Abstract
Objective
The aim of the present study was to evaluate histopathological and biomechanical effects of isotretinoin on Achilles tendon.
Materials & methods
Sixteen rats were divided into two groups including the control group (n = 8) and isotretinoin group (n = 8). The control group received 1.42 ml/kg soy oil per day whereas the isotretinoin group received 15 mg/kg/day (gavage dose 1.42 ml/kg) isotretinoin dissolved in soy oil through gavage method for 6 weeks. Achilles tendons were excised at the end of week 6. The tendon samples were evaluated by hematoxylin-eosin under a light microscope. Quantitative evaluation was performed via Movin and Bonar scoring. A computer-monitored tensile testing machine was utilised for biomechanical testing. Biomechanical characteristics of the tendon samples (elastic modulus, yield force, ultimate tensile force) were measured.
Results
Histopathological evaluation revealed a significantly higher Movin and Bonar scores in histopathological evaluation. Movin score in isotretinoin group was 4.1 ± 2.5 and it was 2.3 ± 1.0 in control group (p = 0.032). Bonar score in isotretinoin group was 2.9 ± 1.4 and it was 1.6 ± 0.7 in control group (p = 0.022). In line with histopathological evaluation, biomechanical measurements in isotretinoin group (elastic modulus, yield force, ultimate tensile force) were significantly lower than the control group. Elastic modulus in isotretinoin group was 227 ± 27.7 N/mm2 and in control group it was 281.7 ± 38.7 N/mm2 (p = 0.006). In isotretinoin group; yield force was 33.7 ± 4.3 Pa and in control group it was 40.8 ± 5.9 Pa (p = 0.021). Ultimate tensile force in isotretinoin group was 35.7 ± 4.2 Pa and in control group it was 44 ± 7 Pa (p = 0.009).
Conclusion
The present study detected histopathological and biomechanical negative effect of isotretinoin on Achilles tendon. Therefore, isotretinoin should be questioned in medical history of patients with tendinopathy.
Keywords: Isotretinoin, Tendon, Tendinopathy, Rat, Biomechanic
Introduction
Isotretinoin (13-cis retinoic acid) is one of the commonly used medications by dermatologists for treatment of severe and treatment-resistant nodular acne for last 3 to 4 decades. Different side effects depending on use of these medications (fetal malformations, mucocutaneous effects, psychiatric disorders etc.) have been reported. Side effects on musculoskeletal system are relatively rare. Musculoskeletal side effects were reported as 15–20% in the literature.1 Since musculoskeletal side effects are less, they are usually ignored by the clinicians.
Although there are studies on development of tendinopathy due to drug use, mechanism could not be explained clearly. There are comprehensive histopathological and biomechanical studies related to tendinopathy development depending on quinolone antibiotics and statins.2, 3, 4, 5 Several case reports exist for isotretinoin-dependent tendinopathy, entesopathy, myopathy.6, 7, 8, 9
Our hypothesis which constitute a basis for the present study is possible negative effect of isotretionin on tendon biology. As far as we know, there is not any comprehensive histopathological and biomechanical study searching effects of isotretinoin on tendons in the literature. Therefore, we aimed to perform a histopathological and biomechanical effects of oral isotretinoin in the rats.
Materials & methods
Animals and treatment
Before commencement of the study, approval of ethical committee for animal experiments was obtained (2015/142).
In the present study, 16 male Sprague Dawley rats which are 4–6 months old with a body weight of 350–400 g were used. The rats were kept under conventional conditions (temperature 22+-2 °C; relative humidity 55+-10, dark–light cycle of 12 h, one rat per cage) and were fed with standard pellet and water ad libitum. Sixteen rats were divided into two groups including 8 rats in each group: one untreated control groups and one treatment group (isotretinoin). Isotretinoin is commercially available in retail pharmacies. Capsule form of isotretinoin were opened and transferred to volumetric flasks and diluted with soybean oil to obtain suspension at desired concentration.10 All drug preparations were conducted in a darkened room and amber bottles were used for storage. The control group received soybean oil only (1.42 ml/kg) whereas isotretinoin group received 15 mg/kg/day (1.42 ml/kg) isotretinoin dissolved in soybean oil through gavage once a day. Studies that evaluate compatible serum isotretinoin concentration to human treatment dose have been refered while determining the doses. Serum isotretinoin concentration was not measured in our study.10, 11 This procedure was conducted for 6 weeks and at the end of week 6, all rats were sacrificed through high dose anesthesia (150 mg/kg pentobarbital). Achilles tendons of all rats were excised with calcaneus bones. Left achilles tendons of 16 rats was used for histopathological evaluation; and right achilles tendons were used for biomechanical evaluation.
Histopathological examination
Eight left achilles tendons in isotretinoin group; eight left achilles tendons in control group were evaluated histopathologically. The Achilles tendon samples were fixed in a 10% buffered formalin for 24 h. After fixation, macroscopically transverse sections were obtained at 2 mm intervals from each tendon sample as complete. Three μm-thick sections obtained from paraffin blocks were prepared by routine tissue processing and they were stained with haematoxylin-eosin (H.E), Masson's trichrome (MT) and Alcian blue (pH 2.5). The slides were examined under a Standard light microscopy (Olympus, BX51,Tokyo, Japan). Interpretation of the slides was performed by semi-quantitative grading scale of Movin and Bonar score for tendon abnormalities.12, 13
The variables included into Movin scale are as follows; 1- fiber structure; 2- fiber arrangement; 3- rounding of the nuclei; 4- regional variations in cellularity; 5- increased vascularity; 6- decreased collagen staining; 7- hyalinization; and 8- GAG content. Each variable is scored between 0 and 3, where 0 represents normal state, 1 represents a slightly abnormal state, 2 for an abnormal state and 3 for markedly abnormal states. The H.E-stained slides were used to assess the first five and seventh variables; Alcian blue-stained slides were used to assess GAG content and Masson's trichrome-stained slides were used to assess the substance of collagen. The total semi-quantitative histological score for a given slide could vary between 0 (normal tendon) and 24 (the most severe abnormality detectable).
The variables of Bonar scale include the following; 1- tenocytes; 2- ground substance; 3-collagen; and 4- vascularity. A four-point scoring system where 0 indicates a normal appearance and 3 indicates a markedly abnormal appearance was used. Overall, the total score for a given slide could vary between 0 (normal tendon) and 12 (the most severe abnormality detectable).
Biomechanical examination
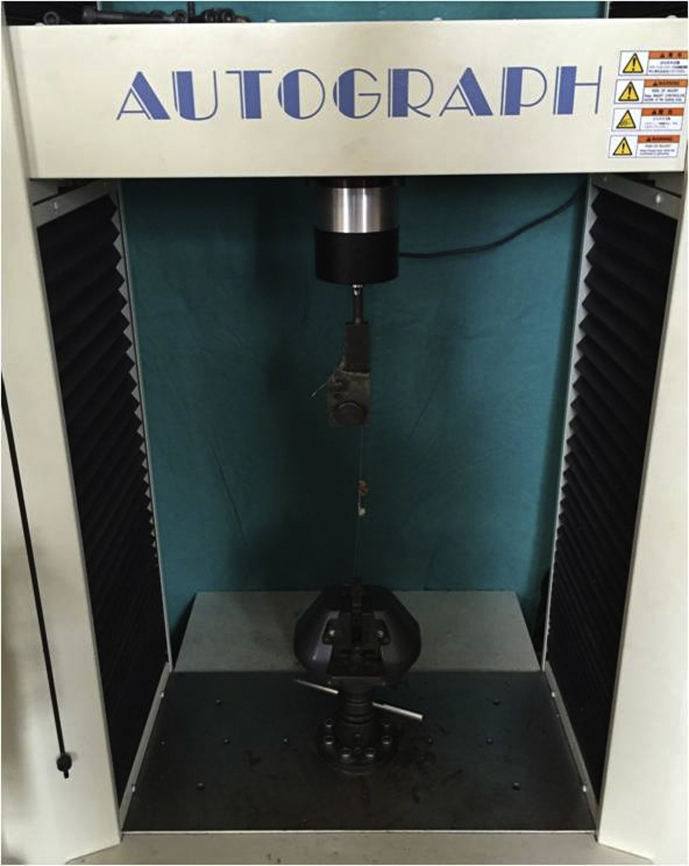
Biomechanical tests were conducted at Department of Mechanical Engineering of Technical University in my country. Eight right achilles tendons in isotretinoin group; eight right achilles tendons of control group were evaluated biomechanically. The samples used in the biomechanical study were covered with serum-soaked gases and frozen at −20 °C until the day of the study. The samples were thawed in room temperature on the day of the study. A preliminary study on the screw driven type tensile testing machine (AG IS-50 KN, Shimadzu, Kyoto, Japan) was performed (Fig. 1). This device is able to perform computer-assisted measurements with very accurate loads. Under stroke and force control, the bottom and top portions of the device were fixed with knots to the proximal and distal sides of Achilles tendon (Fig. 1). Modified sutures were placed using in either proximal or distal parts of Achilles tendon which was connected to the pulling device.2, 3 This connection was made in order to prevent rupture and erosion of the tendon from the connection site. Under stroke control, the strength was measured by pulling at a constant velocity by moving the upper part of the device. This procedure was applied over single axis on an axial plane. A test velocity 10 mm/min was maintained. Rupture point of the Achilles tendon was monitored on a computer screen and recorded. At the end of the test, strength-elongation graphics were obtained and documented.
Fig. 1.
The pulling device used for the rupture test (AG IS-50 kN,Shimadzu).
The biomechanical parameters of tendons used are defined as following: The UTF (Pa) is the force that causes complete rupture of the tendon. The YF (Pa) is the force preceding the rupture or the maximum force that causes permanent damage to the tendon. The EM (N/mm2) is the ratio of the tension to deformation.
Statistical analysis
SPSS 22.0 software program was used for statistical analysis. The average, Standard deviation, median minimum, median maximum, frequency and ratio values were used for data descriptive statistics. The distribution of the variables was measured by the Kolmogorov–Smirnov test. In the quantitative data analysis, a Mann–Whitney U test was used. A p value of <0.05 was considered as statistically significant.
Results
No diseases or severe adverse events were observed in the test animals during the study period. Elastic modulus values in isotretinoin group was significantly lower than the control group (p = 0.006). Furthermore, yield force and ultimate tensile force values were also detected lower with a statistical significance than the control (p = 0.021, p = 0.009). Elastic modulus in isotretinoin group was (n = 8) 227 ± 27.7 N/mm2and in control group (n = 8) it was 281.7 ± 38.7 N/mm2 (p = 0.006). In isotretinoin group; yield force was (n = 8) 33.7 ± 4.3 Pa and in control group it was (n = 8) 40.8 ± 5.9 Pa (p = 0.021). Ultimate tensile force in isotretinoin group was (n = 8) 35.7 ± 4.2 Pa and in control group it was (n = 8) 44 ± 7 Pa (p = 0.009) (Table 1).
Table 1.
Group comparison of elastic modulus, yield force and ultimate tensile force in the isotretionin group and control group.
| İsotretionin Group (n = 8) |
Control Group (n = 8) |
p | |||||
|---|---|---|---|---|---|---|---|
| Mean ± s.d. | Median | Min-Max | Mean ± s.d. | Median | Min-Max | ||
| Biomechanics | |||||||
| Elastic Modulus | 227,0 ± 27,7 | 222 | 192–262 | 281,7 ± 38,7 | 274 | 236–353 | 0,006a |
| Yield Force | 33,7 ± 4,3 | 33,5 | 27,9–39,1 | 40,8 ± 5,9 | 39,3 | 34,1–52,5 | 0,021a |
| Ultimate Tensile Force | 35,7 ± 4,2 | 35,7 | 29,1–40,2 | 44,0 ± 7,0 | 41,9 | 36,1–58,1 | 0,009a |
Significance value of P<0,05.
Mann-whitney u test.
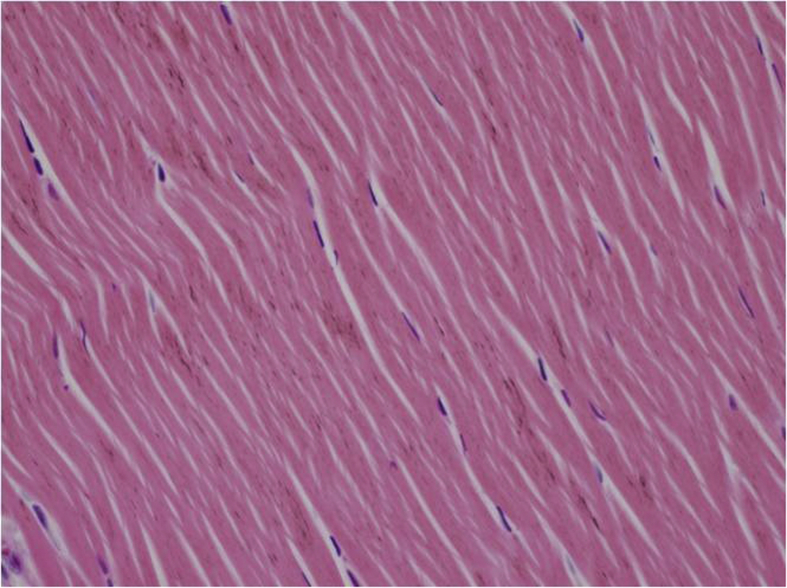
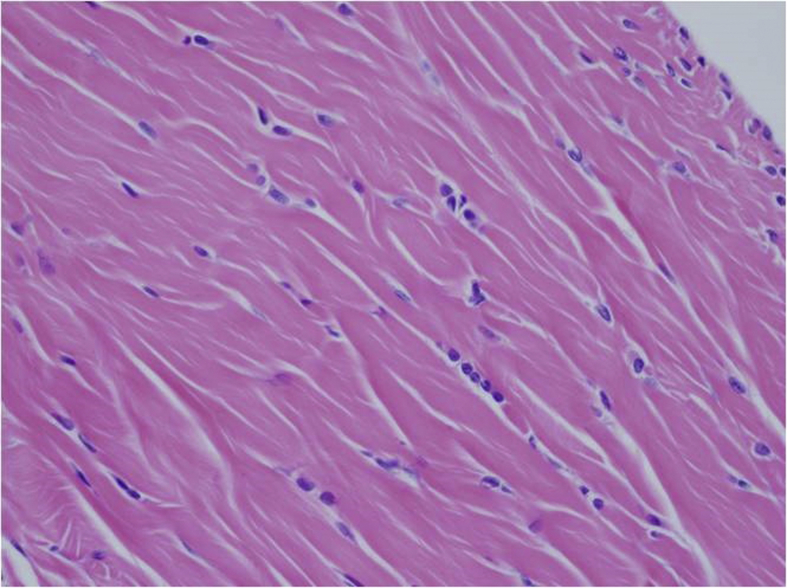
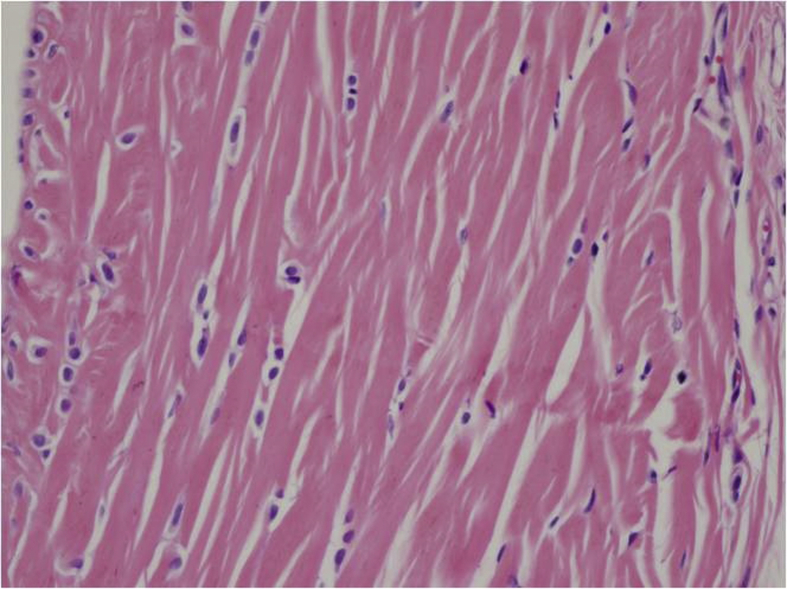
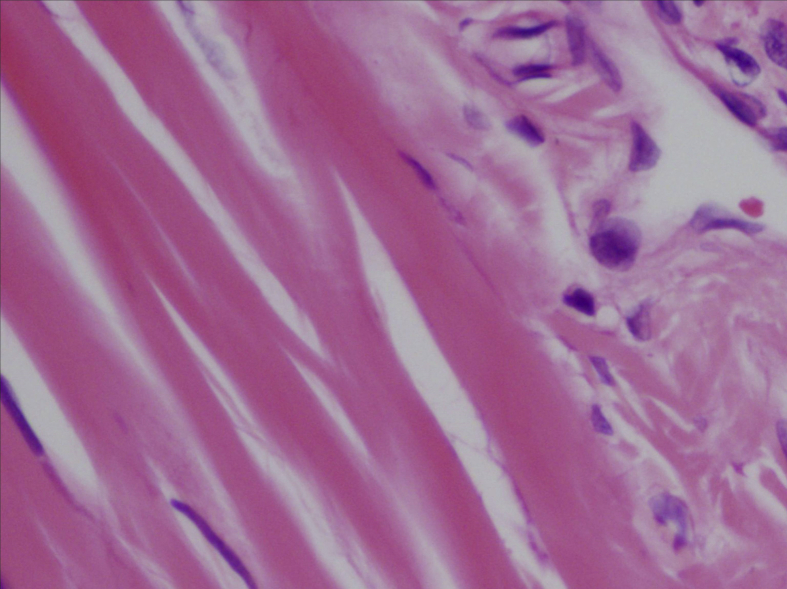
In the histopathological evaluation performed, tenocytes of the control group usually had spindle shaped nuclei and the cytoplasm was in distinct (Fig. 2). In some of the experimental group samples, there was a significant rounding of nuclei and a small amount of cytoplasm was visible (Fig. 3, Fig. 4). Significant cellularity and compelling increase in vascularity was observed in sample 7 (Fig. 5). Movin score in the isotretinoin group was (n = 8) 4.1 ± 2.5 and it was (n = 8) 2.3 ± 1.0 in the control group (p = 0.032). Bonar score in isotretinoin group was (n = 8) 2.9 ± 1.4 and it was (n = 8) 1.6 ± 0.7 in control group (p = 0.022) (Table 2). Movin score in isotretinoin group was significantly higher than the control group (p = 0.032). Bonar score was also detected significantly higher in isotretionin group when compared with the control group (p = 0.022).
Fig. 2.
Spindle shaped nuclei and indistinct cytoplasm in tenocytes (C4- H.E X 400).
Fig. 3.
Increase in cellularity, minimally round, and ovoidnuclei in tenocytes (E1- H.E X 400).
Fig. 4.
Tenocytes with round nuclei and distinct cytoplasm (E3- H.E X 400).
Fig. 5.
Increase in vascularity (E7- H.E X 400).
Table 2.
Group comparison of bonar score and movin score in the isotretionin group and control group.
| İsotretionin Group (n = 8) |
Control Group (n = 8) |
p | |||||
|---|---|---|---|---|---|---|---|
| Mean ± s.d. | Median | Min-Max | Mean ± s.d. | Median | Min-Max | ||
| Pathology | |||||||
| BonarScore | 2,9 ± 1,4 | 2,5 | 2,0–6,0 | 1,6 ± 0,7 | 1,5 | 1,0–3,0 | 0,022a |
| MovinScore | 4,1 ± 2,5 | 4,0 | 0,0–9,0 | 2,3 ± 1,0 | 2,0 | 1,0–4,0 | 0,032a |
Significance value of P<0,05.
Mann-whitney u test.
Discussion
Achilles tendinopathy is a condition of which the etiology could be exactly described. Along with mechanical causes, different medications are also held responsible in development of tendinopathy. History of medications used may be ignored especially in cases with treatment-resistant Achilles tendinitis and this may make the treatment planning difficult, increase the costs and lead loss of labour force. Agents which are held responsible from development of drug-induced tendinopathy include quinolone antibiotics, statins, aromatase inhibitors, glucocorticoids and isotretinoin in rare cases.
Isotretinoin is a synthetic, vitamin A-derivated, retinoid group drug.1, 6, 14 Retinoid group drugs regulate cell proliferation and differentiation, modulate the immune system and has an anti-inflammatory characteristics.14, 15 Retinoids are synthetic vitamins derivates manufactured to avoid severe side effects of vitamin A.6, 14 Isotretinoin is basically used for cases with severe and treatment-resistant acne.14 Furthermore, use of a isotretinoin fort some cancer cases was also reported.14 Along with adverse effects on liver, nervous system and dermatological side effects, different side effects exist on musculoskeletal system.6, 14
Hyperostosis was reported as common side effect on the bone.1, 16, 17 This case was especially reported on cervical vertebrae on the axial skeletal system.14 Nevertheless, the side effects reported include epiphysial closure, periosteal bone resorption and osteoporosis.1, 6, 14, 17 Furthermore, calcification on tendons and ligaments were commonly reported.14, 17, 18
Rate of some side effects such as myalgia, arthralgia was reported as 27% in some studies.13 In the study of Alkan et al where rheumatologic complications dependent to isotretinoin use were evaluated, such complications were detected in 23.1% of 42 patients in isotretinoin group. The most common rheumatologic symptoms included inflammatory low back pain, Achilles tendinopathy and sacroiliitis. In such study, unilateral Achilles tendinitis was detected in 3 patients and bilateral Achilles tendinitis was detected in 1 (9.52%) patient.19 The clinical symptoms in the cases with Achilles tendinitis reported in the literature usually regressed with interruption or termination of the drug therapy.7, 19 Therefore, we believe that adverse event of Achilles tendinopathy dependent to isotretinoin is reversible.
Association of the side effects on musculoskeletal system with the dose was not clearly expressed. Carey et al detected this effect in procedures performed with higher doses in their study where isotretinoin use and hyperostosis development were investigated and they reported that this does not appear with lower doses and the side effect is dose-dependent.20 However, Panney et al reported that side effects on skeletal system is not dose-dependent in their study.21 We also used 15 mg/kg/day used for rats in the present study.10 Since there is not yet any experimental animal study about effect of isotretinoin use on tendon biology in the literature, we could not evaluate different doses because of the number of animals and rules of ethics.
Entesopathy is a case of tendinopathy detected on junction sites of the tendons onto the bones. Besides Achilles tendon, entesopathy cases in different anatomic sites due to isotretinoin use was also reported. In such studies, entesopathy usually develops after long-term use of the drug.22, 23 Nevertheless, there are cases with entesopathy reported due to short-term use in the literature.9
Movin and Bonar scoring are two scoring system providing classification of histopathological findings for evaluation of tendinopathy.12, 13 In Movin scroing; degradation in fibrile alignment, increase in cellularity, increase of nuclear rounding and vascularity indicate development of tendinopathy.13, 24 In the present study, fibrile alignment was regular and nuclei were in spindle form in the control group (Fig. 2) whereas degradated fibrile alignment, increase in nuclear rounding and cellularity as well as increase in vascularization was observed in isotretinoin group (Fig. 3, Fig. 4, Fig. 5). This case indicated an increase in Movin score as well as degradation of normal tendon formation in the drug group. When the parameters used for Bonar scoring were considered, increase in vascularity, tenocytis and degradation in collagen formation were observed in isotretinoin group (Fig. 3, Fig. 4, Fig. 5).
Tendons actually transmit the power from muscles to the bones. Tendons fulfill their tasks even during strong loading due to specific elastic nature. Furthermore, tendon loose its regular elastic nature along with cellular disruption. Histopathological evaluation as well as biomechanical measurements are required to make a comprehensive evaluation. The present study is the first experimental study evaluating histopathological and biomechanical toxicity of isotretinoin on tendon. Histopathological degradation as well as biomechanical disruption was detected in the drug group when compared with the control group of the present study. In biomechanical evaluation, a force was applied onto the tendon by pulling on a single plane until the tendon ruptured. Elastic modulus, yield force, ultimate tensile force values were recorded. Mean elastic modulus, mean yield force and mean ultimate tensile force values were significantly lower in isotretionin group when compared with the control group (p < 0.05). In light of these data, we may say that tendinopathy observed at histopathological level similarly reflects to biomechanical tests.
Experimental studies detected induction of proinflammatory cytokines and matrix metalloproteases in development of tendinopathy.25 Matrix metalloproteinases are main regulators of cellular and extracellular matrix.26 Increase of such enzymes in tendinopathy cases were reported in different studies.25, 26
Limitations of the present study were lack of evaluation of different cytokines and enzymes which are responsible from development of tendinopathy along with biomechanical and histopathological evaluation performed. Another limitation is subject count at optimal level because of animal experiments and rules of ethics. Moreover, relation of side effects to dose were not evaluated in detail; because groups of only one dose were used instead of groups of different dose values. Another limitation is that isotretinoin serum concentration was not measured. Prospective, randomized clinical studies with more subjects should be performed to evaluate effects of isotretinoin ın tendon biology.
Consequently, Movin and Bonar scores used for evaluation of tendinopathy were found significantly higher in isotretinoin group when compared with the control group in the present study where effects of isotretinoin on Achilles tendon was investigated. This histopathological outcome is also in line with the biomechanical studies and elastic modulus, yield force and ultimate tensile force values were found significantly lower in the drug group. In the light of these information, isotretinoin has negative histopathological and biomechanical effects on Achilles tendon. Therefore, use of isotretinoin should also be investigated during medication history investigation especially for the patients with treatment-resistant Achilles tendinopathies and treatment planning should be performed accordingly.
Acknowledgment
For their contribution and support, we would like to thank to Bagcilar Training and Research Hospital Management, to Bagcilar Training and Research Hospital Experimental Research and Skill Development Center (BADABEM). For their help during the biomechanical laboratory studies to Istanbul Technical University, Mechanical Engineering Department member Prof. Dr. Turgut Gülmez.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Ozan Beytemür, Email: beytemur@yahoo.com.
Serdar Yüksel, Email: serdar84yuksel@gmail.com.
Ümit Seza Tetikkurt, Email: umitseza@gmail.com.
Erdinç Genç, Email: erdincgenc@hotmail.com.
Ercan Olcay, Email: ercanolcay@superonline.com.tr.
Akif Güleç, Email: akifgulec@beh.gov.tr.
References
- 1.McLane J. Analysis of common side effects of isotretinoin. J Am Acad Dermatol. 2001;45:S188–S194. doi: 10.1067/mjd.2001.113719. [DOI] [PubMed] [Google Scholar]
- 2.Kaleagasioglu F., Olcay E., Olgac V. Statin-induced calcific Achilles tendinopathy in rats: comparison of biomechanical and histopathological effects of simvastatin, atorvastatin and rosuvastatin. Knee Surg Sports Traumatol Arthrosc. 2017 Jun;25:1884–1891. doi: 10.1007/s00167-015-3728-z. [DOI] [PubMed] [Google Scholar]
- 3.Olcay E., Beytemur O., Kaleagasioglu F. Oral toxicity of pefloxacin, norfloxacin, ofloxacin and ciprofloxacin: comparison of biomechanical and histopathological effects on Achilles tendon in rats. J Toxicol Sci. 2011;36:339–345. doi: 10.2131/jts.36.339. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira L.P., Vieira C.P., Da Re Guerra F. Statins induce biochemical changes in the Achilles tendon after chronic treatment. Toxicology. 2013;311:162–168. doi: 10.1016/j.tox.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira L.P., Vieira C.P., Guerra F.D. Structural and biomechanical changes in the Achilles tendon after chronic treatment with statins. Food Chem Toxicol. 2015;77:50–57. doi: 10.1016/j.fct.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Scuderi A.J., Datz F.L., Valdivia S. Enthesopathy of the patellar tendon insertion associated with isotretinoin therapy. J Nucl Med. 1993;34:455–457. [PubMed] [Google Scholar]
- 7.Bottomley W.W., Cunliffe W.J. Acute Achilles tendonitis following oral isotretinoin therapy for acne vulgaris. Clin Exp Dermatol. 1992;17:250–251. doi: 10.1111/j.1365-2230.1992.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 8.Sameem F., Semira Isotretinoin-induced acute severe myopathy involving pelvic girdle muscles: a case report. Indian J Pharmacol. 2016;48:601–603. doi: 10.4103/0253-7613.190764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stitik T.P., Nadler S.F., Foye P.M. Greater trochanter enthesopathy: An example of “short course retinoid enthesopathy”: a case report 1. Am J Phys Med Rehabil. 1999;78:571–576. doi: 10.1097/00002060-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Abali R., Yuksel M.A., Aktas C. Decreased ovarian reserve in female Sprague-Dawley rats induced by isotretinoin (retinoic acid) exposure. Reprod Biomed Online. 2013;27:184–191. doi: 10.1016/j.rbmo.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson S.A., Siitonen P.H., Cisneros F.J., Gough B., Young J.F. Pharmacokinetics of oral treatment with 13-cis-Retinoic acid or all-trans-Retinoic acid in male and female adult rats. Basic Clin Pharmacol Toxicol. 2006 Jun;98:582–587. doi: 10.1111/j.1742-7843.2006.pto_359.x. [DOI] [PubMed] [Google Scholar]
- 12.Krackow K.A., Thomas S.C., Jones L.C. A new stitch for ligament-tendon fixation. Brief note. J Bone Joint Surg Am. 1986;68:764–766. [PubMed] [Google Scholar]
- 13.Maffulli N., Longo U.G., Franceschi F. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466:1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesher G., Zuckner J. Rheumatologic complications of vitamin A and retinoids. Semin Arthritis Rheum. 1995;24:291–296. doi: 10.1016/s0049-0172(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 15.Orfanos C.E., Ehlert R., Gollnick H. The retinoids. A review of their clinical pharmacology and therapeutic use. Drugs. 1987;34:459–503. doi: 10.2165/00003495-198734040-00003. [DOI] [PubMed] [Google Scholar]
- 16.Archer C., Elias P., Lowe N. Extensive spinal hyperostosis in a patient receiving isotretinoin—progression after 4 years of etretinate therapy. Clin Exp Dermatol. 1989;14:319–321. doi: 10.1111/j.1365-2230.1989.tb01993.x. [DOI] [PubMed] [Google Scholar]
- 17.DiGiovanna J.J., Helfgott R.K., Gerber L.H. Extraspinal tendon and ligament calcification associated with long-term therapy with etretinate. N Engl J Med. 1986;315:1177–1182. doi: 10.1056/NEJM198611063151901. [DOI] [PubMed] [Google Scholar]
- 18.Pehlivan Y., Kisacik B., Sayiner Z.A. Inflammatory back pain in patients treated with isotretinoin. J Rheumatol. 2011;38:2690. doi: 10.3899/jrheum.110703. [DOI] [PubMed] [Google Scholar]
- 19.Alkan S., Kayiran N., Zengin O. Isotretinoin-induced spondyloarthropathy-related symptoms: a prospective study. J Rheumatol. 2015;42:2106–2109. doi: 10.3899/jrheum.150013. [DOI] [PubMed] [Google Scholar]
- 20.Carey B.M., Parkin G.J., Cunliffe W.J. Skeletal toxicity with isotretinoin therapy: a clinico-radiological evaluation. Br J Dermatol. 1988;119:609–614. doi: 10.1111/j.1365-2133.1988.tb03471.x. [DOI] [PubMed] [Google Scholar]
- 21.Pennes D.R., Martel W., Ellis C.N. Evolution of skeletal hyperostoses caused by 13-cis-retinoic acid therapy. AJR Am J Roentgenol. 1988;151:967–973. doi: 10.2214/ajr.151.5.967. [DOI] [PubMed] [Google Scholar]
- 22.Lawson J.P., McGuire J. The spectrum of skeletal changes associated with long-term administration of 13-cis-retinoic acid. Skeletal Radiol. 1987;16:91–97. doi: 10.1007/BF00367754. [DOI] [PubMed] [Google Scholar]
- 23.Ellis C.N., Pennes D.R., Hermann R.C. Long-term radiographic follow-up after isotretinoin therapy. J Am Acad Dermatol. 1988;18:1252–1261. doi: 10.1016/s0190-9622(88)70131-0. [DOI] [PubMed] [Google Scholar]
- 24.Maffulli N., Kader D. Tendinopathy of tendo achillis. J Bone Joint Surg Br. 2002;84:1–8. doi: 10.1302/0301-620x.84b1.12792. [DOI] [PubMed] [Google Scholar]
- 25.Kirchgesner T., Larbi A., Omoumi P. Drug-induced tendinopathy: from physiology to clinical applications. Joint Bone Spine. 2014;81:485–492. doi: 10.1016/j.jbspin.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Vieira C.P., Guerra Fda R., de Oliveira L.P. Alterations in the Achilles tendon after inflammation in surrounding tissue. Acta Ortop Bras. 2012;20:266–269. doi: 10.1590/S1413-78522012000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]