Abstract
Epidermal growth factor receptor (EGFR) gene mutations identify a molecularly defined subset of non-small cell lung cancer (NSCLC) patients who display an excellent sensitivity to EGFR tyrosine kinase inhibitors (TKIs). First-generation reversible EGFR TKIs, gefitinib and erlotinib have been proven to improve the objective response rate and to prolong the progression-free survival compared with standard chemotherapy in large phase III trials. Unfortunately, virtually all patients develop resistance to treatment, usually within 9–12 months. Afatinib is an irreversible ErbB family inhibitor initially designed to overcome the development of resistance. Compared with gefitinib in a first-line setting, afatinib prolonged progression-free survival and time to treatment failure, without impacting on overall survival in the general population of EGFR-mutant patients. However, afatinib has been shown to prolong overall survival in the subset of patients with an EGFR exon 19 deletion compared with chemotherapy. The aim of this review is to summarize the clinical evidence available to date and to critically discuss the place in therapy of afatinib in the rapidly expanding landscape of EGFR-mutant NSCLC first-line therapy.
Keywords: NSCLC, EGFR mutation, osimertinib, brain metastasis
Introduction
Lung cancer represents the leading cause of cancer-related deaths worldwide, accounting for approximately 27% of all cancer-related deaths. Among the 1.8 million cases diagnosed every year, the majority consists into non-small cell lung cancer (NSCLC; 85%) while the remaining fall into the small cell lung cancer group.1 Despite the impressive advancements in the diagnosis and the treatment of lung cancer, the prognosis of patients with advanced NSCLC remains globally poor, with a 5-year survival rate of less than 10%.1 Nonetheless, the discovery that a subset of NSCLC harbors an underlying actionable genetic alteration had a striking impact on the way we treat these molecularly defined subgroups of patients, resulting in an unprecedented survival benefit.2–5
Epidermal growth factor receptor (EGFR) represents the first identified targetable oncogenic driver discovered in NSCLC. EGFR belongs to the ErbB family of tyrosine kinase receptors which includes four members: human epidermal growth factor 1 (HER1; EGFR, ErbB1), HER2 (Neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4). EGFR deregulation has been widely recognized to be involved in cancer cell proliferation, tumor-driven angiogenesis and metastasis.6 Approximately 40% of Asian patients and 10–15% of Whites with newly diagnosed metastatic NSCLC harbor a somatic mutation in the EGFR gene.7 Tyrosine kinase inhibitors (TKIs) against EGFR have increasingly been associated with improved clinical outcomes in patients with advanced EGFR-mutant NSCLC and currently represent the best treatment option for this subset of patients.7,8 To date, three generations of EGFR TKIs are available for clinical use. Gefitinib and erlotinib are adenosine triphosphate (ATP) competitive quinazoline-based derivatives first-generation EGFR TKIs that reversibly bind to the tyrosine kinase pocket of EGFR. Large phase III clinical trials have shown a significant improvement in outcomes for NSCLC patients with activating EGFR mutations (L858R and Del19) treated with TKIs in a first-line setting, in terms of objective response rate (ORR), progression-free survival (PFS) and quality of life (QoL), as compared with standard chemotherapy (Table 1).9–15 Unfortunately, virtually all patients develop resistance to treatment within 9–12 months. In approximately 50% of cases and the mechanism of resistance relies into the emergence of the EGFR T790M secondary mutation.7 In order to overcome this resistance, second-generation EGFR TKIs, including afatinib, were initially designed and developed. Although preclinical data showed promising results of afatinib against the T790M-resistance mutation, in the clinical setting the activity in patients with T790M-mutant NSCLC was unsatisfactory.16 More recently, third-generation EGFR TKIs which are EGFR-mutant selective and wildtype (WT)-sparing inhibitors have been developed to selectively target T790M-resistant secondary mutations. Osimertinib is a third-generation EGFR TKI that binds covalently to EGFR isoforms (del19, L858R and double mutants containing T790M mutation) via a cysteine residue at codon 797 (C797) with minimal activity against WT EGFR17 and is the third-generation EGFR TKI with the most advanced clinical development. Currently osimertinib is recommended worldwide for T790M-mutant patients who progress on or following first-generation EGFR TKIs based on data from the pivotal phase I/II AURA, phase II AURA 2 and from the confirmatory phase III AURA 3 trial which definitely confirmed its superiority over chemotherapy in T790M-positive patients who progress on or following gefitinib or erlotinib in terms of PFS [10.1 versus 4.4 months, hazard ratio (HR): 0.30; 95% confidence interval (CI), 0.23 to 0.41; p < 0.001) and ORR (71% versus 31%, p < 0.001].18–20 More recently, with a doubled median PFS and an encouraging trend towards an improvement in overall survival (OS), osimertinib recently received United States Food and Drug Administration (US FDA) approval as a front-line treatment based on a multicenter, international, randomized, double-blind, active-controlled trial (FLAURA) which compared upfront osimertinib with standard-of-care gefitinib or erlotinib.21
Table 1.
Summary of efficacy of first and second-generation EGFR TKIs in patients with EGFR-mutant NSCLC.
| Study name | Geography | Trial arms | Number of patients | ORR (%) | mPFS (month) | Difference in mPFS HR (95% CI); p-value | Months) | Difference in mOS HR (95% CI); p-value | Difference in mos-del19 mutation HR (95% CI); p-value |
|---|---|---|---|---|---|---|---|---|---|
| IPASS | East Asia | Gefitinib versus carboplatin + paclitaxel | 261 | 71 versus 47 | 9.5 versus 6.3 | 0.48 (0.36–0.64) p < 0.001 |
21.6 versus 21.9 | 1.00 (0.76–1.33) | 0.79 (0.54–1.15) |
| First-SIGNAL | South Korea | Gefinitib versus cisplatin + gemcitabine | 42 | 85 versus 38 | 8.0 versus 6.3 | 0.54 (0.27–1.1) | 27.2 versus 25.6 | 1.04 (0.50–2.18) | n/a |
| WJTOG | Japan | Gefinib versus cisplatin + docetaxel | 177 | 62 versus 32 | 9.2 versus 6.3 | 0.49 (0.34–0.71) p < 0.0001 |
34.8 versus 37.3 | 1.25 (0.88–1.78) | n/a |
| NEJGSG | Japan | Gefitinib versus carboplatin + paclitaxel | 230 | 74 versus 31 | 10.8 versus 5.4 | 0.30 (0.22–0.41) p < 0.001 |
27.7 versus 26.6 | 0.89 (0.63–1.24) | 0.83 (0.52–1.34) |
| OPTIMAL | China | Erlotinib versus carboplatin + gemcitabine | 154 | 83 versus 36 | 13.1 versus 4.6 | 0.16 (0.10–0.26) p < 0.0001 |
22.7 versus 28.9 | 1.04 (0.69–1.58) | n/a |
| EURTAC | France, Italy, Spain | Erlotinib versus cisplatin or carboplatin + docetaxel or gemcitabine | 173 | 58 versus 15 | 9.7 versus 5.2 g | 0.37 (0.25–0.54) p < 0.0001 |
19.3 versus 19.5 | 1.04 (0.65–1.68) | 0.94 (0.57–1.54) |
| LL3 | Global | Afatinib versus cisplatin + pemetrexed | 345 | 56 versus 23 | 13.6 versus 6.9 | 0.47 (0.34–0.65) p = 0.001 |
31.6 versus 28.2 | 0.78 (0.58–1.06) | 0.54 (0.36–0.79) p = 0.0015 |
| LL6 | China, South Korea | Afatinib versus cisplatin + gemcitabine | 364 | 67 versus 23 | 11.0 versus 5.6 | 0.28 (0.20–0.39) p < 0.0001 |
23.6 versus 23.5 | 0.83 (0.62–1.09) | 0.64 (0.44–0.94) p = 0.023 |
| LL7 | Global | Afatinib versus
gefitinib |
319 | 70 versus 56 | 11.0 versus 10.9 | 0.73 (0.57 – 0.95) p = 0.0073 |
27.9 versus
24.5 |
0.86 (0.66–1.12) | 0.83 (0.58–1.17) |
| Archer 1050 | Global | Dacomitinib versus gefitinib | 452 | 75 versus 72 | 14.7 versus 9.2 | 0.59 (0.47–0.74) p < 0.0001 |
34.1 versus 26.8 | 0.76 (0.58–0.99) | 0.88 (0.61–1.26) p = 0.486 |
CI, confidence interval; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; n/a, not available; ORR, objective response rate.
In light of the growing body of evidence which are rapidly accumulating about the optimal management of EGFR-mutant NSCLC, this review aims to summarize the current knowledge about the second-generation EGFR TKI afatinib and to critically discuss the place in therapy of this drug in the contest of the available literature.
EGFR mutation in NSCLC: a brief overview
Sensitizing EGFR mutations occur in approximately 40% of Asians and in 10–15% of North Americans and European patients. Independently from ethnicity, EGFR-activating mutations are more likely to be detected in females, in patients with a never-smoker status and in those with adenocarcinoma histology.22 However, the sole clinical features are not sufficient to rule out the presence of an EGFR mutation, and EGFR testing is currently recommended in all patients with newly diagnosed advanced lung adenocarcinoma.8,23
Mutations in the EGFR gene include a wide range of genetic alterations, such as deletions, insertions, point mutations and amplification. Nonetheless, 85–90% of all sensitizing EGFR mutations in NSCLC consist of in-frame deletions in exon 19 (del19) at the GluLeuArgGluAla sequence (E746-A750) and the exon 21-point mutation Leu858Arg (L858R). The predictive role of these mutations has been largely addressed by randomized phase III clinical trials showing a higher ORR, prolonged PFS and better QoL in patients harboring an EGFR-sensitizing mutation treated with TKIs, as compared with chemotherapy.22–24 A benefit in favor of EGFR TKIs was reported across all studies in terms of PFS, response rates, and disease control rates. The median PFS with gefitinib and erlotinib ranged from 8.0 to 13.1 months, compared with 4.6–6.9 months with chemotherapy (range of HRs 0.16–0.48). In light of nearly doubled PFS achieved with first-generation EGFR TKIs, a consistent OS benefit was expected. However, the median OS was similar for both trial arms across all studies (19.3–34.8 months), as the results of the high rates of treatment crossover (54–95%). Nonetheless, despite the fact that no OS difference was observed between chemotherapy and EGFR TKIs arms in all the aforementioned studies, these data provided the first evidence that, in patients with oncogene-addicted NSCLC, the clinical benefit derived from treatment with TKIs is independent of whether the patients received EGFR TKIs as upfront therapy or upon failure of chemotherapy, in a second or later-line setting.9–15 Detailed results from phase III clinical trials comparing first-generation EGFR TKIs with chemotherapy are reported in Table 1.
In addition to an exon 19 deletion and L858R point mutation, a number of so-called uncommon mutations have been identified so far, including G719X in exon 18 (G719C, G719S, G719A), which account for 3% of EGFR mutations, L861Q in exon 21, which represents approximately 2% of EGFR mutations, or even more rarely the exon 20-point mutation, S768I. In light of their low frequency, the predictive value for EGFR TKI efficacy of uncommon mutations is still poorly understood.22,25 However, afatinib showed a lower half maximal inhibitory concentration (IC50) for uncommon EGFR mutations as compared with first- and third-generation TKIs in preclinical studies.22,25 Consistently, a post-hoc analysis of Lux-Lung 2, 3 and 6 trials confirmed that afatinib was active in patients with NSCLC tumors that harbored certain types of uncommon EGFR mutations, especially Gly719Xaa, Leu861Gln, and Ser768Ile, but was less active in other mutations types.26 A distinct type of EGFR mutation consisting of an in-frame insertion in exon 20 occurs in 3–7% of NSCLC and is known to predict primary resistance to treatment with all clinically available EGFR TKIs.27,28 However, the third-generation EGFR TKI osimertinib and its metabolite AZ5104 have recently shown encouraging antitumor efficacy against the EGFR exon 20 insertion in preclinical models and a preliminary report seems to confirm its activity also in a clinical setting.29,30 Worthy of note, a recent report also demonstrated a promising activity of afatinib in combination with cetuximab in a small cohort of patients with EGFR exon 20 insertion mutant NSCLC, with a reported ORR of 75% (3 out of 4).31
Recently, with the introduction of high-sensitive large-scale mutation analysis, it has increasingly been reported that activating EGFR mutations can occasionally coexist with other dominant mutations or compound EGFR mutations. EGFR compound mutations are usually constituted by an EGFR-TKI sensitizing mutation (such as L858R, L861Q, G719X, del19) plus the coexistence of uncommon mutations occurred in other residues of tyrosine kinase domain (EGFR-TKD) (such as V689L, L833V, E709K, L747V, R776H, A871G) and are likely to associate with EGFR TKI sensitivity, though recent studies have reported a poorer clinical outcome in patients with EGFR compound mutations compared with those with single-site EGFR-sensitizing mutations.32,33
Afatinib: pharmacodynamic and pharmacokinetics
Afatinib (Giotrif, BIBW2992, Boehringer Ingelheim Pharmaceuticals, Inc.) is an orally bioavailable 4-anilino-quinazoline derivative, irreversible, second-generation pan-EGFR TKI.
Afatinib mechanisms of action consist of an irreversible and covalent bond to the ATP-binding site of the EGFR tyrosine kinase domain which is mediated by the acrylamide group of the drug and the cysteine residues in the tyrosine kinase domain of the receptor (Cys797, Cys805, and Cys803 of ErbB-1/EGFR, ErbB-2/HER-2, and ErbB-4/HER-4, respectively) which reduces auto- and transphosphorylation of the receptor blocking the downstream transduction signaling pathways (Figure 1).34,35
Figure 1.
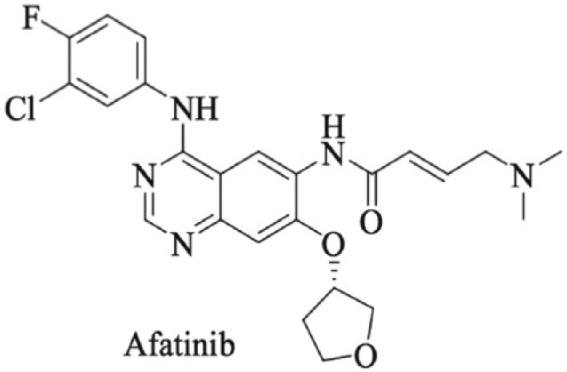
Chemical structure of afatinib (N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl] oxy]-6-quinazolinyl]-4-(dimethylamino)-(2E)-2-butenamide.
In vitro, afatinib was reported to be active against both common activating EGFR mutations (exon 19 deletion and L858R) and uncommon mutations such as G719X (exon 18) and L861Q (exon 21) and S768I (exon 18).35 Besides, in preclinical studies afatinib inhibited tumor growth and induced tumor regression in EGFR-mutant NSCLC murine models (including del19, Leu858Arg and Leu858Arg/Thr790Met).35,36,37 Of note, afatinib has also been reported to inhibit the EGFR T790M gatekeeper mutation, which represents the most common mechanism of resistance to first-generation EGFR inhibitors. However, the activity of afatinib in patients with T790M-mutant NSCLC was unsatisfactory due to the different nanomolar inhibitory concentrations required to inhibit different EGFR mutations [EGFR del19 (IC50 = 0.9), EGFR-L858R (IC50 = 0.4 nM), EGFR T719X (IC50 = 0.9 nM) EGFR del19/T709M (IC50 = 64), and EGFR-L858R/T790M (IC50 = 10 nM)].22,36 A summary of the in-vitro efficacy of each clinically available EGFR TKI against EGFR mutations is reported in Table 2.
Table 2.
In-vitro efficacy of EGFR TKIs against different EGFR mutations (IC50 nM).
| Gefitinib (IC50) | Erlotinib (IC50) | Dacomitinib (IC50) | Osimertinib (IC50) | |
|---|---|---|---|---|
| Del19 (E746-A750) | 4.8 | 4.9 | 0.9 | 1.1 |
| L858R | 26 | 16 | 2.6 | 9 |
| G719X | 213 | 167 | 6 | 53 |
| Del19/T790M | 8300 | >10.000 | 140 | 3 |
| L858R/T790M | >10.000 | >10.000 | 300 | 21 |
EGFR, epidermal growth factor receptor; IC50, half maximal inhibitory concentration; TKI, tyrosine kinase inhibitor.
In 2013, the US FDA approved afatinib for the first-line treatment of metastatic EGFR-mutated NSCLC. Currently afatinib is indicated for the first-line treatment of patients with metastatic NSCLC whose tumors have nonresistant EGFR mutations as detected by a US FDA-approved test (with a limitation of use in cases of resistant EGFR mutations for which the safety and efficacy of the drug have not been established). Afatinib has also been approved for the second-line treatment of patients with squamous NSCLC, having shown to prolong PFS and OS compared with erlotinib in the Lux-Lung 8 trial.38 However, this topic is beyond the scope of this review and will not be further discussed.
The recommended dose of afatinib is 40 mg once daily as a dimaleate salt film-coated tablet. Following oral administration, the maximum plasma concentration is reached approximately after 2–5 h. Afatinib binds both noncovalently and covalently to human plasma proteins producing covalent adducts which represent its main circulating metabolites. Afatinib is eliminated with a half-life of 37 h predominantly via fecal excretion (less than 5% with urine) while the steady-state exposure is reached within 8 days of multiple dosing, resulting in an accumulation of 2.8-fold for area under the curve (AUC) and 2.1-fold for Cmax.34,39.
Afatinib is neither an inhibitor nor an inducer of cytochrome P450 (CYP) enzymes and only 2% of the afatinib dose is metabolized by flavin-containing monooxygenase 3 (FMO3), therefore it has low potential for drug–drug interactions except for drugs that strongly induce or inhibit the P-glycoprotein (P-gp) transporter which can modify its pharmacokinetics properties.40
No starting dose adjustments are required in a special population except for severe renal function impairment in which the recommended dose of afatinib [estimated glomerular filtration rate (eGFR*) 15–29 ml/min /1.73 m2] is 30 mg orally.34 Age, race, hepatic function or smoking history do not affect afatinib pharmacokinetics, while sex and body weight are considered clinically irrelevant variables as they only slightly influence afatinib pharmacokinetics and no adjustments are considered necessary accordingly.34,39 However, these subsets of patients should be closely monitored when receiving afatinib.
Afatinib as an upfront treatment in EGFR-mutant NSCLC
Several phase II and phase III clinical trials have been conducted to assess the efficacy of afatinib as an upfront treatment in patients with EGFR-mutant NSCLC.
Lux-Lung 3 (LL3) and Lux-Lung 6 (LL6) are two large randomized phase III clinical trials that compared afatinib with platinum-based standard chemotherapy. In LL3, 345 patients with advanced EGFR-mutant NSCLC were randomized to receive either afatinib (40 mg daily) or platinum plus pemetrexed at a standard dose every 21 days. Maintenance pemetrexed was not permitted. In this trial, patients were stratified by EGFR mutation (Del19, L858R, or any other mutation) and by race (Asian and non-Asian). The median PFS was significantly longer in the experimental arm, with 11.1 months for afatinib and 6.9 months for chemotherapy (HR: 0.58, 95% CI: 0.43–0.78; p = 0.001). The median PFS among those with exon 19 deletions and L858R EGFR mutations (n = 308) was 13.6 months in the afatinib arm and 6.9 months in the chemotherapy arm (HR: 0.47, 95% CI: 0.34–0.65; p = 0.001) (Table 1).41 In the LL6 trial, 364 were randomly assigned to afatinib (n: 242) or to gemcitabine and cisplatin (122). The median PFS was significantly longer in the afatinib group (11.0 months, 95% CI 9.7–13.7) than in the gemcitabine and cisplatin group (5.6 months, 5.1–6.7, HR: 0.28, 95% CI: 0.20–0.39, p < 0.0001) (Table 1).42 Although mature OS data of LL3 and LL6 were not available at the time of publication of both trials, a preplanned pooled analysis of LL3 and LL6 after 209 deaths in LL3 and 237 deaths in LL6 showed no difference in median OS between afatinib and chemotherapy in the intention to treat population. Specifically, the median OS was 25.8 months (95% CI: 23.1 to 29.3 months) in the afatinib group and 24.5 months (95% CI: 21.1 to 28.1 months) in the combined chemotherapy group (HR: 0.91, p = 0.37).43 However, it should be noticed that the high crossover rate at disease progression may have contributed to hinder the possibility to observe a difference in survival between the two groups.
When OS was analyzed according to the EGFR mutational status, treatment with afatinib was associated with significantly prolonged OS in patients harboring an exon19 deletion (33.3 versus 21.1 months; HR: 0.54, p = 0.0015 in LL3 and 31.4 versus 18.4 months; HR: 0.64, p = 0.0229), suggesting that this molecularly defined subgroup of patients might derive an additional benefit from afatinib.43
On the heels of this data, afatinib was subsequently compared with standard-of-care gefitinib in the Lux-Lung 7 (LL7) trial. LL7 was an exploratory phase IIb open-label trial that randomized 319 EGFR-mutant treatment-naïve NSCLC patient to receive afatinib (n: 160) or gefitinib (n: 159). At a median follow up of 27.3 months, median PFS was 11.0 months (95% CI: 10.6–12.9) with afatinib and 10.9 months (9.1–11.5) with gefitinib [HR: 0.73 (95% CI 0.57–0.95), p = 0.017] and median time to treatment failure (TTF) was 13.7 months (95% CI: 11.9–15.0) with afatinib versus 11.5 months (10.1–13.1) with gefitinib [HR: 0.73 (95% CI: 0.58–0.92), p = 0·0073]. The ORR was also significantly higher in the afatinib arm (70% versus 56%, p = 0.0083).44 In 2017 Pas Arez and colleagues presented the eagerly awaited mature OS data from this study. After a median follow up of 42.6 months, the median OS was 27.9 in the afatinib arm and 24.5 months in the gefitinib arm [HR = 0.86, (95% CI: 0.66–1.12), p = 0.2580]. Different from the LL3-LL6 joint analysis, prespecified subgroup analyses showed a similar OS trends (afatinib versus gefitinib) in patients with an exon 19 deletion [30.7 versus 26.4 months; HR: 0.83 (95% CI: 0.58–1.17), p = 0.2841] and L858R mutations [25.0 versus 21.2 months; HR 0.91, (95% CI 0.62–1.36), p = 0.6585], suggesting that an exon 19 deletion identifies a subgroup of patients with a different natural history and a better sensitivity to EGFR TKIs. Most patients (afatinib, 72.6%; gefitinib, 76.8%) had at least one subsequent systemic anticancer treatment following discontinuation of afatinib/gefitinib; 20 (13.7%) and 23 (15.2%) patients received a third-generation EGFR TKI. Updated PFS (as assessed by independent review), TTF and ORR data were significantly improved with afatinib.45
Overall, the aforementioned data support the use of afatinib as an upfront therapy; however, without providing evidence of a superiority compared with first-generation inhibitors in terms of OS.
Safety profile and tolerability
During the developmental program, afatinib showed a manageable safety profile, with most of drug-related adverse events (AEs) being of grade 1 or 2. In the dose-escalation study, with a 3 week on and 1 week off schedule, 40 patients (93%) experienced at least one AE. The most frequent AEs were diarrhea (65.1%), rash (58.1%), nausea (41.9%), vomiting (34.9%) and fatigue (20.9). However, a dose correlation for incidence and grade of diarrhea emerged, establishing 40 mg once daily as the recommended phase II dose (RP2D).46
The LL3 study evidenced a similar incidence of grade 3 or 4 AEs between the afatinib (49%) and the chemotherapy (48%) arms. The most frequent any-grade AEs for afatinib were diarrhea (95.2%), rash/acne (89.1%), stomatitis/mucositis (72.1%) and paronychia (56.8%), while in the chemotherapy arm were nausea (65.8%), vomiting (42.3%), fatigue (46.8%) and neutropenia (31.5%). With regard to grade 3 or greater AEs, diarrhea (14.4%), rash/acne (16.2%) and stomatitis (8.7%) were the most common in the afatinib arm. On the other hand, neutropenia (18%), anemia (6.3%) and fatigue (12.6%) were the most frequent in the chemotherapy group.41 A similar safety profile was reported in the LL6 study with rash/acne (15%) and diarrhea (15%) being the most common treatment-related grade 3 or 4 events, as compared with neutropenia (27%), vomiting (19%) and leukopenia (15%) which were the most frequent in the gemcitabine-cisplatin group. Afatinib-related AEs leading to treatment discontinuation were diarrhea (1%), paronychia (1%) and interstitial lung disease (1%) in the LL3 trial, and rash (2%) vomiting (14%), nausea (10%) in the LL6 trial.42 Importantly, both LL3 and LL6 studies provided an analysis of QoL based on the European Organisation for Research and Treatment of Cancer (EORTC) QLQLC12 and QLQ-C30 questionnaires. Specifically, treatment with afatinib resulted an improvement of preplanned symptoms with a significantly prolonged time to deterioration for cough and dyspnea and pain in both trials. Globally, longitudinal analysis of LL3 and LL6 trials demonstrated a significant better global health status and QoL with afatinib with respect to chemotherapy across both studies.41,42
In the LL7 trial the most frequent grade 3 or 4 AEs were diarrhea (13%), rash/acne (9%) and fatigue (6%) in the afatinib group, as compared with an elevation of aspartate transaminase and alanine transaminase blood levels (9%) and rash/acne (3%) in the gefitinib group. In 42% of patients receiving afatinib a dose reduction was required due to AE development. In contrast, only 2% of patients treated with gefitinib required a dose reduction. However, the overall discontinuation rate was 6% in both arms.
In conclusion, the toxicity profile of afatinib in patients with NSCLC is predictable and manageable with dose reduction and symptom control, and dose reduction does not seem to affect afatinib efficacy.
Real-life data
The clinical activity of afatinib has been also confirmed in real-life studies. A recent retrospective Taiwanese cohort study reported a 77.2% ORR and a median PFS of 11.8 months in patients with advanced EGFR-mutant NSCLC who received afatinib as an upfront therapy. In this study the clinical outcomes were not affected by dosage (40 mg versus < 40 mg) and EGFR-subtype mutation (del19 versus L858R).47 In contrast, another retrospective analysis involving 165 patients with metastatic NSCLC harboring an EGFR-sensitizing mutation treated with first-line afatinib showed an overall median PFS of 19.1 months with a significant difference according to the EGFR mutation subtype (19.1 months for del19, 15.8 months for L858R, 4.7 months for T790M and ‘not reached’ for uncommon mutations, p = 0.01).48 Further evidence of afatinib activity was derived from a noncomparative phase IIIb study conducted in a broad population of Asian patients with EGFR TKI-naïve advanced EGFR-mutant NSCLC. In this study the authors reported a median PFS of 12.1 months with a median time to symptomatic disease progression (mTTSP) of 15.3 months.49 Of note, the mTTSP [15.3 months (95% CI: 13.4–17.5)] was 3 months longer than PFS [12.1 months (10.8–13.7)], suggesting that afatinib may be safely continued beyond disease progression in selected cases. Intriguingly, both mTTSP and median PFS were longer in patients with common (with/without uncommon) EGFR mutations compared with those harboring uncommon mutations alone (PFS: 12.6 versus 9.1; TTSP: 15.8 versus 10.0 months).49
Activity of afatinib in patients with central nervous system metastases
Patients with oncogene-addicted NSCLC are at increased risk of developing brain metastasis (BMs) compared with the general population of NSCLC, and at least one-third of EGFR-mutant NSCLC patients develop central nervous system (SNC) involvement.50 The increased incidence of BMs in this subset of patients is likely to reflect the pharmacokinetic failure of TKIs and the unprecedented life expectancy achieved with targeted therapies, which eventually results in a higher likelihood of developing BMs during the course of disease.51,52 Several studies have evaluated the intracranial efficacy of reversible first-generation EGFR TKIs, gefitinib and erlotinib. The reported intracranial ORR (IORR) ranged from 43% in unselected patients to 89% in patients with a documented EGFR mutation.53 In addition, available data indicate that central nervous system (CNS) metastases from EGFR-mutant NSCLC respond equally to each of the two first-generation TKIs, despite erlotinib having higher rates of cerebrospinal fluid (CSF) penetration.54–56 Recently, in an attempt to further improve CNS permeability, dose escalation and high-dose ‘pulsatile’ gefitinib/erlotinib has been investigated in early-phase clinical trials.57–59 However, despite initial encouraging results, subsequent studies showed no convincing evidence that these regimens are superior in term of intracranial PFS and OS. Therefore, these approaches and are not currently recommend as a standard option and are still investigational.60 With regard to second-generation EGFR TKIs, afatinib showed an IORR of 35.5% in population molecularly enriched for EGFR mutations who had been pretreated with platinum chemotherapy and progressed following at least 6 months of gefitinib or erlotinib.61 Further evidence of afatinib activity in patients with CNS involvement derives form a subgroup analysis of patients with asymptomatic BMs in the LL3 trial, where afatinib showed a PFS of 11.1 months versus 5.4 months with cisplatin and pemetrexed.62 By contrast, in the head-to-head phase II LL7 trial, afatinib was not superior to gefitinib in the subgroup of patients with asymptomatic BMs (n = 26) and its use was limited by a greater toxicity.44 In contrast, in the FLAURA study patients with baseline CNS involvement benefitted from osimertinib in terms of PFS to a similar extent (HR = 0.47) than patients without CNS metastases (HR = 0.46). Moreover, CNS progression was significantly less frequent in the osimertinib group as compared with standard-of-care gefitinib or erlotinib, regardless of status with respect to known or treated CNS metastases at trial entry. Events of CNS progression were observed in 17 patients (6%) in the osimertinib group and 42 (15%) in the standard EGFR-TKI group.21 The intracranial activity of osimertinib has also been explored in a recent pooled analysis of the phase II extension component of the AURA and AURA2 trials. In this study, the CNS ORR was 54%, with 12% of complete CNS response, and a CNS disease control rate of 92%.63
Based on these data, a CNS-active TKI such as osimertinib should be considered the TKI of choice in patients with newly diagnosed NSCLC harboring an EGFR exon 19 deletion or L858R point mutations in light of the IORR and the durability of CNS control.
Place in therapy
Along with first-generation EGFR TKIs, afatinib is currently considered a standard first-line option for the treatment of patients with NSCLC harboring EGFR-sensitizing mutations. The approval of afatinib in this setting was initially based on the LL3 and LL6 trials, which showed a significantly higher ORR and prolonged PFS compared with standard chemotherapy.41,42 Subsequently, afatinib was compared with the first-generation EGFR TKI gefitinib in a head-to-head trial (LL7) where improved ORR and median PFS were compared with standard of care. However, it should be highlighted that afatinib did not prolong the OS when compared with standard chemotherapy nor with gefitinib.44 Although the lack of benefit in term of OS might be at least partly ascribed to the high rate of crossover in the LL3 and LL6 trials, this does not apply to the phase IIb LL7 study.
In light of the rapidly expanding landscape of treatments for EGFR-mutant NSCLC, defining a specific place for afatinib is challenging. To date, afatinib is the only TKI to have prolonged OS compared with chemotherapy in patients with EGFR exon 19 deletions but not in those with L858R point mutations, suggesting that patients harboring these two different types of mutation belong to different biological subgroups with different sensitivity to EGFR TKIs.43 Besides, in the LL7 trial afatinib significantly prolonged PFS and TTF compared with gefitinib, regardless of the EGRF mutation subtype, which might reflect the potential of afatinib in delaying the development of acquired resistance to treatment in the overall population of EGFR-mutant NSCLC patients.44
On the other hand, Mok and colleagues have recently reported the OS survival data from the ARHCER 1050, a phase III trial comparing gefitinib with the irreversible second-generation EGFR TKI, dacomitinib, in patients with treatment-naïve newly diagnosed EGFR-mutant NSCLC.64 In this study the median OS was 34.1 months with dacomitinib versus 26.8 months with gefitinib [HR: 0.760 (95% CI: 0.582–0.993, p = 0.044)] while the OS probabilities at 30 months were 56.2% and 46.3% with dacomitinib and gefitinib, respectively. This trial is the first to demonstrate a survival advantage for a second-generation EGFR TKI compared with first-generation inhibitors and potentially displaces afatinib as the preferred second-generation agent to be considered up front in patients with EGFR-mutant NSCLC.64
However, some considerations should be taken into account. First of all, patients with BMs were excluded from the trial because of the unknown capacity of dacomitinib to penetrate the blood–brain barrier (BBB), while afatinib has been largely demonstrated to exert activity against BMs in patients with EGFR-mutant NSCLC.65–69 Moreover, in light of the results of the FLAURA trial, in which the upfront third-generation EGFR TKI, osimertinib, significantly prolonged the median PFS compared with gefitinib or erlotinib [18.9 months versus 10.2 months; HR: 0.46 (95% CI: 0.37–0.57), p < 0.001] and proved to be extremely active in the CNS, the absence of data on dacomitinib’s activity within the CNS certainly would have an impact on oncologists’ daily practice.21 In light of these data, the FLAURA trial raises the question whether osimertinib has to be considered the best first-line therapy for patients with del19 or L858R EGFR genotypes or whether it should be used on relapse upon documentation of a T790M resistance mutation. The exclusion of second-generation EGFR TKIs from the comparator arm represents a limitation of the study, as at time of the FLAURA trial, initiation afatinib was not widely used as the standard of care, while dacomitinib was still investigational. In this context, whether the baseline T790M mutation assessment should be used to guide patient’s selection is unknown.
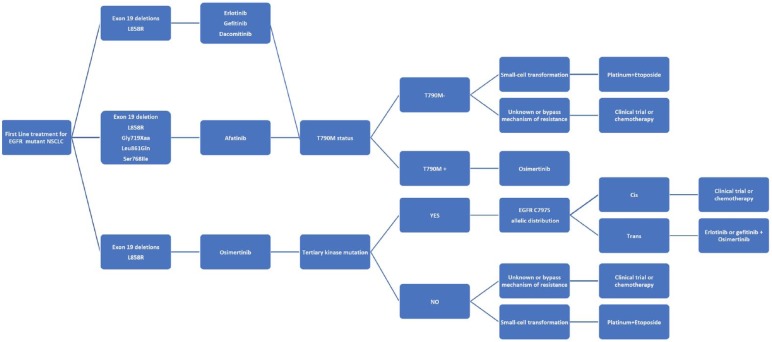
Another issue that still needs to be addressed is optimal therapeutic approach to patients with baseline non-T790M ‘uncommon’ mutations. Against this background, afatinib has demonstrated a substantial grade of activity against EGFR ‘uncommon’ mutations as recently shown by a pooled analysis of the LL2, LL3 and LL6 trials.26 In contrast, there are a lack of data about the efficacy of either dacomitinib or osimertinib in patients with ‘uncommon’ mutations. Nonetheless, preclinical evidence indicates that afatinib has the lowest IC50 for exon 18 mutations (E709X, G719X, del18), S768I at exon 20 and L861Q at exon 21, as compared with other second or third-generation EGFR TKIs, including dacomitinib, neratinib, osimertinib and rociletinib, thus further corroborating the rationale for the use of afatinib in this subset of patients.22,25 A summary of treatment options in EGFR-mutant NSCLC is schematized in Figure 2.
Figure 2.
Treatment options in EGFR-mutant NSCLC.
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer
Conclusion
Afatinib represents an important first-line option for patients with advanced NSCLC harboring an EGFR-sensitizing mutation, having definitely been shown to prolong PFS and improve ORR compared with chemotherapy and the first-generation EGFR TKI, gefitinib. Moreover, preclinical data and clinical evidence support the use of afatinib in patients harboring EGFR ‘uncommon’ mutations (especially G719X, S768I and complex mutations). Certainly, the ARCHER study has set an additional new option for the upfront treatment for patients with EGFR-mutant (del19 and L858R) NSCLC, potentially offering a survival advantage over afatinib as an upfront TKI. In addition, the FLAURA study has confirmed an astounding activity of the third-generation osimertinib in the first-line setting, thus further displacing afatinib form this setting. However, in this study the comparators were only the first-generation TKIs, gefitinib and erlotinib, which leaves unanswered the question of whether starting up front with a third-generation EGFR TKI is superior to the use in sequence of a second-generation TKI (either afatinib or dacomitinib) followed by osimertinib in cases of documented T790M secondary mutations at disease progression. However, these data are still preliminary, and the mature OS will provide us further insight about the optimal initial management of our patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Biagio Ricciuti  https://orcid.org/0000-0002-0651-2678
https://orcid.org/0000-0002-0651-2678
Contributor Information
Biagio Ricciuti, Department of Medical Oncology, Santa Maria della Misericordia Hospital, University of Perugia, Piazzale L. Severi n. 1, 06132 Perugia, Italy.
Sara Baglivo, Department of Medical Oncology, Santa Maria della Misericordia Hospital, University of Perugia, Piazzale Menghini, Perugia, Italy.
Andrea De Giglio, Department of Medical Oncology, Santa Maria della Misericordia Hospital, University of Perugia, Piazzale Menghini, Perugia, Italy.
Rita Chiari, Department of Medical Oncology, Santa Maria della Misericordia Hospital, University of Perugia, Piazzale Menghini, Perugia, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ricciuti B, De Giglio A, Mecca C, et al. Precision medicine against ALK-positive non-small cell lung cancer: beyond crizotinib. Med Oncol 2018; 35: 72. [DOI] [PubMed] [Google Scholar]
- 3. Leonetti A, Facchinetti F, Rossi G, et al. BRAF in non-small cell lung cancer (NSCLC): pickaxing another brick in the wall. Cancer Treat Rev 2018; 66: 82–94. [DOI] [PubMed] [Google Scholar]
- 4. Facchinetti F, Rossi G, Bria E, et al. Oncogene addiction in non-small cell lung cancer: focus on ROS1 inhibition. Cancer Treat Rev 2017. ; 55: 83–95. [DOI] [PubMed] [Google Scholar]
- 5. Schallenberg S, Merkelbach-Bruse S, Buettner R. Lung cancer as a paradigm for precision oncology in solid tumours. Virchows Arch 2017; 471: 221–233. [DOI] [PubMed] [Google Scholar]
- 6. Tsiambas E, Lefas AY, Georgiannos SN, et al. EGFR gene deregulation mechanisms in lung adenocarcinoma: a molecular review. Pathol Res Pract 2016; 212: 672–677. [DOI] [PubMed] [Google Scholar]
- 7. Fogli S, Polini B, Del Re M, et al. EGFR-TKIs in non-small-cell lung cancer: focus on clinical pharmacology and mechanisms of resistance. Pharmacogenomics 2018; 19: 727–740. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN Guidelines): non-small cell lung cancer. version 1. 2018, Fort Washington: National Comprehensive Cancer Network, Inc. [Google Scholar]
- 9. Han JY, Park K, Kim SW, et al. First-signal: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 10. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 11. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 12. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin– paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 13. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non-small cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation–positive non-small-cell lung cancer: analyses from the phase iii, randomized, open-label, ensure study. Ann Oncol 2015; 26: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 15. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation–positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase study. Lancet Oncol 2011; 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 16. Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008; 27: 4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ricciuti B, Baglivo S, Paglialunga L, et al. Osimertinib in patients with advanced epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer: rationale, evidence and place in therapy. Ther Adv Med Oncol 2017; 9: 387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372: 1689–1699. [DOI] [PubMed] [Google Scholar]
- 19. Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Metpositive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016; 17: 1643–1652. [DOI] [PubMed] [Google Scholar]
- 20. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2016; 376: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi Y, and Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci 2016; 107: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl. 5): v1–v27. [DOI] [PubMed] [Google Scholar]
- 24. Antonicelli A, Cafarotti S, Indini A, et al. EGFR-targeted therapy for non-small cell lung cancer: focus on EGFR oncogenic mutation. Int J Med Sci 2013; 10: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi Y, Togashi Y, Yatabe Y, et al. EGFR exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first- or third-generation TKIs. Clin Cancer Res 2015; 21: 5305–5313. [DOI] [PubMed] [Google Scholar]
- 26. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015; 16: 830–838. [DOI] [PubMed] [Google Scholar]
- 27. Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013; 8: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012; 13: e23–e31. [DOI] [PubMed] [Google Scholar]
- 29. Floc’h N, Martin MJ, Riess JW, et al. Antitumor activity of osimertinib, an irreversible mutant-selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR exon 20 insertions. Mol Cancer Ther 2018; 17: 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piotrowska Z, Fintelmann FJ, Sequist LV, et al. Response to osimertinib in an EGFR exon 20 insertion-positive lung adenocarcinoma. J Thorac Oncol 2018; 13: e204–e206. [DOI] [PubMed] [Google Scholar]
- 31. van Veggel B, de Langen AJ, Hashemi SMS, et al. Afatinib and cetuximab in four patients with EGFR Exon 20 insertion–positive advanced NSCLC. J Thorac Oncol 2018; 13: 1222–1226. [DOI] [PubMed] [Google Scholar]
- 32. Kim EY, Cho EN, Park HS, et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 2016; 17: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013; 8: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. European Medicines Agency. Giotrif: summary of product characteristics, version 24 May 2016, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002280/WC500152392.pdf (accessed 1 July 2018).
- 35. Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008; 27: 4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ninomiya T, Takigawa N, Ichihara E, et al. Afatinib prolongs survival compared with gefitinib in an epidermal growth factor receptor-driven lung cancer model. Mol Cancer Ther 2013; 12: 589–597. [DOI] [PubMed] [Google Scholar]
- 37. Solca F, Dahl G, Zoephel A, et al. target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012; 343: 342–350. [DOI] [PubMed] [Google Scholar]
- 38. Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16: 897–907. [DOI] [PubMed] [Google Scholar]
- 39. Boehringer Ingelheim. Gilotrif prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc, http://www.gilotrif.com/ (accessed 1 July 2018). [Google Scholar]
- 40. Wind S, Schnell D, Ebner T, et al. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet 2017; 56: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 42. Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–222. [DOI] [PubMed] [Google Scholar]
- 43. Yang JC-H, Wu Y-L, Schuler M, et al. Afatinib versus cisplatin based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. [DOI] [PubMed] [Google Scholar]
- 44. Park K, Tan E-H, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive nonsmall- cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577–589. [DOI] [PubMed] [Google Scholar]
- 45. Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017; 28: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marshall J, Hwang J, Eskens FA, et al. A phase I, open-label, dose escalation study of afatinib, in a 3-week-on/1-week-off schedule in patients with advanced solid tumors. Invest New Drugs 2013; 31: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim Y, Sun J, Park K, et al. P3.01-023 First-line afatinib for non-small cell lung cancer in real world practice. J Thorac Oncol 2017; 12(Suppl. 2): S2209. [Google Scholar]
- 48. Wu Y, Tu H, Feng J, et al. P3.01-036 A phase IIIb open-label, single-arm study of afatinib in EGFR TKI-naive patients with EGFRm NSCLC: an interim analysis. J Thorac Oncol 2017; 12(Suppl. 2): S2214. [Google Scholar]
- 49. Liang SK, Hsieh MS, Lee MR, et al. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation positive lung adenocarcinoma. Oncotarget 2017; 8: 90430–90443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun M, Behrens C, Feng L, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res 2009; 15: 4829–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010; 16: 5873–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsumoto S, Takahashi K, Iwakawa R, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. In J Cancer 2006; 119:1491–1494. [DOI] [PubMed] [Google Scholar]
- 53. Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res 2012; 18: 938–944. [DOI] [PubMed] [Google Scholar]
- 54. Zhao J, Chen M, Zhong W, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer 2013; 14: 188–193. [DOI] [PubMed] [Google Scholar]
- 55. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012; 70: 399–405. [DOI] [PubMed] [Google Scholar]
- 56. Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2014; 2: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non–small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 2006; 24: 4517–4520. [DOI] [PubMed] [Google Scholar]
- 58. Clarke JL, Pao W, Wu N, et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010; 99: 283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget 2015; 6: 4527–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Metro G, Chiari R, Ricciuti B, et al. Pharmacotherapeutic options for treating brain metastases in non-small cell lung cancer. Expert Opin Pharmacother 2015; 16: 2601–2613. [DOI] [PubMed] [Google Scholar]
- 61. Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015; 10: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016; 11: 380–390. [DOI] [PubMed] [Google Scholar]
- 63. Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 2018; 29: 687–693. [DOI] [PubMed] [Google Scholar]
- 64. Mok TS, Cheng Y, Zhou X, et al. improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018; 36: 2244–2250. [DOI] [PubMed] [Google Scholar]
- 65. Li SH, Liu CY, Hsu PC, et al. Response to afatinib in treatment-naïve patients with advanced mutant epidermal growth factor receptor lung adenocarcinoma with brain metastases. Expert Rev Anticancer Ther 2018; 18: 81–89. [DOI] [PubMed] [Google Scholar]
- 66. Kawaguchi Y, Hanaoka J, Hayashi H, et al. clinical efficacy of afatinib treatment for a patient with leptomeningeal carcinomatosis. Chemotherapy 2017; 62: 147–150. [DOI] [PubMed] [Google Scholar]
- 67. Hochmair M, Holzer S, Burghuber OC. Complete remissions in afatinib-treated non-small-cell lung cancer patients with symptomatic brain metastases. Anticancer Drugs 2016; 27: 914–915. [DOI] [PubMed] [Google Scholar]
- 68. Zhang SR, Zhu LC, Jiang YP, et al. Efficacy of afatinib, an irreversible ErbB family blocker, in the treatment of intracerebral metastases of non-small cell lung cancer in mice. Acta Pharmacol Sin 2017; 38: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Watanabe S, Hayashi H, Nakagawa K. Is afatinib a treatment option for brain metastases in patients with EGFR mutation-positive non-small cell lung cancer? Ann Transl Med 2016; 4: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]