Abstract
Background
Anorexia of ageing may be a precursor to various geriatric syndromes. We elucidated whether anorexia of ageing had a significant impact on incident disability and investigated whether anorexia of ageing had a direct association with future disability or an indirect association with disability via frailty.
Methods
This study employed an observational, longitudinal, cohort design in a community setting. Participants were 4393 older adults (75.9 ± 4.3 years). Anorexia of ageing was assessed by a simplified nutritional appetite questionnaire. Frailty was operationalized as slowness, weakness, exhaustion, low physical activity, and weight loss. Participants who had none of these characteristics were considered robust, those with one or two characteristics were considered pre‐frail, and those with three or more characteristics were considered frail. We examined sociodemographic variables (age, sex, and education), medical history (medication and chronic disease history), lifestyle factors (smoking and drinking habits and living arrangement), body mass index, blood nutrition data, depressive symptoms, physical functioning, and cognitive functioning.
Results
The prevalence of anorexia of ageing was 10.7% (n = 468). The proportion of physical frailty, pre‐frailty, and robustness were 8.4, 52.0, and 39.6%, respectively, in the without anorexia of ageing group, and 20.3, 57.7, and 22.0%, respectively, in the anorexia of ageing group (P < 0.001). During a 2‐year follow‐up, the prevalence proportion of disability was 5.6% in the without anorexia of ageing group and 10.7% in the anorexia of ageing group (P < 0.001). Adjusted for covariates (except for frailty status), the participants with anorexia of ageing had an independently associated higher risk of incident disability compared with those without anorexia of ageing (hazard ratio: 1.43, 95% confidence interval: 1.04–1.95, P = 0.03). However, adjusted for covariates (including frailty status), anorexia of ageing was not significantly associated with incident disability (P = 0.09). Structural equation models revealed that anorexia of ageing had no direct effect on disability; however, anorexia of ageing was associated with frailty.
Conclusions
Older adults with anorexia of ageing had a higher proportion of frailty and a higher prevalence proportion of disability compared with those without anorexia of ageing. Although anorexia of ageing may not have a direct effect on incident disability, the structural equation model suggests an indirect relationship between anorexia of ageing and incident disability via frailty status.
Keywords: Anorexia of ageing, Appetite loss, Disability, Older adults
Introduction
Appetite refers to the natural drive to ingest food, which decreases with ageing. This phenomenon is termed ‘anorexia of ageing’.1 Anorexia of ageing is caused by decreased chemosensory functions, impaired psychological functions, environmental changes, and decreased secretions of the hormones that regulate appetite.1, 2 Anorexia of ageing independently predicts morbidity and mortality among older adults in various clinical settings3, 4 and is associated with cachexia, sarcopenia, poor endurance, and decreased mobility.5
Anorexia of ageing may be a precursor to various geriatric syndromes. Fried proposed a frailty cycle, where a decrease in dietary intake is coupled with a decrease in physical exercise, which leads to a decline in muscle mass, thus making older adults more vulnerable and likely to develop secondary complications (e.g. sarcopenia, frailty, comorbidities, or disability).6 Consequently, anorexia of ageing may lead to frailty and subsequently cause disability, which is consistent with a past study that indicated that anorexia of ageing was associated with frailty among older adults.7
Although it is thought that anorexia of ageing has an indirect effect on disability via the frailty cycle, the association between anorexia of ageing, frailty, and disability remains unclear. Clarification of this association would contribute to the development of interventions for adults with anorexia of ageing, and, in turn, the prevention of frailty and disability. Therefore, we sought to elucidate whether anorexia of ageing had a significant impact on disability using prospective longitudinal data and investigated whether anorexia of ageing had a direct association with future disability or an indirect association with disability via frailty.
Methods
Participants
Potential participants were 5257 people aged ≥70 years from a part of the National Center for Geriatrics and Gerontology—Study of Geriatric Syndromes database.8 After 864 potential participants were excluded, 4393 participants (mean age = 75.9 ± 4.3 years; men = 47.4%, women = 52.7%) were analysed. Exclusion criteria comprised the following: (i) requires support or care [certified by the Japanese public long‐term care insurance (LTCI) system; n = 52], including unconfirmed cases (n = 28); (ii) disabled in basic activities of daily living (n = 5); (iii) having a severe neurological disease (e.g. dementia, cerebrovascular disease, and Parkinson's disease; n = 388); (iv) general cognitive impairment [a Mini Mental State Examination (MMSE) score < 21; n = 163], including unconfirmed cases (n = 16); and (v) missing data [frailty criteria, n = 118; simplified nutritional appetite questionnaire, n = 23; educational level, n = 1; medication, n = 26; blood data, n = 28; body mass index (BMI), n = 1; living arrangement, n = 4; chronic disease, n = 7; Geriatric Depression Scale (GDS), n = 25; Short Physical Performance Battery (SPPB), n = 15; and incident disability, n = 8].
Operationalization of frailty
We operationalized frailty according to Fried's components: slowness, weakness, exhaustion, low physical activity, and weight loss.6 Participants showing none of these components were classified as non‐frail, those showing one or two components were classified as pre‐frail, and those showing three or more components were classified as frail. In this study, both pre‐frail and frail participants were defined as the frailty group.
Slowness was defined using a walking speed cut‐off (<1.0 m/s).9 Participants walked on a flat and straight surface at a comfortable walking speed. Walking time was measured over a 2.4‐m distance in seconds using a stopwatch, and participants' walking speed (m/s) was calculated. Weakness was defined according to a sex‐specific maximum grip strength cut‐off (<26 kg for men and <18 kg for women).10 Grip strength was measured using a Smedley‐type handheld dynamometer (Takei Ltd., Niigata, Japan). Exhaustion was considered present if the participant answered yes to the following question (drawn from the Kihon Checklist11): ‘In the last two weeks, have you felt tired without a reason?’ We evaluated physical activity using the following two questions: (i) ‘Do you engage in moderate levels of physical exercise or sports aimed at health?’ (ii) ‘Do you engage in low levels of physical exercise aimed at health?’ Participants who answered no to both questions were considered as engaging in low physical activity.9 Weight loss was examined with the following question, ‘Have you lost 2 kg or more in the past 6 months?’11 Those who answered yes were defined as having weight loss.
Incident disability
Participants were followed monthly for incident certification of need of care according to the LTCI system during the 2 years after the baseline assessment. Japan implemented a mandatory social LTCI system on 1 April 2000.12, 13 Every Japanese citizen aged 65 years and older is eligible for benefits (institutional and community‐based services, but not cash) in cases of physical and/or mental disability. The computer‐aided standardized needs‐assessment system used by the mandatory social LTCI system categorizes people into seven levels of need.13 To determine an individual's level of nursing care need, a trained local government official visits the individual's home and administers a questionnaire on current physical and mental status (73 items across seven dimensions: paralysis and limitation of joint movement, movement and balance, complex movement, conditions requiring special assistance, activities of daily living/instrumental activities of daily living, communication and cognition, and behavioural problems) and use of medical procedures (12 items). The results of this questionnaire are then entered into the computer to calculate the applicant's standardized scores across the seven dimensions and the estimated time for nine categories of care: grooming/bathing, eating, toileting, transferring, eating, assistance with instrumental activities of daily living, behavioural problems, rehabilitation, and medical services; then, a care needs level based on the total estimated time taken for care is assigned. Next, the Nursing Care Needs Certification Board, which comprises physicians, nurses, and other experts in health and social services, reviews and confirms the care needs level.13 We defined onset of disability as the point when a participant was certified as needing care according to LTCI classification.
Measures
Anorexia of aging
All participants completed the Japanese version of the simplified nutritional appetite questionnaire (SNAQ). Testing indicates that the SNAQ is sufficiently reliable and valid for examining appetite among community‐dwelling older adults in Japan.14 The original SNAQ is a four‐item single‐domain questionnaire; responses were made using a 5‐point, verbally labelled Likert‐type scale: (1) My appetite is (A. very poor, B. poor, C. average, D. good, E. very good); (2) When I eat, (A. I feel full after eating only a few mouthfuls, B. I feel full after eating about a third of a meal, C. I feel full after eating over half a meal, D. I feel full after eating most of the meal, E. I hardly ever feel full); (3) I feel hungry (A. rarely, B. occasionally, C. some of the time, D. most of the time, E. all the time); and (4) Food tastes (A. very bad, B. bad, C. average, D. good, E. very good). The total SNAQ score is the sum of each item score; lower scores indicate more appetite deterioration. Possible scores range from 4 (worst) to 20 (best); a score of 13 and lower was considered to indicate anorexia of ageing.15
Sociodemographic variables and covariates
Using face‐to‐face interviews, we examined participants' sociodemographic characteristics (i.e. age, sex, and educational level), medical history [i.e. number of medications and chronic disease history (e.g. hypertension, diabetes, and hyperlipidaemia)], living arrangement (living alone or not), current smoking habit (current smoker or not), and current alcohol use (current drinker or not). Examined covariates were as follows: BMI, depressive symptoms, physical functioning, cognitive functioning, and blood nutrition data (total protein and albumin). Depressive symptoms were measured at baseline using the 15‐item GDS,16 which contains 15 yes/no question items and provides a score between 0 and 15.17 Higher scores indicate having more depressive symptoms. The SPPB, which incorporates static balance, walking speed, and time for repeated chair rise, was used as an objective assessment of physical functioning. For static standing balance, participants were asked to place their feet sequentially in side‐by‐side, semi‐tandem, and tandem positions and maintain each position for 10 s. For walking speed, participants were asked to walk at their usual pace through a 6.4‐m distance with 2‐m sections of acceleration and deceleration. The time of the faster of the two trials was used for analyses. For the repeated chair rise, participants were required to fold their arms across their chest and stand up from a seated position five times consecutively as quickly as possible. A grade of 0 was given when the participant was unable to perform the task. A summary SPPB score was computed by summing the grades of balance, walking, and chair rise components (range 0–12). Higher scores indicate more ability to maintain physical functioning.18 Cognitive functioning was assessed using the MMSE, which provides scores between 0 and 30. Higher scores indicate more ability to maintain cognitive functioning.19 Venous blood was collected by well‐trained nurses, and serum was prepared from blood samples within 24 h. To obtain serum, whole blood samples could coagulate at room temperature for 30 min and then centrifuged at room temperature for 5 min at 1690 g. Total protein and albumin were measured by trained staff using an automatic analyser (JCA‐BM6070. JEOL Ltd.) in a laboratory at Good Life Design Co., Ltd., Aichi‐ken, Japan.
Statistical analyses
Statistical analyses were performed using SPSS 20.0 in conjunction with AMOS 20.0 Graphics (IBM Japan Tokyo, Japan). Student's t‐tests and chi‐squared tests were used to compare the variables between the participants with/without anorexia of ageing. Cox proportional hazard model was used to examine the longitudinal association between anorexia of ageing and incident disability, with incident disability as the dependent variable. We made the two models to elucidate whether frail status affected the association between anorexia of ageing and disability. Therefore, Model 1 was adjusted for covariates (i.e. age, sex, education, BMI, polypharmacy, chronic disease history, blood data, living arrangement, drinking, and smoking habits, depressive symptoms, and decline in physical and cognitive functioning), except for frailty status, while Model 2 was adjusted for all covariates including frailty status. The hazard ratios (HRs) and 95% confidential intervals (CIs) were calculated. Albumin and total protein levels were treated as dependent variables and categorized into approximate quartiles (albumin, Q1: ≤4.2 g/dL vs. Q2–Q4: >4.2 g/dL; total protein, Q1: ≤7.0 g/dL vs. Q2–Q4: >7.0 g/dL). Polypharmacy, depression symptoms, decline in physical and cognitive functioning were defined using cut‐offs drawn from previous studies (polypharmacy, 5 or more medications;20 depression symptoms, 6 or higher score of GDS;16 decline in physical functioning, 9 or lower score of SPPB;21 and decline in cognitive functioning, 23 or lower score of MMSE19).
Additionally, structural equation modelling is an optimal statistical technique for testing these hypotheses as it can evaluate a priori models, identify mediators, and elucidate direct and indirect paths between variables. Two types of models were considered: first, a direct effects model had direct paths from anorexia of ageing and frailty on incident disability, and second, an indirect effects model had the effects of anorexia of ageing on incident disability be transmitted indirectly through frailty status. Model selection was determined by comparison of the following model fit indices: goodness‐of‐fit index (GFI), adjusted goodness‐of‐fit index (AGFI), standard root mean square residual (SRMR), the root mean square error of approximation (RMSEA), and Akaike's Information Criterion (AIC).22 According to conventional criteria, a ‘good fit’ would be indicated by GFI > 0.95, AGFI > 0.90, SRMR < 0.05, and RMSEA < 0.05; an ‘acceptable fit’ by GFI > 0.90, AGFI > 0.85, SRMR < 0.10, and RMSEA < 0.08.22 The AIC of a good fit model is smaller than comparison model.22
Results
Characteristics of the participants with/without anorexia of ageing
The prevalence of anorexia of ageing among our participants was 10.7% (n = 468). Table 1 presents participants' demographic characteristics and scale scores. Compared with the participants without anorexia of ageing, those with anorexia of ageing were significantly older, less likely to be male, less educated, had a lower BMI, had more prescribed medication, had a higher rate of living alone, more likely to smoke, and less likely to drink. Regarding blood exams, albumin values among the participants with anorexia of ageing were significantly lower than those without anorexia of ageing; however, there was no significant difference in the total protein value. Mean scores on the following variables were significantly worse among participants with anorexia of ageing in comparison with those without: GDS total score, SPPB total score, MMSE total score, and SNAQ score. The proportion of participants who had a history of chronic diseases did not significantly differ between groups.
Table 1.
Participants' characteristics
| Total | Without anorexia of ageing | With anorexia of ageing | P value | |
|---|---|---|---|---|
| N = 4393 | n = 3925 | n = 468 | ||
| Age, years ± SD | 75.8 ± 4.3 | 75.8 ± 4.2 | 76.6 ± 4.4 | <0.001 |
| Sex, number of men (%) | 2084 (47.4) | 1894 (48.3) | 190 (40.6) | 0.002 |
| Education, years ± SD | 12.0 ± 2.6 | 12.1 ± 2.6 | 11.4 ± 2.4 | <0.001 |
| BMI, kg/m2 ± SD | 22.9 ± 3.0 | 23 ± 3.0 | 22.4 ± 3.1 | <0.001 |
| Medication, number (%) | 3.3 ± 2.8 | 3.2 ± 2.7 | 4.2 ± 3.3 | <0.001 |
| Chronic disease | ||||
| Hypertension, number (%) | 2070 (47.1) | 1831 (46.6) | 239 (51.1) | 0.070 |
| Diabetes, number (%) | 548 (12.5) | 492 (12.5) | 56 (12.0) | 0.725 |
| Hyperlipidaemia, number (%) | 1780 (40.5) | 1587 (40.4) | 193 (41.2) | 0.737 |
| Blood exam | ||||
| Total protein, g/dL ± SD | 7.30 ± 0.53 | 7.30 ± 0.53 | 7.30 ± 0.53 | 0.961 |
| Albumin, g/dL ± SD | 4.23 ± 0.43 | 4.23 ± 0.43 | 4.19 ± 0.42 | 0.026 |
| Living arrangement, number of living alone (%) | 660 (15.0) | 567 (14.4) | 93 (19.9) | 0.002 |
| Daily habits | ||||
| Current drinker, number (%) | 1867 (42.5) | 1706 (43.5) | 161 (34.4) | <0.001 |
| Current smoker, number (%) | 283 (6.4) | 242 (6.2) | 41 (8.8) | 0.031 |
| GDS, score ± SD | 2.8 ± 2.7 | 2.7 ± 2.6 | 4.3 ± 3.3 | <0.001 |
| SPPB, score ± SD | 11.3 ± 1.3 | 11.4 ± 1.2 | 11.1 ± 1.5 | <0.001 |
| MMSE, score ± SD | 26.2 ± 2.3 | 26.2 ± 2.3 | 25.6 ± 2.4 | <0.001 |
| SNAQ, score ± SD | 15.3 ± 1.5 | 15.6 ± 1.2 | 12.6 ± 0.8 | <0.001 |
BMI, body mass index; GDS, Geriatric Depression Scale; MMSE, Mini Mental State Examination; SD, standard deviation; SNAQ, simplified nutritional appetite questionnaire; SPPB, Short Physical Performance Battery.
The proportion of frailty and disability between the participants with/without anorexia of ageing and the prevalence proportion of disability
Figure 1 shows the proportion of frailty and the prevalence proportion of disability between the participants with/without anorexia of ageing. The proportions of physical frailty, pre‐frailty, and robustness were 8.4, 52.0, and 39.6% in the without anorexia of ageing group, and 20.3, 57.7, and 22.0% in the anorexia of ageing group, respectively. The participants with anorexia of ageing had a higher proportion of frailty and pre‐frailty than did those without (P < 0.001).
Figure 1.
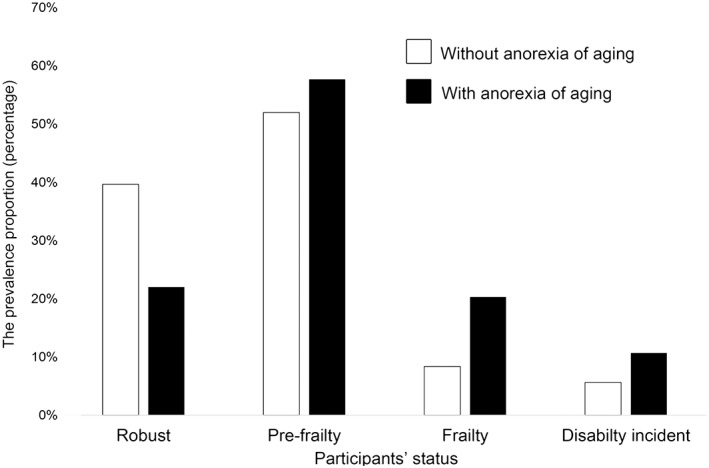
Prevalence of frailty and prevalence proportion of disability between older adults with/without anorexia of ageing. Bar graphs indicate the percentage of older adults with each frailty status (robust, pre‐frailty, or frailty) and the percentage of incident disability between those with/without anorexia of ageing.
At the 2‐year follow‐up assessment, 270 participants (6.1%) developed a disability. The prevalence proportion of disability in each group based on anorexia of ageing is also shown in Figure 1. The prevalence proportion of disability was 5.6% (n = 220) in the without anorexia of ageing group and 10.7% (n = 50) in the anorexia of ageing group. The participants with anorexia of ageing had a higher disability prevalence proportion than did those without (P < 0.001).
The association between anorexia of ageing, frailty, and disability using the Cox proportional hazard model
Results of the Cox proportional hazard models for the impact for association of anorexia on incident disability are shown in Table 2. In Model 1, the participants with anorexia of ageing had an independent association with a higher risk of incident disability compared with those without anorexia of ageing. In addition, being older, male, polypharmacy, having depression symptoms, and declines in physical and cognitive functioning were associated with a higher risk of disability. The other variables were not significantly associated with the risk of disability.
Table 2.
Association between anorexia of ageing and disability incident adjusted for covariates including physical frailty status
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Anorexia of ageing (vs. SNAQ score 14 or more) | 1.43 | (1.04–1.95) | 0.026 | 1.33 | (0.96–1.80) | 0.093 |
| Frailty status | ||||||
| Robust | Reference | |||||
| Pre‐frailty | 1.53 | (1.16–2.32) | 0.007 | |||
| Frailty | 2.44 | (2.00–4.59) | <0.001 | |||
| Age | 1.14 | (1.11–1.17) | <0.001 | 1.12 | (1.10–1.15) | <0.001 |
| Sex (vs. female) | 1.40 | (1.05–1.86) | 0.021 | 0.74 | (0.55–0.98) | 0.033 |
| Education | 1.02 | (0.97–1.07) | 0.427 | 1.03 | (0.98–1.08) | 0.267 |
| BMI | 1.01 | (0.97–1.05) | 0.721 | 1.01 | (0.97–1.05) | 0.643 |
| Polypharmacy (vs. 4 or less medication) | 1.43 | (1.10–1.85) | 0.008 | 1.36 | (1.05–1.76) | 0.021 |
| Hypertension | 1.05 | (0.81–1.37) | 0.693 | 1.07 | (0.82–1.38) | 0.645 |
| Diabetes | 1.37 | (0.99–1.91) | 0.060 | 1.28 | (0.91–1.77) | 0.154 |
| Hyperlipidaemia | 0.83 | (0.64–1.08) | 0.170 | 0.83 | (0.64–1.08) | 0.171 |
| Total protein (vs. 7.1 dL/g or more) | 1.16 | (0.90–1.51) | 0.256 | 1.10 | (0.84–1.43) | 0.487 |
| Albumin (vs. 4.3 dL/g or more) | 1.11 | (0.84–1.46) | 0.461 | 1.10 | (0.84–1.45) | 0.482 |
| Living alone (vs. living with someone) | 1.34 | (1.00–1.80) | 0.053 | 1.36 | (1.01–1.84) | 0.041 |
| Drinking status (vs. nondrinker) | 0.97 | (0.74–1.28) | 0.849 | 1.00 | (0.77–1.31) | 0.989 |
| Smoking status (vs. nonsmoker) | 1.09 | (0.64–1.86) | 0.760 | 1.03 | (0.60–1.76) | 0.923 |
| Depression symptoms (vs. GDS score 5 or less) | 1.57 | (1.19–2.09) | 0.002 | 1.34 | (1.00–1.77) | 0.054 |
|
Decline in physical function (vs. SPPB score 10 or more) |
1.59 | (1.19–2.14) | 0.002 | 1.36 | (1.03–1.86) | 0.032 |
|
Decline in cognitive function (vs. MMSE score 24 or more) |
1.45 | (1.09–1.92) | 0.011 | 1.37 | (1.02–1.81) | 0.037 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; GDS, Geriatric Depression Scale; MMSE, Mini Mental State Examination; SPPB, Short Physical Performance Battery.
Model 1: adjusted for age, sex, education, BMI, polypharmacy, chronic disease, blood data, living arrangement, drinking and smoking habits, depressive symptoms, and decline in physical and cognitive functioning.
Model 2: Model 1 plus adjusted for frailty status.
Model 2 (also see Table 2) showed that frailty was associated with a significantly higher risk of incident disability with reference to being robust; however, anorexia of ageing was not significantly associated with incident disability. The same variables as in Model 1 were associated with a higher risk of disability. However, contrary to Model 1, living alone was independently associated with disability in Model 2, and having depression symptoms was not significantly associated.
In addition, Cox proportional hazard model was made to examine the longitudinal association between anorexia of ageing and incident disability, with anorexia of ageing and each frailty factor as the dependent variables adjusted for all covariates (Table 3). Independent associations were identified between anorexia of ageing and incident disability adjusted for weight loss, exhaustion, physical inactivity, and weakness except for slowness.
Table 3.
Association between anorexia of ageing and disability incident adjusted for covariates including each frailty factor
| Adjusted for weight loss and other covariates | Adjusted for exhaustion and other covariates | Adjusted for physical inactivity and other covariates | Adjusted for weakness and other covariates | Adjusted for slowness and other covariates | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Anorexia of ageing | 1.41 (1.03–1.93) | 0.032 | 1.38 (1.00–1.89) | 0.047 | 1.43 (1.04–1.95) | 0.026 | 1.44 (1.05–1.97) | 0.023 | 1.35 (0.99–1.85) | 0.060 |
| Weight loss | 1.15 (0.84–1.57) | 0.379 | ||||||||
| Exhaustion | 1.39 (1.06–1.83) | 0.019 | ||||||||
| Physical inactivity | 1.08 (0.82–1.43) | 0.591 | ||||||||
| Weakness | 1.77 (1.34–2.33) | <0.001 | ||||||||
| Slowness | 1.99 (1.51–2.61) | <0.001 | ||||||||
All models were adjusted for age, sex, education, body mass index, polypharmacy, chronic disease, blood data, living arrangement, drinking and smoking habits, depressive symptoms, and decline in physical and cognitive functioning apart from each frailty factor. CI, confidence interval; HR, hazard ratio.
Structural equation models for the association between anorexia of ageing, frailty, and disability
The direct effect model indicated a poorly fit model (Table 4). In this model, frailty status was significantly associated with disability, while anorexia of ageing (SNAQ total score) was not significantly associated with disability (Figure 2).
Table 4.
Fit indices of direct/indirect effect model
| GFI | AGFI | SRMR | RMSEA | AIC | |
|---|---|---|---|---|---|
| Direct effect model | 0.972 | 0.944 | 0.368 | 0.084 | 473.25 |
| Indirect effect model | 0.989 | 0.979 | 0.065 | 0.049 | 188.15 |
AIC, Akaike's information criterion; AGFI, adjusted goodness‐of‐fit index; GFI, goodness‐of‐fit index; RMSEA, root mean square error of approximation; SRMR, standardized root mean square residual.
Figure 2.
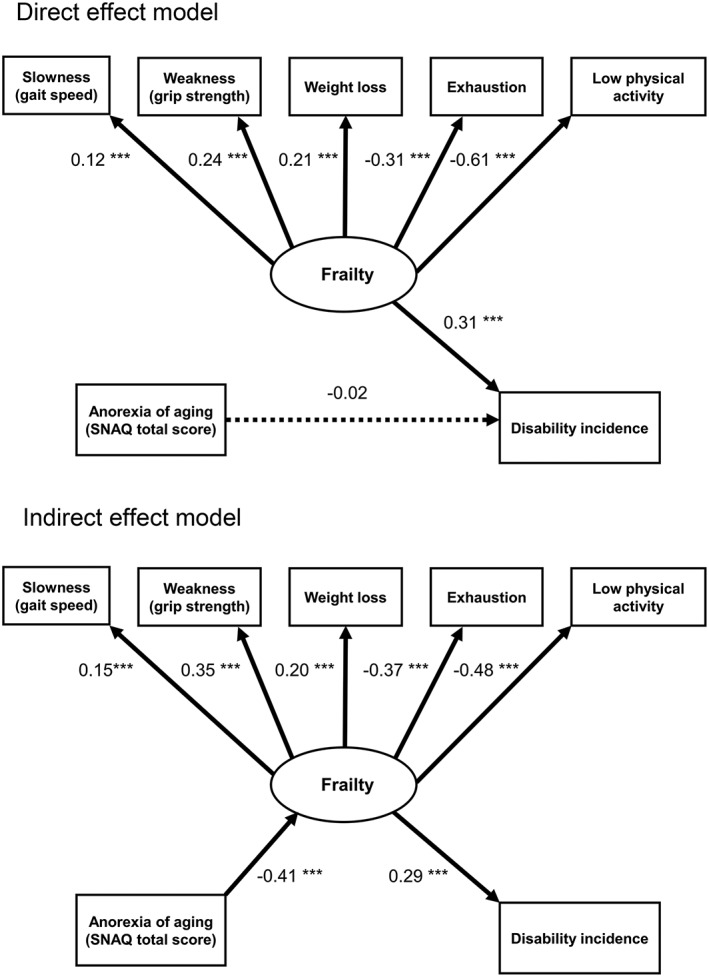
Structural equation modelling for direct/indirect effect model between anorexia of ageing, frailty status, and incident disability. Results of structural equation models of the correlations among anorexia of ageing, frailty status, and incident disability. The direct effects model and the indirect effects model are represented. The estimated standardized coefficients are shown. Latent variables (frailty) are represented with ovals and observed variables are represented with rectangles. The dashed line indicates an association that was not significant (P > 0.05). In the indirect effects model, the indirect effect of anorexia of ageing on incident disability was significant (β = −0.00818, 95% CI [−0.1025, −0.0647], P < 0.001). SNAQ, simplified nutritional appetite questionnaire. *** P < 0.01.
In contrast, the indirect effects model provided a better fit to the data compared with the direct effect model (Table 4). This model showed that anorexia of ageing was not directly associated with disability, but contributed significantly to frailty status, and frailty was also significantly associated with disability (Figure 2). Furthermore, the AIC of the indirect effect model was smaller than the direct one (Table 4). In the indirect effects model, the indirect effect of anorexia of ageing on incident disability was significant (Figure 2).
Discussion
The present study showed that older adults with anorexia of ageing had a higher proportion of frailty and a higher prevalence proportion of disability compared with those without anorexia of ageing. In addition, anorexia of ageing indirectly affected incident disability via frailty status using structural equation models.
The prevalence of senile anorexia in the present study was totally 10.6% among community dwelling older people. There is inconsistency with the previous studies. Donini and his colleagues23 showed that the prevalence of anorexia of ageing was globally 21.2% among Italian older adults, which was higher proportion compared with our results. However, their findings about the prevalence of anorexia included the participants who lived in the rehabilitation and acute wards/the nursing homes apart from free living. In the free living subjects alone, which was the same condition in the current study, the prevalence of anorexia was 11.3% (male) and 3.3% (female), respectively,23 which were the equivalent of our prevalence (10.7%). The other previous study among adults aged 60 and older living in the metropolitan area of Mexico City also showed higher prevalence of anorexia (30.1%) than that of the present study.24 However, their screening of anorexia diffed from ours (SNAQ) and they used a single question ‘Have you been eating less in the last 12 months than you were because of loss of appetite?’ This methodological difference may partly explain the difference in prevalence of anorexia in the two studies. In the future, there may be a need to generalize the definition of senile anorexia so as not to cause a difference.
The participants who had anorexia of ageing were frailer or more pre‐frail than those without, and the disability prevalence proportion was also higher among the participants with anorexia of ageing compared with those without. These results are partially aligned with previous studies.24, 25 A cross‐sectional study indicated an independent association between anorexia of ageing and disability among 1247 people aged 60 years and older.24 A 2‐year follow‐up longitudinal study showed that the presence of anorexia of ageing was associated with a significant increased risk of incident disability among 205 older adults.25 Our results supported these previous reports, and the sample size of the present study may support the evidence of association between anorexia of ageing and incident disability.
The current study indicated that anorexia of ageing had a significantly impact on incident disability adjusted for the covariates excluded frailty status. However, adjusted for the covariates included frailty status, the association between anorexia of ageing and incident disability was not significant. Landi and colleagues reported that the presence of anorexia of ageing led to a significantly higher risk of developing disability (HR: 2.25; 95% CI: 1.15–4.39); however, this association was not significant after adjusting for potential confounders such as the presence of Alzheimer's disease (HR: 1.87; 95% CI: 0.95–3.82).25 A potential explanation for these results is that anorexia of ageing is a predictor of incident disability; however, stronger confounders such as frailty status or Alzheimer's disease may weaken the relationship between anorexia of ageing and disability.
We conducted additional analysis using structural equation modelling to clarify whether anorexia of ageing affected incident disability directly or indirectly. Our results indicated that anorexia of ageing did not have a direct effect on disability; however, it had an indirect effect. Many older adults, even those with an acceptable nutritional status, have been shown to have poor protein intake. This can lead to frailty,26 which is related to poor nutritional quality.27, 28 The decrease in food intake associated with anorexia of ageing leads to a reduction in physical capacity and declines in muscle mass and strength as in sarcopenia,29 and older adults with anorexia of ageing can thus develop frailty syndrome. Furthermore, as suggested by Fried and colleagues,6 sarcopenia plays a vital aetiologic role in frailty, including the latent phase of frailty and explaining many frailty aspects. In fact, community‐dwelling older adults with anorexia of ageing had a significant association with sarcopenia compared with those without.30 According to these findings, anorexia of ageing may affect frailty or sarcopenia at a stage prior to frailty or a pre‐disability stage. In turn, anorexia of ageing may not have a direct pathway to incident disability; however, there may be an indirect pathway from anorexia of ageing to incident disability via sarcopenia or frailty. Our results reinforce this concept.
Our findings indicated the possibility that prevention of senile anorexia of ageing lead to prevention of frailty and incident disability. The study of skeletal muscle biopsy suggested that promoting physical activity is effective for prevention of frailty in the elderly.31 A randomized control trial study showed that patient‐centred strategy was effective in enhancing moderate intensity physical activity and in reducing frailty in older adults.32 In addition to promoting physical activity, the intervention on appetite or nutrition may be able to more effectively prevent frailty and incident disability among older people. In fact, Yamada and his colleagues suggested that physical activity plus nutrition intervention had a significant increase in skeletal muscle index and the serum DHEA‐S and IGF‐1 levels compared with physical activity alone or control group among Japanese older people.33 In future study, it is necessary to investigate whether or not physical activity plus approaching to anorexia of ageing has an effect on prevention on frailty and incident disability among older people.
A major strength of this longitudinal study was the application of monthly disability follow‐up in a large sample using the mandatory social LTCI in Japan, which can address the causality between predisposing factors and new onset of disability. Nevertheless, several limitations should be considered. First, a key limitation is the absence of random recruitment, which could result in underestimating the prevalence of older adults with anorexia of ageing because the participants were older adults who could obtain health check‐ups at their homes. Second, the 2‐year follow‐up might have been too short to sufficiently capture incident disability. Further, the disability end points were too small to investigate prospective associations between anorexia of ageing and incident disability. Third, some variables were self‐reported including anorexia of ageing (e.g. education history, polypharmacy, and chronic disease history), and we could not take the confounder that has been indicated to associate with both frailty and disability into account, such as heart failure.34, 35 These factors and others should be examined in diverse ways in future studies. Fourth, the definition of disability in the present study is in accordance with the Japanese Long‐Term Care System, in which the causes of physical, mental, or cognitive function were intermixed. Fourth, the definition of disability in the present study is in accordance with the Japanese Long‐Term Care System, in which the causes of physical, mental, or cognitive function were intermixed. For example, sarcopenia, which one of the geriatric syndromes that lead to disability, is related not only with thigh skeletal muscle mass, but also are significantly related to cognitive impairment.36 Fifth, we excluded those who were having a severe neurological disease (dementia, cerebrovascular disease, and Parkinson's disease); however, we could not exclude those who were having anorexia secondary to disease in the current study. Finally, the three variables we focused on (i.e. anorexia of ageing, frailty, and disability) comprise several factors, and these factors are interrelated with each other. Therefore, the analytical model may be too simple to describe the association between these variables.
Conflicts of interest
None declared.
Ethical considerations
All participants voluntarily provided a written informed consent before their inclusion in the study. The Ethics Committee of the National Center for Geriatrics and Gerontology approved the study protocol, which was conducted in accordance with the 1964 Declaration of Helsinki.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.37 This work was supported by the Japanese Ministry of Health, Labour and Welfare (Project for Optimizing Long‐Term Care) [grant number: B‐3]; the National Center for Geriatrics and Gerontology (Research Funding for Longevity Sciences) [grant numbers: 22‐16 and 26‐33]; and the Strategic Basic Research Programs (RISTEX Redesigning Communities for Aged Society), Japan Science and Technology Agency. MEXT‐Supported Program for the Strategic Research Foundation at Private Universities, 2015‐2019 from the Ministry of Education, Culture, Sports, Science and Technology (S1511017).
Tsutsumimoto, K. , Doi, T. , Makizako, H. , Hotta, R. , Nakakubo, S. , Makino, K. , Suzuki, T. , and Shimada, H. (2018) Aging‐related anorexia and its association with disability and frailty. Journal of Cachexia, Sarcopenia and Muscle, 9: 834–843. 10.1002/jcsm.12330.
References
- 1. Di Francesco V, Fantin F, Omizzolo F, Residori L, Bissoli L, Bosello O, et al. The anorexia of aging. Dig Dis 2007;25:129–137. [DOI] [PubMed] [Google Scholar]
- 2. Malafarina V, Uriz‐Otano F, Gil‐Guerrero L, Iniesta R. The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013;74:293–302. [DOI] [PubMed] [Google Scholar]
- 3. Landi F, Laviano A, Cruz‐Jentoft AJ. The anorexia of aging: is it a geriatric syndrome? J Am Med Dir Assoc 2010;11:153–156. [DOI] [PubMed] [Google Scholar]
- 4. Cartwright JC, Hickman S, Perrin N, Tilden V. Symptom experiences of residents dying in assisted living. J Am Med Dir Assoc 2006;7:219–223. [DOI] [PubMed] [Google Scholar]
- 5. Morley JE. Anorexia, sarcopenia, and aging. Nutrition 2001;17:660–663. [DOI] [PubMed] [Google Scholar]
- 6. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 7. Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, Makino K, et al. The association between anorexia of aging and physical frailty: results from the National Center For Geriatrics and Gerontology's study of geriatric syndromes. Maturitas 2017;97:32–37. [DOI] [PubMed] [Google Scholar]
- 8. Shimada H, Tsutsumimoto K, Lee S, Doi T, Makizako H, Lee S, et al. Driving continuity in cognitively impaired older drivers. Geriatr Gerontol Int 2015; 10.1111/ggi.12504. [DOI] [PubMed] [Google Scholar]
- 9. Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc 2013;14:518–524. [DOI] [PubMed] [Google Scholar]
- 10. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 11. Fukutomi E, Okumiya K, Wada T, Sakamoto R, Ishimoto Y, Kimura Y, et al. Relationships between each category of 25‐item frailty risk assessment (Kihon Checklist) and newly certified older adults under Long‐Term Care Insurance: a 24‐month follow‐up study in a rural community in Japan. Geriatr Gerontol Int 2014;15:864–871. [DOI] [PubMed] [Google Scholar]
- 12. Tamiya N, Noguchi H, Nishi A, Reich MR, Ikegami N, Hashimoto H, et al. Population ageing and wellbeing: lessons from Japan's long‐term care insurance policy. Lancet 2011;378:1183–1192. [DOI] [PubMed] [Google Scholar]
- 13. Tsutsui T, Muramatsu N. Care‐needs certification in the long‐term care insurance system of Japan. J Am Geriatr Soc 2005;53:522–527. [DOI] [PubMed] [Google Scholar]
- 14. Nakatsu N, Sawa R, Misu S, Ueda Y, Ono R. Reliability and validity of the Japanese version of the simplified nutritional appetite questionnaire in community‐dwelling older adults. Geriatr Gerontol Int 2015;15:1264–1269. [DOI] [PubMed] [Google Scholar]
- 15. Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community‐dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074–1081. [DOI] [PubMed] [Google Scholar]
- 16. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull 1988;24:709–711. [PubMed] [Google Scholar]
- 17. Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15‐item geriatric depression scale in functionally impaired, cognitively intact, community‐dwelling elderly primary care patients. J Am Geriatr Soc 2005;53:1570–1576. [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 19. Folstein MF, Robins LN, Helzer JE. The Mini‐Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 20. Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–995. [DOI] [PubMed] [Google Scholar]
- 21. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schermelleh‐Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness‐of‐fit measures,” MPR‐online. 2003;8:23–74. [Google Scholar]
- 23. Donini LM, Poggiogalle E, Piredda M, Pinto A, Barbagallo M, Cucinotta D, et al. Anorexia and eating patterns in the elderly. PLoS One 2013;8:e63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vazquez‐Valdez OE, Aguilar‐Navarro S, Avila‐Funes JA. Association between anorexia of aging and disability in older community‐dwelling Mexicans. J Am Geriatr Soc 2010;58:2044–2046. [DOI] [PubMed] [Google Scholar]
- 25. Landi F, Russo A, Liperoti R, Tosato M, Barillaro C, Pahor M, et al. Anorexia, physical function, and incident disability among the frail elderly population: results from the ilSIRENTE study. J Am Med Dir Assoc 2010;11:268–274. [DOI] [PubMed] [Google Scholar]
- 26. Jyvakorpi SK, Pitkala KH, Puranen TM, Bjorkman MP, Kautiainen H, Strandberg TE, et al. High proportions of older people with normal nutritional status have poor protein intake and low diet quality. Arch Gerontol Geriatr 2016;67:40–45. [DOI] [PubMed] [Google Scholar]
- 27. Bonnefoy M, Berrut G, Lesourd B, Ferry M, Gilbert T, Guerin O, et al. Frailty and nutrition: searching for evidence. J Nutr Health Aging 2015;19:250–257. [DOI] [PubMed] [Google Scholar]
- 28. Leon‐Munoz LM, Guallar‐Castillon P, Lopez‐Garcia E, Rodriguez‐Artalejo F. Mediterranean diet and risk of frailty in community‐dwelling older adults. J Am Med Dir Assoc 2014;15:899–903. [DOI] [PubMed] [Google Scholar]
- 29. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 30. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Barillaro C, et al. Association of anorexia with sarcopenia in a community‐dwelling elderly population: results from the ilSIRENTE study. Eur J Nutr 2013;52:1261–1268. [DOI] [PubMed] [Google Scholar]
- 31. St‐Jean‐Pelletier F, Pion CH, Leduc‐Gaudet JP, Sgarioto N, Zovile I, Barbat‐Artigas S, et al. The impact of ageing, physical activity, and pre‐frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle 2017;8:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Vries NM, Staal JB, van der Wees PJ, Adang EM, Akkermans R, Olde Rikkert MG, et al. Patient‐centred physical therapy is (cost‐) effective in increasing physical activity and reducing frailty in older adults with mobility problems: a randomized controlled trial with 6 months follow‐up. J Cachexia Sarcopenia Muscle 2016;7:422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail‐based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community‐dwelling Japanese older adults: the INE (Intervention by Nutrition and Exercise) Study. J Am Med Dir Assoc 2015;16:654–660. [DOI] [PubMed] [Google Scholar]
- 34. Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail 2017;4:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vidan MT, Blaya‐Novakova V, Sanchez E, Ortiz J, Serra‐Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non‐dependent elderly patients with heart failure. Eur J Heart Fail 2016;18:869–875. [DOI] [PubMed] [Google Scholar]
- 36. Kohara K, Okada Y, Ochi M, Ohara M, Nagai T, Tabara Y, et al. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program Study. J Cachexia Sarcopenia Muscle 2017;8:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


