Abstract
Background
Low muscle mass occurs in patients with rheumatoid arthritis without weight loss; this condition is referred as rheumatoid cachexia. The aim of the current study was to perform a systematic review with meta‐analysis to determine the rheumatoid cachexia prevalence.
Methods
A systematic review with meta‐analysis of observational studies published in English, between 1994 and 2016, was conducted using MEDLINE (via PubMed) and other relevant sources. Search strategies were based on pre‐defined keywords and medical subject headings. The methodological quality of included studies was assessed using the Newcastle‐Ottawa Scale. Meta‐analysis was used to estimate the prevalence, and because studies reported different methods and criteria to estimate body composition and prevalence of rheumatoid cachexia, subgroup analyses were performed. Meta‐regression adjusted for the 28‐joint disease activity score and disease duration (years) was performed (significance level at P ≤ 0.05).
Results
Of 136 full articles (one duplicate publication) screened for inclusion in the study, eight were included. The estimated overall prevalence of rheumatoid cachexia was 19% [95% confidence interval (CI) 07–33%]. This prevalence was 29% (95% CI 15–46%) when body composition was measured by dual‐energy X‐ray absorptiometry. When the diagnostic criteria were fat‐free mass index below the 10th percentile and fat mass index above the 25th percentile, rheumatoid cachexia prevalence was 32% (95% CI 14–52%). The 28‐joint disease activity score and disease duration had no influence on the estimated prevalence of rheumatoid cachexia (P > 0.05). Most studies were rated as having moderate methodological quality.
Conclusions
Meta‐analysis showed a prevalence of rheumatoid cachexia of 15‐32%, according to different criteria, demonstrating that this condition is a frequent comorbidity of rheumatoid arthritis. To better understand its clinical impact, more studies using standardized definitions and prospective evaluations are urgently needed.
Keywords: Cachexia, Rheumatoid cachexia, Sarcopenia, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory autoimmune disease characterized by chronic, symmetric, and erosive synovitis that may lead to severe disability and premature death.1, 2, 3 In addition to joint damage, changes in body composition have been observed in patients with RA, including reduced fat‐free mass (FFM), of which muscle mass is the major component, with or without loss of fat mass (FM), resulting in no or limited changes in body mass index.4, 5 This condition has been referred to as rheumatoid cachexia (RC).6, 7, 8
The definition of ‘cachexia’ (classic cachexia), ‘sarcopenia’, ‘sarcopenic obesity’, and ‘RC’ is diverse in the available literature. Sarcopenia (Greek ‘sarx’ or flesh and ‘penia’ or loss) is currently used to characterize the combination of low muscle mass and function (strength and performance),9, 10 while sarcopenic obesity refers to the copresence of both sarcopenia and obesity. Classic cachexia (Greek ‘kako's’ or bad and ‘he'xis’ or condition) is a term used to characterize the condition involving severe loss of weight, fat, and muscle mass and increased protein catabolism due to underlying disease(s).11, 12 Roubenoff et al.5, 13 described a common reduction in total body cell mass (BCM) in patients with RA and referred to this condition as RC. BCM consists primarily of muscle mass, with visceral mass (serum proteins, erythrocytes, granulocytes, lymphocytes, liver, kidneys, pancreas, and heart) and immune cell mass contributing lesser amounts. Also, this author considers FM, extracellular water, connective tissue (cartilage, fibrous tissues, and skeletal tissues), and bone, components not included in BCM. However, recently, Engvall et al. described RC as adverse changes in body composition (reduced FFM with or without loss of FM) in patients with RA.14, 15
Excess of pro‐inflammatory cytokines as IL‐1 beta and TNF‐alpha is considered to be the central feature in RC.5 In addition, as for clinical outcomes, RC has been associated with an increased risk of physical disability, morbidity, and mortality.16, 17
Despite several publications on RC, there is no consensus on the clinical criteria for its diagnosis,18 leading to considerable variability in reports of RC prevalence. Attempts have been made to diagnose RC based on body composition measured by different methods, including computed tomography, magnetic resonance imaging, dual‐energy X‐ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), and anthropometric methods. In addition to different methods to evaluate body composition, different criteria have been used to diagnose RC.11, 14, 19 Therefore, the diagnostic criteria used for RC and the method used to measure body composition can greatly impact the estimation of RC prevalence, but this has not been systematically studied. The aim of the current study was to systematically review the literature and estimate the RC prevalence.
Methods
We conducted this systematic review with meta‐analysis in accordance with PRISMA guidelines20 after registering the protocol with PROSPERO (CRD42017073495).
Data sources
An electronic search was performed using MEDLINE (via PubMed) and other relevant sources. We used a comprehensive search strategy tailored to each database.
Search terms
Keywords and medical subject headings for the terms ‘rheumatoid arthritis’, ‘arthritis’, ‘arthritis rheumatoid’ AND ‘cachexia’ AND ‘sarcopenia’ were selected.
Inclusion/exclusion criteria
Cross‐sectional and cohort studies were included in the systematic review and meta‐analysis. Studies on experimental models, randomized controlled trials, and reviews; studies on other diseases or topics; and studies without prevalence data of RC were excluded. In addition, studies that evaluated only muscle mass, without providing data on FM, were excluded.
Data extraction
Title, abstract, and full‐text screening were performed in duplicate by two independent reviewers (Santo, R. C. E. and Fernandes, K. Z.). The reviewers independently extracted data from the studies using a pre‐established data extraction form, which is available upon request. All study data were recorded using a bibliographic management program (Mendeley®, version 1.17.9). Disagreements about data abstraction were resolved by discussion between the two reviewers. If no agreement could be reached, a third and fourth reviewers (Filippin, L. and Lora, P.) provided the final decision. Information extracted during data abstraction included author names, date of publication, journal of publication, number of study participants, age range of population, type of population, definition of RC [with method(s) of body composition assessment, cut‐off points, and reference population], and RC prevalence (percentage or number of participants with and without RC).
Strategy for data synthesis
The Newcastle‐Ottawa Scale, adapted for cross‐sectional studies, was used to assess methodological quality of sampling, selection, exposure, and clinical outcomes of the studies selected. Using the Newcastle‐Ottawa Scale, each study was judged on seven items categorized into three groups: selection of study groups; comparability of groups; and ascertainment of either the exposure or outcome of interest. The maximum possible score was 10 stars, which represented the highest methodological quality.21 Studies awarded 7–10 stars were rated as having high quality; 5–6 stars, as having moderate quality; and <5 stars, as having low quality.22 Studies include independently of the methodological quality calculated. Meta‐analysis was performed using Stata® 11.0. The prevalence of RC [with 95% confidence intervals (CI)] was estimated using random effect adjust and Freeman–Tukey double arcsine transformation (FFT). The I 2 statistic was used to assess heterogeneity among studies. Meta‐regression adjusted for the 28‐joint disease activity score (DAS28) and disease duration (years) was performed, and the significance level was set P ≤ 0.05. All analyses were performed using the metaprop command in Stata.
Results
Search strategy
We identified 136 potentially relevant full articles (one duplicate publication) based on the search strategy described at the initial search stage. Figure 1 shows the flow diagram of study selection. After title, abstract, and full‐text screening, 127 articles were excluded (25 experimental model; 40 reviews; 10 clinical trials; 10 other populations; 26 other topics; and 16 without prevalence of RC) in accordance with inclusion/exclusion criteria. Therefore, eight relevant full articles were included in the review and incorporated into the meta‐analysis.
Figure 1.
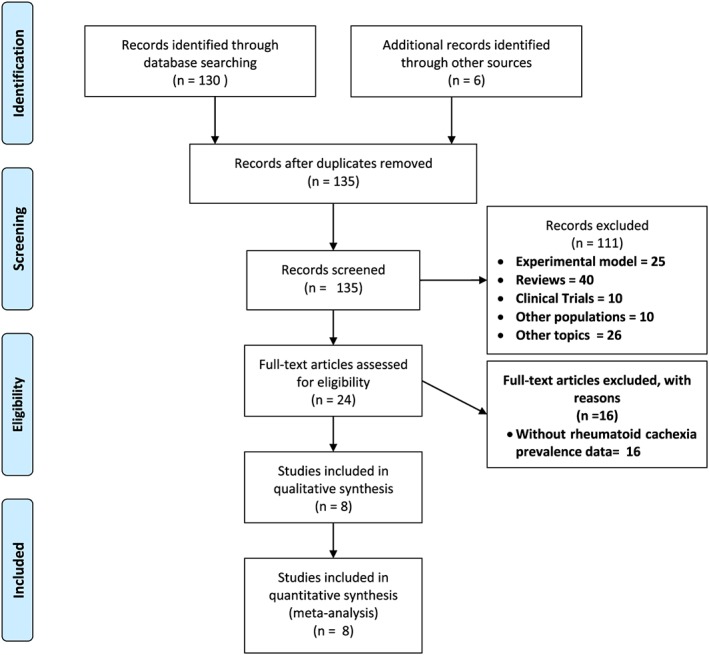
Flow diagram of search results and study selection.
Characteristics of the studies
All included studies were published between 2008 and 2016. Sample sizes ranged from 50 to 400 patients, with a female predominance. Mean age ranged from 54.1 to 65.0 years. DAS28 ranged from 3.1 to 5.2. The characteristics of the included studies are summarized in Table 1. All studies had a cross‐sectional design. Most studies were conducted in European populations, while one was from Morocco, and another was from South Africa.
Table 1.
Characteristics of included studies
| First author name | Country | Sample size | Mean age (years) | Disease duration (years) | Mean DAS28 | Diagnostic criteria | Methods of body composition | Prevalence of cachexia (%) |
|---|---|---|---|---|---|---|---|---|
| Hugo et al.28 | France | (n = 57) | 57.0 ± 13.0 | 3.8 (3.0) | 4.4 ± 1.1 | Engvall et al.14 | DEXA | 18.0 |
| W: 41 | ||||||||
| M: 16 | ||||||||
| El Maghraoui et al.27 | Morocco | (n = 178) | T: 54.1 ± 11.5 | 8.9 (7.4) | T: 4.3 ± 1.6 | Engvall et al.14 | DEXA | T: 53.9 |
| W: 53.7 | ||||||||
| W: 4.7 ± 1.5 | M: 54.8 | |||||||
| W: 147 | W: 51.8 ± 10.3 | M: 4.1 ± 0 1.4 | ||||||
| M: 31 | M: 53.3 ± 10.7 | |||||||
| Lombard et al.26 | South Africa | (n = 246) | 54.7 ± 13.6 | NP | Engvall et al.14 | AM | 10.3 | |
| W: 204 | ||||||||
| M: 42 | ||||||||
| Bokhorst et al.25 | Netherlands | (n = 103) | T: 60.0 (26.0–90.0)a | 8.0a | 3.32 | Evans et al.11 | BIA; handgrip strength; FAACT; VAS fatigue and pain; CRP; ESR; Hb | 1.0 |
| W: 79 | ||||||||
| M: 24 | ||||||||
| Elkan et al.24 | Sweden | (n = 80) | W: 60.8 (57.3–64.4)a | 6.0 | W: 3.3 (3.0–3.6)a | Engvall et al.14 | DEXA | W: 18.0 |
| W: 61 | ||||||||
| M: 63.4 (59.8–66.9)a | ||||||||
| M: 19 | M: 2.6 (2.1–3.0)a | |||||||
| M: 21.0 | ||||||||
| Elkan et al.19 | Sweden | (n = 80) | W: 60.8 (57.3–64.4)a | 6.0 | W: 3.3 (3.0–3.6)a | FFMI below the 25th percentile and FMI above the 50th percentile. | DEXA | W: 18.0 |
| W: 61 | ||||||||
| M: 63.4 (59.8–66.9)a | ||||||||
| M: 2.6 (2.1–3.0)a | ||||||||
| M: 19 | ||||||||
| M: 26.0 | ||||||||
| Metsios et al.23 | UK | (n = 400) | +RC: 68.3 (64.7–73.0)a | +RC: 11.0a | RA + RC: 4.3 ± 1.8 | Engvall et al.14 | BIA | 8.5 |
| W: 292 | −RC: 10.0a | |||||||
| −RC: 62.7 (54.0–69.4)a | ||||||||
| RA − RC: 4.2 ± 1.4 | ||||||||
| M: 108 | ||||||||
| Engvall et al.14 | Sweden | (n = 60) | W: 66.0 (63.0–69.0)a | W: 13.0a | W: 5.7 (5.3–6.1)a | Engvall et al.14 | DEXA | 38.0 |
| W: 50 | ||||||||
| M: 10 | M: 16.0a | |||||||
| M: 60.0 (51.0–70.0)a | M: 4.6 (3.7–5.5)a |
+RC, patients with rheumatoid arthritis and rheumatoid cachexia; −RC, patients with rheumatoid arthritis without rheumatoid cachexia; AM, anthropometric measurements; BIA, bioelectrical impedance analysis; CRP, C‐reactive protein; DAS28, 28‐joint disease activity score; DEXA, total‐body dual‐energy X‐ray absorptiometry; ESR, erythrocyte sedimentation rate; FAACT, the Functional Assessment of Anorexia/Cachexia Therapy questionnaire; FFMI, fat‐free mass index; FMI, fat mass index; Hb, haemoglobin; M, men; NP, not published; T, total; VAS, visual analogue scale; W, women.
Median; ±standard deviation; Engvall et al.14 diagnostic criteria, FFMI below the 10th percentile and FMI above the 25th percentile.
Diagnostic criteria for rheumatoid cachexia
Most studies used only body composition parameters as diagnostic criteria for RC. Fat‐free mass index (FFMI) below the 10th percentile and fat mass index (FMI) above the 25th percentile, as proposed by Engvall et al.,14 were used as diagnostic criteria for RC in six (75.0%) of eight included studies.14, 19, 23, 24, 25, 26, 27, 28 Of the remaining two studies, one used the diagnostic criteria proposed by Elkan et al. of FFMI below the 25th percentile and FMI above the 50th percentile,19 and the other used the diagnostic criteria proposed by Evans et al. that includes another parameters besides body composition {body weight loss of 5% or more within 12 months [or a body mass index ≤20 kg/m2] and at least three of the following factors: decreased muscle strength; fatigue; anorexia; low FMI; and abnormal biochemistry [increased inflammatory markers (CRP, IL‐6), anaemia (Hb <12 g/dL), low serum albumin (<3.2 g/dL)]}.11
Methods of body composition assessment for rheumatoid cachexia
Of eight included articles, five (62.5%) used total‐body DEXA, two (25.0%) used BIA,23, 25 and one (12.5%) used anthropometric measurements.26
Rheumatoid cachexia prevalence
Rheumatoid cachexia prevalence ranged from 1.0% to 53.9% (Table 1). El Maghraoui et al.27 found the highest prevalence using the diagnostic criteria for RC proposed by Engvall et al.,14 while Bokhorst et al.25 found the lowest prevalence using the diagnostic criteria for cachexia proposed by Evans et al.11
Meta‐analysis
In the overall meta‐analysis (body composition as the only criterion), RC prevalence estimated was 19% (95% CI 07–33%) with I 2 = 96.74%, P = 0.00 (Figure 2). Two subgroup analyses were performed. When body composition determine by DEXA, RC prevalence was estimated at 29% (95% CI 15–46%) with I 2 = 92.63%, P = 0.00 (Figure 3), and when FFMI below the 10th percentile and FMI above the 25th percentile were used as diagnostic criteria, RC prevalence estimated was 32% (95% CI 14–52%) with I 2 = 93.24%, P = 0.00 (Figure 4). In the meta‐regression model, neither DAS28 nor age had an influence on the RC estimated prevalence (P = 0.545, SEM = 0.04; and P = 0.614, SEM = 0.02, respectively).
Figure 2.
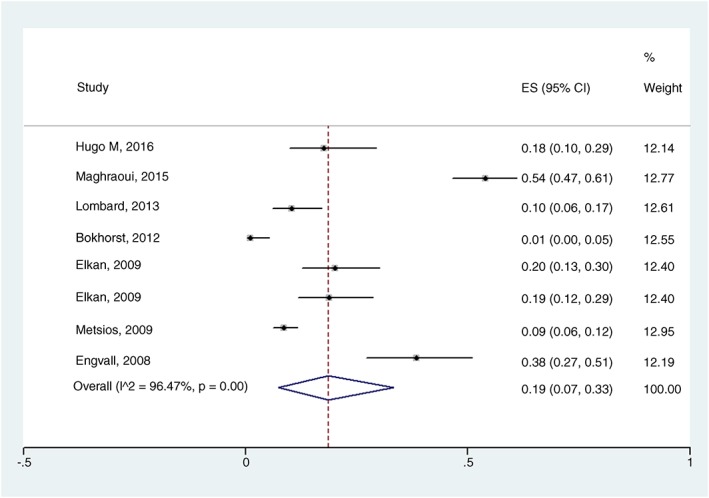
Forest plot of the prevalence of rheumatoid cachexia using body composition (assessed by dual‐energy X‐ray absorptiometry, bioelectrical impedance analysis, or anthropometric measurements) as a diagnostic criterion; ES, estimated; I^2, heterogeneity among studies.
Figure 3.
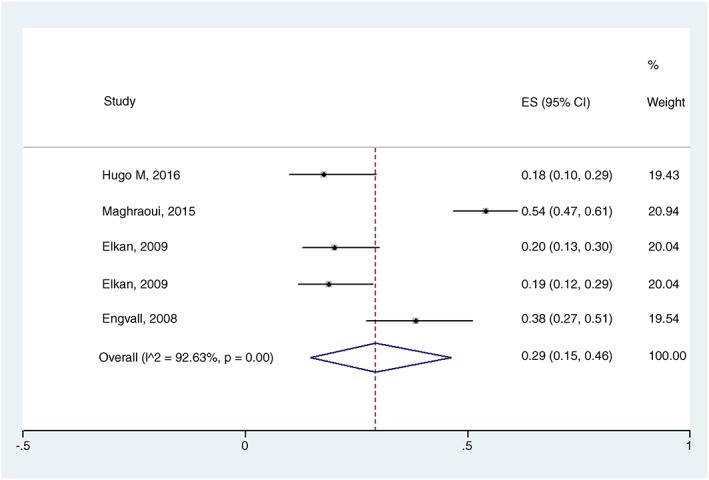
Forest plot of the prevalence of rheumatoid cachexia using dual‐energy X‐ray absorptiometry. ES, estimated; I^2, heterogeneity among studies.
Figure 4.
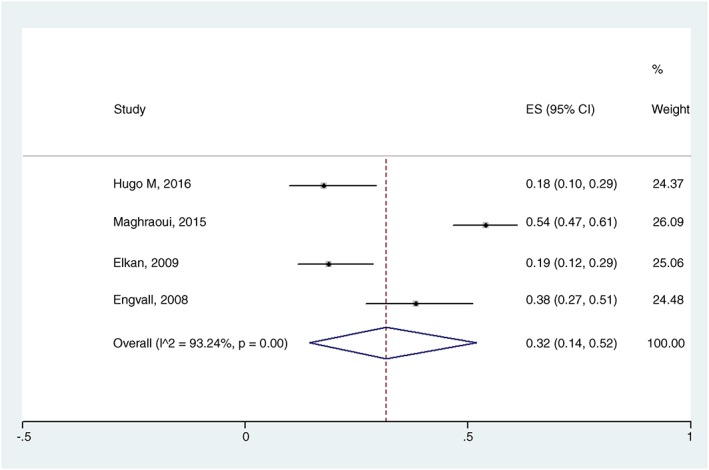
Forest plot of the prevalence of rheumatoid cachexia using dual‐energy X‐ray absorptiometry and fat‐free mass index below the 10th percentile and fat mass index above the 25th percentile as diagnostic criteria. ES, estimated; I^2, heterogeneity among studies.
This prevalence was when body composition was measured by DEXA.
Methodological quality of the studies
The methodological quality of included studies is described in Table 2. Most studies were rated as having moderate quality.
Table 2.
Description of quality assessment using the Newcastle‐Ottawa Scale (NOS)
| First author name | Country | Selection (1–5 stars) | Comparability (1–2 stars) | Outcome (0–3 stars) | Overall NOS (1–10 stars) |
|---|---|---|---|---|---|
| Hugo et al.28 | France | ** | * | ** | 5 |
| El Maghraoui et al.27 | Morocco | ** | * | ** | 5 |
| Lombard et al.26 | South Africa | **** | * | ** | 7 |
| Bokhorst et al.25 | Netherlands | ** | * | 3 | |
| Elkan et al.24 | Sweden | ** | * | ** | 5 |
| Elkan et al.19 | Sweden | ** | * | ** | 5 |
| Metsios et al.23 | UK | ** | * | *** | 6 |
| Engvall et al.14 | Sweden | ** | * | ** | 5 |
Asterisks (*) Represents the number of “stars” of quality Newcastle‐Ottawa Scale (NOS).
Discussion
Rheumatoid cachexia is a term used to characterize adverse changes in body composition that involve reduction in FFM and maintenance or increase in FM in patients with RA. These changes may be related to pro‐inflammatory cytokine‐induced hypermetabolism.13, 14, 15 To our knowledge, this is the first systematic review with meta‐analysis to estimate the prevalence of RC in patients with RA. Of the eight articles included in our study, seven studies used diagnostic criteria developed specifically for RC—six used the criteria proposed by Engvall et al.,14 and one used the criteria proposed by Elkan et al.,19 while one study used the criteria for classic cachexia proposed by Evans et al.11
The estimated RC prevalence was 19% (95% CI 07–33%). However, estimated prevalence varied when studies with different methods of body composition assessment or different cut‐off points were included in the analysis. In the analysis using specific diagnostic criteria for RC and DEXA, the estimated prevalence of RC was 29%. Nevertheless, in the analysis using specific criteria for RC, DEXA, and the cut‐off points proposed by Engvall et al.14 (i.e. FFMI below the 10th percentile and FMI above the 25th percentile), the estimated prevalence of RC was 32% (95% CI 14–52%). Therefore, we can state that RC is influenced by the method of body composition assessment and criteria used for diagnosis.
Body composition analysis refers to the quantification of the main structural components of the human body, divided into specific tissues that compose the total body mass.29, 30 Methods of body composition assessment such as DEXA, BIA, and anthropometric measurements have been used in the clinical and research settings. Using BIA, Bokhorst et al.25 found a very low prevalence of RC (1%). Metsios et al.,23 also using BIA, found a prevalence of RC of 8.5%. Elkan et al.24 compared BIA and DEXA and found good agreement between the two methods, but BIA showed higher lean mass values and lower FM values. Although BIA has lower cost, greater ease of use, and higher measurement speed, the use of this technique requires that the person undergo a set of previous procedures, without which there may be loss of information quality obtained from FM when compared with DEXA.
In addition to these precautions, equipment characteristics and calibration, body position, individual hydration level and food intake, ambient and cutaneous temperature, and use of heavier garments and metal parts may have some influence on the quality of measurements.31, 32 Using anthropometric measurements to assess body composition in patients with RA, Lombard et al.26 found a prevalence of RC of 10.3%. Anthropometric measurement is considered a double indirect method of body composition assessment, and the instruments used, the evaluator's ability, intra‐rater and inter‐rater errors, individual factors (hydration level, physical exercise, and menstrual cycle), and the choice of the anthropometric prediction equation, among other factors, may be a source of error.33, 34, 35, 36
Using DEXA as the method of body composition assessment in patients with RA, Hugo et al.28 found an RC prevalence of 18%, while El Maghraoui et al.27 found a prevalence of 53.9%. A number of techniques are being used to assess body composition as water dilution, anthropometry, DEXA, analysis of computerized tomography and magnetic resonance imaging, and BIA.37 However, DEXA represents a reliable alternative method, non‐invasive, improved feasibility, lower cost, minimal radiation exposure, high accuracy, sensitive and reproducibility for measuring FM and FFM.38, 39 In addition, DEXA is a clinically accessible method that is widely used in bone mineral density measurement for the evaluation of osteoporosis; therefore, it is also suitable for the analysis of body composition.
Rheumatoid cachexia prevalence also varied according to the criteria used for diagnosis, because the diagnostic criteria for RC are not well established. Engvall et al.14 and Elkan et al.19 proposed a RC diagnostic criteria that include changes in FFM and FM. Engvall et al.14 used data from a Swiss population sample of healthy adults (2986 men and 2649 women) to found body composition index that determine RC. Therefore, RC was classified as FFMI below the 10th percentile and FMI below the 25th percentile. Using these cut‐off points, they found an RC prevalence of 38%. Other studies conducted using the same diagnostic criteria (and cut‐off points) found an RC prevalence ranged from 8.5% to 53.9%.
Elkan et al.19 proposed a variation in the same parameters but different cut‐off point and the same Swiss population database used by Engvall et al.14 for RC in order to test the association of RC with dyslipidaemia and risk of cardiovascular disease. Patients with RA were classified as having RC if they had FFMI below the 25th percentile and FMI above the 50th percentile.19 Using these cut‐off points, they found an RC prevalence of 21% for women and 26% for men. Despite the difference between the RC criteria proposed by Engvall et al.14 and by Elkan et al.,19 no apparent difference was observed between prevalence rates.
Evans et al.,11 however, proposed a diagnosis of cachexia to be used in several diseases, including RA. Based on these criteria, Bokhorst et al.25 found a prevalence of RC of 1%. Physical inactivity40 and treatment effects,41, 42, 43 due to the effective reversal of the systemic inflammatory process, seem to affect the gain or maintenance of body weight in patients with RA. Therefore, patients with controlled RA do not lose body weight, and, for this reason, the prevalence of RC using the diagnostic criteria proposed by Evans et al.11 is lower than that reported in the studies that used the diagnostic criteria proposed by Engvall et al.14 or by Elkan et al.19, 24
In fact, no study evaluated the impact of RC on patient outcomes, and no study prospectively validated these criteria. To define the best criterion, such studies are required. Despite the lack of body weight loss, patients with RA lose muscle mass compared with healthy individuals. Loss of muscle mass is commonly evaluated in classic cachexia, RC, and sarcopenia; therefore, these syndromes are often confused with one another.
Using loss of muscle mass as a diagnostic criterion for sarcopenia in patients with RA, studies have reported prevalence rates of sarcopenic patients ranging from 11.0% to 57.1%. However, studies that evaluated only loss of muscle mass were not included in our systematic review with meta‐analysis, because the objective was to specifically evaluate RC according to currently used RC diagnostic criteria. Considering that patients with RA have a decrease in lean mass, maintenance or increase in adipose tissue, and changes in functionality, Weber et al.44, 45 proposed a new parameter of adiposity‐adjusted muscle mass (ALMIFMI). This parameter was defined as appendicular lean mass divided by height squared adjusted for age and body fat (adjZ‐score), and low lean mass was defined as adjZ‐score less than −1.0, leading to stronger positive associations with functional results compared with unadjusted estimates in patients with RA.44, 45 Thus, it is suggested that this new parameter be used in future sarcopenia and cachexia studies of patients with RA.
The systemic inflammatory process of RA has been associated with altered body composition and RC.14 Elkan et al. assessed patients with mild to moderate disease activity and found an RC prevalence of 18.0%19, 24 for women and 21.024–26.0%19 for men. Conversely, studies evaluating patients with moderate to high disease activity found an RC prevalence of 18–53.9%.14, 19, 24, 27, 28 In our meta‐analysis, no association between disease activity and RC was demonstrated, probably because most patients had moderate to high disease activity (DAS28 between 3.1 and 5.2) and because all studies had a cross‐sectional design, thereby preventing the evaluation of changes in body composition and disease activity over time.
Regardless of whether there is agreement between the diagnostic criteria and methods used for body composition assessment, there is common agreement that RC affects the clinical outcome of patients. Patients with RA have increased IL‐6 and TNF within the muscle. These muscle inflammatory markers correlate with physical inactivity and disability.46 Engvall et al.14 demonstrated that patients with RA who had RC and high disease activity had lower physical activity and IGF‐1 levels. In addition to muscle alterations, patients with RA also have endothelial dysfunction and increased risk of atherosclerosis and cardiovascular disease.47, 48, 49 However, the effects of RC on the risk of cardiovascular disease are still controversial.50 Elkan et al.,19 in patients with RA who had RC and moderate disease activity, found high levels of total cholesterol and low‐density lipoprotein, low levels of IgM against phosphorylcholine (anti‐PC IgM), and high frequency of hypertension. However, in patients with RA and moderate RC, disease activity does not appear to be associated with worse cardiovascular disease profile23 or metabolic syndrome.28 Impaired bone metabolism may also be present, because patients with RA and RC have lower hip bone mineral density than patients without RC.27 Considering that RC affects the clinical outcome of patients, longitudinal studies are needed to fill the gaps in the literature regarding the effects of RC on patients with RA.
The main limitation of this study is the small number of RC studies included, making meaningful meta‐analysis difficult. In conclusion, the results of this systematic review with meta‐analysis indicate that the estimated prevalence of RC ranges from 19% to 32% and that there is variability in the prevalence rates according to the diagnostic criteria used for RC. Therefore, there is a need for greater standardization of terms such as RC, cachexia, and sarcopenia, as well as for further prospective studies aiming to clarify the impact of this comorbidity on clinical outcomes.
Conflict of interest
None declared.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.51 We thank the Coordination for the Improvement of Higher Level Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES) institution and the Foundation for Research Support of the Rio Grande do Sul State (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul—FAPERGS) for granting scholarships to the students that helped develop this study. Also, we thank the Service of Rheumatology and Service of Biostatistics of HCPA by financial and scientific support. This work was supported by the Research and Events Incentive Fund (Fundo de Incentivo à Pesquisa e Eventos—FIPE) of HCPA, the Research Support Fund (Fundo de Apoio à Pesquisa da Sociedade de Reumatologia do Rio Grande do Sul) of Rheumatology Society of the Rio Grande do Sul and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq).
Santo, R. C. E. , Fernandes, K. Z. , Lora, P. S. , Filippin, L. I. , and Xavier, R. M. (2018) Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta‐analysis. Journal of Cachexia, Sarcopenia and Muscle, 9: 816–825. 10.1002/jcsm.12320.
References
- 1. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358:903–911. [DOI] [PubMed] [Google Scholar]
- 2. da Mota LM, Cruz BA, Brenol CV, Pereira IA, Fronza LS, Bertolo MB, et al. Consensus of the Brazilian Society of Rheumatology for diagnosis and early assessment of rheumatoid arthritis. Rev Bras Reumatol 2011;51:199–219. [PubMed] [Google Scholar]
- 3. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 4. Roubenoff R, Rall LC. Humoral mediation of changing body composition during aging and chronic inflammation. Nutr Rev 1993;51:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson‐Hughes B, et al. Rheumatoid cachexia: cytokine‐driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 1994;93:2379–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westhovens R, Nijs J, Taelman V, Dequeker J. Body composition in rheumatoid arthritis. Br J Rheumatol 1997;36:444–448. [DOI] [PubMed] [Google Scholar]
- 7. Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol 2002;85:89–99. [DOI] [PubMed] [Google Scholar]
- 8. Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 2004;43:1219–1223. [DOI] [PubMed] [Google Scholar]
- 9. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 12. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 13. Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J Rheumatol 1992;19:1505–1510. [PubMed] [Google Scholar]
- 14. Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin‐like growth factor. Scand J Rheumatol 2008;37:321–328. [DOI] [PubMed] [Google Scholar]
- 15. Summers GD, Deighton CM, Rennie MJ, Booth AH. Rheumatoid cachexia: a clinical perspective. Rheumatology (Oxford) 2008;47:1124–1131. [DOI] [PubMed] [Google Scholar]
- 16. Rajbhandary R, Khezri A, Panush RS. Rheumatoid cachexia: what is it and why is it important? J Rheumatol 2011;38:406–408. [DOI] [PubMed] [Google Scholar]
- 17. Lemmey AB. Rheumatoid cachexia: the undiagnosed, untreated key to restoring physical function in rheumatoid arthritis patients? Rheumatology (Oxford) 2016;55:1149–1150. [DOI] [PubMed] [Google Scholar]
- 18. Masuko K. Rheumatoid cachexia revisited: a metabolic co‐morbidity in rheumatoid arthritis. Front Nutr 2014;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elkan AC, Håkansson N, Frostegård J, Cederholm T, Hafström I. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: a cross‐sectional study. Arthritis Res Ther 2009;11:R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS 2009;6:e1000097 Available at https://www.ncbi.nlm.nih.gov/pubmed/19621072 [PMC free article] [PubMed] [Google Scholar]
- 21. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta‐analysis. PLoS One 2016;25:e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toledano E, Candelas G, Rosales Z, Martínez Prada C, León L, Abásolo L, et al. A meta‐analysis of mortality in rheumatic diseases. Reumatol Clin 2012;8:334–341. [DOI] [PubMed] [Google Scholar]
- 23. Metsios GS, Stavropoulos‐Kalinoglou A, Panoulas VF, Sandoo A, Toms TE, Nevill AM, et al. Rheumatoid cachexia and cardiovascular disease. Clin Exp Rheumatol 2009;27:985–988. [PubMed] [Google Scholar]
- 24. Elkan AC, Engvall IL, Cederholm T, Hafström I. Rheumatoid cachexia, central obesity and malnutrition in patients with low‐active rheumatoid arthritis: feasibility of anthropometry, Mini Nutritional Assessment and body composition techniques. Eur J Nutr 2009;48:315–322. [DOI] [PubMed] [Google Scholar]
- 25. van Bokhorst‐de van der Schueren MA, Konijn NP, Bultink IE, Lems WF, Earthman CP, van Tuyl LH. Relevance of the new pre‐cachexia and cachexia definitions for patients with rheumatoid arthritis. Clin Nutr 2012;31:1008–1010. [DOI] [PubMed] [Google Scholar]
- 26. Lombard LA, du Plessis LM, Visser J. Body composition of rheumatoid arthritis patients in the City of Cape Town, South Africa. Clin Rheumatol 2014;33:467–476. [DOI] [PubMed] [Google Scholar]
- 27. El Maghraoui A, Sadni S, Rezqi A, Bezza A, Achemlal L, Mounach A. Does rheumatoid cachexia predispose patients with rheumatoid arthritis to osteoporosis and vertebral fractures? J Rheumatol 2015;42:1556–1562. [DOI] [PubMed] [Google Scholar]
- 28. Hugo M, Mehsen‐Cetre N, Pierreisnard A, Schaeverbeke T, Gin H, Rigalleau V. Energy expenditure and nutritional complications of metabolic syndrome and rheumatoid cachexia in rheumatoid arthritis: an observational study using calorimetry and actimetry. Rheumatology (Oxford) 2016;55:1202–1209. [DOI] [PubMed] [Google Scholar]
- 29. Heymsfield S. Human Body Composition, 2nd ed. Champaign, IL; Leeds: Human Kinetics; 2005. [Google Scholar]
- 30. Petroski EL. Antropometria: técnicas e padronizações. 5ed. Fontoura; 2007.
- 31. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 2004;23:1226–1243. [DOI] [PubMed] [Google Scholar]
- 32. Ellis KJ, Bell SJ, Chertow GM, Chumlea WC, Knox TA, Kotler DP, et al. Bioelectrical impedance methods in clinical research: a follow‐up to the NIH Technology Assessment Conference. Nutrition 1999;15:874–880. [DOI] [PubMed] [Google Scholar]
- 33. Conde WL, Oliveira DR, Borges CA, Baraldi LG. Consistência entre medidas antropométricas em inquéritos nacionais. Rev Saúde Pública 2013;41:69–76. [DOI] [PubMed] [Google Scholar]
- 34. Perini TA, Oliveira GL, Ornellas JS, Oliveira FP. Cálculo do erro técnico de medição em antropometria. Rev Bras Med Esporte 2005;11:81–85. [Google Scholar]
- 35. Ribeiro G, Lopes A. Análise da Composição corporal: evolução histórica do modelo anatômico de análise tecidual. Rev Bras Prescrição e Fisiol do Exerc 2017;11:620–625. [Google Scholar]
- 36. Cyrino ES, Okano AH, Glaner MF, Romanzini M, Gobbo LA, Makoski AB, et al. Impact of the use of different skinfold calipers for the analysis of the body composition. Rev Bras Med Esporte 2003;9:150–153. [Google Scholar]
- 37. Tewari N, Awad S, Macdonald IA, Lobo DN. A comparison of three methods to assess body composition. Nutrition 2018;47:1–5. [DOI] [PubMed] [Google Scholar]
- 38. Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 2008. Sep;11:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanellakis S, Manios Y. Validation of five simple models estimating body fat in white postmenopausal women: use in clinical practice and research. Obesity (Silver Spring) 2012. Jun;20:1329–1332. [DOI] [PubMed] [Google Scholar]
- 40. Stavropoulos‐Kalinoglou A, Metsios GS, Smith JP, Panoulas VF, Douglas KM, Jamurtas AZ. What predicts obesity in patients with rheumatoid arthritis? An investigation of the interactions between lifestyle and inflammation. Int J Obes 2010;34:295–301. [DOI] [PubMed] [Google Scholar]
- 41. Tournadre A, Pereira B, Dutheil F, Giraud C, Courteix D, Sapin V, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle 2017;8:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baker JF, Sauer BC, Cannon GW, Teng CC, Michaud K, Ibrahim S, et al. Changes in body mass related to the initiation of disease‐modifying therapies in rheumatoid arthritis. Arthritis Rheumatol 2016;68:1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jurgens MS, Jacobs JW, Geenen R, Bossema ER, Bakker MF, Bijlsma JW, et al. Increase of body mass index in a tight controlled methotrexate‐based strategy with prednisone in early rheumatoid arthritis: side effect of the prednisone or better control of disease activity? Arthritis Care Res (Hoboken) 2013;65:88–93. [DOI] [PubMed] [Google Scholar]
- 44. Weber D, Long J, Leonard MB, Zemel B, Baker JF. Development of novel methods to define deficits in appendicular lean mass relative to fat mass. PLoS One 2016;11:e0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baker JF, Giles JT, Weber D, Leonard MB, Zemel BS, Long J, et al. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology (Oxford) 2017; 1;56:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huffman KM, Jessee R, Andonian B, Davis BN, Narowski R, Huebner JL, et al. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthritis Res Ther 2017;23;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flierl U, Bauersachs J, Schäfer A. Modulation of platelet and monocyte function by the chemokine fractalkine (CX3 CL1) in cardiovascular disease. Eur J Clin Investig 2015;45:624–633. [DOI] [PubMed] [Google Scholar]
- 48. Bergholm R, Leirisalo‐Repo M, Vehkavaara S, Mäkimattila S, Taskinen MR, Yki‐Järvinen H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol 2002; 1;22:1637–1641. [DOI] [PubMed] [Google Scholar]
- 49. Berckmans RJ, Nieuwland R, Tak PP, Böing AN, Romijn FP, Kraan MC. Cell‐derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII‐dependent mechanism. Arthritis Rheum 2002;46:2857–2866. [DOI] [PubMed] [Google Scholar]
- 50. Summers GD, Metsios GS, Stavropoulos‐Kalinoglou A, Kitas GD. Rheumatoid cachexia and cardiovascular disease. Nat Rev Rheumatol 2010;6:445–451. [DOI] [PubMed] [Google Scholar]
- 51. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


