Abstract
Introduction
Percutaneous cholecystostomy tube drainage has played a vital role in management of cholecystitis in patients where surgery is not appropriate. However, management differs from unit to unit and even between different consultants in the same unit. We conducted this systematic review to understand which of these resulted in the best patient outcomes.
Methods
We conducted a systematic review using the PubMed database for publication between January 2006 to December 2016. Keyword variants of ‘cholecystostomy’ and ‘cholecystitis’ were combined to identify potential relevant papers for inclusion.
Findings
We identified 46 studies comprising a total of 312,085 patients from 20 different countries. These papers were reviewed, critically appraised and summarised in table format. Percutaneous cholecystostomy tube drainage is an important treatment modality with an excellent safety profile. It has been used successfully both as a definitive procedure and as a bridge to surgery. There continues to be great variation, however, when it comes to the indications, timing and management of these drains. As far as we are aware, this is the only systematic review to cover the past 10 years. It provides a much-needed update, considering all the technological development and new treatment options in laparoscopic surgery and interventional radiology.
Keywords: cholecystostomy, Systematic review, PCT, Gallbladder drain, Cholecystitis
Introduction
Cholecystitis is one of the most common reasons for emergency surgical admission. The majority of patients are treated with antibiotics and laparoscopic cholecystectomy is performed either as an emergency (hot cholecystectomy) or as a delayed interval procedure.
Some patients are high risk for surgery and fail to respond to medical treatment, resulting in rapid clinical deterioration and uncontrolled sepsis. For this group, percutaneous cholecystostomy (PCT) is a very attractive treatment option to drain the source of sepsis and stabilise the patient in the acute setting. PCT has also been used successfully as a bridge to surgery in patients who are too acutely unwell to undergo emergency laparoscopic surgery but may be suitable for a delayed elective procedure should they survive the acute septic episode.
The role of PCT has continued to change over the years, particularly with acute gallbladder surgery becoming a more acceptable and appealing treatment option. On reviewing the literature, it is apparent that there is great variation and contrast across the board in the way PCT is employed. We conducted this review to identify different management practices and interesting strategies over past decade.
Methods
We conducted an electronic PubMed database search from January 2006 to December 2016 using the following keywords: cholecystostomy, biliary drain, gallbladder drain, cholecystitis, PCT, percutaneous cholecystostomy. We excluded non-English studies case reports, case series with less than 20 patients, surgical cholecystostomy studies, gallbladder aspiration studies and non-human studies. The reference lists from the resulting studies were also reviewed and all relevant papers were added on and included in our systematic review.
Results
After identifying all relevant studies from the PubMed search, reviewing the reference lists and carrying out all the necessary exclusions 46 studies remained. These comprised a total of 312,085 patients from 20 different countries. These papers were reviewed and critically appraised by the authors (Tables 1–3).1–46
Table 1.
Studies and demographic data of the included patients.
| Study | City (country) | Design | Duration (years) | Demographic data | |||||
| PCT population | Age years) | Male (%) | Comorbidity index | Severity | ACC (%) | ||||
| Abi–Haidar (2012)1 | Boston (USA) | Retrospective | 9 | 51 | mean 70.4 (SD 13.9) | ASA3–4 88.2% | TG2–3 27.5% | 17.6 | |
| Al–jundi (2012)2 | Chesterfield (UK) | Retrospective | 5 | 30 | mean 76.1 (R 52–90) | 56.7 | – | – | 3.1 |
| Anderson 2013)3 | San Diego (USA) | Retrospective | 11 | 306,747 (CC vs ACC) | CC Gr: mean 54.7 | 40.1 | CC Gr: mean CCS 3.5 | – | 19.07 |
| ACC Gr: mean 57.8 | 49.7 | ACC Gr: mean CCS 3.9 | |||||||
| Atar (2014)4 | Petach Tikva (Israel) | Retrospective | 3 | 81 | median 82 (R 47–99) | 40.7 | – | – | 12.3 |
| Bakkaloglu (2006)5 | Istanbul (Turkey) | Retrospective | 4 | 27 | median 71.4 (R 64–93) | 18.5 | ASA3 74.1%; ASA4 25.9% | – | 7.4 |
| Bickel (2016)6 | Nahariya (Israel) | Retrospective | 6 | 59 | mean 72.5 (R 41–96) | 66.1 | – | – | – |
| Boules (2016)7 | Cleveland (USA) | Retrospective | 14 | 380 | mean 65.3 ± 14.2 | 58.7 | mean CCS 3.2 ± 2.1 | – | 41.8 |
| Carrafiello (2012)8 | Varese (Italy) | Retrospective | 3 | 30 | mean 78.6 (R 57–97) | 56.7 | – | – | – |
| Cha (2014)9 | Jeju (Korea) | Retrospective | 5 | 82 | mean 72.1 ± 13.7 | 52.4 | ASA1–3 59.8%; ASA4 40.2% | – | 44 |
| Chang (2014)10 | Seoul, (South Korea) | Retrospective | 11 | 60a | mean 68.6 ± 13.8 | mean KPS 24.8 ± 9.7 | TG2 65%; TG3 18.3% | 48.3 | |
| Chok (2010)11 | Hongkong, (China) | Retrospective | 8 | 23 | mean 83 (R 71–95) | 47.8 | ASA3 34.8%; ASA4 52.2% | – | 0 |
| Chou (2015)12 | Taipei (Taiwan) | Retrospective | 2 | 209: Gr1 (52.1%) OTD < 24 h | Gr1: mean 76.5 ± 14 | 69.7 | Gr1: ASA3 68.8%; ASA4 25.7% | – | Gr1: 14.7 |
| Gr2 (47.9%) OTD > 24 h | Gr2: mean 72.4 ± 16.3) | 75 | Gr2: ASA3 81%; ASA4 16% | Gr2: 9 (P = 0.2) | |||||
| De Mestral (2013)13 | Toronto (Canada) | Retrospective | 7 | 890 | mean age 75 ± 14 | ADG: 1st 8%; 2nd 16%; 3rd 21%; 4th 55% | – | – | |
| El–Gendi (2016)14 | Alexandria (Egypt) | Prospective | 2 | Gr1: 75 + interval LC | Gr1: mean 50.19 ± 12.01 | 36 | Gr1: ASA1 76%; ASA2 24% | TG2 100% | – |
| Gr2: 75 matched emergency LC onlyb | Gr2: mean 49.65 ± 11.63 | 40 | Gr2: ASA1 73.3%; ASA2 26.7% | ||||||
| Flexer (2014)15 | Bradford (UK) | Retrospective | 3 | 25 | median 66.2 (R 40–95) | 44 | – | – | – |
| Griniatsos (2008)16 | Athens (Greece) | Retrospective | 2 | 24 | median 79 (IR 78–82.5) | 58.3 | ASA3 70.3%; ASA6 29.7% | TG2 83.3% TG3 16.7% | 4.2 |
| Horn (2015)17 | Aarhus (Denmark) | Retrospective | 10 | 278 | median 72.5 (21–99) | 43.5 | – | – | – |
| Hsieh (2012)18 | Taipei (Taiwan) | Retrospective | 1.5 | 160 | mean 75.9 | 72.9 | ASA3 74.1%; ASA4 22.9% | – | 13.7 |
| Jang (2015)19 | Seoul (South Korea) | Retrospective | 6 | 93 | mean 73.8 ± 12.14 | 44.08 | CCS 5.08 ± 1.49 | – | 24.8 |
| Jung (2015)20 | Iksan (South Korea) | Retrospective | 4 | 74: Gr1 (40.4% LC < 10 d | Gr1: mean 67.9 ± 16.4 | 56.7 | Gr1: ASA2 50%; ASA3 40%; ASA6 0% | Gr1: TG2 36.7%; TG3 3.3% | 13.5 |
| Gr2: 61.6% LC > 10 d | Gr2: mean 69.17 ± 11.4 (P = 0.703) | 61.4 (P = 0.686) | Gr2: ASA2 52.3%; ASA3 34.1%; ASA6 2.3% (P = 0.482) | Gr2: TG2 34.1%; TG3 4.5% (P = 0.95) | |||||
| Karakayali (2014)21 | Ankara (turkey) | Prospective | 5 | Gr1: 91 emergency LC onlyb | Gr1: mean 60 ± 10 | 52.1 | Gr1: ASA1 33.3%; ASA2 66.7% | – | – |
| Gr2: 80 + interval LC | Gr2: mean 65 ± 9 | 67.4 | Gr2: ASA1 20.9%; ASA2 79.1% | ||||||
| Khasawneh (2015)22 | Minnesota (USA) | Retrospective | 3 | 202 (excluding died) | median 71 (R 59–80) | 62.5 | CCS median 5.5(R 4–8) | – | 41 |
| Kinkegard (2015)23 | Aarhus (Denmark) | Retrospective | 10 | 56 | median 73.5 (40–95) | 60.7 | – | – | 100 |
| Li (2013)24 | Nanjing(China) | Retrospective | 5 | 73 | median 82 (R 71–94) | 65.8 | ASA3 64.4%; ASA4 35.6% | TG2 6.8% TG3 93.2 | 42.5 |
| Lin (2016)25 | Taiperi (Taiwan) | Retrospective | 15 | 93 | mean 72.4 (R 31–96) | 63.4 | ASA2 13.97%; SA3 61.29%; SA4 24.73% | TG2 67.7% TG3 32.2% | – |
| Marci (2006)26 | Messina (Italy) | Retrospective | 8 | 27 | mean 76 (R 70–88) | ASA3 55.5%; ASA4 7.4% | – | 0 | |
| McKay (2012)27 | Winnipeg (Canada) | Retrospective | 12 | 67 | median 73.5 (R 25–99) | 62.7 | CCS 2 (R 0–7) | – | – |
| Melloul (2011)28 | Lausanne (Switzerland) | Retrospective | 23 on ITU | median 65 (R 37–86) | 78.3 | – | – | 52 | |
| Mizrahi (2015)29 | Jerusalem (Israel) | Retrospective | 10 | Gr1: 163 + interval LC | PCT Gr: mean 64 ± 1; | 66 | PCT Gr: median ASA2 | – | – |
| Gr2: 476 interval LCb | Gr2: mean 48 ± 0.8 (P < 0.001) | 39 | Gr2: ASA2 (P = .21) | ||||||
| Morse (2010)30 | South Caroline (USA) | Retrospective | 6.5 | 50 on ICU | mean 72 ± 11 | 66 | ASA3 32%; ASA4 68% | – | 22 |
| Nasim (2011)31 | Karachi (Pakistan) | Retrospective | 20 | 62 | mean 63.1 | 45 | ASA1–2 29%; ASA3–671% | – | 21 |
| Ni (2015)32 | Shandong (china) | Retrospective | 7 | 62 | mean 73.9 | 45.1 | ASA3–4 72.6% | – | – |
| Nikfarjam (2013)33 | Melbourne (Australia) | Retrospective | 6 | 32 | median 78 (45–97) | 50 | ASA3 72%; ASA4 28% | – | 25 |
| Pang (2016)34 | Singapore (Singapore) | Retrospective | 6 | 71 | mean 73 (R 38–96) | 60.6 | ASA3 47.9%; ASA4 52.1% | TG25.6% TG374.6% | 4.2 |
| Paran (2006)35 | Kfar–Sava (Israel) | Retrospective | 3 | 54 | mean 61 (31–94) | 44.4 | – | – | – |
| Peters (2014)36 | Almelo(Netherlands) | Retrospective | 8 | 111 | mean 72.1 (R 25–93) | 52.3 | ASA3 62%; ASA4 29% | – | 21.6 |
| Rodriguez–Sanjuan (2012)37 | Santander (Spain) | Retrospective | 10 | Gr1: 29 | Gr1: mean 81.8 (R 56–97) | 62.1 | Gr1: ASA3 51.7%; ASA4 41.3% | – | 1.6 |
| Gr2: 32 emergency LC (both groups within 72 h)b | Gr2: mean 83.6 (R 80–93) | 59.4 | Gr II: ASA3 90.6%; ASA4 9.4% | ||||||
| Sanjay (2013)38 | Dundee (UK) | Retrospective | 10 | 53 | median 74(14–93) | 75 | ASA3–4 (92.4%) | – | 37.7 |
| Simorov (2013)39 | Omaha (USA) | Retrospective | 4 | 704 | – | – | – | ACC 100 | |
| T. Smith (2013)40 | Wisconsin (USA) | Retrospective | 11 | 143 | mean 72 ± 13.5 | 65 | CCS mean 3.29 ± 2.77 | – | – |
| Suzuki (2015)41 | New York (USA) | Retrospective | 7 | 82 | mean 73 (R 39–96) | 63 | – | – | – |
| Viste (2015)42 | Bergen (Norway) | Retrospective | 6 | 104 | median 73.5 | 54.8 | ASA1 15.4%; ASA2 42.3%; ASA3 37.5%; ASA4 4.8% | TG2 83.7%; TG3 6.7% | 17.3 |
| Wang (2016)43 | Taipei (Taiwan) | Retrospective | 10 | 184 with clear ducts on CDC | mean 70.1 | 29.9 | CCS 1.4 (SD 1.6) | TG2 41.8%; TG3 7.6% | 25 |
| Yeo (2016)44 | Tan Tock Seng (Singapore) | Retrospective | 10 | 103 | mean 80 (R 43–105) | 55 | median CCI 7 (R 2–14); ASA3 85% | TG2 73%; TG3 27% | – |
| Zehetner (2014)45 | Los Angeles (USA) | Retrospective | 11 | Gr1: 23 PCT; Gr2: matched 1:1 cohort LC after FRMT 72 hb | Gr1: mean 57.3 ± 14.7 | 30 | – | – | 0 |
| Zerem (2014)46 | Mostar, (Bosnia Herzegovina) | Retrospective | 11 | 36 | mean 75 ± 9.7 | 33.3 | ASA3 63.8%; ASA4 5.6% | – | 11.1 |
ACC, acalculous cholecystitis; ASA, American Society of Anaesthesiologists physical status classification; CC, calculous cholecystitis; CCS, Charlson comorbidity score; d, days; Gr, group; h, hours; IR, interquartile range; ITU, intensive care unit; KPS, Karnofsky performance score; LC, laparoscopic cholecystectomy; OTD, onset of symptoms to drain; R, range; SD, standard deviation from the mean; TG, Tokyo severity grade.
a As definitive treatment.
b No prior PCT.
Table 3.
Surgical data.
| Study | Recurrence of acute gallstone-related illness (%) | Post-PCT surgical data | Follow–up | |||
| Surgery (%) | Timing | Difficulty (%) | Mortality (%) | |||
| Abi-Haidar (2012)1 | 25.6 | 58.8 | – | CR 24 | 0 | – |
| Al-jundi (2012)2 | 6.6 | 36.7 | median 58 d (R 1–124 d) | CR 45.5 | – | median 25 m (R 1–52 m) |
| Anderson 2013)3 | – | – | – | – | – | – |
| Atar (2014)4 | – | 44.4 | – | CR 2.8 | 5.8 (small bowel obstruction) 2.8 (sepsis) | mean 4 m (R 3 weeks–8 m) |
| Bakkaloglu (2006)5 | 13.6 | 18.5 | 2 m | Morbidity 0; CR 0 | 0 | – |
| Bickel (2016)6 | – | – | Gr1: 11 W ± 10.9 | Gr1: CR 8.3; severe adhesions 25; fibrotic changes 17 | – | – |
| Gr2: 9.9 W ± 8.6 (P = 0.68) | Gr2: CR 33.3 (P = 0.09); severe adhesion 57 (P = 0.064); fibrotic changes 53.3 ( P = 0.03) | |||||
| Gr3: no data | – | |||||
| Boules (2016)7 | 3.7 | 32.9 | mean 103 d | CR 7.2 | 19 (30-d); 0 (operative) | Minimum 1 year |
| Carrafiello (2012)8 | 0 | – | – | – | – | |
| Cha (2014)9 | 0 | 42.7 | mean 7.43 ± 4.99 d | – | 5.71 (operative) | median 5.2 (5.4–87.7) |
| Chang (2014)10 | 11.7 | 0 | – | – | median 38.1 m ± 24.8 | |
| Chok (2010)11 | 5.3 | 34.8 | – | CR 37.5 | median 35 m | |
| Chou (2015)12 | 9.6 | 45.5 | – | – | Minimum 3 years | |
| De Mestral (2013)13 | 49 (at 1 year) | 50 | – | – | md 1.8 years (IQR 0.6–3.6) | |
| El-Gendi (2016)14 | 0 (within 6 weeks to surgery) | – | ‘All patients operated on 6 weeks after PCT’ | Gr1: CR 2.7; intraoperative bleeding 26.33 ml ± 23.86 ml; operative time 38.09 ± 8.23 minutes; subtotal LC 0 | – | |
| Gr2: CR 24 (P < 0.001); intraoperative bleeding 41.73 ml ± 51.09 ml (P = 0.008); operative time 87.8 ± 33.06 minutes (P < 0.001); subtotal LC 17.3 (P < 0.001) | ||||||
| Flexer (2014)15 | 50 | 52 | – | CBD injury requiring ERCP and stent 7.8 | 7.8 (postoperative) | median 35 m (22–50 m) |
| Griniatsos (2008)16 | 9.5 | 0 | – | – | – | median 17.5 m (IR 13.5–25) |
| Horn (2015)17 | 23.5 | 28.4 | – | – | – | median follow up 5 years |
| Hsieh (2012)18 | 14.4 | 33.1 | – | 18.9; 0; CR 34.3 | – | mean 25.4 m |
| Jang (2015)19 | 19.3 | 66.6 | – | – | – | mean 350 d ± 393 |
| Jung (2015)20 | – | 100 | Gr1: median 6 d (R 2–10 d) | Gr1: mean operative time: 102.6 ± 49.0 minutes; mean LOS postoperative 7.4 ± 5.3 d; CR 33.3; severe inflammation 26.7; bile duct injury 3.3; postoperative morbidity 6.7 | – | – |
| Gr2: median 23 d (R 11–38 d) | Gr2: mean operative time: 94.9 ± 39.9 minutes (P = 0.459); mean LOS postoperative 7.6 ± 6.8 (P = 0.910); CR 25 (P = 0.435); severe inflammation 22.7; bile duct injury 0; postoperative morbidity 13.6 (P = 0.343) | |||||
| Karakayali (2014)21 | – | – | mean 5 weeks (R 4–7 weeks) post-PCT | Gr1: CR 40; intraoperative bleeding > 100 ml 33; morbidity 35 | – | mean 23 m (R 7–29 m) |
| Gr2I: CR 19 (P = 0.029); intraoperative bleeding > 100 ml 9 (P = 0.006) morbidity 9 (P = 0.003) | ||||||
| Khasawneh (2015)22 | – | 35.1 | median 55 d (475) | CR 21 | md 6.1 m | |
| Kinkegard (2015)23 | 1 (1.8) | 4 (7.1) | median 8.8 m (7.7–33.4 m) | – | – | – |
| Li (2013)24 | 4.1 | 0 | – | – | – | md 32.4 m (R 3–58 m) |
| Lin (2016)25 | 27.7 | 61.3 | mean 17.8 d (R 3–69 d) | – | – | – |
| Marci (2006)26 | – | 92.6 (all during index admission) | Gr 1: < 5 d (60%) Gr 2: 5–10 d (40%) | CR 20 | – | – |
| McKay (2012)27 | 41 | 31.3 | – | CR 28.6 | – | median 55 m |
| Melloul (2011)28 | 17.3 | 21.7 | – | – | – | median 16 m (R 2–44) |
| Mizrahi (2015)29 | – | – | PCT Gr: mean 84 ± 5 d | PCT Gr: CR 11; mean operative time 142 ± 4 minutes; bile duct injury 10 | – | – |
| Non-PCT Gr: 102 ± 10 d (P = 0.35) | Non PCT Gr: CR 4 (P = 0.001); operative time 107 ± 4 minutes (P < 0.001); bile duct injury 4% (P = 0.003) | |||||
| Morse (2010)30 | 16 | 22 | mean 49D ± 40 | CR 27.3; CBD injury 9; bile leak 9 | – | – |
| Nasim (2011)31 | 9.67 | 37.08 | – | – | – | mean 403 d (R 4 – 2190) |
| Ni (2015)32 | 3.8 | 41.9 | Operative time mean 116.7 minutes ± 30.8; operative blood loss 32.9ml ± 37.7; CR 19.2; operative morbidity 0 | – | – | |
| Nikfarjam (2013)33 | 48 | 28 | median 73 d (R 32–447 d) | CR 0 (11 planned open procedures) | 11 (30-d postoperative due to sepsis and renal failure) | – |
| Pang (2016)34 | 11.9 | 45.1 | – | CR 9.4 | 0 (operative) | median 37 m (R 0.1–110.8 m) |
| Paran (2006)35 | – | 57.4 | – | CR 8; operative morbidity 16 (collection 12, bile leak 4) | – | – |
| Peters (2014)36 | 19.5 | 45.9 | – | – | – | mean 55 m |
| Rodriguez-Sanjuan (2012)37 | – | 31 | – | – | – | mean 14.6 m (R 1–57 m) |
| Sanjay (2013)38 | 22 | 34 | – | – | – | median 910 d(53 d–9.3 years) |
| Simorov (2013)39 | – | – | – | – | – | |
| T. Smith (2013)40 | – | 41 | – | – | – | |
| Suzuki (2015)41 | – | 34 | mean 7 weeks | CR 32% (75% of these were due to dense adhesions); major postoperative morbidity 17 (CBD obstruction requiring ERCP 6.9, bile leak 6.9, return to theatre for bleeding 4.5) | 0 (postoperative) | – |
| Viste (2015)42 | – | 28.9 | – | – | – | median 12 m (R 0–78 m) |
| Wang (2016)43 | 9.2 | 32.6 | – | – | – | 1 Y |
| Yeo (2016)44 | – | 41 | median 35 (R 2–339) | CR: 15; mean operating time 139.5 minutes (R 60–264 minutes); major postoperative complications 11.9 (including 1 bile duct injury, 2.4) | – | – |
| Zehetner (2014)45 | 39 | 17 | – | Gr1: mean operative time 52 minutes (2.85 ± SD); operative morbidity 4 (abdominal abscess) | – | – |
| Gr2: mean operative time 156 minutes (52.27 ± SD); operative morbidity 4 (wound infection) | ||||||
| Zerem (2014)46 | – | 22.2 | – | CR 12.5 | – | median 13 m (R 5–24 m) |
CBD, common bile duct; CR, conversion rate to open; d, days; ERCP, endoscopic retrograde cholangiopancreatography; Gr, group; m, months; IQR, interquartile range; LC, laparoscopic cholecystectomy; LOS, length of stay; PCT, percutaneous cholecystostomy tube; R, range; SD, standard deviation from the mean.
Table 2.
Percutaneous cholecystostomy data.
| Study | Indication | Timing of placement | Duration of drainage | Protocol for removal | Morbidity (%) | Mortality (%) |
| Abi-Haidar (2012)1 | – | – | – | – | PCT 20.6 (iatrogenic injury 15.7, other PCT related 5.9) | 15.7 (FU) |
| Al-jundi (2012)2 | FRMT at 36–48 h | – | – | – | – | 16.7 (30-day) |
| Anderson 2013)3 | – | – | – | – | CC Gr: 5.8 | 1.1 |
| ACC Gr: 10.4 | 2.6 | |||||
| Atar (2014)4 | – | mean 2 d (6 h–4 d) | – | CDC | PCT 30-day 7.4 (bleeding 1.2, dislodgment 6.2) | 18.5 (24.7 FU) |
| Bakkaloglu (2006)5 | – | – | mean 23 d (R 14–32 ) | CDC | PCT 14.8 (dislodgment 11.1 bleeding 3.7) | – |
| Bickel (2016)6 | FRMT | Gr1 OTD 1–2 d (n = 12) | – | – | – | – |
| Gr2 OTD 3–6 d (n = 30) | ||||||
| Gr3 OTD 7–10 d (n = 17) | ||||||
| Boules (2016)7 | FRMT 48–72 h in patients not fit for surgery due to comorbidity | – | – | CDC | – | 38.5 (30-day); 0 (PCT) |
| Carrafiello (2012)8 | – | – | median 23 d (R 14–34 d) | – | PCT 10 (dislodgement 6.7, haematoma 3.3) | 0 |
| Cha (2014)9 | – | – | – | CDC then no recurrence on 3-d clamp | Failure to remove PCT 34.0 (according to described protocol) | 2.12 |
| Chang (2014)10 | High operative risk + FRMT treatment, impending rupture of distended gallbladder, suspected gallbladder necrosis/perforation | – | 22.9 ± 15.8 | CDC | PCT 3.3 (minor bile leak 1.6, retained T-fastener 1.6) | 0 (FU) |
| Chok (2010)11 | FRMT 2–3 doses of IV antibiotics | ATD All < 30 h | – | CDC | PCT 4.3 (bleeding) | 8.7 (30-day) |
| Chou (2015)12 | Sepsis, localised perforation, progressive intolerance to pain, persistent fever after 48 h medical treatment | Gr1: mean 10.3 ± 6.4 H Gr2: mean 77.9 ± 52.7 H | – | – | Dislodgment 7.7; bleeding 2.4; bile leak 1.4 | Gr1: 7.3; Gr2: 5 (P = 0.572) |
| De Mestral (2013)13 | – | median ATD 2 d (IQR 1–3 d) | – | – | – | 5 (index admission); 18 (1-year); 45 (end of study FU) |
| El-Gendi (2016)14 | – | – | – | – | Gr1: Postoperative 2.7 (bile leak 0; bowel injury 0) | 0 (30-day) |
| Gr2: Postoperative complication 26.7 (P < 0.001); bile leak 10.7 (P = 0.006); bowel injury 2.7 | 0 (30-day) | |||||
| Flexer (2014)15 | – | mean 5.5 d | mean 25.24 d | – | Readmission 50 | 8.3 |
| Griniatsos (2008)16 | FRMT at 36–48 h; deterioration after initial clinical improvement | – | median 19 (IR 17–22) | – | – | 12.5 (PCT 0) |
| Horn (2015)17 | – | – | median 12 (R 0–193) | CDC + satisfactory clinical response | Bile leak 3.9; bleeding 7.2; dislodged catheter 28.1; abscess 3.2 | 4.7 (30-day) |
| Hsieh (2012)18 | Septic shock/severe sepsis; gallbladder perforation; FRMT 48 h | – | mean 16.6 ± 14 | – | PCT 16.6 (dislodgement 10.6, bleeding 3.6, bile leak 1.8, blockage 0.6) | 1.2 (PCT bleeding: refused intervention) 15 (30-day) |
| Jang (2015)19 | SIRS; CVA; CKD; inadequate ASA score; immediate ICU needed | – | – | no recurrence of symptoms on clamping PCT | PCT 2.1 | |
| Jung (2015)20 | – | – | – | – | Complications 10.8 (dislodgment 6.8; bleeding 1.4; bile leak 1.4; obstruction 1.4) | – |
| Karakayali (2014)21 | Consecutively allocated to both groups | – | – | CDC | Gr1: complications 9 (bile leak 10.4; collection 8.3; wound infection 6.3) | |
| Gr2: bile leak 2.3; collection 4.6; wound infection 2.3 | 35 (P = 0.003) | |||||
| Khasawneh (2015)22 | Surgeon discretion | – | – | CDC | – | 17.5 ( during index admission) |
| Kinkegard (2015)23 | – | – | – | – | – | 6 (10.7) |
| Li (2013)24 | FRMT 24 h | – | median 26 d | Absence of AC symptoms for 1 week | PCT 4.2 (bleeding) | 1.4 (30-day) |
| Lin (2016)25 | High risk for surgery due to comorbidity Severe cholecystitis; FRMT; patient refusal; suspected empyema | mean 16.74 d (R 3–49 d) | – | symptom relief; or at surgery | 22.6 (dislodgement 16.1; bleeding 4.3; wound infection 1.1; bile leak 1.1) | 10.75 |
| Marci (2006)26 | – | OTD < 12 h | – | – | – | – |
| McKay (2012)27 | – | – | – | – | PCT 16.2 (tube dislodgment 13.2; tube blockage 1.5; bleeding 1.5) | 15 30-day); 32.8 (FU) |
| Melloul (2011)28 | – | – | median 7 d (R 3–21 d) | CDC | PCT 8.7 (dislodgment 4.3, haematoma 4.3) | 13 (90-day) |
| Mizrahi (2015)29 | – | 3.4+/– 03 D | – | – | PCT Gr: dislodgment 12; leakage 4; further emergency admissions 20 | 1 |
| Non-PCT Gr: further emergency admissions 3 (P < 0.001) | 0.4 (P = 0.27) | |||||
| Morse (2010)30 | – | – | – | – | In hospital 62; PCT 0 | 50 (in hospital); 56 (FU); 0 (PCT) |
| Nasim (2011)31 | – | – | – | CDC | Bleeding 1.6; bile leak 0; non-PCT-related 25.8 (pneumonia, hypotension) | 15 (30-day) |
| Ni (2015)32 | – | – | – | – | Dislodgment 1.6; blockage 1.6 | 0 |
| Nikfarjam (2013)33 | – | ATD md 3 D (R 0–41) | median 49 D (R 2–169) | – | 53 (dislodgment 13; infective collection at PCT site 6; non-PCT-related 34) | 9 (30-day); 0 (PCT) |
| Pang (2016)34 | Declined surgery; multiple comorbidities; severe sepsis/shock; gallbladder perforation | – | – | CDC | Dislodgement 14; drain obstruction 7; bile leak 2.8; bowel perforation 1.4; major haemorrhage requiring embolisation of cystic artery 1.4 | 8.5 ( during index admission); 32.4 (end of follow-up); 0 (PCT) |
| Paran (2006)35 | FRMT 48 h | – | mean 7.4 d (R 3–28 d) | – | PCT 31.5 (dislodged 29.6; 1.8 bile leak and peritonitis requiring emergency surgery) | – |
| Peters (2014)36 | – | – | – | – | Abscess 3.6; fistula 1.8 dislodgment 7.2 | 0 (PCT) |
| Rodriguez-Sanjuan (2012)37 | Sever comorbidity/ASA3–4; advanced age (single patient aged 93 with ASA2) | – | Gr1: mean 12.4 d (R 8–14 d) Gr2: mean 12.7 (R 6–30 d) | – | Gr1: 31 Gr2: 28.1 | 17.2 (MI during tube placement) 0 |
| Sanjay (2013)38 | – | median 3 (R 1–15) | median 43 (R 28–114) | – | 13 (bile leak 9.4; bleeding 1.9; duodenal fistula 1.9) | 22.6 (index admission) |
| Simorov (2013)39 | – | – | – | – | 5 (ICU admission 28.1; 30 d readmission 29) | 2.6 |
| Smith (2013)40 | – | – | median 48.5 d | – | PCT 14.7 (dislodgement 7; bile leak 5; pain 3; occlusion 3; infection 1) | 12 (30-day) |
| Suzuki (2015)41 | – | – | – | – | Drain reinsertion required 34 | 5 (30-day) |
| Viste (2015)42 | – | – | 6.5 d (R 1–75 d) | CDC | Tube related 11.5 (leak, obstruction, dislodgment); serious 0 | 3.8 (30-day) 24.03 (end of FU) |
| Wang (2016)43 | FRMT; impending rupture; severe sepsis/shock | – | mean 20 d (SD 25.7) | CDC or no recurrence of symptoms on clamping PCT | – | 2.7 (1-year) |
| Yeo (2016)44 | – | median 2 d (R 0–15 d) | – | – | Dislodgment 7.8% | 12.6 (in-hospital) |
| Zehetner (2014)45 | FRMT 72 h | – | – | – | Gr1: 17 | 13 (P = 0.665) |
| Gr2: 9 | 0 (P = 0.233) | |||||
| Zerem (2014)46 | Age; comorbidities; acute systemic complications | OTD median 4 d (R 2–6 d) | median 15 d (R 12–20 d) | CDC | PCT 41.7 (dislodgment, leaking, blockage) | 19.4 (30-day) |
ACC, acalculous cholecystitis; ASA, American Society of Anaesthesiologists physical status classification; ATD, admission to drain; CC, Calculous cholecystitis; CDC, cholangiogram duct clearance; CKD, chronic kidney disease; CVA, cerebrovascular accident; d, days; FRMT, failure to respond to medical treatment; FU, follow-up; Gr, group; h, hours; ICU, intensive care unit; OTD, onset of symptoms to drain; PCT, percutaneous cholecystostomy tube; SIRS, systemic inflammatory response syndrome;
Discussion
All the studies in our review were observational; only two were prospective studies and the remaining 44 were retrospective case series, the largest of which was from San Diego, California, and contained 306,747 cholecystostomy patients. There were no randomised controlled trials within our search period. The study population in our review ranged in age from 21 to 99 years, with a higher proportion of females than males (overall combined female patient percentage 57.9%). The vast majority of these patients were classed as American Society of Anesthesiologists physical status classification III or higher, and the incidence of acalculous cholecystitis was reported as ranging from 1.6% to 52% (Table 1).
After reviewing all the papers, the authors considered that the key focus points for the discussion should be centred around the indications for emergency PCT insertion, the long-term role of PCT in the management of cholecystitis, the safety profile, the timing of PCT placement, its removal and subsequent cholecystectomy. These aspects are discussed in detail below.
Indications
In patients who were deemed to be high risk for surgical intervention either due to pre-existing comorbidity or acute illness, the indications for PCT placement were listed as:
failure to respond to medical treatment2,6,7,10,11,16,18,24,25,35,43,45
severe sepsis/septic shock/systemic inflammatory response syndrome or patients requiring immediate intensive care18,19,34,46
suspected or impending necrosis/perforation of gallbladder10,12,18,34
suspected gallbladder empyema25
surgeons discretion22
The most common cause for PCT placement in our review was failure to respond to medical treatment with intravenous antibiotics, but there was no consensus regarding the exact duration of antibiotic treatment between these publications.
Long term role of PCT
PCT has been successfully used as definitive treatment in patients with acalculous cholecystitis,23,39 and also in patients with calculous cholecystitis who are not fit for surgery because of irreversible pre-existing comorbidities and high anaesthesia risk.16,34 In the latter group, it is worthwhile performing a tube cholangiogram and clearing the common bile and cystic ducts prior to drain removal, as this has been associated with low recurrence rates in a number of studies (range 3.7–23.7%).7,10,17,43 In fact, Wang et al. 43 did not consider PCT treatment to be successful unless the cystic duct was patent and they were able to remove the drain. In their study, PCT drains were temporarily clamped after resolution of the cholecystitis episode to check for recurrence of biliary symptoms and a formal cholangiography was performed to check that the cystic duct was clear. They would only consider removing a PCT if both tests were completed successfully, as they considered that a clear duct indicated a low risk of recurrence.43
Alternatively, if a patient failed the above mentioned assessments, the risk of recurrence was expected to be high and the biliary drain was left in place. In these patients, currently, there is no established interventional option for clearance of the cystic duct; however, there have been some promising case series published on percutaneous cystic duct stenting, which has been employed successfully in management of benign gallbladder disease. Stent migration, unfortunately, still remains a problem but with further development and experience, percutaneous cystic duct stenting may very well have a role to play.47,48
PCT has also been employed as a bridge to surgery in patients who are too acutely unwell or haemodynamically unstable to undergo acute gallbladder surgery, but who may have subsequent surgery should they survive the acute septic episode.14 In these patients, early introduction of PCT may allow for easier and significantly shorter subsequent surgery.14,45
Timing of PCT placement, removal and subsequent cholecystectomy
One of the interesting points we identified in our review that needs further clarification was regarding the optimal timing of PCT insertion. There is no consensus and great variation in the literature when it comes to the optimal timing for drainage, ranging from 6 hours to 77 days in various studies, due to contrasting local management practices.
Several authors have suggested that early PCT drainage not only reduces length of hospital stay but will halt the progression of the inflammatory process.12 This prevents the formation of adhesions, severe fibrosis and the destruction of anatomical tissue planes, which can make subsequent gallbladder surgery a challenging and at times dangerous endeavour.14,45 From the available literature, it does seem that the answer to this question is ‘as soon as possible’.
At the time of subsequent cholecystectomy, Bickel et al. 6 reported lower rates of conversion to open surgery in their early PCT group when compared with the delayed PCT group (8.3% vs 33.3%, P = 0.09). They also noticed a considerable difference in the level of intraoperative adhesions (25% vs 57%, P = 0.064) and fibrosis (17% vs 53.3%, P = 0,03).6
It is obvious that a structured universal approach is lacking when it comes to the timing of PCT insertion, although one is indeed needed. In its absence, we recommend setting a clear early point at which to review the efficiency of antibiotics therapy and then promptly introduce a PCT if needed. Chok et al. 11 suggested that this check should be after three to four intravenous antibiotic doses.
Another aspect of PCT management that has been frequently debated is regarding the timing of surgery following PCT insertion. This was examined by a number of studies in our review but none found any significant difference in conversion rates or operative complications.20,26 Lung et al. 20 compared operative data between patients who had laparoscopic cholecystectomy early (less than 10 days) and late (10 days or longer) after PCT. They found no statistical difference when comparing mean operative time (102.6 ± 49.0 minutes vs 94.9 ± 39.9 minutes, P = 0.459), conversion rates (33.3% vs 25%, P = 0.435) and postoperative morbidity (6.7% vs 13.6%, P = 0.343).20
Safety profile
With increasing experience and continued technological development, PCT has become a routine procedure with an excellent safety profile. PCT was completed without incident in a large number of papers in our review. Major PCT-related complications were extremely rare and the majority of patients who deteriorated did so as a result of their pre-existing comorbidities rather than the PCT itself.
The most frequently reported PCT related complications were drain dislodgment (range 7.2–29.6%),35,36 minor bleeding (range 2.4–7.2%),12,17 minor bile leak (range 1.1–10.4%)21,25 and tube blockage (range 0.6–7%).18,34 These were all classed as minor complications and were managed conservatively in the majority of patients, with tube replacement in some cases of blockage and dislodgement.
In our systematic review of 312,085 patients, only four PCT-related mortalities were reported (overall combined PCT mortality 0.001%). One patient suffered a myocardial infarction during drain insertion.37 and The other three deaths were due to bleeding, two of these had refused blood products and further intervention to arrest the bleeding.11,18,38
Conclusion
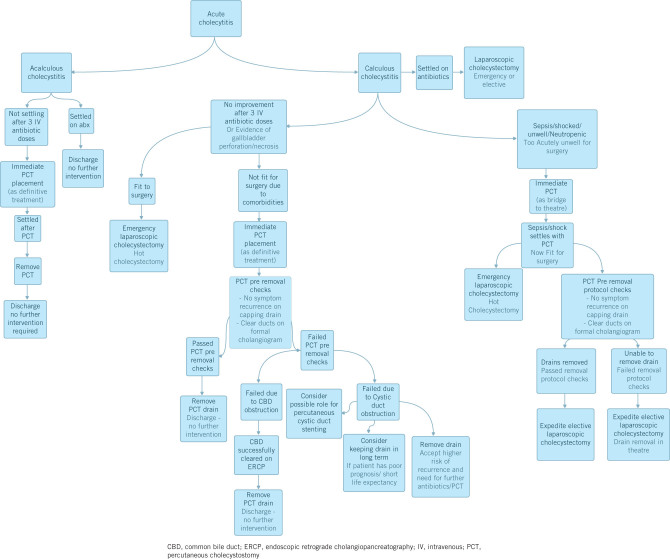
PCT is an efficient treatment modality for severe cholecystitis that is unresponsive to antibiotic treatment in a selected group of patients. It has an excellent safety profile and it can be used both as a bridge to surgery as well as a definitive treatment option. There is great variation in local management protocols in the literature and these need to be considered carefully and consolidated to ensure optimal treatment results and benefits for these patients. The authors would suggest a multidisciplinary approach involving surgeons, interventional radiologists and endoscopists collaborating to agree a clear local treatment pathway for these difficult conditions. Figure 1 outlines our proposed management pathway for this condition.
Figure 1.
Proposed management pathway for acute cholecystitis
References
- 1.Abi-Haidar Y, Sanchez V, Williams SA, Itani KM. Revisiting percutaneous cholecystostomy for acute cholecystitis based on a 10-year experience. Arch Surg 2012; : 416–422. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jundi W, Cannon T, Antakia R et al. Percutaneous cholecystostomy as an alternative to cholecystectomy in high risk patients with biliary sepsis: a district general hospital experience. Ann R Coll Surg Engl 2012; : 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JE, Chang DC, Talamini MA. A nationwide examination of outcomes of percutaneous cholecystostomy compared with cholecystectomy for acute cholecystitis, 1998-2010. Surg Endosc 2013; : 3,406–3,411. [DOI] [PubMed] [Google Scholar]
- 4.Atar E, Bachar GN, Berlin S et al. Percutaneous cholecystostomy in critically ill patients with acute cholecystitis: complications and late outcome. Clin Radiol 2014; : e247–252. [DOI] [PubMed] [Google Scholar]
- 5.Bakkaloglu H, Yanar H, Guloglu R et al. Ultrasound guided percutaneous cholecystostomy in high-risk patients for surgical intervention. World J Gastroenterol 2006; : 7,179–7,182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickel A, Hoffman RS, Loberant N et al. Timing of percutaneous cholecystostomy affects conversion rate of delayed laparoscopic cholecystectomy for severe acute cholecystitis. Surg Endosc 2016; ; 1,028–1,033. [DOI] [PubMed] [Google Scholar]
- 7.Boules M, Haskins IN, Farias-Kovac M et al. What is the fate of the cholecystostomy tube following percutaneous cholecystostomy? Surg Endosc 2017; : 1,707–1,712. [DOI] [PubMed] [Google Scholar]
- 8.Carrafiello G, D’Ambrosio A, Mangini M et al. Percutaneous cholecystostomy as the sole treatment in critically ill and elderly patients. Radiol Med 2012; : 772–779. [DOI] [PubMed] [Google Scholar]
- 9.Cha BH, Song HH, Kim YN et al. Percutaneous cholecystostomy is appropriate as definitive treatment for acute cholecystitis in critically ill patients: a single center, cross-sectional study. Korean J Gastroenterol 2014; : 32–38. [DOI] [PubMed] [Google Scholar]
- 10.Chang YR, Ahn YJ, Jang JY et al. Percutaneous cholecystostomy for acute cholecystitis in patients with high comorbidity and re-evaluation of treatment efficacy. Surgery 2014; : 615–622. [DOI] [PubMed] [Google Scholar]
- 11.Chok KS, Chu FS, Cheung TT et al. Results of percutaneous transhepatic cholecystostomy for high surgical risk patients with acute cholecystitis. A N Z J Surg 2010; : 280–283. [DOI] [PubMed] [Google Scholar]
- 12.Chou CK, Lee KC, Chan CC et al. Early percutaneous cholecystostomy in severe acute cholecystitis reduces the complication rate and duration of hospital stay. Medicine (Baltimore) 2015; : e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mestral C, Gomez D, Haas B et al. Cholecystostomy: a bridge to hospital discharge but not delayed cholecystectomy. J Trauma Acute Care Surg 2013; : 175–180. [DOI] [PubMed] [Google Scholar]
- 14.El-Gendi A, El-Shafei M, Emara D. Emergency versus delayed cholecystectomy after percutaneous transhepatic gallbladder drainage in grade II acute cholecystitis patients. J Gastrointest Surg 2017; : 284–293. [DOI] [PubMed] [Google Scholar]
- 15.Flexer SM, Peter MB, Durham-Hall AC, Ausobsky JR. Patient outcomes after treatment with percutaneous cholecystostomy for biliary sepsis. Ann R Coll Surg Engl 2014; : 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griniatsos J, Petrou A, Pappas P et al. Percutaneous cholecystostomy without interval cholecystectomy as definitive treatment of acute cholecystitis in elderly and critically ill patients. South Med J 2018; : 586–590. [DOI] [PubMed] [Google Scholar]
- 17.Horn T, Christensen SD, Kirkegard J et al. Percutaneous cholecystostomy is an effective treatment option for acute calculous cholecystitis: a 10-year experience. HPB (Oxford) 2015; : 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh YC, Chen CK, Su CW et al. Outcome after percutaneous cholecystostomy for acute cholecystitis: a single-center experience. J Gastrointest Surg 2012; : 1,860–1,868. [DOI] [PubMed] [Google Scholar]
- 19.Jang WS, Lim JU, Joo KR et al. Outcome of conservative percutaneous cholecystostomy in high-risk patients with acute cholecystitis and risk factors leading to surgery. Surg Endosc 2-15; : 2,359–2,364. [DOI] [PubMed] [Google Scholar]
- 20.Jung WH, Park DE. Timing of cholecystectomy after percutaneous cholecystostomy for acute cholecystitis. Korean J Gastroenterol 2015; : 209–214. [DOI] [PubMed] [Google Scholar]
- 21.Karakayali FY, Akdur A, Kirnap M et al. Emergency cholecystectomy vs percutaneous cholecystostomy plus delayed cholecystectomy for patients with acute cholecystitis. Hepatobiliary Pancreat Dis Int 2014; : 316–322. [DOI] [PubMed] [Google Scholar]
- 22.Khasawneh MA, Shamp A, Heller S et al. Successful laparoscopic cholecystectomy after percutaneous cholecystostomy tube placement. J Trauma Acute Care Surg 2015; : 100–104. [DOI] [PubMed] [Google Scholar]
- 23.Kirkegard J, Horn T, Christensen SD et al. Percutaneous cholecystostomy is an effective definitive treatment option for acute acalculous cholecystitis. Scand J Surg 2015; : 238–243. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Li N, Ji W et al. Percutaneous cholecystostomy is a definitive treatment for acute cholecystitis in elderly high-risk patients. Am Surg 2013; : 524–527. [DOI] [PubMed] [Google Scholar]
- 25.Lin WC, Chang CW, Chu CH. Percutaneous cholecystostomy for acute cholecystitis in high-risk elderly patients. Kaohsiung J Med Sci 2016; : 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macri A, Scuderi G, Saladino E et al. Acute gallstone cholecystitis in the elderly: treatment with emergency ultrasonographic percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Surg Endosc 2016; : 88–91. [DOI] [PubMed] [Google Scholar]
- 27.McKay A, Abulfaraj M, Lipschitz J. Short- and long-term outcomes following percutaneous cholecystostomy for acute cholecystitis in high-risk patients. Surg Endosc 2012; : 1,343–1,351. [DOI] [PubMed] [Google Scholar]
- 28.Melloul E, Denys A, Demartines N et al. Percutaneous drainage versus emergency cholecystectomy for the treatment of acute cholecystitis in critically ill patients: does it matter? World J Surg 2011; : 826–833. [DOI] [PubMed] [Google Scholar]
- 29.Mizrahi I, Mazeh H, Yuval JB et al. Perioperative outcomes of delayed laparoscopic cholecystectomy for acute calculous cholecystitis with and without percutaneous cholecystostomy. Surgery 2015; : 728–735. [DOI] [PubMed] [Google Scholar]
- 30.Morse BC, Smith JB, Lawdahl RB, Roettger RH. Management of acute cholecystitis in critically ill patients: contemporary role for cholecystostomy and subsequent cholecystectomy. Am Surg 2010; : 708–712. [DOI] [PubMed] [Google Scholar]
- 31.Nasim S, Khan S, Alvi R, Chaudhary M. Emerging indications for percutaneous cholecystostomy for the management of acute cholecystitis: a retrospective review. Int J Surg 2011; : 456–459. [DOI] [PubMed] [Google Scholar]
- 32.Ni Q, Chen D, Xu R, Shang D. The efficacy of percutaneous transhepatic gallbladder drainage on acute cholecystitis in high-risk elderly patients based on the tokyo guidelines: a retrospective case-control study. Medicine (Baltimore) 2015; : e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikfarjam M, Shen S, Fink MA et al. Percutaneous cholecystostomy for treatment of acute cholecystitis in the era of early laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 2013; : 474–480. [DOI] [PubMed] [Google Scholar]
- 34.Pang KW, Tan CH, Loh S et al. Outcomes of percutaneous cholecystostomy for acute cholecystitis. World J Surg 2016; : 2,735–2,744. [DOI] [PubMed] [Google Scholar]
- 35.Paran H, Zissin R, Rosenberg E et al. Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg 2006; : 101–105. [DOI] [PubMed] [Google Scholar]
- 36.Peters R, Kolderman S, Peters B et al. Percutaneous cholecystostomy: single centre experience in 111 patients with an acute cholecystitis. JBR-BTR 2014; : 197–201. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Sanjuan JC, Arruabarrena A, Sanchez-Moreno L et al. Acute cholecystitis in high surgical risk patients: percutaneous cholecystostomy or emergency cholecystectomy? Am J Surg 2012; : 54–59. [DOI] [PubMed] [Google Scholar]
- 38.Sanjay P, Mittapalli D, Marioud A et al. Clinical outcomes of a percutaneous cholecystostomy for acute cholecystitis: a multicentre analysis. HPB (Oxford) 2013; : 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simorov A, Ranade A, Parcells J et al. Emergent cholecystostomy is superior to open cholecystectomy in extremely ill patients with acalculous cholecystitis: a large multicenter outcome study. Am J Surg 2013; : 935–941. [DOI] [PubMed] [Google Scholar]
- 40.Smith TJ, Manske JG, Mathiason MA et al. Changing trends and outcomes in the use of percutaneous cholecystostomy tubes for acute cholecystitis. Ann Surg. 2013; : 1,112–1,115. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki K, Bower M, Cassaro S et al. Tube cholecystostomy before cholecystectomy for the treatment of acute cholecystitis. JSLS 2015; : e2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viste A, Jensen D, Angelsen JH, Hoem D. Percutaneous cholecystostomy in acute cholecystitis; a retrospective analysis of a large series of 104 patients. BMC Surg 2017; : 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang CH, Wu CY, Yang JC et al. Long-term outcomes of patients with acute cholecystitis after successful percutaneous cholecystostomy treatment and the risk factors for recurrence: a decade experience at a single center. PLoS One 2016; : e0148017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo CS, Tay VW, Low JK et al. Outcomes of percutaneous cholecystostomy and predictors of eventual cholecystectomy. J Hepatobiliary Pancreat Sci 2016; : 65–73. [DOI] [PubMed] [Google Scholar]
- 45.Zehetner J, Degnera E, Olasky J et al. Percutaneous cholecystostomy versus laparoscopic cholecystectomy in patients with acute cholecystitis and failed conservative management: a matched-pair analysis. Surg Laparosc Endosc Percutan Tech 2014; : 523–527. [DOI] [PubMed] [Google Scholar]
- 46.Zerem E, Omerovic S. Can percutaneous cholecystostomy be a definitive management for acute cholecystitis in high-risk patients? Surg Laparosc Endosc Percutan Tech 2014; : 187–191. [DOI] [PubMed] [Google Scholar]
- 47.Hersey N, Goode SD, Peck RJ, Lee F. Stenting of the cystic duct in benign disease: a definitive treatment for the elderly and unwell. Cardiovasc Intervent Radiol 2015; : 964–970. [DOI] [PubMed] [Google Scholar]
- 48.Comin JM, Cade RJ, Little AF. Percutaneous cystic duct stent placement in the treatment of acute cholecystitis. J Med Imaging Radiat Oncol 2010; : 457–461. [DOI] [PubMed] [Google Scholar]