Abstract
Background:
Cortical lesions (CLs) are typical of multiple sclerosis (MS) and have been recently incorporated in MS diagnostic criteria. Thus, the ‘no evidence of disease activity’ (NEDA) definition should now include CLs. The aim of this study was to evaluate the NEDA3 + CL status in natalizumab- or fingolimod-treated relapsing remitting MS (RMS) patients.
Methods:
Natalizumab- or fingolimod-treated RMS patients were enrolled in a 2-year longitudinal study based on clinical and magnetic resonance imaging (MRI) evaluations performed respectively biannually and annually. CLs were detected by double inversion recovery. The NEDA3 + CL condition was evaluated at baseline (T0) and at the end of the first (T1) and second (T2) year.
Results:
Of the 137 RMS patients included in the study, 86 were propensity-matched. At T2, the annualized relapse rate was lower on natalizumab (p = 0.021), but the effect on white matter lesions (p = 0.29) and the proportion of NEDA-3 patients (p = 0.14) were similar in the two treatment arms. At T2, 11.6% natalizumab- and 62.8% fingolimod-treated patients had new CLs (p < 0.001) and a higher proportion of natalizumab-treated patients (55.8% versus 11.6%, p < 0.001) achieved the NEDA3 + CL status (hazard ratio 5.2, p < 0.001).
Conclusion:
The incorporation of CLs in the NEDA-3 definition highlighted the higher efficacy of natalizumab versus fingolimod in suppressing disease activity in RMS patients.
Keywords: cortical lesions, fingolimod, multiple sclerosis, natalizumab, NEDA
Introduction
Natalizumab (Tysabri®. Biogen, Cambridge, MA, USA), an anti-very late antigen monoclonal antibody, and fingolimod (Gilenya®, Novartis Pharmaceutical, Basel, Switzerland), a sphingosine 1-phosphate receptor modulator, are widely used for the treatment of multiple sclerosis (MS).1–5 Although no head-to-head randomized clinical trial has been conducted to date, multicentre observational studies, based on large MS databases and on propensity score matching, have tried to compare the efficacy of these two drugs, but the results were discordant6–12 and magnetic resonance imaging (MRI) data, which constitute a relevant outcome in clinical practice, were only rarely available.
Recently, cortical lesions (CLs)13 have been definitively recognized to be a typical aspect of brain pathology in MS and, thus, included in the MS diagnostic criteria14 to acquire the dissemination in space (DIS) criterion of lesions. Up to date, no study has compared the ability of natalizumab and fingolimod in preventing the accumulation of CLs or has investigated the occurrence of the ‘not evidence of disease activity 3’ (NEDA-3) status after incorporation of CLs in the neuroradiological parameters included in the NEDA definition. Thus, we performed a propensity score-matched comparative analysis of the NEDA-3 status including CLs in relapsing MS (RMS) treated for 2 years with natalizumab or fingolimod.
Material and methods
Study population
All patients with a diagnosis of RMS, in agreement with the 2010 McDonald criteria,15 who started therapy with natalizumab or fingolimod after the 1 July 2012, were enrolled in a 2-year longitudinal prospective study. Patients were treated according to the rules defined by the Italian Medicines Agency (AIFA; www.aifa.it). Namely, one of these two conditions were respected: (1) lack of clinical response to a first-line immune-modulating therapy taken for 1 year (criterion A) or (2) severe and rapidly evolving disease, defined by the presence of at least two clinical relapse in the last year associated with gadolinium-enhancing or new white matter lesions, even in untreated patients (criterion B). The study was approved by the ‘Comitato Etico per la Sperimentazione Clinica della Provincia di Padova’ and informed written consent was obtained.
Clinical follow up
All patients were assessed every 6 months by means of a complete neurological and Expanded Disability Status Scale (EDSS) evaluation. Physicians were blinded to MRI findings but not to the on-going treatment. Clinical NEDA was achieved in the absence of both clinical relapse and disability accumulation. A clinical relapse was defined as the occurrence of new symptoms or exacerbation of existing symptoms that lasted for 24 h or longer, in the absence of concurrent illness or fever, and occurring 30 days or more since a previous relapse. The development of any cortical symptom was also carefully evaluated. The definition of relapse used in our study did not require confirmation by change in EDSS. Disability accumulation was defined as an increase in EDSS by 1 step (1.5 steps if baseline EDSS was 0 and 0.5 steps if baseline EDSS was >5.5) confirmed at 6 months.16
MRI protocol and follow up
Images were acquired annually (at baseline, T0, and after one, T1, and 2, T2, years) using a 1.5 T scanner (Achieva, Philips Medical Systems, Best, The Netherlands) with 33 mT/m power gradient and a 16-channel head coil. The following images were acquired for each subject: (a) fast fluid attenuated inversion recovery: repetition time (TR) = 10,000 ms; echo time (TE) = 120 ms; inversion time (TI) = 2500 ms; echo train length (ETL) = 23; 50 contiguous axial slices with thickness = 3.0 mm; matrix size = 172 × 288; and field of view (FOV) = 250 × 200 mm2; (b) three-dimensional (3D) fast field echo (FFE): TR = 25 ms; TE = 4.6 ms; 120 contiguous axial slices with the off-centre positioned on zero with thickness = 1.2 mm; flip angle = 30°; matrix size = 256 × 256; and FOV = 250 × 250 mm2; (c) post-contrast T1-weighted spin echo: TR = 618 ms; TE = 10 ms; 20 contiguous axial slices with thickness = 5.5 mm; flip angle 90°; matrix size = 224 × 256; and FOV = 230 × 230 mm2; acquired 5 min after intravenous administration of polycyclic gadolinium-ethylenediaminetetraacetic acid (EDTA) acid (0.1 mMol/kg); (d) double inversion recovery sequences: TR = 15,631 ms; TE = 25 ms; TI = 3400 ms; delay = 325 ms; ETL = 17; 50 contiguous axial slices with thickness = 3 mm; matrix size = 130 × 256; and FOV = 250 × 200 mm2. Participants were positioned for serial measurements according to published guidelines for serial MRI studies in MS.17 MRI NEDA was defined as the absence of new/enlarging white matter lesions or gadolinium-enhancing lesions. NEDA3 + CLs included no evidence of new CLs, which were identified by a consensus of two experienced observers (SZ and CC) accordingly with current guidelines.18 Follow-up MRI sequences were compared with baseline by the same observers, who independently evaluated the presence of any new or enlarging white or grey matter lesion.
Statistical analysis
The propensity-matched population was identified by the means of a multivariable logistic regression model of treatment allocation that used the following demographic and clinical variables at the time of treatment assignment as independent variables: sex, age, time from first MS symptom (hereafter referred as disease duration), EDSS, number of relapses in the previous 12 months and prescription criteria (A or B). For normally distributed variables the Student’s t test was performed. For ordinal categorical variables, the Mann–Whitney U test was performed, while for no ordinal variables, the Pearson’s Chi-square test was used. A total of three timespans were evaluated: T0–T1, T1–T2, and T0–T2. Chi-square was applied to compare the percentage of patients reaching clinical NEDA, radiological NEDA, NEDA-3 and NADA-3 + CL status in the two cohorts of patients at each time points. Kaplan–Meier and Cox regression analyses were applied to compare the two drugs and to identify basal clinical and demographic variables eventually associated with NEDA status. The significance level was set at 0.05.
Results
Propensity-matched study population
A total of 137 RMS patients were included in the study. Propensity matching retained 86 patients (63%) in the analysis, 43 treated with natalizumab and 43 with fingolimod. These two cohorts did not differ in age at onset (p = 0.7), sex (p = 0.4), disease duration (p = 0.9), criteria for drug prescription (p = 0.3), clinical relapses in the previous 12 months (p = 0.4) and baseline EDSS (p = 1.0) as shown in Table 1.
Table 1.
Clinical and demographic features of natalizumab- and fingolimod-treated MS patients.
| Natalizumab | Fingolimod | p-value | |
|---|---|---|---|
| Age at disease onset (y) | 27.7 ± 9.4 | 28.7 ± 9.8 | 0.7 |
| Sex (female/male) | 32/11 | 28/15 | 0.4 |
| Disease duration at study inclusion (m) | 114.0 ± 94.0 | 110.4 ± 98.4 | 0.9 |
| Criterion of prescription (A/B) | 32/11 | 36/7 | 0.3 |
| Relapses during the 12 months before study inclusion | 1.4 ± 0.8 | 1.2 ± 0.6 | 0.4 |
| EDSS at study inclusion | 2.0 (1.0–6.5) | 2.0 (1.0–6.5) | 1.0 |
| Number of previous treatments | 1 (0–5) | 1 (0–6) | 0.3 |
| Previous treatment: none | 11 | 7 | 0.5 |
| Glatiramer acetate | 5 | 7 | |
| Interferon | 25 | 25 | |
| Cyclophosphamide | 1 | 3 | |
| Mitoxantrone | 0 | 1 | |
| Azathioprine | 1 | 0 |
EDSS, Expanded Disability Status Scale; m, months; MS, multiple sclerosis; y, years.
Clinical outcome
At T2, the annualized relapse rate (ARR) was reduced from 1.2 ± 0.6 to 0.1 ± 0.3 (p < 0.001) in natalizumab-treated patients and from 1.3 ± 0.8 to 0.4 ± 0.5 in fingolimod-treated patients (p < 0.001), and the difference was significant (p = 0.021). At T2, the percentage of relapse-free patients was also higher on natalizumab (95% versus 70%, p < 0.005). This finding was further confirmed by Cox regression analysis [hazard ratio (HR): 13.5; 95% confidence interval (CI): 1.8–103.9; p < 0.05; Figure 1(a)].
Figure 1.
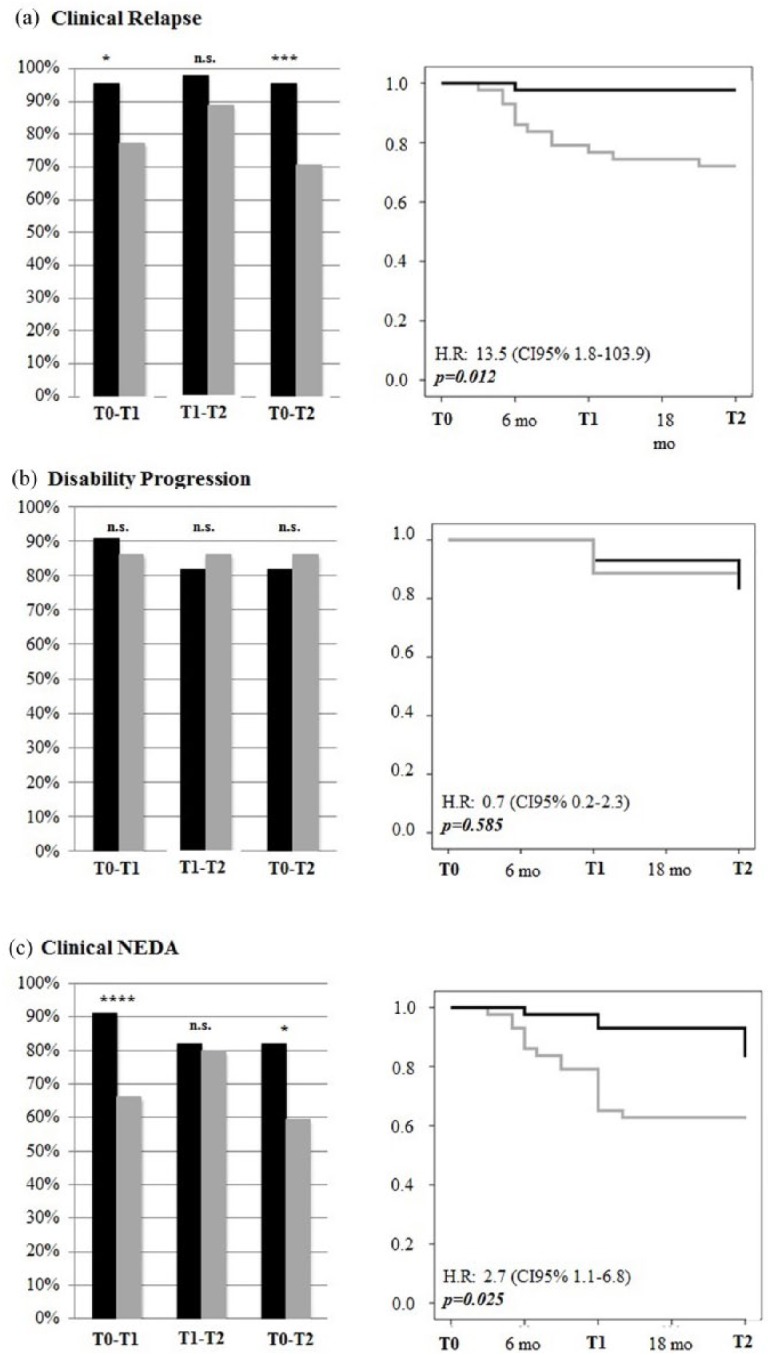
Clinical outcomes. Left panel: Percentage of patients treated with natalizumab (black bars) or fingolimod (grey bars) that achieved relapse-free (a), no increase in EDSS (b) or clinical NEDA (c) status at each timespan (T0–T1, T1–T2, T0–T2). *p < 0.05; **p < 0.01; ***p < 0.005. Right panel: Survival curves demonstrating the risk of relapse (a), EDSS increase (b) or both, that is, clinical NEDA (c) in natalizumab- (black line) or fingolimod-treated (grey line) patients.
EDSS, Expanded Disability Status Scale; NEDA, no evidence of disease activity.
No difference in the 6-month sustained disability progression rate was observed between the two treatments. Indeed, increased EDSS values were observed only in four natalizumab-treated and three fingolimod-treated patients (HR: 0.7; 95% CI 0.2–2.3; p = 0.59) as shown in Figure 1(b).
At T2, the percentage of natalizumab-treated patients that had clinical NEDA was significantly higher compared with fingolimod-treated patients (82% versus 59%, p < 0.05). The proportion of patients achieving clinical NEDA was also higher on natalizumab [p < 0.05; Figure 1(c)], a finding clearly supported by the more rapid effect of natalizumab observed in the timespan T0–T1 (p < 0.01)
Radiological outcome
The percentage of patients with radiological NEDA was identical at T1, but significantly differed (p < 0.05) in T1–T2 in favour of natalizumab (Figure 2). Accordingly, the survival curves were almost identical at T1 and started to differ at T2, but the difference was not significant (p = 0.29; Figure 2).
Figure 2.
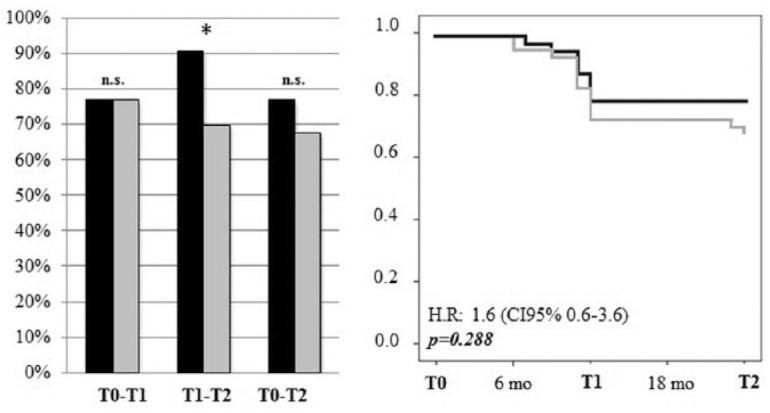
Radiological NEDA. Left panel: Percentage of patients treated with natalizumab (black bars) or fingolimod (grey bars) that achieved radiological NEDA at each timespan (T0–T1, T1–T2, T0–T2). *p < 0.05; Right panel: Survival curves demonstrating the risk of new/enlarging white matter lesions or gadolinium-enhancing lesions (natalizumab: black line, fingolimod: grey line).
NEDA, no evidence of disease activity.
NEDA-3
A higher percentage of natalizumab-treated patients achieved NEDA-3 status in all the timespans, but the difference with fingolimod-treated patients was not significant (Figure 3). Moreover, survival curves revealed only a trend in favour of natalizumab (p = 0.144) sustained by the effect of the drug in the second year of therapy (Figure 3). Cox regression analysis showed that the NEDA-3 status was significantly associated with a lower disease duration (HR: 0.995; 95% CI: 0.990–0.999; p = 0.025) and lower baseline EDSS score (HR: 1.30; 95% CI: 1.053–1.608; p = 0.015), but not with sex, age, number of relapses in the previous 12 months, and criterion A or B.
Figure 3.
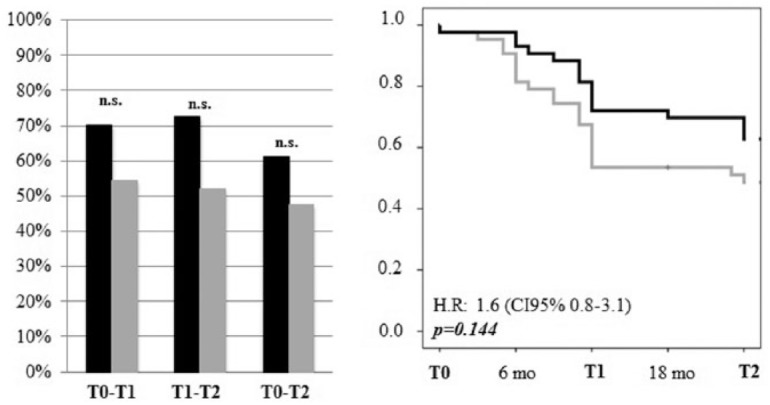
NEDA-3. Left panel: Percentage of patients treated with natalizumab (black bars) or fingolimod (grey bars) that achieved NEDA-3 status at each timespan (T0–T1, T1–T2, T0–T2). Right panel: Survival curves demonstrating the proportion of patients that achieved the NEDA-3 status (natalizumab: black line, fingolimod: grey line).
NEDA, no evidence of disease activity.
NEDA-3 + CLs
At T2, new CLs were observed in 11.6% of natalizumab and 62.8% of fingolimod-treated patients (p < 0.005) and NEDA-3 + CL status was achieved in 55.8% of natalizumab- and in 11.6% of fingolimod-treated patients (p < 0.001; Figure 4). These findings were further confirmed by Cox regression analysis (p < 0.001; Figure 4).
Figure 4.
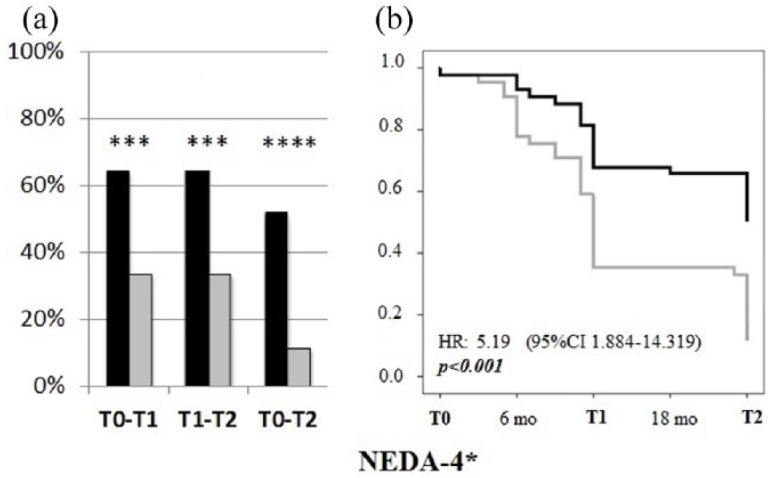
NEDA-3+CLs. Left panel: Percentage of patients treated with natalizumab (black bars) or fingolimod (grey bars) that achieved NEDA-3 + CL status at each timespan (T0–T1, T1–T2, T0–T2). *p < 0.05; ***p < 0.005. Right panel: Survival curves showing the proportion of patients that achieved the NEDA-3 + CL status (natalizumab: black line, fingolimod: grey line).
CL, cortical lesion; NEDA, no evidence of disease activity.
Discussion
No randomized controlled head-to-head trial assessing the efficacy of natalizumab versus fingolimod has been carried out, and the comparative observational studies published to date have yielded contradictory results that can be explained by the different methodologies applied, the retrospective collection of data and the baseline characteristics of the patients.
In the retrospective United States database analysis, natalizumab and fingolimod had similar effects on the relapse rate, but relapse identification was based on database claims rather than on clinical assessment, and no EDSS and MRI findings were reported.19 In line with these findings, in the Austrian nationwide observational analysis, despite a lower mean ARR in natalizumab-treated patients, no difference was observed analysing the log-transformed ARR, the probability of experiencing a relapse and EDSS progression.9 Finally, no significant difference in clinical efficacy between the two drugs was also observed in the propensity score matching based observational study that analysed data of the Danish Multiple Sclerosis Treatment Register,12 which, however, lacked MRI information.
We observed that in natalizumab-treated patients the mean ARR was lower and the proportion of relapse-free patients was higher. These findings are in line with those of other observational studies. For instance, in the large international, observational prospective study based on MSBase, natalizumab was more effective than fingolimod in active RMS (i.e. not responding to injectable first-line therapies).20 This study suggested that the impact of these two drugs on more active disease courses might be different. A further study based on propensity score-matched patients from the same MSBase and six other cohorts21 confirmed the superiority of natalizumab on clinical parameters. However, as stated by the authors, limitations of this study were the absence of systematic and comparable acquisition of data, and the lack of radiological outcomes. More recently, a study based on the registry of the Swiss Federation for Common Tasks of Health Insurances, disclosed that natalizumab-treated patients had a lower risk of relapse and were more likely to experience EDSS improvement compared with fingolimod-treated patients.22 Finally, three observational studies which included MRI parameters of white matter pathology into the propensity score-based model,6,23,24 in patients switching from first-line therapies for lack of efficacy, showed a higher effectiveness of natalizumab in both clinical and MRI parameters.
Since an active MRI may induce clinicians to prefer natalizumab than fingolimod even under the same clinical parameters, MRI data are important in studies aimed at comparing the efficacy of these two drugs. In particular, on-treatment evaluation of T2 white matter lesion accumulation is an essential MRI parameter of efficacy and helps to achieve the evidence of NEDA. Certainly, comparable and reproducible MRI data cannot be obtained by a nationwide register or large MS databases, while single centre studies, based on well controlled and homogeneous clinical and MRI data, may help to figure out the efficacy profile of disease modifying drugs, especially in comparison studies.
In our prospective, observational, single centre, propensity score-matching-based study, we analysed, for the first time, the efficacy of natalizumab and fingolimod in terms of inducing a NEDA-3 status that incorporated CLs, a definitely recognized typical element of brain pathology in MS25,26 which is currently used to achieve the criterion of DIS of lesions.14 In the majority of clinical and radiological parameters, natalizumab was superior to fingolimod, and in some comparisons the difference was significant. Interestingly, the incorporation of CLs in the NEDA-3 definition further increased the evidence of a higher efficacy of natalizumab in suppressing brain inflammation in RMS.
The most original aspect of our study is the differential effect of the two drugs on focal grey matter inflammation. Histological studies have disclosed significant qualitative and quantitative differences between grey and white matter inflammation in MS.13,27 To what extent these differences may account for the higher efficacy of natalizumab is unknown and deserves further investigation. However, our data suggest that the suppression of CLs, which are characterized by a lower degree of inflammation and normal (or only mildly impaired) blood–brain barrier compared with white matter lesions, is better achieved by natalizumab. Indeed, this drug efficiently blocks the entry of lymphocytes into the brain independently of the blood–brain barrier condition.
Although whole brain and grey matter atrophy constitutes a relevant aspect of MS pathology,28 no data on cortical atrophy are here presented. Indeed, controversial findings on the effects of natalizumab on brain atrophy are available in the literature. These discrepancies can be primarily explained by the marked pseudo-atrophy effect observed in natalizumab-treated MS patients, especially in those with very high disease activity and coming from first-line treatment failure, as the majority of the patients included in our study. Moreover, in patients with very high disease activity, baseline white matter inflammation was found to strongly influence brain volume measures up to 18–24 months after natalizumab initiation.29,30 On the other hand, data from two phase III randomized controlled clinical trials showed that fingolimod was able to reduce the progression of deep grey matter and the thalamus, but not cortical atrophy compared with placebo over 24 months. In addition, ventricular volume enlargement was also less significant in fingolimod-treated patients compared with placebo, thus suggesting a less prominent pseudo-atrophy effect.31 For all these reasons, a correct comparison of natalizumab versus fingolimod effects on brain or grey matter atrophy cannot be achieved with a 2-year study. Thus, the patients enrolled in this study will be followed for as long as possible in order to achieve reliable data on this aspect of MS-related pathology.
From a therapeutic point of view, the influence of both disease duration and EDSS on NEDA-3 status indicates the opportunity of a switch to second-line therapies as soon as possible, even in presence of a mild physical disability. Moreover, considering that relapses and MRI lesions were found to be surrogate markers of EDSS progression in a 2-year follow-up period,32 the greater effects of natalizumab, especially on grey matter focal damage, suggests natalizumab is a better treatment in patients with more active disease, especially in those having evidence of clinical or MRI cortical pathology.
A limitation of our study might be the restricted number of patients analysed after propensity matching. However, the significant effect of natalizumab on NEDA-3 + CLs and the homogeneity of all the other findings, although obtained in a limited cohort of patients, highlight the strength of our observations.
In summary, in our prospective, observational, single centre, propensity-matched score-based study, natalizumab was found to be more effective than fingolimod in RMS, especially when CLs were included in the NEDA definition (NEDA-3 + CLs).
Acknowledgments
We thank all the patients recruited in this study and the staff of our Multiple Sclerosis Centre (Mrs Elisa Quaggia, Mrs Irene Boscariol, Mrs Silvia Frigato and Mrs Elena Lazzaretto).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: MP reports grants and personal fees from Novartis, grants and personal fees from Almirall, grants and personal fees from Biogen Idec, grants and personal fees from Sanofi Genzyme, grants from Teva, outside the submitted work. CC, ES and MA have nothing to disclose. SZ reports grants from Sanofi Genzyme, grants from Almirall, outside the submitted work. FR serves as an advisory board member of Biogen Idec and Sanofi Genzyme and has received funding for travel and speaker honoraria from Merck Serono, Biogen Idec, Sanofi-Aventis, Teva and Bayer Schering Pharma. PP has received funding for travel and speaker honoraria from Merck Serono, Biogen Idec, Sanofi-Aventis, and Bayer Schering Pharma and has been consultant for Merck Serono, Biogen Idec and Teva. PG reports grants and personal fees from Novartis, grants and personal fees from Almirall, grants and personal fees from Biogen Idec, grants and personal fees from Sanofi Genzyme, grants and personal fees from Teva, grants and personal fees from Merck Serono, grants from University of Padova, grants from the Italian Ministry of Public Health, grants from the Veneto Region of Italy, and grants from the Italian Association for Multiple Sclerosis, outside the submitted work.
ORCID iD: Marco Puthenparampil  https://orcid.org/0000-0002-2313-8462
https://orcid.org/0000-0002-2313-8462
Contributor Information
Marco Puthenparampil, Multiple Sclerosis Centre, Department of Neuroscience DNS, Univeristà Degli Studi di Padova, Via Giustinaini 2, 35128, Padova, Italy.
Chiara Cazzola, Multiple Sclerosis Centre, Department of Neuroscience DNS, University of Padua, Padua, Italy.
Sofia Zywicki, Multiple Sclerosis Centre, Department of Neuroscience DNS, University of Padua, Padua, Italy.
Lisa Federle, Multiple Sclerosis Centre, Ospedale San Bortolo, ULSS8 Berica, Vicenza, Italy.
Erica Stropparo, Multiple Sclerosis Centre, Department of Neuroscience DNS, University of Padua, Padua, Italy.
Mariagiulia Anglani, Neuroradiology Unit, University Hospital of Padua, Padova, Italy.
Francesca Rinaldi, Neurology Clinic, University Hospital of Padua, Padova, Italy.
Paola Perini, Neurology Clinic, University Hospital of Padua, Padova, Italy.
Paolo Gallo, Multiple Sclerosis Centre, Department of Neuroscience DNS, University of Padua, Padua, Italy.
References
- 1. Rudick RA, Lublin FD, Weinstock-Guttman B, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354: 911–923. [DOI] [PubMed] [Google Scholar]
- 2. Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 3. Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007; 68: 1390–1401. [DOI] [PubMed] [Google Scholar]
- 4. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 5. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 6. Barbin L, Rousseau C, Jousset N, et al. Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology 2016; 86: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 8. Lorscheider J, Benkert P, Lienert C, et al. Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing–remitting multiple sclerosis. Mult Scler 2018; 24: 777–785. [DOI] [PubMed] [Google Scholar]
- 9. Guger M, Enzinger C, Leutmezer F, et al. Real-life clinical use of natalizumab and fingolimod in Austria. Acta Neurol Scand 2018; 137: 181–187. [DOI] [PubMed] [Google Scholar]
- 10. Bergvall N, Lahoz R, Reynolds T, et al. Healthcare resource use and relapses with fingolimod versus natalizumab for treating multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin 2014; 30: 1461–1471. [DOI] [PubMed] [Google Scholar]
- 11. Braune S, Lang M, Bergmann A; NTC Study Group. Second line use of fingolimod is as effective as natalizumab in a German out-patient RRMS-cohort. J Neurol 2013; 260: 2981–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koch-Henriksen N, Magyari M, Sellebjerg F, et al. A comparison of multiple sclerosis clinical disease activity between patients treated with natalizumab and fingolimod. Mult Scler 2017; 23: 234–241. [DOI] [PubMed] [Google Scholar]
- 13. Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011; 365: 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2017; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 15. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain 2015; 138: 3287–3298. [DOI] [PubMed] [Google Scholar]
- 17. Miller D H, Barkhof F, Berry I, et al. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry 1991; 54: 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geurts JJ, Roosendaal SD, Calabrese M, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 2011; 76: 418–424. [DOI] [PubMed] [Google Scholar]
- 19. Bergvall N, Lahoz R, Reynolds T, et al. Healthcare resource use and relapses with fingolimod versus natalizumab for treating multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin 2014; 30: 1461–1471. [DOI] [PubMed] [Google Scholar]
- 20. Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol 2015; 77: 425–435. [DOI] [PubMed] [Google Scholar]
- 21. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 22. Lorscheider J, Benkert P, Lienert C, et al. Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing–remitting multiple sclerosis. Mult Scler 2018; 24: 777–785. [DOI] [PubMed] [Google Scholar]
- 23. Prosperini L, Saccà F, Cordioli C, et al. Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naïve patients with multiple sclerosis. J Neurol 2017; 264: 284–294. [DOI] [PubMed] [Google Scholar]
- 24. Baroncini D, Ghezzi A, Annovazzi PO, et al. Natalizumab versus fingolimod in patients with relapsing-remitting multiple sclerosis non-responding to first-line injectable therapies. Mult Scler 2016; 22: 1315–1326. [DOI] [PubMed] [Google Scholar]
- 25. Puthenparampil M, Poggiali D, Causin F, et al. Cortical relapses in multiple sclerosis. Mult Scler 2016; 22: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 26. Rinaldi F, Calabrese M, Grossi P, et al. Cortical lesions and cognitive impairment in multiple sclerosis. Neurol Sci 2010; 31: 235–237. [DOI] [PubMed] [Google Scholar]
- 27. Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011; 134: 2755–2771. [DOI] [PubMed] [Google Scholar]
- 28. Klaver R, De Vries HE, Schenk GJ, et al. Grey matter damage in multiple sclerosis. Prion 2013; 7: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magraner M, Coret F, Casanova B. The relationship between inflammatory activity and brain atrophy in natalizumab treated patients. Eur J Radiol 2012; 81: 3485–3490. [DOI] [PubMed] [Google Scholar]
- 30. Sastre-Garriga J, Tur C, Pareto D, et al. Brain atrophy in natalizumab-treated patients: a 3-year follow-up. Mult Scler 2015; 21: 749–756. [DOI] [PubMed] [Google Scholar]
- 31. Gaetano L, Häring DA, Radue EW, et al. Fingolimod effect on gray matter, thalamus, and white matter in patients with multiple sclerosis. Neurology 2018; 90: e1324–e1332. [DOI] [PubMed] [Google Scholar]
- 32. Sormani MP, Li DK, Bruzzi P, et al. Combined MRI lesions and relapses as a surrogate for disability in multiple sclerosis. Neurology 2011; 77: 1684–1690. [DOI] [PubMed] [Google Scholar]


