Abstract
Objectives
Antioxidants can reduce oxidative radicals that affect the early phase of atherogenesis, that is endothelial dysfunction. Polysaccharide Peptide (PsP) derived from Ganoderma lucidum has an active substance in the form of β-glucan. Previous studies have proven the PsP of Ganoderma lucidum as an effective antioxidant in atherosclerotic rats and shows no toxicity in animal model. This study aims to prove the effect of PsP as potent antioxidant in high risk and stable angina patients.
Method
This is a clinical trial conducted to 37 high risk and 34 stable angina patients, which were determined based on ESC Stable CAD Guidelines and Framingham risk score, with pre and post test design without control group. The parameters are superoxide dimustase (SOD) and malondialdehyde (MDA) concentration, circulating endothelial cell (CEC) and endothelial progenitor cell (EPC) counts. The patients were given PsP 750 mg/day in 3 divided dose for 90 days. Paired t-test was performed for normally distributed data, and Wilcoxon test for not normally distributed data, and significant level of p ≤ 0,05.
Results
SOD level in high risk patients slightly increased but not statistically significant with p = 0,22. Level of SOD in stable angina group significantly increased with p = 0,001. MDA concentration significantly reduced in high risk and stable angina patients with p = 0.000. CEC significantly reduced both in high risk and stable angina patients, with p = 0.000 in both groups. EPC count significantly reduced in high risk and stable angina with p = 0.000.
Conclusion
PsP of Ganoderma lucidum is a potent antioxidant against pathogenesis of atherosclerosis in stable angina and high risk patients
Abbreviations: CEC, circulating endothelial cell; EPC, endothelial progenitor cell; MDA, malondialdehyde; PsP, polysaccharide peptide; SOD, superoxide dismutase; LDL, low density lipoprotein; NO, nitric oxide; CVCU, cardio vascular care unit; HSC, hematopoietic stem cell; SDF, stromal-derived factor; VEGF, vascular endothelial growth factor
Keywords: Polysaccharide peptide, Ganoderma lucidum, Antioxidant, Stable angina, High risk patients
1. Introduction
Coronary heart disease is the first cause of death in the world, accountable for approximately 48%of world populace. In 2008, 17.3 millions people die because of heart attack, and World Health Organization predicted that in the year of 2030, there will be 23.6 millions people that will die because of cardiovascular disease.1 Atherosclerosis − as leading cause of coronary heart disease − has endothelial dysfunction as one phase of its development. Endothelial dysfunction is caused by free oxidative radicals.2
Smoking, hyperlipidemia, hypertension, and high blood sugar are the source of free radicals, which if not anticipated with antioxidant will cause oxidative stress. Oxidative stresswill leads to dysfunction of respiration chain inside mitochondria, causes tissue damage and endothelial dysfunction.3
Antioxidant systems in the body include superoxide dismutase or SOD. This enzyme catalyzes the breakdown of superoxide anion into oxygen and hydrogen peroxide, and nullifies free radicals.4 On another end, increasing malondialdehyde or MDA is known to increase free radicals production and decreases antioxidant activity. Imbalance between free radicals and antioxidants can cause oxidative stress, endothelial dysfunction and finally atherosclerosis.5, 6 Thus, it’s important to find a potent antioxidative agent that can lower oxidative stress, endothelial dysfunction and prevent or improve atherosclerotic cardiovascular disease.
Ganoderma lucidum can be the agent of choice for this condition. Ganoderma lucidum is a type of mushroom that has been used for years throughout Asia, and it is known for the antioxidative, anti inflammatory, and anti cancer properties.7 It is called “Lingzhi” in China and “Reishi” in Japan. Health benefit of Ganoderma lucidum impressed some observers and researchers to investigate the components within it, and found β-d-Glucan as the active component, which is also found in the polysacharide peptide (PsP). Ganoderma lucidum has high concentration of β-d-glucan compared to other organism producing β-d-glucan, such as Saccharomyces cerevisiae.
Previous study has proven the PsP of Ganoderma lucidum as an effective antioxidant and anti inflammation in atherosclerotic rats after 5 weeks treatment.8Another previous study for toxicity of PsP is also conducted for acute and chronic side effects in animal model in 5 doses variation and shows no toxic effect in the histopathologic, immunologic, and blood test. It is also not harmful for liver, kidney, heart, aorta, and lung.9 This study aimed to prove the effect of PsP as potent antioxidant in high risk and stable angina patients.
2. Method
This is a quasi experimental research with pre- and post-test design, single-blinded, to know the effect of PsP to 34 stable angina patients and 37 high risk patients, which were determined based on ESC Stable CAD Guidelines and Framingham risk score, without control group.
This research was conducted at Cardiology outpatient and Cardiovascular Care Unit of Saiful Anwar General Hospital Malang, assisted by Lavalette Hospital Malang, Indonesian Heart Association and geriatric association in Malang, Indonesia. Along with cooperations from Biomedical Laboratory and Physiology Laboratory of Faculty of Medicine, Brawijaya University Malang. In addition, there are Prodia and Proclinic Laboratories for the blood sampling for each patient.
2.1. Patients
Patients who participated in this research were patients that come to CVCU, Cardiology outpatient at Saiful Anwar General Hospital Malang, Indonesian Heart Association Malang, and who agreed to participate in research and signed the informed consents.
High risk patients are patients without ischemic symptoms and classified by Framingham Score as high risk. Stable angina patients are patients with ischemic symptoms at exercises, activities, or emotional stress, but resolved at rest.
Patients who disagreed to participate in the study, with Medium Moderate Risk according to criteria of Framingham Cardiovascular Risk Score, acute hepatitis or renal failure were exempted from the research. Meanwhile, patients who did not consume PSP for 3 months (no recovery required every month to take the drug) and patients with new cardiac symptoms during the study were dropped out of the research.
All of the protocols in this research have already been approved through informed consents by the patients and by the Ethical Committee of Saiful Anwar General Hospital Malang, Indonesia (no.400/79/K.3/302/2015).
2.2. Polysaccharide peptide
PsP derived from Ganoderma lucidum was supplied by Sahabat Lingkungan Hidup company in Surabaya. Each was in the form of freeze dried preparation in which 250 mg PsP contains about 180 mg β-D glucan. The patients were given PsP 750 mg/day in 3 divided doses for 90 days. Stable angina patients continued their previous medications beside PsP until the end of study with the same dose.
ELISA - SOD was measured using ELISA kits (Elabsciensce, Wuhan) applied in vitro quantitative determination of human SOD1 concentrations in plasma collected from pre- and post-tests. SOD1 can be detected at least at 37.5 pg/mL. This kit uses a competitive ELISA as a method. Microtiter plate on this kit has been precoated with SOD1. SOD1 in the sample or standard will compete with SOD1 in solid phase to place in the specific SOD1 Biotinylated Detection antibody. Conjugates and non-sticking samples are discarded, and Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate and incubated. Then TMB substrate solution is added at each well. The enzyme and substrate reactions at termination with the addition of sulfuric acid solution and color change were measured spectrophotometrically with a wavelength of 450 nm ± 2 nm. The SOD1 concentration in the sample will be compared with the OD in the sample on the standard curve. The samples were collected using Ethylenediaminetetraacetic acid (EDTA) as anticoagulant, centrifuged for 15 min at 1000 × g at 2–8 °C within 30 min of collection. The supernatant was collected and assayed. MDA was measured by ELISA kit (Elabscience, Wuhan) with Competitive-ELISA method. MDA in the samples competed with a fixed amount of MDA on the solid phase supporter for sites on the Biotinylated Detection Ab specific to MDA. Excess conjugate and unbound sample or standard are washed from the plate, and Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then a TMB substrate solution is added to each well. The enzyme-substrate reaction is terminated by the addition of a sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of MDA in the samples is then determined by comparing the OD of the samples to the standard curve. Samples were collected from blood samples which were centrifuged for 15 min at 1000 × g at 2–8 °C within 30 min of collection. The supernatant was collected and assayed.
2.3. Flow cytometry
CECs (Circulating Endothelial Cells) were assayed using CD45 and CD146 antibodies. Plasma was freshly collected from the blood samples and assayed using PE anti-human CD45 and anti-human CD146 antibodies (BioLegend, USA) according to the manufacturer’s instructions and analyzed using flowcytometry. EPCs (Endothelial Progenitor Cells) were assayed using CD133 and CD34 antibodies. Plasma was freshly collected from the blood samples and assayed using PE anti-human CD133 and anti-human CD34 antibodies (BioLegend, USA) according to the manufacturer’s instructions and analyzed using flowcytometry.
2.4. Statistical analysis
Data are given in mean ± SD. To see the differences of pre- and post-test of high risk patients, paired t-test was performed. If the normality test indicated the data was not homogeneous, Wilcoxon will be used. The statistical calculation used SPSS version 21 (SPSS Inc). The differences considered statistically significant at p ≤ 0.05.
3. Results
3.1. Subject characteristics
There were no new ischemic symptoms occurred to the patients during this study. Systolic and diastolic blood pressure decreased with the average of 9.72 mmHg and 5.43 mmHg, respectively, in stable angina group. In high risk group, the average of reduction in systolic and dyastolic between pre and post test is 11.89 mmHg and 6.76 mmHg, respectively, as can be seen in Fig. 1.
Fig. 1.
Blood pressure measurement: pre and post treatment of PsP in high risk and stable angina patients.
Both of this data are not normally distributed, and not significantly decreased, with p = 0.22 and p = 0.27 for systolic and diastolic of stable angina group, and p = 0.11 and p = 0.10 for systolic and diastolic of high risk group.
3.2. SOD (Superoxide dismutase)
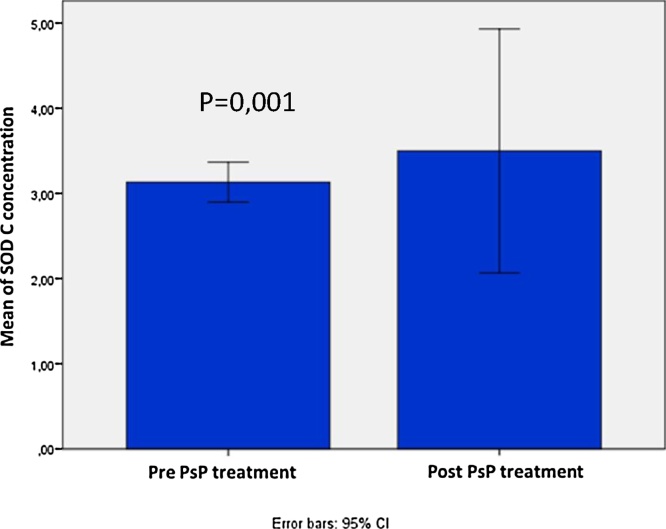
Level of SOD in high risk patients slightly increased from 3.12 ± 0.70 U/mL to 3.62 ± 4.26 U/mL, but not statistically significant, with p = 0.22. Level of SOD in stable angina group significantly increased from 3.41 ± 0.46 U/ml to 5.79 ± 4.19 U/ml with p = 0.001 as can be seen in Fig. 2, Fig. 3.
Fig. 2.
SOD concentration: pre and post treatment of PsP in high risk patients.
Fig. 3.
SOD concentration: pre and post treatment of PsP in stable angina patients.
3.3. MDA (Malondialdehyde)
MDA concentration significantly reduced in stable angina patients after PsP administration, with average from 95.63 ± 21.27 U/mL to 44.84 ± 50.95 U/mL, with p = 0.000. MDA concentration in high risk patients are also reduced significantly, with p = 0.000, and the average is 114.13 ± 24.56 U/mL in pre administration of PsP, and become 36.84 ± 28.39 U/mL after PsP administration, as seen in Fig. 4, Fig. 5.
Fig. 4.
MDA concentration: pre and post treatment of PsP in high risk patients.
Fig. 5.
MDA concentration: pre and post treatment of PsP in stable angina patients.
3.4. CEC (Circulating endothelial cell)
CEC significantly reduced both in high risk and stable angina patients, with p = 0.000 in both groups. The average count of CEC reduced from 7.91 ± 9.11 cells/μl to 1.76 ± 1.56 cells/μl in stable angina patients, and from 7.38 ± 4.44cells/μl to 2.23 ± 3.05cells/μl in high risk patients (see Fig. 6, Fig. 7).
Fig. 6.
CEC count: pre and post treatment of PsP in high risk patients.
Fig. 7.
CEC count: pre and post treatment of PsP in stable angina patients.
3.5. EPC (Endothelial progenitor cell)
EPC count significantly reduced in stable angina patients after PsP administration, with average from 15.11 ± 7.44cells/μl to 6.14 ± 5.30 cells/μl, with p = 0.000. EPC count in high risk patients are also reduced significantly, with p = 0.000, and the average is 12.94 ± 6.97cells/μl in pre administration of PsP, and become 6.10 ± 3.95 cells/μl after PsP administration (see Fig. 8, Fig. 9).
Fig. 8.
EPC count: pre and post treatment of PsP in high risk patients.
Fig. 9.
EPC count: pre and post treatment of PsP in stable angina patients.
4. Discussion
β-Glucans from different sources have different linkage types. β-Glucans extracted from both barley or oats were found to comprise mainly β-(1.3–1.4)-d-glucan. On the other hand, β-glucan extracted from yeasts and mushrooms comprised mainly β-(1.3–1.6)-d-glucan.10 Beside these differences in linkage type, the properties of β-glucans are influenced by the molecular weight, degree of branching, and conformation.11, 12 Although the mechanisms by which β-glucan scavenges hydroxyl radicals are not yet clarified, the different structures of β-glucan may influence its antioxidant activity, which may be associated with the source and extraction method.13 The molecular weight of β-glucan extracted from barley with warm water is 40,000–100,000 Da; the oligomer prepared from the macromolecule β-glucan by enzymatic degradation with lichenase has a molecular weight of approximately 2000 Da.
The study resulted in the hydroxyl radical scavenging activity of β-glucan was reduced with a decrease in molecular size. However, even when the molecular weight was reduced by about 1/20–1/50, antioxidant activity was reduced only about half. This finding indicates that β-glucan exerts hydroxyl radical scavenging activity across a wide range of molecular sizes.14
In this study, the molecular weight of immunomodulatory protein in PsP, from Ganoderma lucidum, was between 14.000-17.000 Da through SDS PAGE method.15As stated above that the properties of β-glucan are influenced by the molecular weight. The molecular weight of β-glucan extracted from Ganoderma lucidum are supporting its antioxidant effects.
Meanwhile, β-glucan could better modulate MnSOD-related angiogenesis if the mechanisms underlying it could be studied. Endothelial cells express Dectin-1, a C-type lectin-like receptor, and the engagement of Dectin-1 with β-glucan induces MnSOD expression via histone acetylation. This process shows the binding of Dectin-1 to β-glucan can induces antioxidant properties in endothelial.16 These results shows Ganoderma lucidum as potent antioxidant.
In this study, the level of SOD after given PsP 750 mg/day in 3 divided dose for 90 days in high risk patients slightly increased from 3.12 ±0.70U/mL to 3.62 ± 4.26U/mL, but not statistically significant, with p = 0.22. Level of SOD in stable angina group significantly increased from 3.41 ± 0.46 U/ml to 5.79 ± 4.19U/ml with p = 0.001. The medication that the stable angina patients consumed, such as statin, has antioxidative effect that possibly affected high risk and stable angina patients’ results.17 These results can also due to the effect of β-glucan that increase antioxidant enzyme activity of SOD, whereas in stable angina patients the activity of SOD would be greatly boosted due to the present pathology, while in high risk patients the levels of SOD have not reduced too far and then increased just slightly due to this effect.
It has been proven in some studies that common risk factors for atherosclerosis, including: hyperlipidemia, diabetes mellitus, hypertension, smoking, and aging, increase the production of free radicals, from the endothelial cells, smooth muscle cells, and also the adventitial cells. Those factors also trigger the expression of adhesion molecules, proliferation and migration of smooth muscle cells, and the apoptosis of endothelial cells, lipid oxidation, and alteration of vasomotor activity.2 Subjects in this clinical trial are stable angina and high risk patients, many of them have hypertension, diabetes mellitus, hypercholestrolemia, old age and smoking, with an increased oxidative radicals.
In endothelial dyfunction, free oxidative radicals work as follow: Superoxide anion (O2−) reacts with endothelium-derived nitric oxide (NO) via a radical–radical reaction at a diffusion-limited rate to generate peroxynitrite (ONOO−), a potent oxidant and mediator of vascular tissue injury. Peroxynitrit oxidize lipoproteins and interupt lipid membranes. The oxidized LDL in the vessel wall then cause cytotoxicity, excess of superoxide anion also increase platelet aggregation, adhesion and migration of monocytes, and matrix degradation.18 Thus, the endothelial dysfunction begins with imbalance of oxidant and anti-oxidants. SOD which is known as antioxidant, is an enzyme other than catalase, glutathione peroxidase and paraoxonase, acts in blood vessel walls and reduce the damage due to oxidative stress.19 SOD catalyzes dismutation of superoxide anion to hydrogen peroxide, catalase (CAT) which detoxifies hydrogen peroxide and converts lipid hydro-peroxides to non-toxic substances.20
The active component of Ganoderma lucidum’s polysaccharide is β-d-Glucan. Some studies suggested that β-d-glucan can improves SOD and some other antioxidants, increases SOD activity and inhibits lipid peroxidation in animal models.21, 22, 23 In the previous study, PsP can increases SOD in atherogenic mice with dose of 150 mg/kgBW. Another preliminary study on diabetes mellitus mice showed a significant increase in SOD at a PsP dose of 300 mg/kgBW.8 These previous studies, along with this study, resulted in the potent antioxidant properties of PsP for the improvement of atherosclerosis.
This study also showed that polysaccharide of Ganoderma lucidum reduced the pro-oxidant significantly in both high risk and stable angina patients from 114.13 ± 24.56 U/L to 36.84 ± 28.39U/L and 95.63 ± 21.27U/mL to 44.84 ± 50.95U/mL, respectively. MDA, which is one of the products of lipid peroxidation and also one of oxidative markers has been the most extensively studied marker. According to Ayala, MDA, which is the main product during lipid peroxidation, appears to be the most mutagenic product and involved in many diseases such as Alzheimer’s disease, cancer, diabetes, and also cardiovascular diseases.24
Increased MDA levels enhance the production of free radical, reduce the antioxidant activity and mark the index of assessing oxidative stress.6 Amrita (2016) observed the increase of MDA in both hyperlipidemic and normolipidemic menopausal women which indicated the degree of oxidation in heart disease.25 Another study by Sener et al showed that β-glucan can lower MDA level in the liver, kidney, heart, lung, diaphragm and brain tissues on sepsis model rats.26 Alp (2012) also described the effect of β-glucan as a lowering agent for MDA in diabetic rats with 21 days treatment.27 Other studies also indicate that MDA lowering agents and SOD increasing agents could be beneficial and considered as therapeutic agents for cardiovascular disease, because they can attenuate the oxidative injuries in the body.28, 29, 30
PsP of Ganoderma lucidum is a potential agent for improving endothelial dysfunction in atherosclerosis by its antioxidant effects. This is also confirmed with the result of CEC as one of endothelial dysfunction parameters. CEC significantly reduced both in high risk and stable angina patients, with p = 0.000 in both groups. The average count of CEC reduced from 7.91 ± 9.11 to 1.76 ± 1.56 in stable angina patients, and from 7.38 ± 4.44 to 2.23 ± 3.05 in high risk patients.
The CEC counts in patients with acute coronary syndromes correlate with endothelial dysfunction and with the prothrombotic state, hence, CECs can act as a blood-based biomarker of cardiovascular diseases.31, 32 Endothelial cells adhere to a basement membrane in the vascular tree, and these cells would be expected to remain in this location, with a very low level of cell loss into the blood, with consequent clearance by the reticuloendothelial system. Damage to the endothelium causes endothelial cell detachment, resulting in increased numbers of CECs within the bloodstream. CECs are considered to reflect the occurrence of endothelial defects and vascular disruption, and serve as a biomarker that answers to both diagnostic and prognostic needs. Because of the close temporal relationship with vascular injury, CECs are superior to classic cardiac markers such as CK-MB or cardiac troponin, which show a delayed rise after the actual coronary event.30 CECs are rarely found in normal healthy individuals, in the order of <3 cells/ml.
There are various factors that can induced detachment of CEC from the vascular wall, including mechanical injury to the vascular wall, after arterial or venipuncture, and acute plaque rupture. ROS activity along with prolonged inflammation that disrupt the integrity and adhesion factors of endothelial cells may also induce endothelial cells detachment from the vasculature.31 The significant reduction of CEC in this study, for both high risk and stable angina patients suggest the improvement of endothelial dysfunction in atherosclerosis after PsP administration.
In parallel is the hypothesis that EPCs are restorative or regenerative cells, possibly destined to replace or renew damaged areas of the intima.
This study shows that EPC count significantly reduced in stable angina patients after PsP administration, with average from 15.11 ± 7.44cells/μl to 6.14 ± 5.30 cells/μl, with p = 0.000. EPC count in high risk patients are also reduced significantly, with p = 0.000, and the average is 12.94 ± 6.97cells/μl in pre administration of PsP, and become 6.10 ± 3.95 cells/μl after PsP administration.
In patients with cardiovascular disease, the disease process might be the factor that drives mural endothelial cells into the blood to become CECs and also reduces levels of EPCs. In acute myocardial infarction, there were also elevated numbers of both CECs and EPCs. Furthermore, stimulatory/therapeutic factors (e.g., statins and endurance training) among patients with CAD leads to both improved endothelial function and increased EPC counts, although the relationship between therapeutic factors and decreasing CEC counts has not been studied for cardiovascular disease.
Endothelial turnover rates are different in individuals. Low shear stress area has high endothelial death rate suggesting that cells need a high turnover rate to maintain vessel homeostasis.32 Endothelial cells in the presence of hyperlipidemia and disturbed blood flow undergo death and proliferation cycle.33 This study result shows a significant reduce of CEC but also EPC. As the CEC is reduced, so there is minimal detachment of endothel and injury in endothelial cells, suggesting that the endothelial cells do not need high turnover rate to maintain vessel homeostasis, and will not induce the mobilization of EPC. A study focused on EPC shows that there is no significant increase of EPC due to the acute impact of high dose lipid lowering agents. Another study summarized that EPC and CEC counts in acute myocardial infarction patients were higher at baseline after 7, 30, and 180 days, compared to healthy controls.34
Lee (2014) reported a decreased EPCs in patients with coronary artery disease. Impairment and reduced number of EPCs can also due to exhaustion of endothelial progenitor cells in bone marrow, reduced nitric oxide bioavailability, and long term statin treatment.35
According to a study conducted by Alessio et al (2013) in deep vein thrombosis (DVT) patients, CEC levels were increased significantly 24 h after induction and decreased after 72 h. The same phenomenon occurred for EPC levels. In myocardial infarction, EPC markedly increased with peak values on day 7, and returned to baseline within 60 days.36
Mobilization of EPC from bone marrow into the peripheral circulation and to the site of injury has complex mechanism. The majority of EPC remain quiescent in the bone marrow. Circulating hematopoietic stem cell (HSC) migrate towards bone marrow, proliferate and differentiate within these quiescent cells. Integrin tethered HSC into stromal cells. Stimulation of these cells induces proliferation and conversion into functional cells capable of migration. Translocation of SDF1 (stromal-derived factor 1) from the plasma to the bone marrow further activates matrix metalloproteinase 9 (MMP9) and causes the release of soluble Kit Ligand (sKitL). After ligand binding, the receptor autophosphorylates for a prerequisite of migrating EPC from bone marrow. Proteinases then break the adhesive bonds between EPC and stromal cells, allowing EPC to exit. Beside these factors, there are also other factors that modulate EPC mobilization from the bone marrow. These factors include vascular endothelial growth factor (VEGF), IL-8, Groβ, nitric oxide (NO), erythropoietin, and other inflammatory agents or pharmacological modulation.37 The anti-inflammatory properties of β-glucan as reported on the previous study in atherosclerotic rats, can also reduce the inflammatory factors that modulate the mobilization of EPC.8
Hence, the EPC mobilization patterns and other contributing factors for release of EPC also have impact on the study outcome.
Since this study was only 90 days, the significant reduction of EPC remains a debate whether because of the minimal endothelial injury that was not enough to induce EPC mobilization, or decreasing EPC mobilization due to effect of β-glucan.
5. Conclusion
Polysaccharide Peptide of Ganoderma lucidum is a potent antioxidant against pathogenesis of atherosclerosis in stable angina and high risk patients.
5.1. Limitations
(1)This intervention population is too small to assess a community issue. (2) 90 days duration of intervention may not be sufficient enough.
5.2. Future prospective
New drug for improving atherosclerosis by its antioxidative properties.
Funding
Ministry of Research, Technology and Higher Education of the Republic of Indonesia.
Conflicts of interest
The authors have none to declare.
Acknowledgments
Financial support by: Ministry of Research, Technology and Higher Education of the Republic of Indonesia and the authors would like to thank PT. Sahabat lingkungan Hidup, Surabaya, Indonesia for the products that support this research. We also would thank to all high risk and stable angina patients who agreed to volunteer in this research.
References
- 1.WHO . 2011. Global Atlas on Cardiovascular Disease Prevention and Control. [Geneva] [Google Scholar]
- 2.Vogiatzi G., Tousoulis D., Stefanadis C. The role of oxidative stress in atherosclerosis. Hellenic J Cardiol HJC=Hellenike kardiologike epitheorese. 2009;50(5):402–409. [PubMed] [Google Scholar]
- 3.Bonomini F., Tengattini S., Fabiano A., Bianchi R., Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol. 2008;23(3):381–390. doi: 10.14670/HH-23.381. [DOI] [PubMed] [Google Scholar]
- 4.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedralli M., Waclawovsky G., Lehnen A. Impact of exercise training on blood pressure and endothelial function in individuals with systemic hypertension. Austin Sports Med. 2016;1(2):1006. [Google Scholar]
- 6.Li X., Hu Y., Zhang F. Unbalanced oxidant-antioxidant status: a potential therapeutic target for Coronary chronic total occlusion in very old patients. Oxidat Med Cell Longevity. 2016;2016:4910829. doi: 10.1155/2016/4910829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Chen X., Zhong Z., Chen L., Wang Y. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39(1):15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- 8.Sargowo Polysaccharide Peptide D. Acta Medica; Indonesia: 2014. A Promising Anti Inflamation and Anti Oxidant in Atherosclerosis. [Google Scholar]
- 9.Sargowo D. Penelitian Unggulan Perguruan Tinggi; Indonesia: 2015. Pengembangan Potensi Peptida Polisakarida (Ganoderma Lucidum) Sebagai Antioksidan Dan Antiinflamasi: Upaya Penanganan Komprehensif Penyakit Kardiovaskuler. [Google Scholar]
- 10.Rahar S., Swami G., Nagpal N., Nagpal M.A., Singh G.S. Preparation, characterization, and biological properties of beta-glucans. J Adv Pharmaceut Technol Res. 2011;2(2):94–103. doi: 10.4103/2231-4040.82953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan G.C., Chan W.K., Sze D.M. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choromanska A., Kulbacka J., Harasym J., Oledzki R., Szewczy A., Saczko J. High- and low-molecular weight oat beta-glucan reveals antitumor activity in human epithelial lung cancer. Pathol Oncol Res. 2016 doi: 10.1007/s12253-017-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Many J., Vizhi K. Analysis of different extraction methods on the yield and recovery of β-dlucan from baker’s yeast (Saccharomyces cerevisiae) Int J Innov Sci Eng Technol. 2014;1(6) [Google Scholar]
- 14.Kofuji K., Aoki A., Tsubaki K., Konishi M., Isobe T., Murata Y. Antioxidant activity of beta-glucan. ISRN Pharm. 2012;2012:125864. doi: 10.5402/2012/125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SLH Labs . Sahabat Lingkungan Hidup Biopharmaceutical Co; Indonesia: 2016. Advanced Technology in the Production of PsP. [Google Scholar]
- 16.Agostini S., Chiavacci E., Matteucci M., Torelli M., Pitto L., Lionetti V. Barley beta-glucan promotes MnSOD expression and enhances angiogenesis under oxidative microenvironment. J Cell Mol Med. 2015;19(1):227–238. doi: 10.1111/jcmm.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broncel M., Koter-Michalak M., Chojnowska-Jezierska J. The effect of statins on lipids peroxidation and activities of antioxidants enzymes in patients with dyslipidemia. Przegl Lek. 2006;63(9):738–742. [PubMed] [Google Scholar]
- 18.Foresman E.L., Miller F.J., Jr. Extracellular but not cytosolic superoxide dismutase protects against oxidant-mediated endothelial dysfunction. Redox Biol. 2013;1:292–296. doi: 10.1016/j.redox.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Horke S., Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira I.C.F.R., Heleno S.A., Reis F.S., Stojkovic D., Queiroz M.J.R., Vasconcelos M.H., Sokovic M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor andantimicrobial activity. Phytochem Rev. 2014 doi: 10.1016/j.phytochem.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Kabasakal L., Sener G., Balkan J., Dogru-Abbasoglu S., Keyer-Uysal M., Uysal M. Melatonin and beta-glucan alone or in combination inhibit the growth of dunning prostatic adenocarcinoma. Oncol Res. 2011;19(6):259–263. doi: 10.3727/096504011x13021877989748. [DOI] [PubMed] [Google Scholar]
- 22.Pacheco R., Ascencio F., Zarain M., Gomez G., Campa A. Enhancement of superoxide dismutase and catalase activity in juvenile brown shrimp, Farfantepenaeus californiensis (Holmes, 1900), fed β-1.3 glucan vitamin E, and β-carotene and infected with white spot syndrome virus. Lat Am J Aquat Res. 2011;39(3):534–543. [Google Scholar]
- 23.Kim Y.S., Ke F., Zhang Q.Y. Effect of beta-glucan on activity of antioxidant enzymes and Mx gene expression in virus infected grass carp. Fish Shellfish Immunol. 2009;27(2):336–340. doi: 10.1016/j.fsi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidat Med Cell Longevity. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrita J., Mahajan M., Bhanwer A.J., Mohan G. Oxidative stress an effective prognostic tool for an early detection of cardiovascular disease in menopausal women. Biochem Res Int. 2016;2016:6157605. doi: 10.1155/2016/6157605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sener G., Toklu H., Ercan F., Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int Immunopharmacol. 2005;5(9):1387–1396. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Alp H., Varol S., Celik M.M. Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozosin. Exp Diabetes Res. 2012;2012:230–342. doi: 10.1155/2012/230342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araujo V.B., de Melo A.N., de Souza N.T. Oral intake of carboxymethyl-glucan (CM-G) from yeast (Saccharomyces uvarum) reduces malondialdehyde levels in healthy men. Molecules. 2015;20(8):14950–14958. doi: 10.3390/molecules200814950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boos C.J., Lip G.Y., Blann A.D. Circulating endothelial cells in cardiovascular disease. J Am CollCardiol. 2006;48(8):1538–1547. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 30.Li C., Wu Q., Liu B. Detection and validation of circulating endothelial cells, a blood-based diagnostic marker of acute myocardial infarction. PloS one. 2013;8(3):e58478. doi: 10.1371/journal.pone.0058478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt D.E., Manca M., Hoefer I.E. Circulating endothelial cells in coronary artery disease and acute coronary syndrome. Trends Cardiovasc Med. 2015;25(7):578–587. doi: 10.1016/j.tcm.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Tricot O., Mallat Z., Heymes C., Belmin J., Leseche G., Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101(21):2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 33.Xu Q. Disturbed flow-enhanced endothelial turnover in atherosclerosis. Trends Cardiovasc Med. 2009;19(6):191–195. doi: 10.1016/j.tcm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Regueiro A., Cuadrado-Godia E., Bueno-Beti C. Mobilization of endothelial progenitor cells in acute cardiovascular events in the PROCELL study: time-course after acute myocardial infarction and stroke. J Mol Cell Cardiol. 2015;80:146–155. doi: 10.1016/j.yjmcc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Lee P.S., Poh K.K. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6(3):355–366. doi: 10.4252/wjsc.v6.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alessio A.M., Beltrame M.P., Nascimento M.C. Circulating progenitor and mature endothelial cells in deep vein thrombosis. Int J Med Sci. 2013;10(12):1746–1754. doi: 10.7150/ijms.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilling L., Chowienczyk P., Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009;68(4):484–492. doi: 10.1111/j.1365-2125.2009.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]