.Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. 10.1016/j.lungcan.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 19.Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6. 10.1016/j.ejcts.2009.07.032 [DOI] [PubMed] [Google Scholar]
- 20.Azzouqa AG, Stevenson JP. The evolution of the diminishing role of extrapleural pneumonectomy in the surgical management of malignant pleural mesothelioma. Onco Targets Ther 2016;9:7247-52. 10.2147/OTT.S100214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. 10.1016/S1470-2045(11)70149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. 10.1016/j.lungcan.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 23.Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. 10.1016/j.athoracsur.2014.09.056 [DOI] [PubMed] [Google Scholar]
- 24.Kostron A, Friess M, Inci I, et al. Propensity matched comparison of extrapleural pneumonectomy and pleurectomy/decortication for mesothelioma patients. Interact Cardiovasc Thorac Surg 2017;24:740-6. 10.1093/icvts/ivw422 [DOI] [PubMed] [Google Scholar]
- 25.Infante M, Morenghi E, Bottoni E, et al. Comorbidity, postoperative morbidity and survival in patients undergoing radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:1077-82. 10.1093/ejcts/ezw215 [DOI] [PubMed] [Google Scholar]
- 26.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3. [DOI] [PubMed]
- 27.Paul S, Neragi-Miandoab S, Jaklitsch MT. Preoperative assessment and therapeutic options for patients with malignant pleural mesothelioma. Thorac Surg Clin 2004;14:505-16, ix. 10.1016/j.thorsurg.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 28.Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. 10.1097/JTO.0b013e31824ab6c5 [DOI] [PubMed] [Google Scholar]
- 29.Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5. 10.1016/S1470-2045(11)70307-2 [DOI] [PubMed] [Google Scholar]
- 30.Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. 10.1016/j.jtcvs.2013.01.039 [DOI] [PubMed] [Google Scholar]
- 31.Filosso PL, Guerrera F, Lausi PO, et al. Pleurectomy/decortication versus extrapleural pneumonectomy: a critical choice. J Thorac Dis 2018;10:S390-4. 10.21037/jtd.2018.01.116 [DOI] [PMC free article] [PubMed] [Google Scholar]


