Abstract
Hormonal contraception (HC), particularly injectable depot-medroxyprogesterone acetate (DMPA), has been associated with increased HIV acquisition and higher levels of cervical regulated upon activation, normal T-cell expressed, and secreted (RANTES), also associated with HIV seroconversion. Longitudinal changes in cervical immunity associated with DMPA and combined oral contraceptives (COCs) have not been studied. Cervical samples from 216 HIV seroconverters in Uganda and Zimbabwe with matched samples from 727 HIV-uninfected controls were collected at two quarterly visits before (t − 2, t − 1), at (t0), and two visits following (t + 1, t + 2) HIV seroconversion and corresponding visits for HIV-negative controls. We measured 10 biomarkers of inflammation and immunity and used generalized linear models to estimate and compare biomarker levels across HIV status, contraceptive, and pregnancy groups. Biomarkers remained relatively stable across visits for controls, while in HIV-infected women cervical immunity started to change before seroconversion with RANTES and BD-2 increased and secretory leukocyte protease inhibitor (SLPI) decreased at t − 1 and continued to change at t0 with ICAM-1 up and IL-8 down and with more biomarkers after seroconversion (IL-1β, IL-6, MIP-3α, VEGF, and IL-1RA down and IL-1RA:IL-1β ratio up). In multivariable analyses, seroconverters had higher BD-2 at t − 1, higher RANTES and lower SLPI from t − 1 through t + 2, and lower IL-8 and IL-1RA at and/or after seroconversion compared to nonseroconverters. Compared to non-HC users, DMPA users had higher RANTES at all visits and lower BD-2 at t − 2 through t0, while COC users and pregnant women had higher IL-8 and SLPI at all visits; COC users also had lower BD-2 preseroconversion; pregnant women had lower RANTES at t0 − t + 2. Longitudinal patterns of cervical immunity differ between HIV seroconverters and HIV-negative women; seroconverters demonstrate increased RANTES and decreased SLPI starting before and continuing postseroconversion. Furthermore, these patterns are differentially regulated by DMPA, COC, and pregnancy.
Keywords: : HIV-1, hormonal contraception, cervical inflammation, immunity, pregnancy
Introduction
Over 150 million women worldwide use hormonal contraception. The most commonly used contraceptives in Sub-Saharan African countries are depot-medroxyprogesterone acetate (DMPA), a 3-monthly progestin injectable, and combined oral contraceptives (COCs). DMPA and COCs, and pregnancy have been associated with increased HIV acquisition and other sexually transmitted infections (STIs) in some studies,1–8 but not others.9–11 One prospective study suggests that hormonal contraception might also contribute to a higher risk of HIV transmission to the male partner.5
In February 2017, the World Health Organization (WHO) issued a guidance statement regarding hormonal contraceptive eligibility for women at high risk of HIV. The WHO changed the guidance for women at high risk of HIV using injectable progestins from a medical eligibility criteria (MEC) rating of 1 (the method can be used without restriction) to a MEC rating of 2 (the advantages of using the method generally outweigh the theoretical or proven risks).12 The guidance stated “There continues to be evidence of possible increased risk of acquiring HIV among progestogen-only injectable users. Uncertainty exists about whether this is due to methodological issues with the evidence or a real biological effect.”12
To further explore the biological mechanisms that might increase HIV susceptibility, we used specimens and data from the hormonal contraception (HC)-HIV Study—a prospective study of 4,500 women in Uganda and Zimbabwe specifically designed to examine the association between hormonal contraception and HIV acquisition.1 The study found that women who used DMPA, but not COCs, were at significantly increased risk of HIV acquisition compared to women not using hormonal contraception.1 Data and stored samples from this study were available for more in-depth evaluation and were used in the analysis reported in this study.
Biomarkers of cervical inflammation and immunity are influenced by menstrual cycle associated hormonal changes13–17; however, limited data exist on the impact of HC and pregnancy on these biomarkers. Stored HC-HIV study samples provided a rare opportunity to assess the impact of hormonal contraceptives and pregnancy on biomarkers of innate immunity.
We previously found in cross-sectional analyses that HC use, pregnancy, and young age altered biomarkers of cervical immunity in ways associated with increased risk of HIV acquisition (through increased levels of pro-inflammatory cytokines, including regulated upon activation, normal T-cell expressed, and secreted [RANTES], and decreased levels of secretory leukocyte protease inhibitor [SLPI]).18 In subsequent analyses we also found that alteration of cervical immunity by hormonal contraceptives is modified by the presence of genital tract infections19 and that higher levels of RANTES and lower SLPI and MIP-3α are associated with increased HIV shedding among HIV-infected women.20
We now present the results of a longitudinal analysis, including data from two preseroconversion (t − 2, t − 1), the seroconversion (t0), and two postseroconversion (t + 1, t + 2) visits, and matched visits for women remaining seronegative. The objectives of this study were to: (1) evaluate intra-women variation in biomarkers of cervical immunity over time in HIV-uninfected and HIV-infected women and (2) determine whether biomarker levels over time are influenced by use of hormonal contraception and pregnancy. A better understanding of how hormonal contraception and pregnancy modify the genital mucosal environment to influence HIV infection risk is critical to informing contraceptive guidelines in regions of high HIV prevalence, as well as the development of improved contraceptive methods.
Materials and Methods
Study population and variables
The study design and methods have been previously described.7,11,19 Briefly, data and samples were from a longitudinal study of hormonal contraception and HIV acquisition (HC-HIV Study) and a follow-on study of women with primary HIV infection (Hormonal Contraception and HIV Genital Shedding and Disease Progression Study). Women used DMPA (150 mg, every 3 months, administered by study staff), COCs (30 μg ethinylestradiol and 150 μg levonorgestrel provided by study staff as a 3-month supply), or no hormonal contraception. We enrolled 4,531 HIV-uninfected women ages 18–35 years from family planning clinics in Uganda and Zimbabwe.11 Time-varying contraceptive group was assigned based on the primary contraceptive method women used during the time between the previous and current study visit. Women in the nonhormonal (NH) group used only condoms or no contraception. Participants were seen every 12 weeks for up to 24 months while HIV negative and at 4, 8, and 12 weeks after HIV seroconversion and quarterly thereafter for HIV-positive women.
We identified 216 women with incident HIV infection as cases (62 Ugandan and 154 Zimbabwean) and matched 727 HIV-uninfected controls (up to four controls per case) on study site, age, a composite STI/reproductive tract infection (RTI) variable (presence of Chlamydia trachomatis [CT], Neisseria gonorrhoeae [NG], Trichomonas vaginalis [TV], and/or bacterial vaginosis [BV] either at the seroconversion visit or the visit before), and time in study.21 Samples were taken from the HIV seroconversion visit and the two visits before and after the seroconversion visit and corresponding matched visits for HIV-uninfected controls.
The study protocol received a nonhuman subject determination (use of deidentified data) from the Office of International Research Ethics at FHI 360 and the Institutional Review Board at Brigham and Women's Hospital.
Sample collection and processing
We used endocervical swab specimens collected in Uganda and Zimbabwe and stored these as frozen after STI analyses were completed.22 Sample collection, processing, STI diagnostics, and testing have been previously described.18–20 Swab collection followed the Roche Diagnostics (Amplicor CT/NG) protocol established for detection of STI pathogens. In brief, following removal of mucus and loose cellular material from the ectocervix, a swab was inserted into the endocervical canal, gently rotated 3–5 s, withdrawn, and placed in 1 mL Amplicor lysis medium, and after agitation discarded. Each sample was mixed with 1 mL diluent (Roche Diagnostics) and processed for STI diagnosis as per manufacturer's instruction.
Residual of the Amplicor-extracted solutions was shipped on ice to Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) and stored at −80°C until shipped to the Laboratory of Genital Tract Biology, Brigham and Women's Hospital, where it was aliquoted and stored at −80°C in air-tight coded micronic tubes (USA Scientific) until analyzed. Laboratory diagnosis of STI/RTIs included PCR (Roche Amplicor) for CT and NG, antibody enzyme-linked immunosorbent assay (ELISA) for herpes simplex virus-2 (HSV-2), wet mount for TV and candida, and Nugent scoring for BV. HIV status was ascertained by ELISA and PCR.
Measurement of biomarkers
The rationale for the measurement of these specific biomarkers has been presented previously.19 The Laboratory of Genital Tract Biology at Brigham and Women's Hospital measured protein levels of immune biomarkers under rigorous quality control procedures and accreditation by the College of American Pathologists. A Meso Scale Discovery electrochemiluminescence multiplex assay was used to measure IL-1β, IL-1RA, IL-6, IL-8, RANTES, MIP-3α, VEGF, and soluble ICAM-1, and ELISA was used to measure SLPI (R&D Systems, Minneapolis, MN) and BD-2 (Phoenix Pharmaceuticals, Burlingame, CA) followed by normalization to total protein as previously described.18–20
All samples were run on plates of the same assay batch. Interplate reproducibility was ascertained by applying a quality control pool run in duplicate on each plate which showed good intra- and interplate comparability (typically less than 15% coefficient of variation which is standard for immune-enzyme assays).
Statistical analyses
We compared demographic characteristics among HC exposure groups using Cochran–Mantel–Haenszel and Fisher's exact tests. Descriptive statistics, including medians and interquartile ranges, was used to summarize nontransformed biomarker levels; between-group differences were evaluated using the Wilcoxon–Mann–Whitney test. We explored the patterns of biomarkers among HC exposure groups and HIV status over time by a weighted local regression smoothing approach (LOESS procedure).23 We used Box–Cox power transformation19 to normalize biomarker data used in multivariable mixed-effect models. Covariates evaluated as potential confounders included age, country, breastfeeding, number of sexual partners, number of unprotected sexual acts, current STIs/RTIs (BV, candidiasis, CT, NG, HSV-2, and TV), STI signs and symptoms, and vaginal practices. We note that all analyses in the study were considered hypothesis generating (i.e., exploratory) and were not necessarily powered to detect clinically meaningful effect sizes. p-Values less than 0.05 were considered statistically significant and were not adjusted for multiple comparisons. Thus, results should thus be interpreted with caution. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Participant characteristics
This analysis included 943 women composed of 216 HIV seroconverters and 727 women remaining HIV negative who contributed 3,721 specimens at five study visits. At the seroconversion (t0) visit, there were twice as many Zimbabwean (68%) as Ugandan women (32%) and roughly equal numbers of women using COCs, DMPA, and not using HC (36%, 33%, and 31%, respectively) (Table 1). While there were no statistically significant differences in HC use, pregnancy, or numbers of sex partners between HIV seroconverters and the HIV-negative controls, HIV seroconverters reported somewhat fewer unprotected sex acts but higher levels of HSV-2 and any STIs/RTIs (CT, NG, TV, HSV-2, BV, and candida).
Table 1.
Participant Characteristics by HIV Status at the HIV Seroconversion Visit (t0)
| Characteristic | Remaining, HIV negative (n = 727) n (%) | Becoming, HIV positive (n = 216) n (%) | Total (n = 943) n (%) | p-Valuea |
|---|---|---|---|---|
| Site | ||||
| Uganda | 240 (33.01) | 62 (28.7) | 302 (32.03) | |
| Zimbabwe | 487 (66.99) | 154 (71.3) | 641 (67.97) | .234 |
| Age at screening | ||||
| 18–24 | 411 (56.53) | 120 (55.56) | 531 (56.31) | |
| 25+ | 316 (43.47) | 96 (44.44) | 412 (43.69) | .799 |
| HC group (last 3 months) | ||||
| COC | 273 (37.66) | 66 (30.56) | 339 (36.03) | |
| DMPA | 233 (32.14) | 76 (35.19) | 309 (32.84) | |
| NH | 219 (30.21) | 74 (34.26) | 293 (31.14) | .159 |
| Currently pregnant | 53 (7.35) | 10 (4.63) | 63 (6.72) | .162 |
| Currently breastfeeding | 101 (13.91) | 30 (13.89) | 131 (13.91) | .993 |
| 2+ sexual partners (last 3 months) | 17 (2.34) | 6 (2.78) | 23 (2.44) | .801 |
| No. of unprotected acts (typical month) | ||||
| 15+ | 154 (21.21) | 38 (17.59) | 192 (20.38) | |
| 8–14 | 196 (27) | 49 (22.69) | 245 (26.01) | |
| 1–7 | 184 (25.34) | 61 (28.24) | 245 (26.01) | |
| 0 or no sex act | 192 (26.45) | 68 (31.48) | 260 (27.6) | .050 |
| HSV-2 infected | 393 (54.51) | 182 (85.45) | 575 (61.56) | <.001 |
| Any RTI/STIb | 523 (75.14) | 202 (95.28) | 725 (79.85) | <.001 |
Chi-square or Fisher's exact or Cochran–Mantel–Haenszel test for categorical variables.
Any RTI/STI includes chlamydia, gonorrhea, trichomoniasis, HSV-2, BV, and candida.
BV, bacterial vaginosis; HC, hormonal contraception; COC, combined oral contraceptive; DMPA, depot-medroxyprogesterone acetate; HSV-2, herpes simplex virus-2; NH, nonhormonal; RTI, reproductive tract infection; STI, sexually transmitted infection.
Mean levels and biomarker trends across time by HIV status (t − 2 to t + 2 visits)
We first evaluated biomarker trends over all visits by comparing mean levels across time by HIV status (Table 2). Among HIV seroconverters, IL-1β, IL-6, IL-8, MIP-3α, VEGF, IL-1RA, SLPI, and BD-2 all declined significantly across time (test of trend: p < .0001), while RANTES and the IL-1RA:IL-1β ratio (p < .0001) and ICAM-1 (p < .01) increased across visits. Among women remaining HIV negative, only IL-6, VEGF, and BD-2 decreased across time (test of trend: p < .001), while other biomarkers remained relatively stable. All biomarker values were positively correlated across visits with the intraclass correlation coefficient (ICC) being somewhat higher for women remaining HIV negative than for HIV seroconverters (e.g., for IL-6, ICC = 0.31 and 0.20 for HIV negative and HIV seroconverters, respectively). Among HIV-negative women, biomarker values were more strongly correlated among DMPA users than the NH group with COC users falling in between (e.g., for SLPI, ICC = 0.42, 0.26, and 0.36 for DMPA, NH, and COC users, respectively).
Table 2.
Mean (Standard Error) of Vaginal Immunity Biomarker Values by HIV Status Across All Visits
| Biomarkera | t − 2, Mean (SE) (n = 703) | t − 1, Mean (SE) (n = 825) | T0, Mean (SE) (n = 824) | t + 1, Mean (SE) (n = 728) | t + 2, Mean (SE) (n = 641) | Across visits, mean (SE) (n = 3721) | Test of trendb, p-value |
|---|---|---|---|---|---|---|---|
| IL-1β | |||||||
| HIV− | 4.37 (0.20) | 5.93 (0.20) | 4.77 (0.18) | 4.51 (0.20) | 4.70 (0.21) | 4.85 (0.11) | .1263 |
| HIV+ | 4.53 (0.36) | 5.62 (0.33) | 4.13 (0.28) | 2.23 (0.18)* | 2.44 (0.19)* | 3.66 (0.15)* | <.0001 |
| IL-6 | |||||||
| HIV− | 18.57 (0.65) | 21.95 (0.68) | 18.40 (0.61) | 16.98 (0.63) | 17.68 (0.76) | 18.54 (0.40) | .0022 |
| HIV+ | 18.73 (1.19) | 20.73 (1.13) | 18.15 (1.06) | 10.01 (0.62)* | 9.51 (0.64)* | 14.87 (0.56)* | <.0001 |
| IL-8 | |||||||
| HIV− | 770.03 (39.42) | 879.95 (39.53) | 731.13 (32.55) | 683.38 (31.71) | 749.09 (37.80) | 758.89 (22.25) | .0244 |
| HIV+ | 827.58 (75.79) | 791.49 (63.73) | 522.58 (43.07)* | 267.10 (24.52)* | 278.61 (26.50)* | 489.27 (27.24)* | <.0001 |
| RANTES | |||||||
| HIV− | 40.33 (1.84) | 40.67 (1.74) | 40.46 (1.68) | 38.05 (1.78) | 38.88 (1.98) | 38.88 (1.07) | .1536 |
| HIV+ | 44.11 (3.68) | 52.67 (4.14)** | 92.66 (7.58)* | 107.32 (10.07)* | 98.87 (9.43)* | 74.08 (3.96)* | <.0001 |
| MIP-3α | |||||||
| HIV− | 354.02 (13.20) | 372.58 (13.72) | 358.30 (12.58) | 355.75 (13.30) | 360.06 (14.60) | 358.50 (8.12) | .6108 |
| HIV+ | 322.19 (21.42) | 359.66 (23.31) | 335.98 (20.74) | 207.98 (12.70)* | 200.10 (12.46)* | 278.06 (10.93)* | <.0001 |
| ICAM-1 | |||||||
| HIV− | 459.79 (16.87) | 501.83 (16.07) | 466.53 (15.54) | 482.65 (17.64) | 508.07 (21.91) | 471.17 (10.85) | .0578 |
| HIV+ | 456.01 (30.26) | 501.60 (28.39) | 561.21(32.04)** | 577.70 (35.81)*** | 529.41 (37.57) | 523.22 (21.28)*** | .0076 |
| VEGF | |||||||
| HIV− | 1218.77 (31.26) | 1418.46 (32.11) | 1199.68 (28.44) | 1178.71 (31.06) | 1186.11 (36.42) | 1245.79 (18.13) | .0001 |
| HIV+ | 1226.30 (56.84) | 1313.86 (52.09) | 1126.54 (46.97) | 759.17 (33.14)* | 769.36 (37.08)* | 1023.44 (25.92)* | <.0001 |
| IL-IRA | |||||||
| HIV− | 798.83 (17.25) | 925.17 (17.26) | 847.21 (17.96) | 801.73 (16.82) | 800.88 (17.33) | 839.69 (9.26) | .0083 |
| HIV+ | 765.22 (29.41) | 925.77 (30.54) | 806.19 (29.78) | 619.59 (20.85)* | 614.14 (20.13)* | 742.82 (13.94)* | <.0001 |
| IL-1RA/IL-1β ratio | |||||||
| HIV− | 184.40 (6.25) | 171.52 (4.61) | 184.52 (5.16) | 186.27 (6.06) | 174.25 (5.57) | 181.26 (2.85) | .7806 |
| HIV+ | 170.67 (10.22) | 177.75 (8.53) | 200.40 (10.16) | 245.61 (15.45)* | 214.44 (12.38)** | 199.63 (5.76)** | <.0001 |
| SLPI | |||||||
| HIV− | 131166.6 (9490.20) | 176735.3 (9731.15) | 143549.9 (9153.32) | 127387.2 (9702.52) | 127300.6 (10575.78) | 135600.6 (6041.26) | .1171 |
| HIV+ | 116705.8 (15167.68) | 115983.3 (11082.28)* | 84311.68 (9298.93)* | 51208.56 (6539.65)* | 63744.94 (8498.92)* | 81323.49 (6369.13)* | .0003 |
| BD-2 | |||||||
| HIV− | 1496.27 (121.12) | 1435.98 (95.52) | 1380.48 (104.65) | 1121.45 (91.74) | 1140.64 (98.22) | 1359.97 (66.07) | <.0001 |
| HIV+ | 1944.50 (291.19) | 2118.37 (258.20)** | 1491.94 (201.74) | 888.87 (124.53) | 891.90 (124.36) | 1374.43 (120.07) | <.0001 |
Concentration values for each biomarker are presented in pg/mL adjusted to mg/mL. Total protein. Biomarkers include IL-1β, IL-1 RA, IL-6, IL-8, RANTES, MIP-3α, VEGF, soluble ICAM-1; SLPI, BD-2.
Limited to ±52 weeks.
p < .001, **p < .01, ***p < .05 for testing the difference of biomarker between ‘becoming/HIV+’ and ‘remain HIV’ groups for each visit and overall.
BD, beta-defensin; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; MIP, macrophage inflammatory protein; RA, receptor antagonist; RANTES, regulated upon activation, normal T-cell expressed, and secreted; SLPI, secretory leukocyte protease inhibitor; VEGF, vascular endothelial growth factor.
Multiple biomarkers differed between the HIV-uninfected and HIV-seroconverter groups (Table 2) with differences starting before seroconversion for some and postseroconversion for others. For example, HIV seroconverters had significantly lower IL-1β, IL-6, IL-8, MIP-3α, VEGF, IL-1RA, and SLPI and significantly higher RANTES, ICAM-1, and 1RA:IL-1β ratio at some or all postseroconversion visits (t0, t + 1, t + 2) compared with HIV-negative controls. Among seroconverters, increases in RANTES and decreases in SLPI started before and continued postseroconversion.
Biomarker changes associated with seroconversion (t − 1 vs. t0 visits)
When we analyzed the changes in biomarker levels between the t − 1 and t0 visits, we identified IL-8, RANTES, IL-1RA, SLPI, and BD-2 to be most strongly associated with HIV seroconversion (Table 3). Among HIV-negative controls IL-8, IL-1RA, and SLPI decreased (Table 3), while among HIV seroconverters RANTES increased and IL-8, IL-1RA, SLPI, and BD-2 all decreased. There were few statistically significant changes by HC use among women remaining HIV negative from t − 1 to t0. However, among HIV seroconverters, all contraceptive groups had increased RANTES and decreased IL-8, while DMPA users alone had decreased IL-1RA during the seroconversion period (Table 3).
Table 3.
Change in Biomarker Level Between Immediate Pre- and Post-HIV Acquisition Visits (T − 1 vs. T0)
| Characteristic | IL-8 (p-value) | RANTES (p-value) | IL-1RA (p-value) | SLPI (p-value) | BD-2 (p-value) |
|---|---|---|---|---|---|
| Remaining HIV negative | |||||
| Overall | −131.51 (.002) | −0.29 (.883) | −76.29 (.001) | −28695.30 (.008) | −64.64 (.595) |
| Site | |||||
| Uganda (n = 211) | −95.47 (.241) | 1.64 (.461) | −117.43 (.004) | −39735.74 (<.001) | 469.94 (.267) |
| Zimbabwe (n = 481) | −143.50 (.005) | −1.34 (.630) | −63.44 (.020) | −17267.97 (.286) | −157.67 (.165) |
| Age | |||||
| 18–24 (n = 389) | −112.12 (.043) | 1.63 (.530) | −70.61 (.028) | −25551.05 (.063) | −47.20 (.774) |
| 25+ (n = 303) | −154.94 (.023) | −2.64 (.380) | −84.42 (.011) | −32649.86 (.063) | −88.32 (.627) |
| Contraception | |||||
| COC (n = 245) | −239.56 (.005) | 1.78 (.563) | −90.50 (.024) | −17652.97 (.531) | −58.16 (.735) |
| DMPA (n = 227) | −64.23 (.329) | 2.47 (.624) | −15.19 (.702) | −22146.46 (.087) | −27.79 (.882) |
| NH (n = 174) | −97.57 (.252) | 0.77 (.770) | −104.38 (.031) | −35228.92 (.055) | −434.99 (.296) |
| Current pregnancy | |||||
| Yes (n = 29) | −426.48 (.305) | 11.07 (.112) | −297.11 (.002) | −10144.56 (.879) | −1426.21 (.198) |
| No (n = 632) | −142.21 (.001) | −0.20 (.927) | −64.26 (.007) | 31701.69 (.004) | −48.70 (.698) |
| Current breastfeeding | |||||
| Yes (n = 87) | −101.29 (.340) | 3.60 (.601) | −43.22 (.552) | 5693.59 (.761) | −354.07 (.416) |
| No (n = 567) | −144.22 (.003) | −0.18 (.933) | −83.36 (.001) | −33688.06 (.011) | −49.24 (.717) |
| Becoming HIV positive | |||||
| Overall | −269.14 (<.001) | 39.66 (<.001) | −117.20 (.001) | −30678.34 (.018) | −620.75 (.033) |
| Site | |||||
| Uganda (n = 59) | −163.85 (.142) | 28.28 (.007) | −151.59 (.069) | −58433.35 (.011) | 446.58 (.547) |
| Zimbabwe (n = 154) | −314.10 (<.001) | 44.13 (<.001) | −106.71 (.008) | −19225.39 (.220) | −796.10 (.009) |
| Age | |||||
| 18–24 (n = 118) | −300.66 (<.001) | 32.77 (<.001) | −172.83 (<.001) | −22035.86 (.229) | −855.15 (.039) |
| 25+ (n = 95) | −228.60 (.006) | 47.28 (<0.001) | −48.56 (.388) | −43272.31 (.015) | −325.02 (.407) |
| Contraception | |||||
| COC (n = 62) | −316.93 (.021) | 23.32 (.003) | −81.07 (.248) | −63032.17 (.062) | 112.39 (.823) |
| DMPA (n = 71) | −342.74 (<.001) | 64.94 (<.001) | −173.73 (.002) | −36816.86 (.087) | −534.71 (.084) |
| NH (n = 62) | −211.80 (.048) | 34.78 (.003) | −19.50 (.807) | −29399.63 (.087) | −1149.93 (.268) |
| Current pregnancy | |||||
| Yes (n = 5) | N/A | N/A | N/A | N/A | N/A |
| No (n = 203) | −284.71 (<.001) | 42.02 (<.001) | −113.43 (.002) | −30906.95 (.016) | −656.60 (.023) |
| Current breastfeeding | |||||
| Yes (n = 26) | −291.47 (.025) | 35.35 (.073) | −125.38 (.169) | 10539.37 (.655) | −403.75 (.534) |
| No (n = 173) | −271.39 (<.001) | 41.35 (<.001) | −103.88 (.011) | −45352.15 (.005) | −695.41 (.040) |
Change in biomarker concentration levels between t − 1 and t0 visits standardized by total protein: IL-8, RANTES, IL-1RA, SLPI, BD-2.
Variability of biomarkers associated with HIV seroconversion across visits by contraceptive use (t − 2 to t + 2 visits)
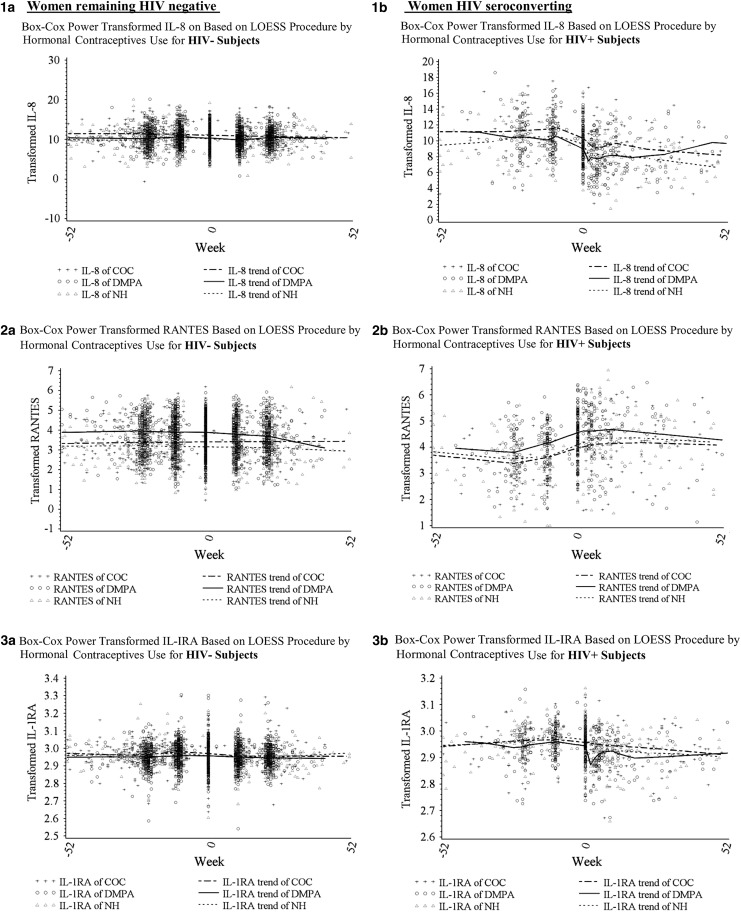
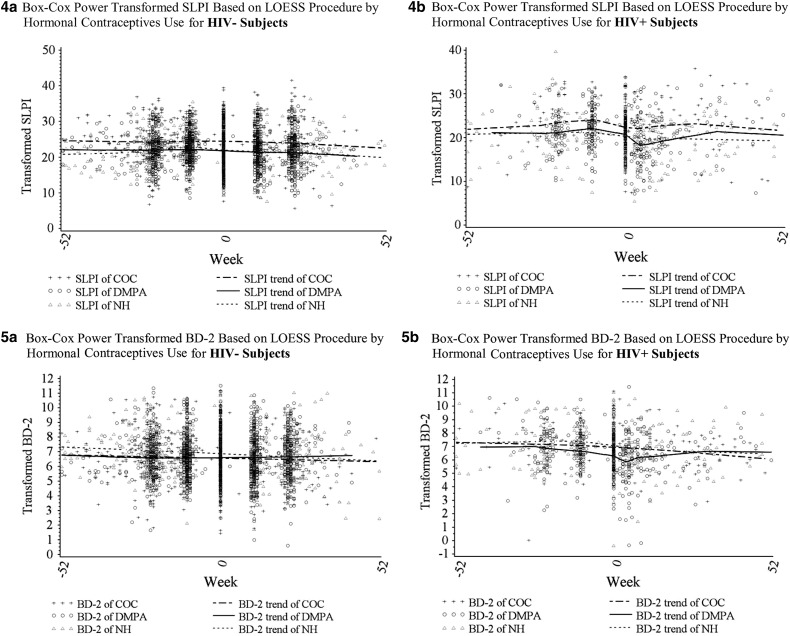
To visually assess overall trends in the five biomarkers before and after HIV seroconversion and by HC use, we first plotted Box–Cox transformed levels of biomarkers using the LOESS procedure (Fig. 1). For HIV-negative women the levels of most biomarkers appeared relatively stable across the five visits. In contrast, immune biomarkers appeared to vary considerably across time for HIV-seroconverting women, with changes occurring most notably from the t − 1 to the t0 (seroconversion) visit.
FIG. 1.
Overall trends in biomarkers by HIV status and contraceptive use (COC users, DMPA users, and women not using hormonal contraception [NH]) groups across five study visits (time 0 equals the seroconversion visit for HIV seroconverters) using a weighted local regression smoothing approach (LOESS procedure). (1a–5a) For women remaining HIV-negative—levels of IL-8, RANTES, IL1-RA, SLPI, and BD-2, respectively. (1b–5b) For women seroconverting at t0—levels of IL-8, RANTES, IL1-RA, SLPI, and BD-2, respectively. BD, beta-defensin; COC, combined oral contraceptive; DMPA, depot-medroxyprogesterone acetate; IL-8, interleukin-8; IL1-RA, IL1-receptor antagonist; RANTES, regulated upon activation, normal T-cell expressed, and secreted; SLPI, secretory leukocyte protease inhibitor.
We next conducted separate multivariable analyses for each of the five (t − 2 through t + 2) study visits (Table 4). We found that HIV seroconverters (compared to HIV-negative controls) continued to have significantly higher RANTES and lower SLPI levels starting before seroconversion (t − 1) and continuing throughout the postseroconversion visits controlling for age, site, contraceptive use, pregnancy, and breastfeeding status. In addition, HIV seroconverters had lower IL-8 and IL-1RA postseroconversion than HIV-negative controls. DMPA users (compared with the NH group) had higher RANTES at all visits and lower BD-2 from t − 2 through t0. Conversely, COC users (compared to the NH group) and pregnant (compared to nonpregnant) women had higher IL-8 and SLPI at all visits and higher IL-1RA at some visits. COC users had lower BD-2 at t − 2 and t − 1, while pregnant women had lower RANTES at t0 through t + 2 compared to nonpregnant women.
Table 4.
Multivariable Models for Selected Biomarkers by Visit (from T − 2 to T + 2)
| Mediators and visits | Becoming HIV+c(nd = 825) | DMPac(nd = 824) | COCc(nd = 824) | Current pregnantc(nd = 825) | Current breastfeedingc(nd = 825) | Zimbabwec(nd = 825) | Age: 18–24c(nd = 825) |
|---|---|---|---|---|---|---|---|
| IL-8ab | |||||||
| t − 2 | ↑ | ↑ | ↑ | ||||
| t − 1 | ↑ | ↑ | ↑ | ↓ | |||
| t0 | ↓ | ↑ | ↑ | ↑ | ↓ | ||
| t+1 | ↓ | ↑ | ↑ | ↑ | |||
| t+2 | ↓ | ↑ | ↑ | ↑ | ↓ | ↑ | |
| RANTESab | |||||||
| t − 2 | ↑ | ↑ | |||||
| t − 1 | ↑ | ↑ | ↑ | ||||
| t0 | ↑ | ↑ | ↓ | ↑ | |||
| t+1 | ↑ | ↑ | ↓ | ↑ | ↑ | ||
| t+2 | ↑ | ↑ | ↓ | ↑ | ↑ | ||
| IL-1Rab | |||||||
| t − 2 | ↓ | ||||||
| t − 1 | ↑ | ↓ | |||||
| t0 | ↓ | ||||||
| t+1 | ↓ | ↑ | ↑ | ||||
| t+2 | ↓ | ↑ | |||||
| SLPIab | |||||||
| t − 2 | ↑ | ↑ | ↓ | ↑ | |||
| t − 1 | ↓ | ↑ | ↑ | ↓ | ↑ | ||
| t0 | ↓ | ↑ | ↑ | ↓ | ↑ | ||
| t+1 | ↓ | ↑ | ↑ | ↓ | ↑ | ||
| t+2 | ↓ | ↑ | ↑ | ↓ | ↑ | ||
| BD-2ab | |||||||
| t − 2 | ↓ | ↓ | |||||
| t − 1 | ↑ | ↓ | ↓ | ↓ | |||
| t0 | ↓ | ↓ | |||||
| t+1 | ↓ | ↓ | |||||
| t+2 | ↓ | ||||||
↑ Indicates statistically significant increase and ↓ indicates statistically significant decrease in levels of these mediators (p < .05).
Multivariable model adjusting for HIV status, contraceptive use, current pregnancy, current breastfeeding, site, and age.
Based on Box–Cox transformation.
Reference groups are, in order left to right, remaining HIV negative; not using hormonal contraception at the visit, not pregnant at the visit, not breastfeeding at the visit, Uganda site, age ≥25 years.
Number of maximum visits used among all visits.
Breastfeeding women had lower SLPI at all visits and higher RANTES and lower BD-2 levels at the t + 1 and t + 2 visits compared to nonbreastfeeding women. There were also important differences by site; Zimbabwean women had higher IL-8, RANTES, and SLPI than Ugandan women at all visits. They also had lower IL-1RA and BD-2 at some visits. Younger women (18–24 years) had lower IL-8 at t − 1 and t0 than older women. When we adjusted additionally for STIs/RTIs (chlamydia, gonorrhea, trichomonas, HSV-2, and BV infection), there was little change to these results.
Discussion
We measured 10 biomarkers of inflammation and immunity in 943 HIV seroconverters and HIV-negative women from Zimbabwe and Uganda at five visits, including two before and three after HIV seroconversion and analogous visits by the HIV-negative controls.
Among the HIV-negative women we found that while some biomarkers changed modestly over time (IL-6, IL-8, VEGF, IL-1RA, and BD-2), most biomarkers remained relatively stable across the five study visits (Table 2). In contrast, all 10 markers changed significantly across the five visits among HIV seroconverters (test of trend p < .01). Most mediators, including IL-1β, IL-6, IL-8, MIP-3α, VEGF, IL-1RA, SLPI, and BD-2, decreased significantly starting at (t0) or before HIV seroconversion (t − 1), while several others, including RANTES, ICAM-1, and the 1RA:IL-1β, increased significantly across time—also starting at the seroconversion visit (Table 2). In addition, levels of most biomarkers were lower at analogous visits among HIV seroconverters than among HIV-negative women except for RANTES, ICAM, and the 1RA:IL-1β ratio, which were higher postseroconversion compared with values for women remaining HIV negative. RANTES was also higher and SLPI lower in HIV seroconverters compared to HIV-negative controls at the t − 1 visit (Tables 2 and 4). Levels of biomarkers at the t − 2 visit did not differ between participants remaining HIV negative and those that later seroconverted suggesting that immune mediator changes underlying increased HIV susceptibility occur in temporal proximity to primary HIV infection.
Decreased levels of innate immunity mediators in those who became HIV positive may be explained with the overall immunosuppressive syndrome following HIV infection especially in the absence of antiretroviral therapy. The t + 1 time interval where we saw the most suppressed cytokine levels corresponds chronologically to the early chronic phase of infection. During this phase a significant depletion of mucosal CD4+ T cells has already occurred, the initial immune activation and cytokine storm that distinguish the acute phase of infection have already passed, and the immunosuppressive effects of immune-cell derived apoptotic bodies may dominate the cervical mucosal environment.24
We previously found that elevated RANTES was associated with increased risk of subsequent HIV acquisition.18,25 In addition, our group and others have found that higher RANTES both before and after HIV infection was associated with increased genital shedding of HIV.20,26 It is plausible that RANTES might be associated with HIV acquisition risk as it is both a chemoattractant for HIV target cells and can promote inflammatory pathways associated with HIV replication.27 In addition, it is consistent that RANTES levels were increased at all HIV-positive visits among seroconverters as RANTES is one of the major viral stress response genes upregulated upon HIV infection as demonstrated by in vitro studies.28
We also found that women who seroconverted (compared HIV-negative women) had significantly lower SLPI starting at the visit before seroconversion (t − 1) and continuing throughout postseroconversion after controlling for other variables. In addition, HIV seroconverters had lower IL-8 and IL-1RA postseroconversion than women remaining HIV negative. Our finding of lower SLPI among HIV seroconverters just before HIV acquisition agrees with prior research suggesting that SLPI is an antiviral protein protective against HIV acquisition.29 Nevertheless, other studies have reported no association between SLPI levels and HIV acquisition.30,31 The finding that lower SLPI also continues postseroconversion compared to women remaining HIV negative has not been shown previously.
DMPA users had higher levels of RANTES at all five visits and lower levels of BD-2 from the t − 2 through t0 visits than the NH group. Increased RANTES among DMPA users has also been reported in a cross-sectional analysis by Deese25 and a cohort study by Francis.32 These epidemiologic data are further supported by two in vitro studies that reported upregulation of RANTES by DMPA in genital epithelial cells.33,34 Our finding of decreased levels of the antiviral peptide BD-2 at some visits has not been reported by other smaller epidemiologic studies.32,35 While our finding of no association between SLPI and DMPA use agrees with previous epidemiologic research,25,32,35,36 we did not find the increases in other pro-inflammatory markers such as IL-6, IL-8, and IL-1β among DMPA users reported in some studies25,32,37 but not in others.15,38–41 The reasons for these differences are unclear but may be due to differences in study design, study population, or specimen collection timing and methods. Nevertheless, the increased RANTES and decreased BD-2 results suggest that DMPA may create an immune environment conducive to HIV target cell recruitment and inhibitory for antiviral activity.31
Conversely, we found that COC users experienced higher IL-8 and SLPI at all five visits and higher IL-1RA but lower BD-2 at some (t − 2, t − 1) visits than the NH group. Similarly, pregnant women had higher IL-8 and SLPI at all visits and higher IL-1RA at some visits. However, in contrast to COC users they had lower RANTES at some visits and no differences in BD-2 levels (compared to nonpregnant women). The increase of both pro- and anti-inflammatory immune biomarkers in both COC users and pregnant women is consistent with an established condition of inflammation. Both pro- (e.g., cytokines and chemokines) and anti-inflammatory mediators (e.g., SLPI and IL-1RA) are upregulated by inflammatory signaling pathways (e.g., NF-kB) in an attempt to render protection from infection. The fact that IL-8, a downstream marker of inflammation associated with inflammatory cell influx and activation, is increased despite the higher levels of IL-1RA suggests that the anti-inflammatory response, although present, was insufficient to counterbalance the stronger pro-inflammatory responses.
We identified important differences in immune biomarkers between Zimbabwean and Ugandan women: Zimbabwean women had higher levels of IL-8, RANTES, and SLPI than Ugandan women at all visits and lower levels of IL-1RA and BD-2 at some pre- and postseroconversion visits. Differences in female genital tract immune biomarkers by location have been recorded by others, for example, among Kenyan and Zimbabwean women compared to U.S. women,42,43 and since standardized collection procedures were used and all biomarkers were assessed in one central laboratory, we have no reasons to believe that the differences are an artifact of laboratory procedures. However, it is important to emphasize that the observed geographic differences may not be necessarily attributable to ethnicity and genetic background. They may be due to differences in STI/HIV prevalence and pathogen subtypes or to behavioral differences between Zimbabwean and Ugandan women. For example, a recent study showed that both vaginal douching and vaginal drying practices influence cervicovaginal immunity, including some of the biomarkers measured in this study (RANTES, IL-8, and SLPI) in both women at risk of HIV and among HIV-positive women.44,45 Different HIV subtypes prevalent in these two countries may also contribute to the immune differences we observed and should be further investigated.46
Our study has multiple strengths. First, we followed large number of women longitudinally over 5 visits (3,700 visits in total) with large numbers of HIV seroconverters included both before and after seroconversion. This is a unique dataset that allows comparisons in the same women before and after seroconversion. Second, women were roughly evenly divided between contraceptive groups with large numbers of DMPA, COC, and NH users allowing us to study contraceptive effects with some precision. The study provided women with their contraceptive method so we have confidence in the measurements of contraceptive exposure, particularly for DMPA which was administered on site. In addition, data from multiple sites ensured a more representative sample of East and Southern African women. Finally, all biomarkers were measured at the same accredited laboratory with methods previously validated for technical accuracy and clinical content.19,20,47,48
Our study also had some important limitations. First, the study was observational, and biases may have been introduced by women's original contraceptive choices. However, this is an issue with all published in vivo studies of the effects of contraception on immunological biomarkers in the female genital tract (FGT). Second, although our analyses were adjusted for a composite STI variable through matching, they were not adjusted by individual STI, and thus, matching on STI status was imperfect. A separate analysis and article are underway to address the effect of each individual STI on the immunological biomarkers. Third, our study did not measure hormone levels and, thus, was not able to correlate cervical immunity with hormone levels nor assess the accuracy of self-reported OC use. However, the large sample size and longitudinal character with random variation of menstrual cycle within the non-HC group help minimize confounding by menstrual cycle phase and endogenous hormones. Paired serum samples were obtained and are currently being analyzed. Subsequent publications will compare systemic and cervical immunity and will correlate these measures with hormonal levels. Finally, our study did not use biological markers of semen exposure, and semen is known to influence FGT immunity in vitro.49,50 We believe that this weakness does not significantly detract from the merits of our study, given that none of the biological markers currently available is able to precisely measure the timing of sexual intercourse and semen exposure. Moreover, sexual intercourse may have an impact on FGT immunity even when condoms are used due to sexual trauma. The large sample size should help to minimize the effect of confounders randomly across the long follow-up period of up to 300 weeks.
In summary, we found that markers of immunological risk (increased RANTES and decreased SLPI) occurred both before and after HIV infection in a large cohort of Zimbabwean and Ugandan women. Changes preceding HIV seroconversion appear to occur close to the time of infection. The longitudinal patterns of cervical immunity differed between HIV seroconverters, assessed both before and after HIV acquisition, and nonseroconverters, and DMPA, COC use, and pregnancy differentially affected the cervical immunoinflammatory mediators associated with HIV risk.
Acknowledgments
This study was funded with U.S. federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health through an Interagency Agreement with the United States Agency for International Development (USAID) (GHO-A-00-09-00016-00) and from funds from USAID provided to CONRAD (GPO-A-00-08-00005-00). The study was also funded by NIH/NICHD 5R01HD077888.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. The content of this publication does not necessarily reflect their views or policies nor does mention of trade names, commercial products, or organizations imply endorsement by FHI 360, Brigham's and Women's Hospital, CONRAD, Case Western Reserve University, or the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Morrison CS, Chen PL, Kwok C, et al. : Hormonal contraception and HIV acquisition: Reanalysis using marginal structural modeling. AIDS 2010;24:1778–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison CS, Turner AN, Jones LB: Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best Pract Res Clin Obstet Gynaecol 2009;23:263–284 [DOI] [PubMed] [Google Scholar]
- 3.Mugo NR, Heffron R, Donnell D, et al. : Increased risk of HIV-1 transmission in pregnancy: A prospective study among African HIV-1-serodiscordant couples. AIDS 2011;25:1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RH, Li X, Kigozi G, et al. : Increased risk of incident HIV during pregnancy in Rakai, Uganda: A prospective study. Lancet 2005;366:1182–1188 [DOI] [PubMed] [Google Scholar]
- 5.Heffron R, Donnell D, Rees H, et al. : Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. Lancet Infect Dis 2012;12:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Nyange PM, Richardson BA, et al. : Hormonal contraception and risk of sexually transmitted disease acquisition: Results from a prospective study. Am J Obstet Gynecol 2001;185:380–385 [DOI] [PubMed] [Google Scholar]
- 7.Lancaster KE, Kwok C, Rinaldi A, et al. : Incident pregnancy and pregnancy outcomes among HIV-infected women in Uganda and Zimbabwe. Int J Gynaecol Obstet 2015;131:255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polis CB, Curtis KM, Hannaford, et al. : An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS 2016;30:2665–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myer L, Denny L, Wright TC, Kuhn L: Prospective study of hormonal contraception and women's risk of HIV infection in South Africa. Int J Epidemiol 2007;36:166–174 [DOI] [PubMed] [Google Scholar]
- 10.Reid SE, Dai JY, Wang J, et al. : Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: Relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr 2010;53:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison CS, Richardson BA, Mmiro F, et al. : Hormonal contraception and the risk of HIV acquisition. AIDS 2007;21:85–95 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization: Hormonal contraceptive eligibility for women at high risk of HIV. WHO, Geneva, 2017. Available at http://apps.who.int/iris/bitstream/10665/254662/1/WHO-RHR-17.04-eng.pdf?ua=1 Accessed August20, 2018 [Google Scholar]
- 13.Kyongo JK, Jespers V, Goovaerts O, et al. : Searching for lower female genital tract soluble and cellular biomarkers: Defining levels and predictors in a cohort of healthy caucasian women. PLoS One 2012;7:e43951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Harthi L, Kovacs A, Coombs RW, et al. : A menstrual cycle pattern for cytokine levels exists in HIV-positive women: Implication for HIV vaginal and plasma shedding. AIDS 2001;15:1535–1543 [DOI] [PubMed] [Google Scholar]
- 15.Al-Harthi L, Wright DJ, Anderson D, et al. : The impact of the ovulatory cycle on cytokine production: Evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res 2000;20:719–724 [DOI] [PubMed] [Google Scholar]
- 16.Wira CR, Fahey JV: A new strategy to understand how HIV infects women: Identification of a window of vulnerability during the menstrual cycle. AIDS 2008;22:1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gargiulo AR, Fichorova RN, Politch JA, Hill JA, Anderson DJ: Detection of implantation-related cytokines in cervicovaginal secretions and peripheral blood of fertile women during ovulatory menstrual cycles. Fertil Steril 2004;82 Suppl 3:1226–1234 [DOI] [PubMed] [Google Scholar]
- 18.Morrison C, Fichorova RN, Mauck C, et al. : Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014;66:109–117 [DOI] [PubMed] [Google Scholar]
- 19.Fichorova RN, Chen PL, Morrison CS, et al. : The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio 2015;6:e00221-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauck C, Chen PL, Morrison CS, et al. : Biomarkers of cervical inflammation and immunity associated with cervical shedding of HIV-1. AIDS Res Hum Retroviruses 2016;32:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Pol B, Kwok C, Pierre-Louis B, et al. : Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis 2008;197:548–554 [DOI] [PubMed] [Google Scholar]
- 22.Nowak RG, Gravitt PE, Morrison CS, et al. : Increases in human papillomavirus detection during early HIV infection among women in Zimbabwe. J Infect Dis 2011;203:1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleveland WS, Grosse E, Shyu W, M: Local regression models. In: Statistical Models in S (Chambers JM, Hastie TJ,eds.) Wadsworth, Pacific Grove, CA, 1992, pp. 309–376 [Google Scholar]
- 24.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF: The immune response during acute HIV-1 infection: Clues for vaccine development. Nat Rev Immunol 2010;10:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deese J, Masson L, Miller W, et al., eds.: Genital cytokine elevation is associated with HIV acquisition among African women. HIVR4P, Chicago, IL: 2016 [Google Scholar]
- 26.Roberts L, Passmore JA, Mlisana K, et al. : Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J Infect Dis 2012;205:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haase AT: Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010;464:217–223 [DOI] [PubMed] [Google Scholar]
- 28.Cota M, Kleinschmidt A, Ceccherini-Silberstein F, et al. : Upregulated expression of interleukin-8, RANTES and chemokine receptors in human astrocytic cells infected with HIV-1. J Neurovirol 2000;6:75–83 [DOI] [PubMed] [Google Scholar]
- 29.Hocini H, Becquart P, Bouhlal H, Adle-Biassette H, Kazatchkine MD, Bélec L: Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin Diagn Lab Immunol 2000;7:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dezzutti CS, Richardson BA, Marrazzo JM, et al. : Mucosal Escherichia coli bactericidal activity and immune mediators are associated with HIV-1 seroconversion in women participating in the HPTN 035 trial. J Infect Dis 2012;206:1931–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deese J, Masson L, Miller W, et al. : Injectable progestin-only contraception is associated with increased levels of pro-inflammatory cytokines in the female genital tract. Am J Reprod Immunol 2015;74:357–367 [DOI] [PubMed] [Google Scholar]
- 32.Francis SC, Hou Y, Baisley K, et al. : Immune activation in the female genital tract: Expression profiles of soluble proteins in women at high risk for HIV infection. PLoS One 2016;11:e0143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira VH, Dizzell S, Nazli A, et al. : Medroxyprogesterone acetate regulates HIV-1 uptake and transcytosis but not replication in primary genital epithelial cells, resulting in enhanced T-cell infection. J Infect Dis 2015;211:1745–1756 [DOI] [PubMed] [Google Scholar]
- 34.Irvin SC, Herold BC: Molecular mechanisms linking high dose medroxyprogesterone with HIV-1 risk. PLoS One 2015;10:e0121135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guthrie BL, Introini A, Roxby AC, et al. : Depot medroxyprogesterone acetate (DMPA) use is associated with elevated innate immune effector molecules in cervicovaginal secretions of HIV-1-uninfected women. J Acquir Immune Defic Syndr 2015;69:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roxby AC, Fredricks DN, Odem-Davis K, et al. : Changes in vaginal microbiota and immune mediators in HIV-1-seronegative kenyan women initiating depot medroxyprogesterone acetate. J Acquir Immune Defic Syndr 2016;71:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quispe Calla NE, Vicetti Miguel RD, Boyaka PN, et al. : Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol 2016;9:1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huijbregts RP, Michel KG, Hel Z: Effect of progestins on immunity: Medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception 2014;90:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z: Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J Acquir Immune Defic Syndr 2015;68:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hapgood JP, Ray RM, Govender Y, Avenant C, Tomasicchio M: Differential glucocorticoid receptor-mediated effects on immunomodulatory gene expression by progestin contraceptives: Implications for HIV-1 pathogenesis. Am J Reprod Immunol 2014;71:505–512 [DOI] [PubMed] [Google Scholar]
- 41.Goode D, Aravantinou M, Jarl S, et al. : Sex hormones selectively impact the endocervical mucosal microenvironment: Implications for HIV transmission. PLoS One 2014;9:e97767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen CR, Moscicki AB, Scott ME, et al. : Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS 2010;24:2069–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Achilles S, Mhlanga F, Dezzutti C, et al. : Differences in genital tract immune cell populations and innate cervicovaginal fluid anti-HIV activity among women from Zimbabwe and the United States. HIV Research for Prevention, Chicago, IL, 2016 [Google Scholar]
- 44.Alcaide ML, Rodriguez VJ, Brown MR, et al. : High levels of inflammatory cytokines in the reproductive tract of women with BV and engaging in intravaginal douching: A cross-sectional study of participants in the women interagency HIV study. AIDS Res Hum Retroviruses 2017;33:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birse KD, Romas LM, Guthrie BL, et al. : Genital injury signatures and microbiome alterations associated with depot medroxyprogesterone acetate usage and intravaginal drying practices. J Infect Dis 2017;215:590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison CS, Demers K, Kwok C, et al. : Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS 2010;24:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fichorova RN, Richardson-Harman N, Alfano M, et al. : Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: A multicenter study. Anal Chem 2008;80:4741–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison CS, Chen PL, Kwok C, et al. : Hormonal contraception and the risk of HIV acquisition: An individual participant data meta-analysis. PLoS Med 2015;12:e1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doncel GF, Anderson S, Zalenskaya I: Role of semen in modulating the female genital tract microenvironment–implications for HIV transmission. Am J Reprod Immunol 2014;71:564–574 [DOI] [PubMed] [Google Scholar]
- 50.Joseph T, Zalenskaya IA, Sawyer LC, Chandra N, Doncel GF: Seminal plasma induces prostaglandin-endoperoxide synthase (PTGS) 2 expression in immortalized human vaginal cells: Involvement of semen prostaglandin E2 in PTGS2 upregulation. Biol Reprod 2013;88:13. [DOI] [PubMed] [Google Scholar]