Abstract
Background
Building both strength and endurance has been a challenge in exercise training in the elderly, but dietary supplements hold promise as agents for improving muscle adaptation. Here, we test a formulation of natural products (AX: astaxanthin, 12 mg and tocotrienol, 10 mg and zinc, 6 mg) with both anti‐inflammatory and antioxidant properties in combination with exercise. We conducted a randomized, double‐blind, placebo‐controlled study of elderly subjects (65–82 years) on a daily oral dose with interval walking exercise on an incline treadmill.
Methods
Forty‐two subjects were fed AX or placebo for 4 months and trained 3 months (3×/week for 40–60 min) with increasing intervals of incline walking. Strength was measured as maximal voluntary force (MVC) in ankle dorsiflexion exercise, and tibialis anterior muscle size (cross‐sectional area, CSA) was determined from magnetic resonance imaging.
Results
Greater endurance (exercise time in incline walking, >50%) and distance in 6 min walk (>8%) accompanied training in both treatments. Increases in MVC by 14.4% (±6.2%, mean ± SEM, P < 0.02, paired t‐test), CSA by 2.7% (±1.0%, P < 0.01), and specific force by 11.6% (MVC/CSA, ±6.0%, P = 0.05) were found with AX treatment, but no change was evident in these properties with placebo treatment (MVC, 2.9% ± 5.6%; CSA, 0.6% ± 1.2%; MVC/CSA, 2.4 ± 5.7%; P > 0.6 for all).
Conclusions
The AX formulation improved muscle strength and CSA in healthy elderly in addition to the elevation in endurance and walking distance found with exercise training alone. Thus, the AX formulation in combination with a functional training programme uniquely improved muscle strength, endurance, and mobility in the elderly.
Keywords: Skeletal muscle, Interval training, Fatigue, Sarcopenia, Dynapenia
Introduction
Age is characterized by a progressive loss of mobility, with muscle wasting (sarcopenia) and reduced endurance as key factors in this exercise intolerance. Exercise training has been the gold standard for slowing or reversing sarcopenia. Resistance training (RT) has been most effective at treating muscle wasting,1 while endurance training has the greatest benefits to exercise intolerance.2, 3 However, combining training modes to treat both declines simultaneously typically yields a lower response than either mode applied separately.4, 5
Recent evidence indicates that dietary supplements may lead to improvements in both strength and endurance, which is a combination of adaptations that have not been possible with a traditional training programme alone.5, 6 For example, anti‐inflammatory supplements yield greater strength improvements when combined with training in elderly subjects,7 while antioxidant supplements lead to greater endurance improvements.8 Astaxanthin is a natural product with both antioxidant and anti‐inflammatory properties9, 10, 11, 12 that has been found to increase strength and endurance. For example, 6 months of feeding in the healthy young resulted in ‘strength endurance’ gains of 55% as measured by the number of weighted knee bends completed by the end of the study.13 Muscle adaptations, such as these, in an elderly population would counter key muscle functional losses that are found with age.14 Additional benefit would come from combining a dietary treatment with functional training, which has the potential to not only reverse muscle wasting and improve exercise intolerance but also to increase mobility with a single intervention.
Astaxanthin accumulates in tissues of animals in the food chain, such as salmon, that feeds on the marine algae, Haematococcus pluvialis 9 and is estimated to have been consumed at levels of 6 mg per day in populations with a salmon‐based diet.15 Two factors supplied in the diet, vitamin E and zinc, also have antioxidant properties, but intake is often inadequate, especially in the elderly.16 Vitamin E has the additional benefit of enhancing the antioxidant effect of astaxanthin in vitro 17 and zinc stabilizes SOD1 activity.18 Formulating these dietary supplements into a single treatment offers the possibility of combining or synergizing the beneficial effects of the individual natural compounds with antioxidant and anti‐inflammatory properties.
Here, we test a dietary formulation of astaxanthin, vitamin E, and zinc in combination with exercise training as an approach to elevate mobility, endurance, and strength in the elderly.6, 8 Previous intervention studies have focused on astaxanthin alone in young animals and humans, but there are few studies on the elderly.19 Evidence for the beneficial impact of AX on old muscle comes from a pilot study in mice, reported here, that found that AX accumulated in muscle with feeding and was associated with elevated muscle quality (specific force) after exercise training on a treadmill. The approach of this study was to build on this pilot study using a formulation of dietary factors with antioxidant and anti‐inflammatory properties paired with exercise training. Our hypothesis was that this astaxanthin‐based formulation in combination with a functionally based exercise training programme would activate improvements in both endurance and muscle strength, thereby providing a single approach to reverse loss of mobility and muscle properties in the elderly.
Methods
Pilot study
Twenty‐nine‐month‐old male mice were treated with either 300 mg/(kg × day) astaxanthin (n = 10, Astareal, Inc. Moses Lake, WA, USA) or standard chow alone (n = 9). These mice were very old at the start of training given that 50% survivorship generally occurs about 28 months of age for this strain20 (vs. mean life expectancy of 78 years old for humans in the USA in 201321). The AX dose was determined for mice by scaling22 from the level found to be effective in rat studies.23 Exercise training occurred 3×/week on a 20° inclined treadmill at 10 m/min for 5 min at the start reaching 15 min in the final 4 weeks of training. In vivo muscle force of the gastrocnemius was measured as the maximum twitch and tetanic force during electric stimulations (200 Hz for 300 ms) at baseline and at 8 weeks in anaesthetised mice as described.24 The quadriceps muscle was frozen at the end of training to determine the level of astaxanthin. The Institutional Animal Care and Use Committee of the University of Washington Animal approved this experimental protocol.
Human study
This randomized, double‐blind, placebo‐controlled study was conducted at the University of Washington Medical Center and the Fred Hutchison Cancer Research Center. Adults age 65–85 years old were recruited through public lectures, mailers, posted advertisements, and referrals from prior studies. To be included in the study, subjects had to be healthy and not under treatment for serious chronic conditions, ambulatory and able to perform activities of daily living without assistance, and able to speak and read English fluently. Exclusion criteria are listed in Table S1. A total of 365 subjects were phone screened; 58 subjects enrolled in the study and were randomly assigned to groups. Each subject had a physical examination, resting and exercise electrocardiogram, and blood testing to ensure that they were healthy and free from orthopaedic and neuromuscular problems.
Treatment and dosing
The dietary formulation consisted of astaxanthin (12 mg), tocotrienol (10 mg), and zinc (6 mg; Astamed, Bellevue, WA) and was ingested as two capsules per day. The astaxanthin dose was determined based on safety studies showing that a dose up to 45 mg per day for 4 weeks is well tolerated without serious side effects.19 Long‐term intervention studies (4 mg/day for 52 weeks25; 12 mg/day26 and 16 mg/day for 12 weeks27) reported no serious adverse side effects. The 12 mg daily dose resulted in significant improvement in cognitive measurements.26 Two additional components were included in the formulation because they provide additional antioxidant benefits and are often ingested below the recommended intakes.16 Tocotrienols (vitamin E) reduce lipid peroxidation in combination with astaxanthin more than astaxanthin alone17 and quench reactive oxygen species in liposomes 40% better than the sum of the two antioxidants acting alone.28 In addition, zinc stabilizes SOD1 activity.18 Thus, this formulation has more antioxidant protection than the individual ingredients as well as supplementing nutrients that are often ingested below recommended levels.
Randomization and blinding
The subjects were assigned to the two treatment groups by an individual not associated with the study. A random number generator provided the assignments. Copies of the codes were held in separate locations that were not accessible to the investigators. Thus, the assignment of the individual subjects to the treatment groups was not known to the participants or to the investigators until the study was completed.
Figure 1 shows a chart of the subject flow through the study. Sixteen subjects dropped out after randomization: 8 for medical reasons unrelated to the treatment, 6 for protocol non‐adherence, and 2 for personal reasons. Forty‐three subjects (n = 19, placebo and n = 23, astaxanthin formulation) aged 65–82 years completed the study. The characteristics of the subjects that completed the study are shown in Table 1. All participants gave written informed consent consistent with the Declaration of Helsinki in a project approved by the University of Washington and Western Institutional Review Boards.
Figure 1.
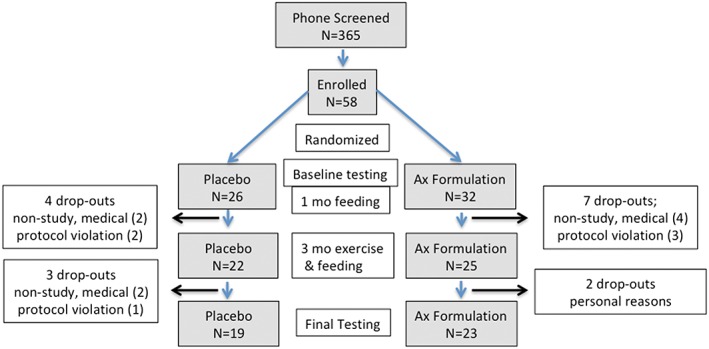
The study flow diagram. Schema of subject progress through the phases of the parallel randomized study of the two treatment groups.
Table 1.
Subjects' characteristics
| Placebo | AX | |
|---|---|---|
| Age, year | 72.2 ± 5.2 | 69.1 ± 3.4 |
| F, n | 9 | 13 |
| M, n | 10 | 10 |
| Height, cm | 66.6 ± 3.4 | 66.4 ± 4.1 |
| Weight, kg | ||
| Pre | 71.1 ± 14.8 | 73.8 ± 13.4 |
| Post | 71.3 ± 14.8 | 72.4 ± 13.9 |
| BMI | ||
| Pre | 24.7 ± 3.1 | 26.3 ± 3.2 |
| Post | 25.4 ± 3.1 | 25.8 ± 3.2 |
Value are means ± SD. BMI, body mass index.
Exercise training
The 12 week training programme met 3× per week with a 10 min warm‐up before and 5–10 min cool down period at the end of each session. Treadmill training involved walking at ~1.3 m/sec with periods at a high treadmill incline of 9–12% grade (interval training) separated by periods of low incline walking at 5–7% grade (recovery). Table S2 contains the time and incline grade (%) used at baseline, at the end of training, and the overall change with training. The training level was incremented based on the heart rate (HR) response (HR targets listed in the succeeding text) as well as relative perceived exertion during the treadmill test. Training progressed in three steps: (i) familiarization with the treadmill protocol (weeks 1 and 2), which involved 8–10 intervals at the high incline for ~1 min each and recovery for 2 min, (ii) baseline interval training (weeks 3–7), which involved 1–1.5 min exercise in 8–10 intervals to achieve 70–80% HRmax with 2–3 min of recovery exercise between intervals, and (iii) ramping up (weeks 8–12), which involved 1.5–2 min exercise in 10–12 intervals to achieve 80–85% HRmax with 0.5–1.0 min of recovery exercise between intervals. All exercise training was overseen by an American College of Sports Medicine certified exercise physiologist at the Fred Hutchinson Cancer Research Center.
Magnetic resonance imaging
Tibialis anterior (TA) muscle cross‐sectional area (CSA) was determined from magnetic resonance images (Bruker 4.7‐T magnet with Biospin console; Bruker Corporation, Billerica, MA) acquired as axial plane T1‐weighted, 2‐D gradient‐echo images collected with the following parameters: 500 ms repetition time, 2.5 ms echo time, 3 mm slice thickness, 1 mm inter‐slice interval, 192 × 192 matrix, and number of excitations = 2. Five slices of each right limb were analysed with NIH Image software (Image J, version 1.50 e) using manual planimetry29 to determine the muscle CSA. The measurements by two independent investigators agreed to within 2.5% on average.
Single muscle test: isometric ankle dorsiflexion
The TA muscle strength and contractile properties were determined on the right leg using a custom‐built isometric exercise apparatus, as previously described30 (Figure S1A). The TA muscle is important in ankle dorsiflexion and represents a simple system connecting muscle mechanics and energetics to foot movement30 and is a critical part of walking mobility. The subject performed a maximal voluntary contraction (MVC) using ankle dorsiflexion for ~5 sec by pulling on a strap that secured the foot to a force transducer platform. Three successive bouts were separated by 5 sec each (Figure S1B). A test, retest evaluation found no significant difference in MVC in two determinations separated by 30 days (−0.01 ± 0.04 N, mean ± SEM, n = 24, paired t‐test). The relative standard deviation for the difference scores was 21% (SD of difference/mean × 100).
Statistical analysis
An unpaired Student's t‐test was used to evaluate treatment vs. placebo in the pilot mouse study. To explore for measurements that change with treatment in the human study, a paired, 2‐tailed t‐test (pre‐training vs. post‐training change) was used with significance assigned at α = 0.05 (P < 0.05). No correction for multiple comparisons was used as is consistent with a proof of concept study.31 Data are reported as means ±SEM in figures and text and ±SD in the tables.
Results
Pilot study
The level of astaxanthin in muscle after the 8 week exercise programme was significantly elevated in the AX (236.7 ± 123.4 ng/g, n = 4) vs. the placebo (9.2 ± 9.2 ng/g, n = 6) treatment group. Specific force (maximum twitch force/muscle cross‐sectional area) was significantly greater in AX vs. placebo‐treated mice after training (P < 0.004, Table 2).
Table 2.
Mouse muscle force, size, and specific force (MVC/mass) after 8 weeks of training with the astaxanthin formulation (AX) or placebo (PL) supplementation
| PL | AX | P (PL vs. AX) | |
|---|---|---|---|
| Strength (max. force, mN) | |||
| Pre | 395 ± 63 | 366 ± 41 | 0.32 |
| Post | 351 ± 73 | 406 ± 74 | 0.12 |
| Muscle size (mass, g) | |||
| Post | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.49 |
| Specific force (max. force/CSA, N/cm2) | |||
| Pre | 4.6 ± 1.0 | 4.7 ± 0.9 | 0.73 |
| Post | 4.0 ± 0.6 | 5.0 ± 0.6 | 0.004 |
Values are mean ± SD: Max. force: maximum twitch force elicited by electrical stimulation; CSA, cross sectional area; P: α level in a Student's t‐test. MVC, maximal voluntary force.
Human study
The subjects' physical characteristics are reported in Table 1. Figure 2A shows that the time in the interval stage (high % grade incline walking) was the predominant change with training (Table S2). The increased interval stage exercise time demonstrates that the subjects in both treatment groups could exercise longer (greater time) and at a higher intensity (higher % grade) after training. Walking distance in the 6 min walk also significantly improved by ~8% in both groups with training (Figure 2B, Table S3).
Figure 2.
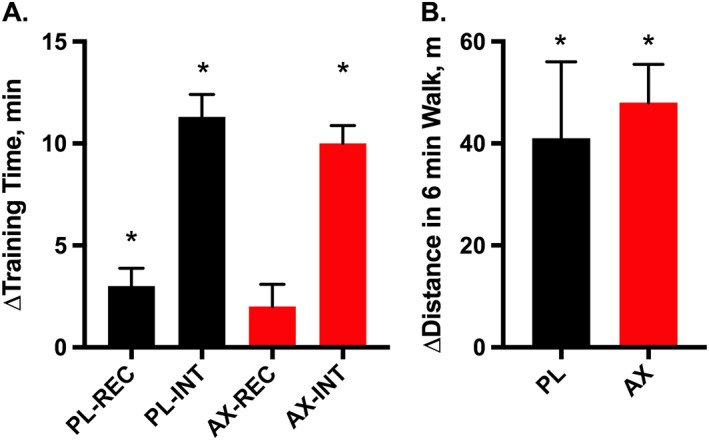
Changes in endurance (training time) and mobility (walking distance) after 3 months of training in placebo (PL) and astaxanthin formulation (AX) treated elderly subjects. (A) Change in training time (min) in the recovery (REC) and interval (INT) exercise periods in the training session and (B) change in distance (m) in the 6 min walk test. Values are mean ± SEM.
* P < 0.05.
Figure 3 shows the relative change in muscle strength and size in the two groups with training (Table S4 presents the absolute changes). A significant change in human muscle strength, as measured by MVC (∆14.4 ± ∆6.2% mean ± SEM, P < 0.02), is shown for the AX treatment group alone. The TA muscle CSA (∆2.7 ± ∆1.0%) also only increased in the AX treatment group (both image analysers found CSA differences at P < 0.01). The ratio of these measures provides the muscle specific force (MVC/CSA), which trended to a higher value (∆11.6 ± ∆6.1%, P = 0.053) in the AX treatment group alone. No significant change in muscle properties was found in the placebo treatment group (MVC, ∆2.9% ± ∆5.6%; CSA, ∆0.6% ± ∆1.2%; MVC/CSA, ∆2.4 ± ∆5.7%; P > 0.6 for all).
Figure 3.
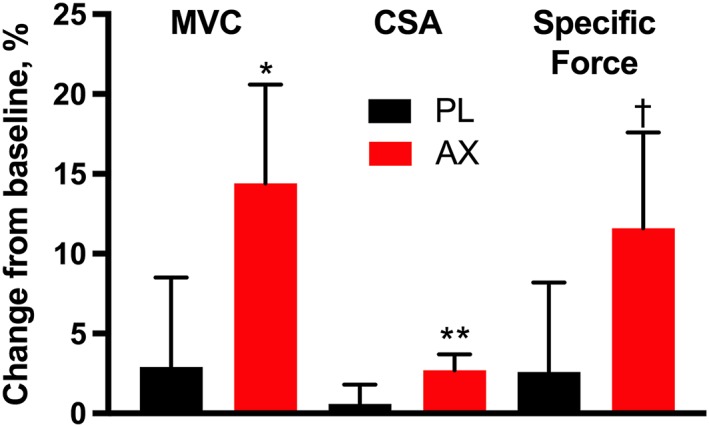
Changes in muscle properties after 3 months of training in AX formulation and placebo‐treated elderly subjects. Changes in maximum voluntary contraction (MVC), muscle cross‐sectional area (CSA), and specific force (MVC/CSA) are shown. Values are mean ± SEM. * P < 0.02, ** P < 0.01, † P = 0.053 for paired 2‐tailed t‐test.
Discussion
The key findings from this study are that the astaxanthin formulation in combination with functionally based exercise training improved endurance and walking distance (Figure 2), as well as elevated muscle strength and size (sarcopenia) in elderly subjects (Figure 3). Thus, mobility, endurance, and strength were all improved with a single training approach in elderly humans. This approach of pairing a dietary formulation with functionally based training holds promise as a single mode to reverse key deficits in muscle and mobility that limit the elderly.14
The pilot study involving exercise training of aged mice on an incline treadmill provided two results important for the human study. The first result was that AX accumulated in the muscle and was significantly above the levels found with placebo treatment at the end of exercise training. The second result was that this AX accumulation was associated with significantly greater specific force in the AX‐treated vs. placebo‐treated muscle after incline training (Table 2), which points to the biological activity of AX. The human study emulated this design with the addition of interval training that mixed level with incline treadmill walking to provide both endurance and strength training stimuli. Figure 2 shows that total walking time on the treadmill increased (endurance) due predominantly to more time in high incline (interval) walking vs. low incline (recovery) walking after 3 months of training. The similarity of the response of the two groups indicates that the antioxidant properties of the AX formulation did not inhibit the endurance training adaptation as has been found with other antioxidant treatments in exercise training interventions.32 Instead, both training groups increased walking distance in a 6 min test, thereby demonstrating both greater endurance (treadmill time in interval training) and mobility (walking distance) with this approach.
Accompanying these functional improvements were adaptations in both strength and muscle CSA in the group treated with the formulation of dietary supplements with antioxidant and anti‐inflammatory properties (astaxanthin, vitamin E, and zinc) (Figure 3).9, 11 The strength gain (14.4%) in the AX formulation group was similar to that found with RT in studies of the elderly (10–15%,1, 7, 33) that used isokinetic or isometric methods to measure MVC. This strength gain was accompanied by an increase in muscle size (2.5%), which indicates that a greater muscle mass was a part of the gains in strength. Although this CSA gain is smaller than found in RT studies of the elderly (~10%,1, 7, 33, 34), the muscle size gain was one of a suite of changes that accompanied the training stimulus with the AX formulation. This muscle adaptive response has been found with anti‐inflammatory treatments7 and is consistent with the anti‐inflammatory properties reported for AX.10, 11
The difference in the strength (14.4%) vs. the CSA change (2.5%) points to an improved muscle quality with the AX formulation plus training, as measured by specific force (MVC/CSA; 11.5%, P = 0.053). Remarkably, this specific force gain was similar in size to the change found in many RT studies of the elderly (Figure S2). These results point to specific force as a key contributor to the strength increases with the AX formulation treatment in the human study and to the difference between placebo and AX treatment in the mice after exercise training (Table 2).
Many factors have been implicated as contributors to a rise in specific force with training, including neuromuscular,35 muscle architecture,36 and cellular mechanisms.37 Our testing approach and the observed changes with training and treatment minimize the impact of several of the factors and highlight one factor that could raise strength. For example, direct muscle electrical stimulation (mouse) and ballistic contractions of the TA muscle38 are designed to recruit all fibres and thereby reduce the impact of muscle and fibre recruitment on strength measures. Also, the ankle dorsiflexion exercise protocol primarily activates the TA muscle with little contribution by other muscles39 indicating a minimal impact of co‐activation of antagonist muscles to the strength measure. A second factor, muscle architecture (fibre length and pennation angle), is reported to change strikingly with RT in old subjects33 but, despite these changes, CSA (or related size measures) remains the predominant factor determining MVC.36 The results of these RT studies suggest that other factors, in addition to muscle architecture, contribute to the 11.5% improvement in specific force found in this study.
Reversal of the denervation that comes with age is a potential mechanism for increasing specific force with AX formulation feeding. Such a reversal in neuromuscular function with RT was demonstrated by two studies: a 4% rise in MVC relative to an electrically stimulated maximum in healthy elderly33 and improvement in skeletal muscle innervation reported in obese older adults.40 The neuromuscular junction (NMJ) is the interface between the muscle and nervous system; it is known to be disrupted by oxidative stress, leading to degeneration and denervation with age.41 Reduction of oxidative stress has been shown to improve the NMJ and specific force in mouse studies.42 The AX formulation is reported to act as an antioxidant in vitro in rodent models and in vivo in humans.11 This antioxidant property may reduce oxidative stress so that the biosynthetic signals raised by exercise training can activate rebuilding of degenerative NMJs with AX treatment. Rejuvenated NMJ's could permit activation of more muscle fibres within the TA43 and could account for the improved specific force of ankle dorsiflexion found in this study.
Limitations
Three improvements in the experimental design of future studies would provide insight into the mechanism of action of astaxanthin beyond this ‘proof of concept’ study. First, adding an untrained control group to the mouse study would reveal whether age‐related, rather than training‐related, changes were responsible for the lower specific force in the placebo group after the 8 week experiment. An age‐related decline would point to a mechanism of action that elevates specific force with astaxanthin feeding and exercise training in mice as was found in the human study. Second, this study provides the statistical information needed to power a future clinical trial to directly test AX formulation treatment vs. placebo for effectiveness in reversing the strength and muscle size declines that come with age. Third, a muscle biopsy would allow a direct test of the mechanism of action of the AX formulation using biomarkers of inflammation and oxidative stress. Despite the limitations of a ‘proof of concept’ study, the clear responses of the AX formulation group to exercise training provide a template for interventions designed to counter sarcopenia, exercise intolerance, and low mobility in elderly subjects.
Conclusion
Here, we show that functionally based exercise training combined with a formulation of natural anti‐inflammatory and antioxidant compounds improved muscle strength and size in elderly subjects more than exercise training alone. This was done without sacrificing the improvements in walking distance and endurance that typically accompany endurance training. These results suggest that the potential for strength and endurance improvements in elderly muscle is realized when natural products that promote adaptation are combined with exercise training incorporating both resistance and aerobic components. The end result is an approach involving functional exercise and a dietary formulation that can improve endurance, strength, and function to remedy the deficits associated with sarcopenia that limit mobility in the elderly.
Author contributions
K.C., D.M, M.C, S.L., and Astavita, Inc. designed the research; S.L., A.A., M.C., K.C., and E.S. performed research; B.R. provided physician oversight; S.L., A.A., M.C., K.C., and D.M. analysed data; and K.C. and S.L. wrote the paper.
Conflict of interest
S.Z.L. declares she has no conflict of interest, A.S.A. declares he has no conflict of interest, M.D.C. declares he has no conflict of interest, K.K. declares he has no conflict of interest, E.G.S. declares he has no conflict of interest, B.R. declares he has no conflict of interest, D.J.M. declares he has no conflict of interest, and K.E.C. has received research funds and is a Scientific Advisor for Astavita, Inc.
Supporting information
Table S1. Exclusion Criteria
Table S2. Treadmill time (min) and incline grade (%) during training at baseline (Pre), at the end (Post) and the change (∆Post‐Pre) with the study.
Table S3. Walking distance at baseline (Pre), at the end (Post) and the change (∆Post‐Pre) with the study in the placebo (PL) and astaxanthin formulation (AX) fed groups.
Table S4: Human TA muscle properties pre and post training and treatment in the placebo (PL) and astaxanthin formulation (AX) fed groups.
Figure S1. A) Apparatus for measuring muscle strength in ankle dorsiflexion, B) Example of a single maximum voluntary contraction at baseline and after three months of training in an AX formulation treated human elderly subject.
Figure S2. Specific force changes with resistance training (RT) and AX treatment with exercise training (red arrow). Note: “Tracy/M” represents the older male average and “Tracy/W” represents the older female data. Farup et al. (black bar) training data from young adults; Tracy, Ferrir, and Trappe et al. data points all represents elderly (>65 yr) subject training studies.
Acknowledgements
We thank the volunteers who participated in this study for their time and dedication. Chessa Goss coordinated the study. Will Siegell analysed muscle CSA. Matt van Doren, Claudia Kumai, and Angela Modelski provided exercise training and clinical assessments. Nelly Nicklason provided comments. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.44 This work was supported by Astavita, Inc.; National Institutes of Health grants (T32 AG000057, UL1TR000423, 1S10OD016201, K23DK099442); Department of Radiology and Office of the Provost of the University of Washington, and the Prevention Center at the Fred Hutchison Cancer Research Center. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Liu, S. Z. , Ali, A. S. , Campbell, M. D. , Kilroy, K. , Shankland, E. G. , Roshanravan, B. , Marcinek, D. J. , and Conley, K. E. (2018) Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. Journal of Cachexia, Sarcopenia and Muscle, 9: 826–833. 10.1002/jcsm.12318.
References
- 1. Lastayo PC, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual‐care with older cancer survivors: the impact on muscle and mobility—an exploratory pilot study. BMC Geriatr 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunjes DL, Kennel PJ, Christian Schulze P. Exercise capacity, physical activity, and morbidity. Heart Fail Rev 2017;22:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conley KE, Jubrias SA, Cress ME, Esselman PC. Elevated energy coupling and aerobic capacity improves exercise performance in endurance‐trained elderly subjects. Exp Physiol 2013;98:899–907. [DOI] [PubMed] [Google Scholar]
- 4. Coffey VG, Hawley JA. Concurrent exercise training: do opposites distract? J Physiol 2017;595:2883–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nader GA. Concurrent strength and endurance training: from molecules to man. Med Sci Sports Exerc 2006;38:1965–1970. [DOI] [PubMed] [Google Scholar]
- 6. Alway SE, Mccrory JL, Kearcher K, Vickers A, Frear B, Gilleland DL, et al. Resveratrol enhances exercise‐induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci 2017;72:1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trappe TA, Carroll CC, Dickinson JM, Lemoine JK, Haus JM, Sullivan BE, et al. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 2011;300:R655–R662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mankowski RT, Anton SD, Buford TW, Leeuwenburgh C. Dietary antioxidants as modifiers of physiologic adaptations to exercise. Med Sci Sport Exer 2015;47:1857–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell JG, Mcevoy J, Tocher DR, Sargent JR. Depletion of alpha‐tocopherol and astaxanthin in Atlantic salmon (Salmo salar) affects autoxidative defense and fatty acid metabolism. J Nutr 2000;130:1800–1808. [DOI] [PubMed] [Google Scholar]
- 10. Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ, Ha KS, et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I (kappa) B kinase‐dependent NF‐kappaB activation. Mol Cells 2003;16:97–105. [PubMed] [Google Scholar]
- 11. Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polotow TG, Vardaris CV, Mihaliuc AR, Goncalves MS, Pereira B, Ganini D, et al. Astaxanthin supplementation delays physical exhaustion and prevents redox imbalances in plasma and soleus muscles of Wistar rats. Nutrients 2014;6:5819–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malmsten CL, Lignell A. Dietary supplementation with Astaxanthin‐rich algal meal improves strength and endurance—a double blind placebo‐controlled study on male students. Carotenoid Science 2008;13:20–22. [Google Scholar]
- 14. Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care 2010;13:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Efsa . Opinion of the scientific panel on additives and products or substances used in animal feed on the request from the European commission on the safety of using coloring agents in animal nutrition. PART 1. General principles and Astaxanthin. The EFSA Journal 2005;291:1–40. [Google Scholar]
- 16. Tur JA, Colomer M, Monino M, Bonnin T, Llompart I, Pons A. Dietary intake and nutritional risk among free‐living elderly people in Palma de Mallorca. J Nutr Health Aging 2005;9:390–396. [PubMed] [Google Scholar]
- 17. Ravena VG, Shimasaki H, Ueta N, Takahashi J. Interaction between a‐tocopherol, tocotrienols and astaxantin in lipisomes, subject to lipid peroxidation. J Oleo Sci 2003;52:347–352. [Google Scholar]
- 18. Homma K, Fujisawa T, Tsuburaya N, Yamaguchi N, Kadowaki H, Takeda K, et al. SOD1 as a molecular switch for initiating the homeostatic ER stress response under zinc deficiency. Mol Cell 52:75–86. [DOI] [PubMed] [Google Scholar]
- 19. Brown DR, Gough LA, Deb SK, Sparks SA, Mcnaughton LR. Astaxanthin in exercise metabolism, performance and recovery: a review. Front Nutr 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer KE, Hoffman JM, Sloane LB, Gelfond JA, Soto VY, Richardson AG, et al. A cross‐sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated. Aging (Albany NY) 2016;8:2370–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Health US, 2016 . National Center for Health Statistics In Health, United States, 2016: With Chartbook on Long‐term Trends in Health. Hyattsville, Maryland: CDC/National Center for Health Statistics/Office of Analysis and Epidemiology; 2017. [PubMed] [Google Scholar]
- 22. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Preuss HG, Echard B, Yamashita E, Perricone NV. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: are these effects interdependent? Int J Med Sci 2011;8:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, et al. Mitochondrial‐targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 2013;12:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S, et al. Carotenoids and antioxidants in age‐related maculopathy italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology 2008;115:324–333, e322. [DOI] [PubMed] [Google Scholar]
- 26. Satoh A, Tsuji S, Okada Y, Murakami N, Urami M, Nakagawa K, et al. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin‐rich Haematococcus pluvialis extract. J Clin Biochem Nutr 2009;44:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: a double blind, randomized trial. Asian J Androl 2005;7:257–262. [DOI] [PubMed] [Google Scholar]
- 28. Kamezaki C, Nakashima A, Yamada A, Uenishi S, Ishibashi H, Shibuya N, et al. Synergistic antioxidative effect of astaxanthin and tocotrienol by co‐encapsulated in liposomes. J Clin Biochem Nutr 2016;59:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trappe TA, Lindquist DM, Carrithers JA. Muscle‐specific atrophy of the quadriceps femoris with aging. J Appl Physiol (1985) 2001;90:2070–2074. [DOI] [PubMed] [Google Scholar]
- 30. Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 2003;553:589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998;316:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 2016;594:5135–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reeves ND, Maganaris CN, Longo S, Narici MV. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol 2009;94:825–833. [DOI] [PubMed] [Google Scholar]
- 34. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol (1985) 2001;90:1663–1670. [DOI] [PubMed] [Google Scholar]
- 35. Mckinnon NB, Connelly DM, Rice CL, Hunter SW, Doherty TJ. Neuromuscular contributions to the age‐related reduction in muscle power: mechanisms and potential role of high velocity power training. Aging Res Rev 2017;35:147–154. [DOI] [PubMed] [Google Scholar]
- 36. Reeves ND, Narici MV, Maganaris CN. Musculoskeletal adaptations to resistance training in old age. Man Ther 2006;11:192–196. [DOI] [PubMed] [Google Scholar]
- 37. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol 2014;5:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiology 1977;264:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol 2007;100:543–551. [DOI] [PubMed] [Google Scholar]
- 40. Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci 2016;71:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez‐Freire M, De Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci 2014;6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, et al. Dietary restriction attenuates age‐associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell 2012;11:770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 2014;49:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Exclusion Criteria
Table S2. Treadmill time (min) and incline grade (%) during training at baseline (Pre), at the end (Post) and the change (∆Post‐Pre) with the study.
Table S3. Walking distance at baseline (Pre), at the end (Post) and the change (∆Post‐Pre) with the study in the placebo (PL) and astaxanthin formulation (AX) fed groups.
Table S4: Human TA muscle properties pre and post training and treatment in the placebo (PL) and astaxanthin formulation (AX) fed groups.
Figure S1. A) Apparatus for measuring muscle strength in ankle dorsiflexion, B) Example of a single maximum voluntary contraction at baseline and after three months of training in an AX formulation treated human elderly subject.
Figure S2. Specific force changes with resistance training (RT) and AX treatment with exercise training (red arrow). Note: “Tracy/M” represents the older male average and “Tracy/W” represents the older female data. Farup et al. (black bar) training data from young adults; Tracy, Ferrir, and Trappe et al. data points all represents elderly (>65 yr) subject training studies.


