Abstract
Denosumab is a fully human monoclonal antibody to receptor activator of nuclear factor kappa-B ligand (RANKL), a cytokine expressed by cells of the osteoblast lineage that is a key regulator of osteoclastic bone resorption. By binding and neutralizing RANKL, denosumab inhibits osteoclast differentiation, activity, and survival. Clinical trials in postmenopausal women with osteoporosis have shown that it reduces the risk of vertebral fractures, nonvertebral fractures, and hip fractures, with a generally favorable safety profile. With a dose of 60 mg subcutaneously every 6 months, it is approved for: treatment of postmenopausal women and men with osteoporosis, and for women and men with glucocorticoid-induced osteoporosis who are at high risk for fracture; treatment to increase bone mass in men at high risk for fracture receiving androgen-deprivation therapy for nonmetastatic prostate cancer; and treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer. Atypical femur fractures and osteonecrosis of the jaw have been reported in patients treated with denosumab. Discontinuation of denosumab is followed by rapidly rising bone turnover markers, decreasing bone density, and vertebral fracture risk that returns to baseline, with a possible increase in the risk of multiple vertebral fractures. Further study is needed to clarify this potential risk. After stopping long-term denosumab, patients should be switched to another antiresorptive agent to maintain the benefit achieved with denosumab.
Keywords: bone density, discontinuation, fracture, osteoporosis, treatment
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mineral density (BMD) and degradation of other determinants of bone strength, resulting in an increased risk of fractures.1 It is a global public health concern with serious consequences due to fractures. There are an estimated 200 million individuals with osteoporosis worldwide and 9 million osteoporotic fractures each year, with a fracture occurring every 3 s somewhere in the world.2 Fractures can cause pain, disability, loss of independence, and death, and are associated with high healthcare costs.3 Many pharmacological agents for treating osteoporosis have been evaluated in large clinical trials and proven to reduce fracture risk.4 However, despite their availability, there is a large osteoporosis treatment gap, with most patients who could benefit from treatment not receiving it.5 Among the proposed strategies to reduce the osteoporosis treatment gap are development of new medications and more effective use of the ones we already have.6 This is an update on the development, clinical applications, and emerging concepts (Table 1) on the use of denosumab (Prolia®, Amgen Inc., Thousand Oaks, CA, USA) for the management of osteoporosis.
Table 1.
Noteworthy features of denosumab in the management of osteoporosis.
| Feature | Reference |
|---|---|
| The only approved drug in its class (monoclonal antibody to RANKL) | Amgen Inc.7 |
| Parenteral administration (SC) with long dosing interval (6 months) | Cummings et al.8 |
| Reduces risk of vertebral fractures, hip fractures, and nonvertebral fractures in women with PMO | Cummings et al.8 |
| Greatest BMD increase of any single osteoporosis drug (21.7% at lumbar spine over 10 years) | Bone et al. 9 |
| Higher total hip T score with treatment is associated with lower incidence of nonvertebral fractures | Ferrari et al.10 |
| Additive effect on BMD when combined with teriparatide | Leder et al.11 |
| BMD loss when followed by teriparatide | Leder et al.11 |
| BMD gain when given after teriparatide | Leder et al.11 |
| Rapid rise of bone turnover markers and decrease of BMD after discontinuation | Miller et al.12 |
| Increased risk of multiple vertebral fractures after discontinuation | Cummings et al.13 |
| Patients stopping denosumab should be transitioned to another antiresorptive medication | Cummings et al.13 |
| Zoledronic acid after denosumab may be most effective given 7–8 months after last denosumab dose | Horne et al.14 |
| Rare reports of ONJ and AFF with denosumab | Bone et al.9 |
AFF, atypical femur fracture; BMD, bone mineral density; ONJ, osteonecrosis of the jaw; PMO, postmenopausal osteoporosis; RANKL, receptor activator of nuclear factor kappa-B ligand; SC, subcutaneously.
Denosumab is a fully human monoclonal immunoglobulin G2 (IgG2) antibody that binds and inhibits receptor activator of nuclear factor kappa-B ligand (RANKL), the principal regulator of osteoclastic bone resorption. It was first approved by the United States Food and Drug Administration (FDA) in 2010 for the treatment of postmenopausal women with osteoporosis at high risk for fracture, with a dose of 60 mg subcutaneously (SC) every 6 months (Q6M). It has subsequently received FDA approval with the same dose for: treatment to increase bone mass in men with osteoporosis at high risk for fracture; treatment of glucocorticoid-induced osteoporosis in men and women at high risk for fracture; treatment to increase bone mass in men at high risk for fracture receiving androgen-deprivation therapy for nonmetastatic prostate cancer; and treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.7 The FDA definition of high risk for fracture is history of osteoporotic fracture, multiple risk factors for fracture, or failure or intolerance to other available osteoporosis therapy. Another preparation of denosumab (Xgeva®, Amgen Inc., Thousand Oaks, CA, USA), not discussed here, using dosages different than for osteoporosis, is approved by the FDA for the following skeletal disorders: prevention of skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors (120 mg SC every 4 weeks); treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity (120 mg SC every 4 weeks with additional 120 mg doses on days 8 and 15 of the first month of therapy); and treatment of hypercalcemia of malignancy refractory to bisphosphonate therapy (120 mg SC every 4 weeks with additional 120 mg doses on days 8 and 15 of the first month of therapy).15 Denosumab is primarily marketed in the US and Europe, but also available in many other countries worldwide. Denosumab biosimilars are in development.
Pharmacological properties and histomorphometric effects of denosumab
The pharmacokinetics and pharmacodynamics of denosumab, previously described in this journal,16 were evaluated in a phase I, dose-escalation study with 49 healthy postmenopausal women who received a single dose of denosumab (0.01, 0.03, 0.3, or 1.0 mg/kg SC) or placebo and were then followed for 6–9 months.17 Administration of denosumab was followed by a rapid, dose dependent, sustained (lasting as long as 6 months) decrease in urinary N-telopeptide (NTX), a marker of bone resorption. There was a decrease of serum bone-specific alkaline phosphatase (BSAP), a marker of bone formation, although this occurred slower than with NTX and the change was not as great. There were 3 phases of serum levels of denosumab, with a prolonged absorption phase having a maximum serum concentration (Cmax) 5–21 days after dosing, a prolonged β-phase with serum half-life up to 32 days with the maximum dose, and a more rapid terminal phase at concentrations less than 1000 ng/ml with half-life increasing from 5 to 10 days as the dose increased from 0.01 to 3.0 mg/kg. These nonlinear pharmacokinetics contributed to the eventual selection of the dose of 60 mg SC Q6M for study in phase III clinical trials.
Denosumab has a high affinity and specificity for RANKL, a cytokine expressed by osteoblasts, activated T cells, and cells in other tissues that include lymph nodes, thymus, lung, and mammary glands.18 By binding to RANKL, denosumab prevents the interaction between RANKL and its receptor, RANK, which is located on the cell surface of preosteoclasts and mature osteoclasts. Denosumab thereby inhibits osteoclast differentiation, activity, and survival. Denosumab acts in much the same ways as osteoprotegerin (OPG), an endogenous product of cells of the osteoblast lineage that is a ‘decoy receptor’ for RANKL, serving as a natural modulator of osteoclastic bone resorption.
Bone histomorphometry has been helpful in assessing the quality of bone tissue in patients treated with denosumab.19 In phase III clinical trials, bone was qualitatively normal, with up to 3 years of continuous denosumab; biopsies showed normal lamellar bone, normal mineralization with no osteoid accumulation and no marrow fibrosis, with normal cortical and trabecular microarchitecture. Indices of bone resorption and formation were greatly reduced, consistent with a profound decrease in the rate of bone remodeling. Double tetracycline labeling was observed in 19% of patients treated with denosumab compared with 94% of those treated with placebo. Bone biopsies in patients treated with denosumab for 10 years showed normal bone histology, low bone remodeling, increased matrix mineralization, and lower mineralization heterogeneity compared with placebo-treated patients.20 Double fluorochrome labeling of cortical or trabecular bone was found in 32% of biopsy specimens after 10 years of continuous treatment with denosumab. There was progression of the increase in bone mineralization and reduction of mineralization heterogeneity over the first 5 years of treatment with denosumab but not thereafter.
Phase II clinical trial
Efficacy and safety of denosumab were evaluated in a phase II, placebo-controlled, dose-ranging study in 412 postmenopausal women with low BMD.21 Patients were randomized to receive denosumab 6, 14, or 30 mg SC every 3 months (Q3M) or 14, 60, 100, or 210 mg SC Q6M, open-label oral alendronate 70 mg weekly, or placebo. The primary endpoint was percentage change in lumbar spine BMD at 12 months compared with baseline. Secondary endpoints included percentage change from baseline in BMD at the femoral neck, total hip, and one-third radius, as well as change in bone turnover markers (BTMs) urinary NTX, serum C-telopeptide (CTX), and serum BSAP. At 12 months, there were significant lumbar spine BMD increases (3.0–6.7%, depending on the dose and dosing interval), with smaller BMD increases at other skeletal sites.21 BTM decreases with denosumab were dose dependent, rapid, sustained, and reversible. Adverse events (AEs) and serious adverse events (SAEs) were similar in all treatment groups except for dyspepsia being more common with open-label alendronate. The study was extended beyond 12 months for a total of 8 years, with reports of findings at intervals of 2 years,22 4 years,12 6 years,23 and 8 years.24
The objective of the first study extension was to evaluate the efficacy and safety of extended exposure of denosumab for 2 years, with all study groups remaining the same as in the first 12 months.22 After 2 years of treatment with denosumab, there were further increases in BMD and continued suppression of BTMs. AEs continued to be generally similar in the placebo, denosumab, and alendronate groups. There were six cases (1.9%) of SAEs, which were infections in the denosumab group (two patients with diverticulitis, three with pneumonia, and one with labyrinthitis) compared with none in the placebo group or open-label alendronate group. No neutralizing antibodies to denosumab were observed. In the study extension from 2 years to 4 years, patients treated with denosumab were reassigned.12 Those originally treated with denosumab 6 and 14 mg SC Q3M and 14, 60, and 100 mg SC Q6M were switched to denosumab 60 mg Q6M, while those originally treated with 210 mg SC Q6M were switched to placebo. Patients originally treated with 30 mg SC Q3M were switched to placebo for 12 months, followed by denosumab 60 mg SC Q6M for the next 12 months. Patients receiving open-label alendronate were terminated from the study after 24 months and received no additional treatment. The original placebo group was maintained for the entire 4 years. Continuous denosumab treatment for 4 years was associated with additional increases in BMD at the lumbar spine (9.4–11.8% compared with baseline) and total hip (4.0–6.1% compared with baseline), with continuing reduction of BTMs. Discontinuation of denosumab after 2 years of treatment was followed by BMD decreases of 6.6% at the lumbar spine and 5.3% at the total hip, occurring within 1 year of discontinuation. Retreatment with denosumab 1 year after discontinuation was associated with a BMD increase similar to initial treatment. BTMs rose to levels higher than baseline after discontinuation of denosumab and declined after retreatment. SAEs were 10.9% (5/46) in the placebo group, 17.8% (56/314) in the denosumab group, and 17.4% (8/46) in the alendronate group. The overall incidence of infections was similar in all treatment groups. Infections requiring hospitalization were reported in 3.2% (10/314) of denosumab-treated patients and none receiving placebo or alendronate. Patients completing the first 4 years of the phase II study (n = 262) were invited to continue in a single-arm extension for an additional 4 years, with all receiving denosumab 60 mg SC Q6M. Of the 200 patients continuing beyond 4 years, there were 178 who completed 6 years23 and 138 who completed 8 years;24 90 patients received 8 years of continuous denosumab. After 8 years of continuous denosumab, BMD increase at the lumbar spine was 16.5% and BMD increase at the total hip was 6.8%. AEs were consistent with previous reports.
The findings of the phase II clinical trial provided the foundation for further investigation of denosumab as a potential treatment for osteoporosis and other skeletal diseases associated with bone loss. This led to a phase III registration trial to evaluate antifracture efficacy and long-term safety of denosumab 60 mg Q6M in postmenopausal women with osteoporosis.
Phase III clinical trials
FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis every 6 Months) was a phase III, randomized, placebo-controlled clinical trial comparing denosumab 60 mg SC Q6M and placebo in 7868 postmenopausal women with osteoporosis.8 The primary endpoint was new vertebral fractures at 36 months, with secondary endpoints that included nonvertebral and hip fractures. All patients received elemental calcium 1000 mg and vitamin D 400–800 IU daily. Compared with women receiving placebo, those treated with denosumab for 36 months had a statistically significant 68% relative risk reduction (RRR) of new radiographic vertebral fractures, 40% RRR of hip fractures, and 20% RRR of nonvertebral fractures. Denosumab treatment for 3 years was associated with a BMD increase of 9.2% at the lumbar spine and 6.0% at the total hip compared with placebo. There were no significant differences in total AEs, SAEs, or treatment discontinuation rates between patients receiving denosumab or placebo. There was no increase in the risk of cancer, infection, cardiovascular disease, or hypocalcemia. There were no reported cases of osteonecrosis of the jaw (ONJ) or atypical femur fractures (AFFs) with denosumab. No patients were found to have neutralizing antibodies to denosumab. Eczema was reported in 3.0% of patients in the denosumab group compared with 1.7% in the placebo group (p < 0.001). Cellulitis as an SAE was reported in 12 patients (0.3%) in the denosumab group compared with one patient (<0.1%) in the placebo group (p = 0.002); there was no significant difference in overall incidence of cellulitis as an AE. Denosumab did not impair fracture healing, including in patients who received it near the time of the fracture.25
To assess the long-term effects of denosumab in postmenopausal women with osteoporosis, FREEDOM was extended for an additional 7 years.9,26,27 Patients initially randomized to denosumab received open-label denosumab for the next 7 years (long-term group; total of 10 years continuous denosumab) and those initially randomized to placebo were switched to open-label denosumab for the next 7 years (crossover group; total of 7 years continuous denosumab). The primary objective of the extension study was to monitor safety; secondary endpoints included changes in BMD and BTMs. In patients receiving 10 years of continuous denosumab, BMD increased by 21.7% at the lumbar spine, 9.2% at the total hip, 9.0% at the femoral neck, and 2.7% at the one-third radius compared with FREEDOM baseline. Serum CTX and procollagen type 1 N-terminal propeptide (P1NP) levels remained low. The incidence of new vertebral fractures and new nonvertebral fractures was low during the extension, comparable with rates observed in FREEDOM in the denosumab group. Exposure-adjusted annual incidence of all AEs was stable. There were reports of five subtrochanteric or diaphyseal femur fractures in the long-term group and four in the crossover group; of these, there were two (0.8 per 10,000 participant-years) adjudicated as AFFs, one in the long-term group and one in the crossover group. There were 13 adjudicated cases of ONJ (5.2 per 10,000 participant-years), 7 in the long-term group and 6 in the crossover group. There were no reports of neutralizing antibodies to denosumab. The progressive increase in BMD over 10 years of continuous treatment with denosumab is in marked contrast to the plateau of BMD that is typically seen after several years of treatment with bisphosphonates. Possible mechanisms for this observation include more robust antiresorptive effects, greater access to cortical bone, reduction of cortical porosity, modeling-based bone formation at some skeletal sites, and greater increases in serum parathyroid hormone levels compared with bisphosphonates.
A phase III trial evaluated the efficacy and safety of denosumab in postmenopausal women with low bone mass (osteopenia). Postmenopausal women (n = 332) with lumbar spine T scores between −1.0 and −2.5 who were randomized to receive SC denosumab 60 mg Q6M (n = 166) or placebo (n = 166).28 The primary efficacy endpoint was the percentage change from baseline in lumbar spine BMD measured by dual-energy X-ray absorptiometry (DXA) at 24 months compared with placebo. It was found that denosumab significantly increased BMD at the lumbar spine compared with placebo at 24 months (denosumab 6.5% versus placebo −0.6%; p < 0.0001). There was a significant decrease in markers of bone resorption and formation compared with placebo. Despite these findings, denosumab has not received FDA approval for prevention of postmenopausal osteoporosis.
The effects of denosumab in men were evaluated in ADAMO (A multicenter, randomized, double-blind, placebo-controlled study to compare the efficacy and safety of DenosumAb versus placebo in Males with Osteoporosis).29,30 There were 242 eugonadal and hypogonadal men (age 30–85 years) with low baseline BMD (T score with male reference database ⩽ −2.0 and ⩾ −3.5 at the lumbar spine or femoral neck, or previous major osteoporotic fracture and T score ⩽ −1.0 and ⩾ −3.5) enrolled in this study. Patients were randomized to receive denosumab 60 mg SC Q6M or placebo, with a primary endpoint of percentage change of lumbar spine BMD at 12 months compared with baseline. In men treated with denosumab, BMD increased by 5.7% at the lumbar spine, 2.4% at the total hip, and 0.6% at the one-third radius compared with baseline adjusted (p ⩽ 0.0144 for all skeletal sites compared with placebo).29 Serum CTX was significantly reduced at day 15 for men in the denosumab group compared with placebo (p < 0.0001). The effects of denosumab on BMD and CTX were independent of baseline testosterone levels, baseline BMD, age, and estimated fracture risk. AEs were similar in the study groups. Clinical fractures were reported in two patients in the placebo group and one in the denosumab group. Increases in BMD and BTM changes in men treated with denosumab were similar to those in women receiving denosumab in FREEDOM, providing the basis for FDA approval of denosumab in men with osteoporosis at high risk for fracture. ADAMO was extended for a second year, with all participating patients (n = 228) receiving open-label denosumab 60 mg SC Q6M.30 Exploratory endpoints were BMD changes, CTX changes, and safety through month 24. In the group receiving long-term continuous denosumab for 24 months, there was a total BMD increase of 8.0% at the lumbar spine, 3.4% at the total hip, and 0.7% at the one-third radius compared with baseline (p < 0.01 for all). CTX levels were significantly decreased in both groups compared with baseline. AEs were similar in both groups; no new safety signals were identified.
Denosumab has also been evaluated for the treatment of patients with glucocorticoid-induced osteoporosis. In a phase III, double-blind, active-control, double-dummy, non-inferiority trial, denosumab 60 mg SC Q6M was compared with oral risedronate 5 mg weekly in 795 patients with low BMD or fragility fracture on chronic glucocorticoid therapy.31 Patients were men and women age 18 years or older receiving glucocorticoids (prednisone ⩾7.5 mg daily or equivalent) for at least 3 months (‘glucocorticoid continuing’ group) or less than 3 months (‘glucocorticoid initiating’ group) before screening. The primary endpoint was non-inferiority of denosumab to risedronate in terms of percentage change from baseline in lumbar spine BMD at 12 months based on non-inferiority margins of −0.7 and −1.1 percentage points for the glucocorticoid-continuing and glucocorticoid-initiating subpopulations, respectively. Superiority was assessed as a secondary outcome measure. Denosumab was found to be non-inferior and superior to risedronate at 12 months for effect on lumbar spine BMD in the glucocorticoid continuing group [4.4% (95% confidence interval (CI) 3.8–5.0) versus 2.3% (1.7–2.9); p < 0.0001] and the glucocorticoid initiating group [3.8% (3.1–4.5) versus 0.8% (0.2–1.5); p < 0.0001]. The incidence of AEs, SAEs, and fractures was similar between treatment groups.
Safety issues of special interest
Possible adverse immune effects of denosumab
Because RANKL and RANK are expressed by immune cells (e.g. activated T cells, B cells, dendritic cells) and gene ablation studies in mice have shown that complete absence of RANKL during embryogenesis is followed by total absence of lymph nodes,32 there is theoretical concern that denosumab might have adverse immune effects in humans. However, investigations of RANKL inhibition in rodents, cynomolgus monkeys, and humans have found no significant impairment of immune function.33,34 Numerical imbalances in the incidence of some infections, such as cellulitis as an SAE, were found in FREEDOM, leading to a more thorough analysis of the data.35 It was found that SAEs of infections in the gastrointestinal tract, urinary tract, ear, and endocarditis were numerically higher in patients treated with denosumab compared with placebo, with a small number of events and no relationship between the events and timing of dosing or duration of exposure to denosumab. It was concluded that there was no evidence for a causal relationship between denosumab and adverse immune effects resulting in infections. Additional safety observations in the FREEDOM extension, with patients receiving up to 6 years of continuous denosumab, have been reported, with no evidence of increasing imbalances of these infections.36
Infection risk combining denosumab with another biologic agent
Reports of increased risk of serious infections in patients treated with more than one biologic agent for rheumatoid arthritis37,38 have raised concerns of potential harm by combining denosumab with another biologic agent. Rheumatoid arthritis, the prototype chronic inflammatory disease, is associated with skeletal effects that include focal joint erosions, subchondral joint erosions, periarticular osteoporosis, and systemic osteoporosis.39 Denosumab added to ongoing treatment with methotrexate in patients with rheumatoid arthritis has been shown to inhibit structural damage, improve BMD, and suppress bone turnover without increasing the rate of AEs compared with placebo.40 In a retrospective review of Medicare claim data of 5814 patients with rheumatoid arthritis, the rate of hospitalization for infections was not increased in patients receiving denosumab combined with a biologic agent (e.g. infliximab, abatacept) compared with patients treated with zoledronic acid.41 In a chart review of 308 patients with rheumatoid arthritis in two Canadian rheumatology practices, there was a low risk of serious and opportunistic infections in patients with rheumatoid arthritis and in patients treated with denosumab plus another biologic.42 Taken as a whole, recognizing the limitations of these studies, there is no convincing evidence for increased infection risk by combining denosumab and another biologic. Large prospective randomized trials are needed to settle the issue with certainty.
Risk of atypical femur fractures
AFFs have been reported in patients treated with denosumab. In the FREEDOM extension, two patients with adjudicated AFF (0.8 per 10,000 participant-years) were identified, one in the long-term group after 7 years of continuous denosumab and one in the crossover group after 3 years of continuous denosumab.9 In addition, there have been case reports of AFF in patients treated with denosumab.43–47 The pathophysiology of AFF associated with denosumab and bisphosphonates is poorly understood, but the risk appears to be low, with other apparent risk factors that include Asian ethnicity, lateral bowing of the femur, autoimmune disease, and glucocorticoid use.48 Strategies for managing AFF include limiting medication exposure in patients with other risk factors for AFF, early identification with an extended view of the femur by DXA or conventional X-ray, and possibly the use of teriparatide to enhance healing of AFF.48
Risk of osteonecrosis of the jaw
ONJ has been reported in patients treated with denosumab. There were 13 adjudicated cases of ONJ in the FREEDOM extension, 7 in the long-term group and 6 in the crossover group (5.2 per 10,000 participant-years).9 A systematic review of 35 randomized clinical trials reported seven cases of ONJ in patients treated with denosumab 120 mg SC every 4 weeks or Q6M, a dose higher than that used for osteoporosis.49 Risk factors for ONJ in that analysis included dental extraction, use of removable dental apparatus, poor oral hygiene, and cancer chemotherapy. The low risk of ONJ with osteoporosis therapy may be minimized by maintaining good oral hygiene and avoiding unnecessary invasive oral surgery.50 As ONJ is such a rare event, there are no evidence-based guidelines for the management of patients treated with denosumab who require invasive oral surgery. One strategy that might minimize the risk of ONJ is to schedule elective oral surgery near the end of the dosing period (e.g. 5 months after the last denosumab dose), with the intention of giving the next dose on schedule or with a delay of no more than 1 month. A longer delay may expose the patient to undesirable loss of BMD and increase in the risk of multiple vertebral fractures (see section on ‘Vertebral fracture risk after stopping denosumab’).
Hypocalcemia after denosumab dosing
Small asymptomatic reductions in serum calcium levels have been observed, with corresponding elevations of serum parathyroid hormone levels, in phase II and III clinical trials.8,21 Symptomatic hypocalcemia is a clinical concern in patients with low renal function, especially patients with creatinine clearance less than 30 ml/min and those with end-stage renal disease on dialysis.51,52 Patients with hypoparathyroidism, previous thyroid surgery, parathyroid surgery, malabsorption syndromes, or small bowel resection are at high risk for hypocalcemia after denosumab and should be closely monitored.7 It is prudent to measure serum calcium before administration of denosumab and to evaluate and correct baseline hypocalcemia, if present.
Denosumab compared with other antiresorptive agents for treating osteoporosis
Denosumab was compared with alendronate in the DECIDE (Determining Efficacy: Comparison of Initiating Denosumab versus alEndronate) study. This was a phase III, double-blind, double-dummy, non-inferiority trial in postmenopausal women (n = 1189) with low BMD (lumbar spine or total hip T score ⩽ −2.0).53 Patients were randomized to receive denosumab 60 mg SC Q6M (plus weekly oral placebo) or oral alendronate 70 mg weekly (plus placebo SC injections Q6M). The primary endpoint was percentage change from baseline of total hip BMD at month 12 in patients treated with denosumab versus alendronate. Changes in BMD, BTMs, and safety measures were assessed. At month 12, there was a greater BMD increase at the total hip with denosumab compared with alendronate (treatment difference 0.9%, p < 0.0001) as well as at other measured skeletal sites (p ⩽ 0.0002 for all sites). Safety assessments were similar for both groups. The effects of switching from alendronate to denosumab versus continuing alendronate were assessed in the STAND (Study of Transitioning from AleNdronate to Denosumab) study. This was a phase III, double-blind, active-controlled, double-dummy study in postmenopausal women (n = 504) with low BMD (lumbar spine or total hip T score −2.0 to −4.0) who had previously been treated with alendronate for at least 6 months (median 36 months).54 The primary endpoint was the percentage change in total hip BMD from baseline to month 12. Patients were randomized to receive denosumab 60 mg SC Q6M once every 6 months (plus weekly placebo tablets) or to continue oral alendronate 70 mg once weekly (plus placebo SC Q6M). At 12 months, there was a significantly greater total hip BMD increase of 1.90% in the denosumab group compared with 1.05% in the alendronate group (p < 0.0001). AEs and SAEs were similar in both groups.
Denosumab has been compared with other bisphosphonates (i.e. risedronate, ibandronate, zoledronic acid). The effects of denosumab were compared with risedronate in a randomized, open-label study in postmenopausal women (n = 870), age 55 years and older, who were previously suboptimally adherent to treatment with alendronate.55 Patients were randomized to receive denosumab 60 mg SC Q6M or oral risedronate 150 mg once monthly. The primary endpoint was the mean percentage change from baseline in total hip BMD at month 12. The total hip BMD increase was significantly greater with denosumab compared with risedronate (2.0% versus 0.5%, respectively; p < 0.0001). AEs and SAEs were similar in the two groups. In a similar study, postmenopausal women (n = 833) who had discontinued or were poorly adherent to daily or weekly oral bisphosphonate therapy were randomized to receive open-label denosumab 60 mg SC Q6M or oral ibandronate 150 mg once monthly.56 The primary endpoint was the percentage change from baseline in BMD at the total hip at month 12. After 12 months, there was a significantly greater BMD gain from baseline with denosumab compared with ibandronate at the total hip (2.3% compared with 1.1%; p < 0.001). AEs were similar in the two groups. Another study evaluated the effect of switching from an oral bisphosphonate to denosumab or zoledronic acid.57 This was a randomized, double-blind study in postmenopausal women (n = 643), with patients randomized to receive denosumab 60 mg SC Q6M for 12 months plus intravenous (IV) placebo or zoledronic acid 5 mg IV plus placebo SC Q6M. The primary endpoint was mean percentage change from baseline in lumbar spine BMD at month 12. After 12 months, the BMD change from baseline at the lumbar spine was significantly greater with denosumab than zoledronic acid (3.2% versus 1.1%, respectively; (p < 0.0001). AEs were similar in both groups. There were three reported patients with adjudicated AFF, two in the denosumab group and one in the zoledronic acid group.
Persistence and compliance with denosumab were compared with other osteoporosis therapies in a retrospective, observational cohort study involving 43,543 women with mean age 65 years using US Medicare and commercial insurance administrative claims data.58 Persistence and compliance over 24 months were higher in women initiating denosumab SC Q6M compared with women starting more frequently dosed (e.g. daily and weekly) oral or injectable agents. Persistence was better with denosumab SC Q6M than with annual dosing of IV zoledronic acid.
Combination and sequential therapy with denosumab and teriparatide
The skeletal effects of denosumab and teriparatide administered individually or combined were evaluated in the DATA (Denosumab And Teriparatide Administration) study,59 in which postmenopausal women with osteoporosis (n = 94) were randomized in a 1:1:1 ratio to receive denosumab 60 mg SC Q6M, teriparatide 20 µg SC daily, or a combination of both. The primary endpoint was percentage change from baseline in lumbar spine BMD at 12 months. At 12 months, lumbar spine BMD increased more in the combination group (9.1%) compared with denosumab alone (5.5%, p = 0.0005) or teriparatide alone (6.2%, p = 0.0139). A similar pattern of BMD changes was observed at the total hip and femoral neck. In the DATA extension study, the three treatment groups were continued for an additional 12 months.60 At 24 months, lumbar spine BMD increased more in the combination group (12.9%) compared with denosumab alone (4.1%, p = 0.008) or teriparatide alone (9.5%, p = 0.003), with a similar pattern at the hip. The additive effects on BMD with the combination of denosumab and teriparatide is distinct from the lack of additive effect observed in combining alendronate and teriparatide.61,62
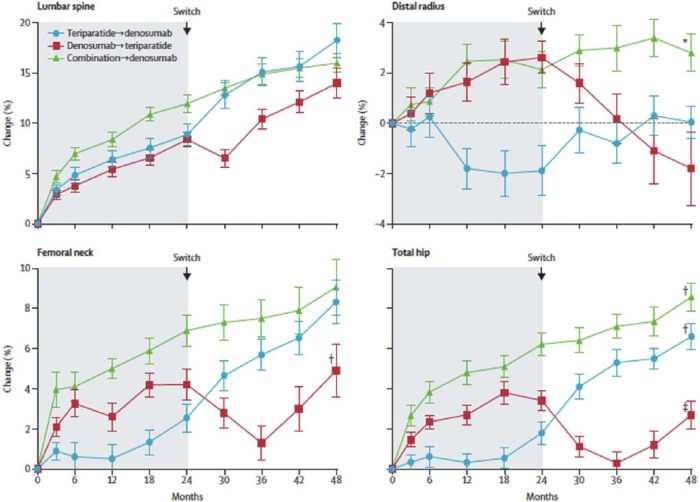
In DATA-Switch, a preplanned extension of the DATA study, patients in the combination group (denosumab + teriparatide) for 24 months were switched to denosumab for an additional 24 months (‘combination to denosumab’ group, n = 23), those treated with denosumab for 24 months were switched to teriparatide for an additional 24 months (‘denosumab to teriparatide’ group, n = 27), and those treated with teriparatide for 24 months were switched to denosumab for an additional 24 months (‘teriparatide to denosumab’ group, n = 27).11 The primary outcome was change in lumbar spine BMD compared with DATA study baseline (4 years total). It was found that mean lumbar spine BMD increased by a total of 16.0% (95% CI 14.0–18.0%) in the combination to denosumab group, 14.0% (98% CI 10.9–17.2%) in the denosumab to teriparatide group, and 18.3% (95% CI 14.9–21.8%) in the teriparatide to denosumab group over 4 years (Figure 1). When switching from combination or teriparatide to denosumab, there were also increases in BMD at all other measured skeletal sites. However, a different pattern was observed when switching from denosumab to teriparatide: a transient decrease in BMD at the total hip and femoral neck, and progressive bone loss at the one-third radius. Although the study has insufficient power to assess fracture risk, this raises concern that switching from denosumab to teriparatide in clinical practice may be problematic, and that it might be preferable to switch from denosumab to a bisphosphonate or a combination of denosumab + teriparatide in patients at high risk for fracture.
Figure 1.
Mean percentage change (standard error of the mean; error bars) of bone mineral density from baseline to 48 months in the DATA-Switch study.11
Figure 1 reproduced with permission from the publisher.
There is increasing recognition regarding the importance of sequence of therapy in high risk patients. Optimal therapeutic effect may be achieved with an anabolic agent followed by an antiresorptive medication, while an anabolic agent after a potent antiresorptive may result in a transient decrease in hip BMD, with possible adverse consequences for fracture risk.63 The common practice of switching from an antiresorptive medication to an anabolic agent after a patient is deemed to be a poor responder may not be the best use of treatment options.
Vertebral fracture risk after stopping denosumab
In the phase II trial in postmenopausal women with low BMD, discontinuation of denosumab after 2 years of therapy was followed by a BMD decline of 6.6% at the lumbar spine and 5.3% at the total hip within 12 months and a rise of BTM levels above baseline.12 Accordingly, there is a possibility that the risk of fractures might rapidly return to baseline (before treatment with denosumab) or to greater than at baseline. This possibility was highlighted in recent years by case reports of vertebral fractures, including multiple vertebral fractures, soon after discontinuation of denosumab.64–67 The best available data, although limited in scope, is from a post hoc analysis of women discontinuing denosumab in the FREEDOM and FREEDOM extension study.13 There were 1475 patients who discontinued treatment after receiving at least two doses of denosumab or placebo and remained in the study at least 7 months after receiving the last dose. Vertebral fracture risk increased after denosumab discontinuation to the level observed in untreated patients, consistent with fracture risk returning to baseline. However, a majority (61%) of those who experienced a vertebral fracture after discontinuing denosumab had multiple vertebral fractures, compared with 39% having multiple vertebral fractures after discontinuing placebo. The risk of multiple vertebral fractures was 3.4% after stopping denosumab and 2.2% after stopping placebo (p = 0.049), with the risk being 3.9 (95% CI 2.1–7.2) times higher in those with a prior vertebral fracture before or during treatment compared with those having no prior vertebral fracture. It was concluded that patients who discontinue denosumab should rapidly switch to another antiresorptive agent. A limitation of this study was the short follow-up time, with a median of 11 months after the last dose of denosumab or placebo in FREEDOM and a median of 8.4 months after the last dose of denosumab in FREEDOM extension. A longer follow-up time, as reported in published case series, might have found more off-treatment fractures. A ‘drug holiday,’ which is a consideration for some patients after long-term bisphosphonate therapy, is not an appropriate strategy for patients treated with denosumab.68
In response to the apparent increase in the risk of multiple vertebral fractures after denosumab discontinuation, the European Calcified Tissue Society (ECTS) conducted a systematic review of the scientific literature and developed recommendations for managing patients.69 They suggest that fracture risk be assessed after 5 years of treatment with denosumab. For those at high risk of fracture, treatment should be continued for up to 10 years total or switched to alternative treatment. For patients at low risk of fracture, consider stopping denosumab and switching to a bisphosphonate therapy to reduce or prevent the rebound increase in bone turnover. However, the optimal postdenosumab bisphosphonate regimen is not known. The ECTS cautioned that patients may be ill advised to stop denosumab prior to dental procedures. They suggest that patients starting denosumab be clearly informed that the benefits and risks of stopping treatment for any reason be discussed with the treating physician.
Treatment after denosumab
The effects of alendronate after denosumab were assessed in the DAPS (Denosumab Adherence Preference Satisfaction) study.70 This was a 24-month, randomized, open-label crossover study in which 250 postmenopausal women with low BMD were randomized to receive denosumab for 1 year followed by alendronate for 1 year, or alendronate for 1 year followed by denosumab for 1 year. The primary endpoint was adherence in the first 12 months, which was significantly better with denosumab (87.3%) than with alendronate (76.6%). An exploratory analysis of the data at 2 years provided an opportunity to assess the effects of treatment crossover on BMD. Patients who received 1 year of alendronate after 1 year of denosumab had stability of BMD increases achieved with denosumab in the first year, and those who received 1 year of denosumab after 1 year of alendronate benefited from additional BMD increases after the first year.
The effects of zoledronic acid and risedronate after denosumab have been evaluated. In a case series of six women with postmenopausal osteoporosis treated with 7 years of continuous denosumab in FREEDOM and FREEDOM extension at a single investigative site, IV zoledronic acid 5 mg was administered 6 months after the last dose of denosumab.71 A significant decrease in BMD at the lumbar spine and total hip was observed 18–23 months later, with lumbar spine BMD remaining significantly above pretreatment baseline, while hip BMD was not significantly different than baseline. The authors suggested that the disappointing treatment effect of zoledronic acid on BMD may have been due to diminished skeletal uptake of the bisphosphonate due to extreme suppression of bone remodeling by denosumab. In another case series at the same investigative site, women involved in the FRAME (Fracture Study in Postmenopausal Women) study, a phase III clinical trial with romosozumab or placebo administered for 1 year followed by open-label denosumab for 2 years, were offered an opportunity to receive IV zoledronic acid or oral risedronate to prevent post-trial bone loss.14 Eleven woman chose to receive IV zoledronic acid as a single 5 mg infusion, five chose oral risedronate 35 mg per week, and three chose no additional treatment. Zoledronic acid was administered after a median delay of 65 days (range 15–165 days) from end of trial, corresponding to a median delay of 241 days (range 191–353 days) since the last dose of denosumab. BMD was measured 12 months after discontinuation of denosumab. In women opting for zoledronic acid, there was a modest BMD decrease at the lumbar spine representing 73% retention of the increase in BMD achieved during FRAME compared with baseline, and 87% retention of the total hip BMD increase at the end of FRAME (Figure 2). Those choosing no treatment at the conclusion of FRAME had only 10–20% retention of the FRAME BMD increase, while those receiving risedronate had an intermediate response, with 41–64% retention of the FRAME BMD increase.
Figure 2.
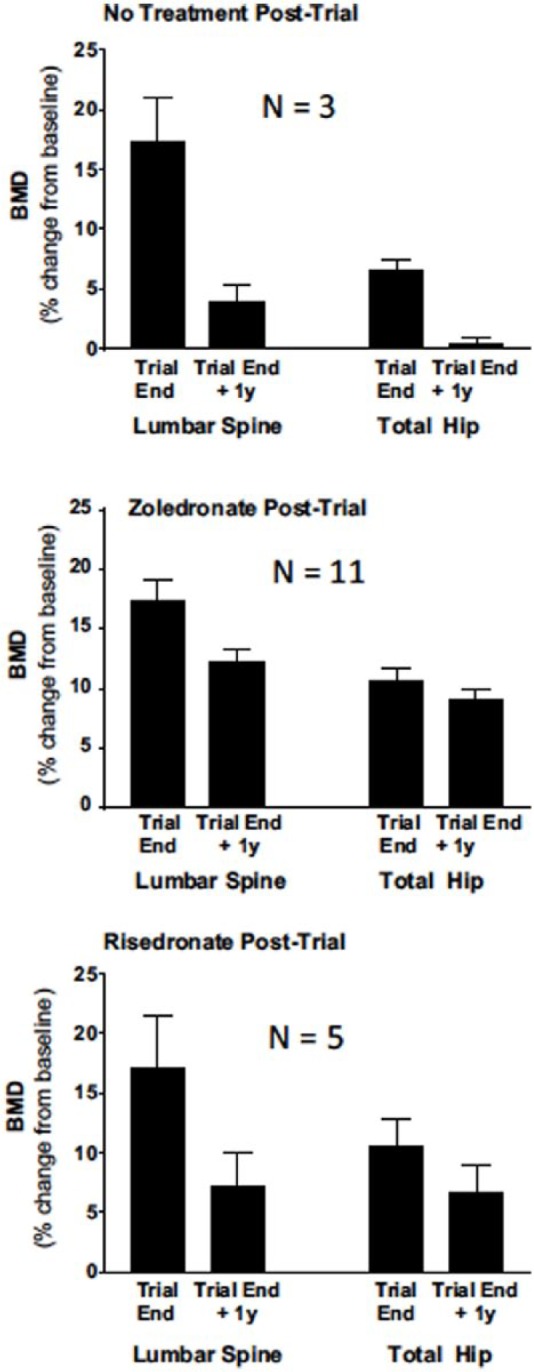
Mean percentage change (±95% confidence intervals) from baseline at the beginning of the FRAME study to end of trial when denosumab was discontinued and 1 year later after post-trial treatment, with nothing, intravenous zoledronate administered after end of trial, or weekly oral risedronate.14
Intravenous zoledronate (zoledronic acid) 5 mg was administered 15–165 days after end of trial (mean 65 days), or weekly oral risedronate 35 mg. There was significant bone loss after end of trial to 1 year in all treatment groups (p < 0.05) except for hip in those taking risedronate. Figure 2 reproduced with permission from the publisher.
BMD, bone mineral density.
Although the optimal interval from the last dose of denosumab to receiving IV zoledronic acid is yet to be determined, the data currently available suggest that administration 7–8 months after the last dose of denosumab may be appropriate. The delay beyond 6 months may allow for an opening of the bone remodeling space and allow greater attachment of zoledronic acid than when it is given 6 months after the last dose of denosumab, but may also expose the patient to a risk of multiple vertebral fractures. Some decrease in BMD may be unavoidable due to changing treatment to another antiresorptive agent with less suppression of bone turnover. If an oral bisphosphonate is used after denosumab, alendronate may be preferable to risedronate, with dosing that begins 6 months after the last dose of denosumab. Although there may be limited skeletal attachment of the oral bisphosphonate when first started, as the remodeling space opens, the drug will become more effective. The strategy of using an oral bisphosphonate 6 months after the last dose of denosumab rather than zoledronic acid with a delay beyond 6 months might be expected minimize the risk of multiple vertebral fractures.
Treat to target with denosumab
‘Treat to target’ (treat to goal) for osteoporosis is a concept based on the premise that treatment should be selected according to the likelihood of achieving an acceptable level of fracture risk; response to therapy is necessary but not always sufficient to reach this goal.72,73 In other words, a patient may respond well to treatment yet still have an undesirably high level of fracture risk. If a greater increase in BMD with a therapeutic agent can provide more fracture risk reduction, then BMD might be a useful treatment target, at least for some patients with some medications. An American Society for Bone and Mineral Research-National Osteoporosis Foundation (ASBMR-NOF) working group on goal-directed treatment for osteoporosis has suggested a T score target of greater than −2.5 for patients started on treatment for a T score no more than −2.5, with a higher level of confidence for a T score target of greater than −2.0.74 More data are needed and consensus must be achieved before treat to target can be universally embraced.75
In FREEDOM, increases in total hip BMD explained much of the effect of denosumab in reducing the risk of vertebral fractures. In an assessment of the relationship between time-dependent BMD changes at the total hip and new or worsening vertebral fractures in FREEDOM patients, BMD change explained 51% (95% CI 39–66%) of fracture risk reduction.76 For nonvertebral fractures, time-dependent total hip BMD changes explained 72% of treatment effect. The relationship between total hip T score and nonvertebral fracture risk was evaluated in an analysis of women in FREEDOM and FREEDOM extension receiving up to 8 years of denosumab.10 Higher total hip T scores achieved with treatment were associated with lower incidence of nonvertebral fractures, with the lowest risk of these fractures when T score was between −2.0 and −1.0. The data on the relationship between BMD increases with denosumab and reductions in fracture support the concept of treat to target for osteoporosis. This is consistent with the correlation between BMD increases with treatment and the magnitude of fracture risk reduction with other medications, including alendronate,77 zoledronic acid,78 and meta-regressions of multiple therapeutic agents.79,80
Summary
Denosumab is a fully human monoclonal antibody to RANKL, the principal osteoclastic bone regulator. By inhibiting the differentiation, activity, and survival of osteoclasts, it reduces the rate of bone remodeling, increases BMD, and reduces fracture risk, with a favorable balance of benefits over risks in appropriately selected patients. AFF and ONJ have been reported in patients treated with denosumab. It is an effective treatment for postmenopausal women with osteoporosis, men with osteoporosis, and individuals with glucocorticoid-induced osteoporosis. Discontinuation of denosumab is followed by a rise in BTMs above baseline, decrease in BMD, increase in vertebral fracture risk to pretreatment levels, and an apparent increase in the risk of multiple vertebral fractures. Switching from denosumab to teriparatide has been associated with bone loss at some skeletal sites and therefore may not be an effective sequence of therapy in high risk patients. Treating with a bisphosphonate after stopping denosumab can stabilize BMD or mitigate bone loss, with uncertainties regarding the best bisphosphonate to use and optimal timing of administration. Limited evidence suggests that zoledronic acid given 7–8 months after the last dose of denosumab or alendronate starting 6 months after the last dose of denosumab may be the preferred clinical strategy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author has received institutional grant/research support from Amgen, PFEnex, and Mereo; he has served on scientific advisory boards for Amgen, Radius, Shire, Alexion, Ultragenyx, and Sandoz; he serves on the speakers’ bureau for Shire, Alexion, and Radius; he is a board member of the National Osteoporosis Foundation, International Society for Clinical Densitometry, and Osteoporosis Foundation of New Mexico.
ORCID iD: E. Michael Lewiecki  https://orcid.org/0000-0003-2026-9587
https://orcid.org/0000-0003-2026-9587
References
- 1. Klibanski A, Adams-Campbell L, Bassford T, et al. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785–795. [DOI] [PubMed] [Google Scholar]
- 2. International Osteoporosis Foundation. Facts and statistics. https://www.iofbonehealth.org/facts-statistics (accessed 29 May 2017).
- 3. Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med 1997; 103: 12S–17S; discussion 17S–19S. [DOI] [PubMed] [Google Scholar]
- 4. Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther 2015; 37: 1837–1850. [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Borgstrom F, Compston J, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 2013; 8: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khosla S, Cauley JA, Compston J, et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res 2017; 32: 424–430. [DOI] [PubMed] [Google Scholar]
- 7. Amgen Inc. Prolia prescribing information. https://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/prolia/prolia_pi.ahsx (2018, accessed 16 July 2018).
- 8. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]
- 9. Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5: 513–523. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari S, Libanati C, Lin CJF, et al. Relationship between total hip BMD T-score and incidence of nonvertebral fracture with up to 8 years of denosumab treatment. J Bone Miner Res 2015; 30: S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 2015; 386: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43: 222–229. [DOI] [PubMed] [Google Scholar]
- 13. Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res 2018; 33: 190–198. [DOI] [PubMed] [Google Scholar]
- 14. Horne AM, Mihov B, Reid IR. Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif Tissue Int 2018; 103: 55–61. [DOI] [PubMed] [Google Scholar]
- 15. Amgen Inc. Xgeva prescribing information. https://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/xgeva/xgeva_pi.pdf (2018, accessed 5 May 2018).
- 16. Lewiecki EM. Denosumab in postmenopausal osteoporosis: what the clinician needs to know. Ther Adv Musculoskelet Dis 2009; 1: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 18. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008; 473: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reid IR, Miller PD, Brown JP, et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res 2010; 25: 2256–2265. [DOI] [PubMed] [Google Scholar]
- 20. Dempster DW, Brown JP, Fahrleitner-Pammer A, et al. Effects of long-term denosumab on bone histomorphometry and mineralization in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 2018; 103: 2498–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354: 821–831. [DOI] [PubMed] [Google Scholar]
- 22. Lewiecki EM, Miller PD, McClung MR, et al. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low bone mineral density. J Bone Miner Res 2007; 22: 1832–1841. [DOI] [PubMed] [Google Scholar]
- 23. Miller PD, Wagman RB, Peacock M, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a phase 2 clinical trial. J Clin Endocrinol Metab 2011; 96: 394–402. [DOI] [PubMed] [Google Scholar]
- 24. McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adami S, Libanati C, Boonen S, et al. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg Am 2012; 94: 2113–2119. [DOI] [PubMed] [Google Scholar]
- 26. Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res 2012; 27: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM extension study. Osteoporos Int 2015; 26: 2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93: 2149–2157. [DOI] [PubMed] [Google Scholar]
- 29. Orwoll E, Teglbjaerg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97: 3161–3169. [DOI] [PubMed] [Google Scholar]
- 30. Langdahl BL, Teglbjaerg CS, Ho PR, et al. A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 2015; 100: 1335–1342. [DOI] [PubMed] [Google Scholar]
- 31. Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6: 445–454. [DOI] [PubMed] [Google Scholar]
- 32. Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999; 397: 315–323. [DOI] [PubMed] [Google Scholar]
- 33. Stolina M, Dwyer D, Ominsky MS, et al. Continuous RANKL inhibition in osteoprotegerin transgenic mice and rats suppresses bone resorption without impairing lymphorganogenesis or functional immune responses. J Immunol 2007; 179: 7497–7505. [DOI] [PubMed] [Google Scholar]
- 34. Stolina M, Kostenuik PJ, Dougall WC, et al. RANKL inhibition: from mice to men (and women). Adv Exp Med Biol 2007; 602: 143–150. [DOI] [PubMed] [Google Scholar]
- 35. Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watts NB, Brown JP, Papapoulos S, et al. Safety observations with 3 years of denosumab exposure: comparison between subjects who received denosumab during the randomized FREEDOM trial and subjects who crossed over to denosumab during the FREEDOM extension. J Bone Miner Res 2017; 32: 1481–1485. [DOI] [PubMed] [Google Scholar]
- 37. Weinblatt M, Schiff M, Goldman A, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis 2007; 66: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004; 50: 1412–1419. [DOI] [PubMed] [Google Scholar]
- 39. Walsh NC, Crotti TN, Goldring SR, et al. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev 2005; 208: 228–251. [DOI] [PubMed] [Google Scholar]
- 40. Cohen SB, Dore RK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 2008; 58: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 41. Curtis JR, Xie F, Yun H, et al. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol 2015; 67: 1456–1464. [DOI] [PubMed] [Google Scholar]
- 42. Lau AN, Wong-Pack M, Rodjanapiches R, et al. Occurrence of serious infection in patients with rheumatoid arthritis treated with biologics and denosumab observed in a clinical setting. J Rheumatol 2018; 45: 170–176. [DOI] [PubMed] [Google Scholar]
- 43. Selga J, Nunez JH, Minguell J, et al. Simultaneous bilateral atypical femoral fracture in a patient receiving denosumab: case report and literature review. Osteoporos Int 2016; 27: 827–832. [DOI] [PubMed] [Google Scholar]
- 44. Khow KS, Yong TY. Atypical femoral fracture in a patient treated with denosumab. J Bone Miner Metab 2015; 33: 355–358. [DOI] [PubMed] [Google Scholar]
- 45. Schilcher J, Aspenberg P. Atypical fracture of the femur in a patient using denosumab–a case report. Acta Orthop 2014; 85: 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aspenberg P. Denosumab and atypical femoral fractures. Acta Orthop 2014; 85: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cating-Cabral MT, Clarke BL. Denosumab and atypical femur fractures. Maturitas 2013; 76: 1–2. [DOI] [PubMed] [Google Scholar]
- 48. Starr J, Tay YKD, Shane E. Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr Osteoporos Rep 2018; 16: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boquete-Castro A, Gomez-Moreno G, Calvo-Guirado JL, et al. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Implants Res 2016; 27: 367–375. [DOI] [PubMed] [Google Scholar]
- 50. Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22: 1479–1489. [DOI] [PubMed] [Google Scholar]
- 51. Ungprasert P, Cheungpasitporn W, Srivali N, et al. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31: 756 e1–e2. [DOI] [PubMed] [Google Scholar]
- 52. Talreja DB. Severe hypocalcemia following a single injection of denosumab in a patient with renal impairment. J Drug Assess 2012; 1: 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24: 153–161. [DOI] [PubMed] [Google Scholar]
- 54. Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25: 72–81. [DOI] [PubMed] [Google Scholar]
- 55. Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 2014; 58: 48–54. [DOI] [PubMed] [Google Scholar]
- 56. Recknor C, Czerwinski E, Bone HG, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 2013; 121: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 57. Miller PD, Pannacciulli N, Brown JP, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab 2016; 101: 3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Durden E, Pinto L, Lopez-Gonzalez L, et al. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 2017; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab 2014; 99: 1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Finkelstein JS, Wyland JJ, Lee H, et al. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 2010; 95: 1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 2003; 349: 1216–1226. [DOI] [PubMed] [Google Scholar]
- 63. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 2017; 32: 198–202. [DOI] [PubMed] [Google Scholar]
- 64. Anastasilakis AD, Polyzos SA, Makras P, et al. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res 2017; 32: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 65. Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int 2016; 27: 1917–1921. [DOI] [PubMed] [Google Scholar]
- 66. Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, et al. Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 2016; 27: 1923–1925. [DOI] [PubMed] [Google Scholar]
- 67. Lamy O, Gonzalez-Rodriguez E, Stoll D, et al. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 2017; 102: 354–358. [DOI] [PubMed] [Google Scholar]
- 68. McClung MR. Cancel the denosumab holiday. Osteoporos Int 2016; 27: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 69. Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 2017; 105: 11–17. [DOI] [PubMed] [Google Scholar]
- 70. Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reid IR, Horne AM, Mihov B, et al. Bone loss after denosumab: only partial protection with zoledronate. Calcif Tissue Int 2017; 101: 371–374. [DOI] [PubMed] [Google Scholar]
- 72. Lewiecki EM, Cummings SR, Cosman F. Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab 2013; 98: 946–953. [DOI] [PubMed] [Google Scholar]
- 73. Cummings SR, Cosman F, Eastell R, et al. Goal-directed treatment of osteoporosis. J Bone Miner Res 2013; 28: 433–8. [DOI] [PubMed] [Google Scholar]
- 74. Cummings SR, Cosman F, Lewiecki EM, et al. Goal-directed treatment for osteoporosis: a progress report from the ASBMR-NOF working group on goal-directed treatment for osteoporosis. J Bone Miner Res 2017; 32: 3–10. [DOI] [PubMed] [Google Scholar]
- 75. Kanis JA, McCloskey E, Branco J, et al. Goal-directed treatment of osteoporosis in Europe. Osteoporos Int 2014; 25: 2533–2543. [DOI] [PubMed] [Google Scholar]
- 76. Austin M, Yang YC, Vittinghoff E, et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 2012; 27: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296: 2927–2938. [DOI] [PubMed] [Google Scholar]
- 78. Cosman F, Cauley JA, Eastell R, et al. Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment? J Clin Endocrinol Metab 2014; 99: 4546–4554. [DOI] [PubMed] [Google Scholar]
- 79. Black DM, Vittinghoff E, Eastell R, et al. Hip BMD by DXA can reliably estimate reduction in hip risk in osteoporosis trials: a meta-regression. J Bone Miner Res 2015; 30: S49. [Google Scholar]
- 80. Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000; 85: 231–236. [DOI] [PubMed] [Google Scholar]