Abstract
Background:
Recent investigations on the biochemical pathways after a musculoskeletal injury have suggested that vitamin C (ascorbic acid) may be a viable supplement to enhance collagen synthesis and soft tissue healing.
Purpose:
To (1) summarize vitamin C treatment protocols; (2) report on the efficacy of vitamin C in accelerating healing after bone, tendon, and ligament injuries in vivo and in vitro; and (3) report on the efficacy of vitamin C as an antioxidant protecting against fibrosis and promoting collagen synthesis.
Study Design:
Systematic review; Level of evidence, 2.
Methods:
A systematic review was performed, with the inclusion criteria of animal and human studies on vitamin C supplementation after a musculoskeletal injury specific to collagen cross-linking, collagen synthesis, and biologic healing of the bone, ligament, and tendon.
Results:
The initial search yielded 286 articles. After applying the inclusion and exclusion criteria, 10 articles were included in the final analysis. Of the preclinical studies evaluating fracture healing, 2 studies reported significantly accelerated bone healing in the vitamin C supplementation group compared with control groups. The 2 preclinical studies evaluating tendon healing reported significant increases in type I collagen fibers and scar tissue formation with vitamin C compared with control groups. The 1 preclinical study after anterior cruciate ligament (ACL) reconstruction reported significant short-term (1-6 weeks) improvements in ACL graft incorporation in the vitamin C group compared with control groups; however, there was no long-term (42 weeks) difference. Of the clinical studies evaluating fracture healing, 1 study reported no significant differences in the rate of fracture healing at 50 days or functional outcomes at 1 year. Vitamin C supplementation was shown to decrease oxidative stress parameters by neutralizing reactive oxygen species through redox modulation in animal models. No animal or human studies reported any adverse effects of vitamin C supplementation.
Conclusion:
Preclinical studies demonstrated that vitamin C has the potential to accelerate bone healing after a fracture, increase type I collagen synthesis, and reduce oxidative stress parameters. No adverse effects were reported with vitamin C supplementation in either animal models or human participants; thus, oral vitamin C appears to be a safe supplement but lacks clinical evidence compared with controls. Because of the limited number of human studies, further clinical investigations are needed before the implementation of vitamin C as a postinjury supplement.
Keywords: ascorbic acid, collagen synthesis, fracture healing, ACL reconstruction, collagen cross-linking, oxidative stress
The healing of musculoskeletal tissues, such as bone, tendons, and ligaments, is dependent on the capacity of collagen synthesis and cross-linking.7,20,34 Poorly developed extracellular matrices derived from collagen can lead to inadequate tissue structures and biomechanical strength, which can result in unsatisfactory outcomes and an increased risk for reinjuries. Basic science investigations on the biochemical pathways after a musculoskeletal injury have suggested that vitamin C, also known as ascorbic acid, may enhance collagen synthesis and soft tissue healing.22,24,28,31
Vitamin C has an essential role in connective tissue healing, being a cofactor for prolyl hydroxylase and lysyl hydroxylase.22,28 These enzymes catalyze the hydroxylation of proline and lysine residues of procollagen, promoting the proper folding of the stable collagen triple-helix conformation.9,22 In addition to its role in collagen synthesis, vitamin C acts as a powerful antioxidant by neutralizing deleterious reactive oxygen species (ROS) responsible for cell apoptosis during the inflammatory phase.1,14,16 Cell culture studies have also reported that vitamin C can induce tendon-derived stem cell mobilization, osteoblast growth and differentiation, and fibroblast stimulation.8,11,18,21,26 Therefore, vitamin C has been increasingly studied for its contributions to the treatment of musculoskeletal injuries in both clinical and in vitro trials.
Although laboratory studies have reported that vitamin C is essential for the formation of collagen fibers and cell differentiation, there is controversy surrounding the efficacy of vitamin C as a supplement for clinical treatment. Therefore, the purposes of this systematic review were to (1) summarize vitamin C treatment protocols; (2) report on the efficacy of vitamin C in accelerating healing after bone, tendon, and ligament injuries in vivo and in vitro; and (3) report on the efficacy of vitamin C as an antioxidant protecting against fibrosis and promoting collagen synthesis. It was hypothesized that vitamin C supplementation would result in reduced oxidative stress and accelerated tissue healing when compared with control groups.
Methods
Article Identification and Selection
A systematic review of articles, using the PRISMA (Preferred Reporting Items for Systematic Meta-Analyses) guidelines, on the efficacy of vitamin C in promoting collagen synthesis, reducing inflammation, and improving bone/ligament/tendon healing after musculoskeletal injuries was performed using the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, PubMed (1980-2017), MEDLINE (1980-2017), and Embase (1980-2017); the queries were performed in November 2017. Registration of this systematic review was performed in January 2018 using the PROSPERO international prospective register of systematic reviews (CRD42018086378). The search terms utilized were “vitamin C” OR “ascorbic acid” AND “musculoskeletal injury”; “vitamin C” OR “ascorbic acid” AND “collagen cross-linking”; “vitamin C” OR “ascorbic acid” AND “orthopaedics”; “vitamin C” OR “ascorbic acid” AND “bone healing”; “vitamin C” OR “ascorbic acid” AND “ligament healing”; “vitamin C” OR “ascorbic acid” AND “tendon healing”; and “vitamin C” OR “ascorbic acid” AND “postoperative recovery.”
The inclusion criteria consisted of English-language studies and both animal and human studies on vitamin C supplementation after musculoskeletal injuries specific to collagen cross-linking, collagen synthesis, and biologic healing of the bone, ligament, and tendon. Exclusion criteria were vitamin C supplementation specific to nonmusculoskeletal-related injuries or surgery, healthy/noninjured populations, scurvy, vitamin C deficiency, effect of vitamin C on mesenchymal stem cells, genetic studies, cellular studies, epidemiological studies, dietary intake without vitamin C supplementation, editorial articles, review articles, case reports (level 5 evidence), and surveys. Two investigators (N.N.D., Z.S.A.) independently reviewed the abstracts from all identified articles. If necessary, full-text articles were obtained for a review to allow for further application of inclusion and exclusion criteria. Additionally, reference lists from the included studies were reviewed and reconciled to verify that all eligible articles were considered.
Data Collection
Both animal and human studies were collected. These findings included experimental focus, abnormality, animal model type, collagen type (ie, bone, ligament, tendon), treatments, postoperative restrictions, route of vitamin C administration, and vitamin C dosage (including frequency and duration). The level of evidence of all available clinical studies was assigned according to the classification as specified by Wright et al.38 Patient demographics, supplementation dosage, treatment details, follow-up, and subjective and objective patient outcomes were recorded for all clinical studies. For continuous variables (age, outcome scores, etc), the mean and range were obtained if reported.
Results
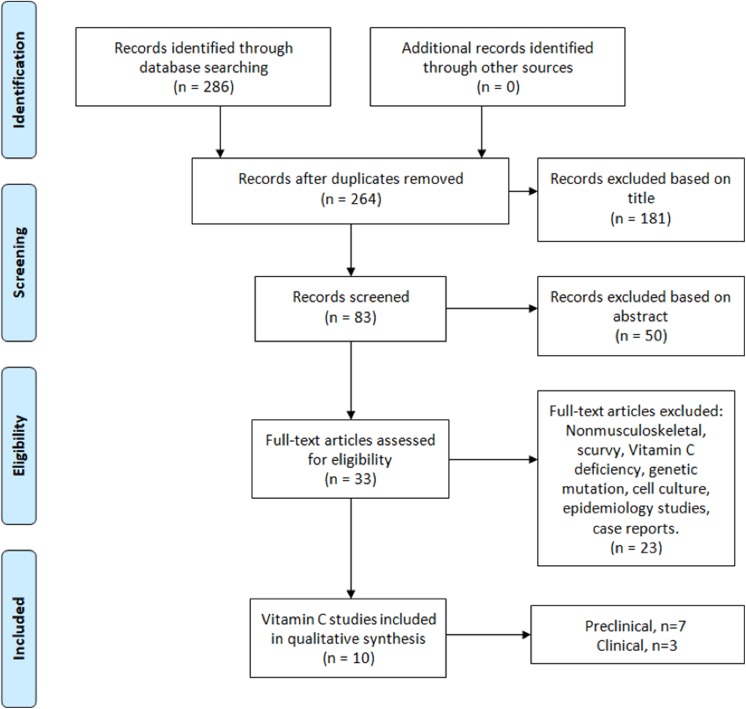
The literature search identified 286 studies from the aforementioned databases. After duplicates were removed, 264 articles were screened, and 10 articles met the inclusion criteria (Figure 1). There were 7 studies that evaluated the effects of vitamin C supplementation in animal models: 4 after bone fractures (nonoperative treatment), 2 after tendon ruptures (nonoperative and surgical treatment), and 1 after anterior cruciate ligament (ACL) reconstruction (ACLR) (Table 1). Six of the animal studies used a rat model, and 1 study used a chicken model. There were 3 studies that evaluated the effects of vitamin C supplementation in human models: 1 after ACLR and 2 after bone fractures (nonoperative and surgical treatment). Two of the clinical studies were level 1 evidence, while 1 study was level 2 evidence.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart showing the selection criteria used to identify studies with the search strategy.
TABLE 1.
Preclinical Studies: Demographics, Study Design, and Experimental Focus
| Study | Study Design (Level of Evidence) | Participants | Abnormality | Experimental Groups | Experimental Focus |
|---|---|---|---|---|---|
| Omeroglu et al25 (2009) | Randomized controlled trial (level 1) | Female, nonpregnant, albino Wistar rats (N = 42) | Achilles tendon rupture | (1) Vitamin C (n = 21) and (2) control (n = 21) with subgroups (3rd day, 10th day, and 21st day [n = 7 each]) | Tendon healing |
| Sarisozen et al33 (2002) | Randomized controlled trial (level 1) | Sprague-Dawley rats (N = 48) | Tibial fracture | (1) Vitamin E only (n = 12), (2) vitamin C only (n = 12), (3) vitamin C and E (n = 12), and (4) no treatment (n = 12) | Bone healing |
| Hung et al19 (2013) | Controlled trial (level 2) | Female Kamei chickens (N = 57) | Flexor digitorum profundus tendon tear | Part 1: (1) Operated (n = 9) with subgroups (day 0, 2 weeks, and 6 weeks [n = 3 each]) and (2) sham (n = 3) with subgroups (day 0, 2 weeks, and 6 weeks [n = 1 each]) Part 2: (1) 5 mg/mL of vitamin C (n = 12), (2) 50 mg/mL of vitamin C (n = 12), and (3) control (n = 12) |
Reduction of tendon adhesion by antagonizing oxidative stress |
| Giordano et al15 (2012) | Controlled trial (level 2) | Male Wistar rats (N = 30) | Tibial fracture | (1) Vitamin C (n = 15) and (2) control (n = 15) | Bone healing and effect on osteogenesis |
| Duygulu et al5 (2007) | Randomized controlled trial (level 1) | Male, albino Wistar rats (N = 50) | Radial and ulnar fracture | (1) Control (n = 10), (2) zymosan only (n = 10), (3) zymosan + DMSO (n = 10), (4) zymosan + EGb 761 (n = 10), and (5) zymosan + vitamin C (n = 10) | Effect of oxidative stress and protective effect of antioxidants on bone healing |
| Yilmaz et al41 (2001) | Randomized controlled trial (level 1) | Albino Wistar rats (N = 16) | Tibial fracture | (1) Control (n = 8) and (2) vitamin C (n = 8) | Bone healing |
| Fu et al10 (2013) | Controlled trial (level 2) | Male Sprague-Dawley rats (N = 114) | Anterior cruciate ligament tear | Part 1: (1) Control (n = 26), (2) 3 mg/mL of vitamin C (n = 26), (3) 10 mg/mL of vitamin C (n = 26), and (4) 30 mg/mL of vitamin C (n = 26) with subgroups (day 1, day 2, day 3, and week 6 [n = 4 each]) Part 2: (1) Sham (n = 10) and (2) operated (n = 10) |
Ligament graft healing and reduction of C-reactive protein plasma levels |
Preclinical Studies
Vitamin C Dosage
Four studies determined vitamin C dosage as a function of animal weight, ranging from 0.5 mg/kg to 500 mg/kg.5,15,33,41 The remaining 3 studies applied standard doses between 0.005 mg and 150 mg.10,19,25 The delivery of vitamin C varied, including intraperitoneally (n = 4),5,15,25,33 locally (n = 1),19 intramuscularly (n = 1),41 or by intraoperative irrigation (n = 1).10 The intervention was initiated on postinjury day 0 in all preclinical studies (n = 7). The frequency of vitamin C dosage varied between once at the time of injury to once per day until the end of the trial. The duration of individual trials ranged from 3 days to 6 weeks. Four studies allowed free access to standard diet and water,5,25,33,41 1 study provided a diet that did not contain vitamin C,15 and 2 studies did not report food and water restrictions.10,19 Five studies allowed animals to move freely without immobilization after the procedure,10,15,25,33,41 1 study immobilized the injured limb,19 and 1 study did not report on immobilization5 (Table 2).
TABLE 2.
Preclinical Studies: Treatment Protocols After Musculoskeletal Injuriesa
| Study | Treatment and Restrictions | Route of Exposure | Dosage | Frequency | Duration | Conditions | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Omeroglu et al25 (2009) | None; weightbearing without immobilization | Intraperitoneally | 150 mg vitamin C (1.5 mL) | Once on day of injury and then once every 2 days | 3 days, 10 days, and 21 days | Allowed to move freely in cages with free access to food and water | Significant difference in type I collagen production on 10th day, mean collagen fiber diameter and active fibroblasts higher in vitamin C group, and more evident angiogenesis on 3rd day | Low dose of vitamin C irrigation may be of potential use to promote healing |
| Sarisozen et al33 (2002) | None; weightbearing without immobilization | Intraperitoneally | 200 mg/kg vitamin C | Once per day for 3 days and then 3 times per week | 14 days and 21 days | Free access to food and water | Vitamin C group had accelerated bone matrix mineralization and increased amount of collagen | Vitamin C supplementation accelerated fracture healing |
| Hung et al19 (2013) | Tendon repair; immobilization for 2 weeks | Local injection | 5 mg/mL or 50 mg/mL vitamin C (50 μL total volume) | Once | 2 weeks and 6 weeks | N/A | No significant difference at 2 weeks between groups, significant improvement in gliding resistance at 6 weeks in vitamin C group, 5 mg/mL of vitamin C had significant reduction in fibrotic size at 6 weeks compared with control group, and less peritendinous adhesion in vitamin C group | Local injection of vitamin C solution can reduce extent of tendon adhesion after tendon repair |
| Giordano et al15 (2012) | None; weightbearing without immobilization | Intraperitoneally | 200 mg/kg vitamin C | Once per day | 2 weeks, 4 weeks, and 6 weeks | Caged with access to water and standard feed ad libitum containing no vitamin C | No significant histological or histomorphological differences | Vitamin C supplementation did not accelerate fracture healing process |
| Duygulu et al5 (2007) | None; N/A | Intraperitoneally | 500 mg/kg vitamin C (+ 100 mg/kg zymosan per day for first 5 days) | Once per day | 21 days | Caged laboratory conditions with standard diet and water | Oxidative stress impaired bone healing; histopathological, radiographic (bony union), and electromyographic (collagen fibrils) evaluations for vitamin C group were similar to that of control group; and significant difference in zymosan-only group for improved fracture healing | Free oxygen radicals have role in disruption of fracture healing, and vitamin C can partially prevent negative effects |
| Yilmaz et al41 (2001) | None; weightbearing | Intramuscularly | 0.5 mg/kg vitamin C | Once | 5 days, 10 days, 15 days, and 20 days | Caged with rat food, unlimited access to water, and unrestricted activity | No significant difference between groups overall; and vitamin C group was faster in chondroid cell development, chondrocyte hypertrophy, and fibrocartilaginous callus development than control group (P > .05) | Although there was no difference in quality of fracture healing, vitamin C–supplemented group was faster in healing process compared with controls |
| Fu et al10 (2013) | ACL reconstruction; weightbearing without immobilization | Intraoperative irrigation solution | 3 mg/mL, 10 mg/mL, or 30 mg/mL (10 mL) vitamin C | Once | 1 day, 4 days, 7 days, and 6 weeks | Caged | Vitamin C group had significantly reduced serum C-reactive protein levels at day 1, 3 mg/mL of vitamin C led to better restoration of anteroposterior knee stability at 6 weeks compared with control group, 3 and 10 mg/mL of vitamin C significantly reduced graft deterioration at 6 weeks, and no significant difference between groups at 42 weeks for graft incorporation | Low dose of vitamin C demonstrated short-term improvements after ACL reconstruction compared with controls, but there were no significant differences in ACL graft incorporation between groups |
aThe intervention was initiated on postinjury day 0 in all studies (n = 7). ACL, anterior cruciate ligament; N/A, not available.
Efficacy of Vitamin C on Collagen Synthesis and Tissue Healing
Overall, the preclinical studies supported the use of vitamin C for tendon, bone, and ligament graft healing. Three of the 4 studies evaluating the effects of vitamin C on fracture healing reported an increase or acceleration in collagen synthesis when compared with control groups.5,33,41 One study reported that there were no significant histological or histomorphological score differences between the vitamin C and control groups for fracture healing.15 Both studies evaluating tendon healing after ruptures reported improved structural quality with vitamin C supplementation.19,25 Omeroglu et al25 reported that there was a significant increase in type I collagen formation and a higher rate in active fibroblasts in low-dose vitamin C groups. Hung et al19 reported similar structural integrity in tendons, as vitamin C significantly reduced the fibrotic size and peritendinous adhesion. However, the authors did not report on tendinous collagen composition.19 Fu et al10 reported that low-dose vitamin C restored anteroposterior knee stability and significantly reduced graft deterioration after ACLR compared with the control group after 6 weeks; however, there were no significant differences in long-term outcomes (42 weeks) or ACL graft incorporation between groups. All of the studies evaluating the role of vitamin C as an antioxidant and collagen synthesizer (n = 3) suggested that vitamin C positively influences bone, tendon, and graft healing by antagonizing oxidative stress.5,10,19 No adverse effects were reported in any preclinical study (n = 7).
Efficacy of Vitamin C on the Reduction of Oxidative Stress Parameters
Three preclinical studies5,10,19 evaluated oxidative stress after vitamin C supplementation. All 3 preclinical studies reported that vitamin C was effective in reducing oxidative stress after injuries by decreasing endogenous or exogenous ROS, signified by an improved tissue composition in ligaments, tendons, and bone. However, only 1 of the 3 studies reported vitamin C as an antagonist for oxidative stress after 2 weeks.19 Furthermore, 1 clinical study evaluated oxidative stress and muscle recovery after vitamin C and E supplementation after ACLR.2 The authors reported that vitamin C and E supplementation had no significant effects on oxidative stress parameters compared with controls after ACLR.2
Clinical Studies
Patient Demographics and Vitamin C Dosage
The patient demographics, study design, and experimental focus of all clinical studies are reported in Table 3. Of the 3 clinical studies, 2 studies evaluated a combined supplement of vitamin C and other antioxidants,2,32 while 1 study evaluated exclusively the effects of vitamin C.6 The dosage of orally supplemented vitamin C ranged from 60 mg twice daily to 500 mg twice daily for a minimum of 7 days and maximum of 3 months. The 2 studies evaluating bone fracture healing began vitamin C supplementation on day 1 after the fracture.6,32 The study evaluating oxidative stress and muscle recovery began vitamin C supplementation 2 weeks before the planned ACLR.2
TABLE 3.
Clinical Studies: Demographics, Study Design, and Experimental Focus
| Study | Study Design (Level of Evidence) | Participants | Abnormality | Experimental Groups | Experimental Focus |
|---|---|---|---|---|---|
| Barker et al2 (2009) | Randomized controlled trial (level 1) | Male with preoperative/postoperative supplementation (N = 20) | Anterior cruciate ligament tear | (1) Vitamins C and E (n = 10) and (2) placebo (n = 10) | Muscle atrophy/recovery and oxidative stress parameters |
| Ekrol et al6
(2014) |
Controlled trial (level 2) | Male and female with postinjury supplementation (N = 336) | Distal radius fracture | Displaced fractures: (1) Vitamin C (n = 94) and (2) placebo (n = 92) Nondisplaced fractures: (1) Vitamin C (n = 75) and (2) placebo (n = 75) |
Bone healing |
| Sandukji et al32 (2011) | Randomized controlled trial (level 1) | Male with postoperative supplementation (N = 55) | Long bone fracture (humerus, radius, ulna, tibia, femur) | (1) Vitamins A, C, and E and selenium (n = 20) and (2) control (n = 35) | Bone healing and oxidative stress parameters |
Clinical Outcomes
The clinical trial evaluating muscle recovery after ACLR reported no significant differences between the vitamin C and control groups.2 However, baseline vitamin C status was associated with significant improvements in strength, suggesting that long-term dietary habits may be more effective than short-term supplements.2 The results of the 2 clinical trials evaluating bone healing after fractures were conflicting. Sandukji et al32 reported significant increases in osteocalcin levels and the activity of alkaline phosphatase in the blood plasma of patients who received antioxidants for 2 weeks (P < .05), both of which are bone markers previously reported to be positively correlated with bone mineral density (BMD).13,35 However, these authors did not report on the long-term results of fracture healing. In contrast, Ekrol et al6 noted no significant difference in the time to fracture healing in patients with long bone fractures who were supplemented with vitamin C compared with controls. Furthermore, there were no significant differences between the 2 treatment groups at 1-year follow-up.6 No adverse effects were reported in any clinical study (n = 3) regarding the use of vitamin C, and 1 study reported a dropout rate of 26% and a compliance rate of 84%.6 Despite this high dropout rate, the authors still managed to include a large sample size (n = 162) in their final analysis.6 Further results are detailed in Table 4.
TABLE 4.
Clinical Studies: Treatment Protocols After Musculoskeletal Injuriesa
| Study | Treatment and Restrictions | Start of Vitamin C | Route of Exposure | Dosage | Frequency | Duration | Conditions | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Barker et al2 (2009) | ACLR with double-bundle hamstring autograft (n = 19 [95%]) and bone–patellar tendon–bone autograft (n = 1 [5%]); nonweightbearing for 5 days (n = 20 [100%]) | 2 weeks before ACLR | Orally | 500 mg vitamin C and 200 IU vitamin E |
Twice daily | 3 months | Diet not restricted, and restricted NSAID use for duration of study | Significant increase in peak isometric force of injured limb in both groups (P < .05), vitamin C/E did not augment strength gains (P > .05), and significant correlation between baseline vitamin C and strength recovery after ACLR (P < .05) | Vitamin E and C supplementation was ineffective in augmenting improvement in force production by injured limb; however, baseline vitamin C status was associated with beneficial outcomes in strength, suggesting that long-term dietary habits are more effective than short-term supplements |
| Ekrol et al6 (2014) | Surgical (displaced fracture): open reduction internal/external fixation (n = 186 [55%]) Conservative (nondisplaced fracture): cast (n = 150 [45%]) |
Day 1 after fracture | Orally | 500 mg vitamin C | Daily | 50 days | Diet not restricted but monitored, and excluded patients currently taking vitamin C before fracture |
Time to fracture healing did not differ between treatment groups (P = .42 and .23), and no difference in functional outcomes at 1 year (P > .05) | No difference in DASH score, other functional outcomes, occurrence of CRPS, or fracture healing with vitamin C treatment in patients with distal radius fracture |
| Sandukji et al32 (2011) | Surgery: open reduction internal fixation (n = 55 [100%]) | Day 1 after fracture | Orally and intravenously | Groups 1/2: 300 mg vitamin A, 10 mg vitamin E, 60 mg vitamin C, and 75 mg selenium Groups 3/4: 1000 mg/d cephalosporin for 3 days, 50 mg oral diclofenac, and 500 mg paracetamol twice daily for 15 days |
Twice daily | Group 1: 7 days Group 2: 15 days Groups 3/4: 15 days |
N/R | Osteocalcin level and activity of alkaline phosphatase were markedly increased in plasma of patients who received antioxidants for 2 weeks (P < .05), and level of plasma glutathione was significantly increased only after 2 weeks in patients who received antioxidants (P < .05) | Elevation of osteocalcin levels might increase BMD and thus could accelerate healing of bone fractures because high levels of osteocalcin and alkaline phosphatase are positively correlated with BMD; and antioxidant vitamins A, C, and E and selenium could accelerate bone healing after long-bone fixation surgery |
aStatistical significance was indicated at P < .05. ACLR, anterior cruciate ligament reconstruction; BMD, bone mineral density; CRPS, chronic regional pain syndrome; DASH, Disabilities of the Arm, Shoulder and Hand; N/R, not reported; NSAID, nonsteroidal anti-inflammatory drug.
Discussion
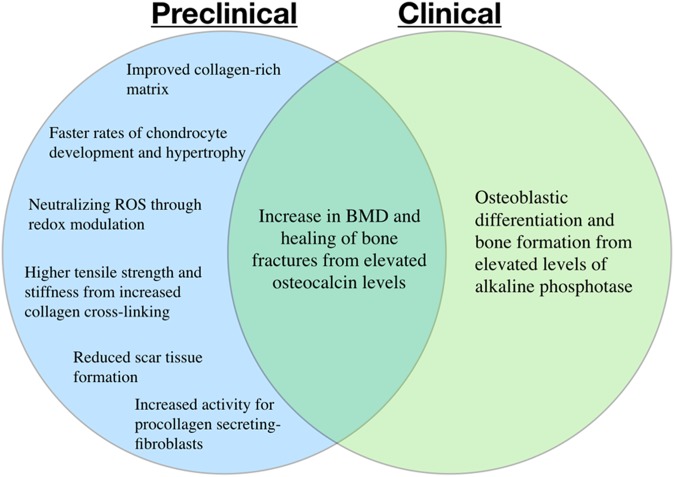
The most important finding of this systematic review was that there is preclinical evidence that vitamin C supplementation accelerates bone healing after fractures, increases type I collagen synthesis, and reduces oxidative stress parameters (detailed in Figure 2). However, clinical evidence does not replicate the results seen in animal models to date. Furthermore, high doses (ie, ≥1000 mg/d) of orally administered vitamin C had no direct benefit compared with controls. Conversely, low doses (ie, 60 mg/d) of orally administered vitamin C had a significant difference of increased bone biomarkers compared with controls. Overall, there are limited data regarding the efficacy of vitamin C supplementation after musculoskeletal injuries. No adverse effects were reported with vitamin C supplementation in either animal models or human participants.
Figure 2.
Reported effects of vitamin C supplementation after musculoskeletal injuries according to preclinical and clinical evidence. BMD, bone mineral density; ROS, reactive oxygen species.
After a musculoskeletal injury, the activation of inflammatory cells has been observed to overproduce ROS, causing deleterious oxidative stress. Oxidative stress has been described as an imbalance between ROS and antioxidants,19 resulting in a hostile healing environment that negatively affects the viability and proliferation of recruited collagen-producing cells and ultimately promoting apoptosis.14,16,17,21 Vitamin C as an antioxidant is capable of neutralizing ROS through redox reactions, relieving oxidative stress due to inflammation. All 3 preclinical studies evaluating oxidative stress reported that vitamin C was effective in reducing oxidative stress after injuries by decreasing endogenous or exogenous ROS, signified by an improved tissue composition in ligaments, tendons, and bone.5,10,19 However, only 1 of the 3 studies reported vitamin C as an antagonist for oxidative stress after 2 weeks,19 limiting preclinical support for the clinical implications as a postinjury supplement.
Four of 5 studies investigating the effects of vitamin C on collagen production suggested that vitamin C was effective by stimulating biochemical pathways associated with collagen synthesis. Omeroglu et al25 reported that vitamin C increased the activity for procollagen-secreting fibroblasts and overall type I collagen production. The stimulation of collagen-producing cells was also reported by Yilmaz et al,41 who observed accelerated rates of chondrocyte development and hypertrophy. Sandukji et al32 reported elevations in alkaline phosphatase, a vitamin C–dependent enzyme involved in osteoblastic differentiation and bone formation.8,11,18,26 Sarisozen et al33 also suggested that the observed increase in callus formation due to vitamin C resulted from osteoblast proliferation, although cellular activity was not quantified. Overall, these studies provide evidence that vitamin C may be effective in promoting collagen synthesis in vivo, although further clinical studies are needed to strengthen the implications for postoperative vitamin C supplementation.
Barker et al2 reported that vitamin C and E supplementation was ineffective in potentiating the improvement in force production of the injured limb after ACLR. However, baseline vitamin C status was associated with beneficial outcomes in strength, suggesting that long-term dietary habits may be more effective than short-term supplements. Ligamentous integrity at follow-up (3 months postoperatively) was not examined2; thus, collagen synthesis and ligament healing with vitamin C supplementation have not been investigated clinically in the current literature.
Ekrol et al6 found no significant difference in functional outcomes at 1 year between patients treated with vitamin C or a placebo after either a nondisplaced or displaced distal radius fracture. Although other studies have suggested a beneficial effect of vitamin C on fracture healing in animal models, Ekrol and colleagues6 found no difference in the Disabilities of the Arm, Shoulder and Hand (DASH) score, other functional outcomes, occurrence of chronic regional pain syndrome, or fracture healing with vitamin C treatment in patients with a distal radius fracture. It is important to note that functional outcomes after a distal radius fracture are also influenced by the condition of soft tissues in addition to bone healing.
Sandukji et al32 found that osteocalcin levels significantly increased in the plasma of patients treated with vitamin C for 2 weeks compared with those of the non–antioxidant-treated group. An elevation of osteocalcin levels might increase BMD and consequently could accelerate healing of bone fractures because high levels of osteocalcin and alkaline phosphatase have been reported to be positively correlated with BMD.12,23,37,39 The authors concluded that the administration of the antioxidant vitamins A, E, and C, in addition to selenium, could accelerate bone healing after long-bone fixation surgery; therefore, antioxidants could be considered in designing therapeutic protocols for postoperative bone fracture surgery.32
Previous research has demonstrated that the intravenous administration of vitamin C produces a plasma concentration 30- to 70-fold higher than the maximum tolerated oral doses.27 This suggests that high doses given through intravenous administration may not be necessary, if not deleterious to musculoskeletal healing. However, low doses of vitamin C administered intravenously allows for optimal bioavailability of the supplement, which may provide the means for musculoskeletal healing.4,27 With regard to supplementation after musculoskeletal injuries, the majority of preclinical studies administering vitamin C parenterally observed significant benefits in the rate of healing and reduced oxidative stress parameters compared with controls.5,10,19,25,33,41
Although there is a paucity of clinical studies in the literature, 2 studies administering vitamin C orally (500 mg/d2 and 1000 mg/d6) did not observe significant benefits of vitamin C supplementation after musculoskeletal injuries compared with controls. However, no current studies have evaluated the efficacy of only vitamin C administered intravenously after musculoskeletal injuries. We recognize that there is difficulty administering intravenous medications over long durations and rather suggest that intravenous vitamin C administration immediately postoperatively may have beneficial effects on musculoskeletal healing. We encourage future studies that attempt to delineate the effects of intravenous vitamin C during these recovery periods before intravenous doses can be recommended.
A complete understanding of the mechanism of action of vitamin C remains unclear. Several basic science and clinical studies investigating tumor development have reported that the overexpression of antioxidant enzymes and high doses of vitamin C (ie, ≥1000 mg/d) may impair wound healing and angiogenesis.29,30,36,40 Although high concentrations of ROS can be deleterious to tissue healing, low concentrations of ROS have been demonstrated to promote angiogenesis and mesenchymal stem cell proliferation.3 Therefore, it has been hypothesized that overscavenging of ROS by vitamin C may impair healing in musculoskeletal injury settings.19 Sandukji et al32 reported increased biomarkers of bone with 60 mg/d of vitamin C, which provides clinical evidence for the use of low doses of vitamin C as a postinjury supplement. Therefore, we recommend that the low-dose oral administration (ie, 60 mg/d) of vitamin C may be an effective form of treatment after musculoskeletal injuries. However, there are currently no definitive treatment protocols for the administration of vitamin C. Further clinical studies analyzing a broader range of oral doses, the efficacy of vitamin C administration via parental routes, and the synergistic actions of combined antioxidant administration are encouraged.
Limitations
We acknowledge some limitations to this systematic review. Although vitamin C is a well-known supplement that is used frequently, only 10 studies were included after the specific inclusion criteria were applied, which reflects the overall lack of evidence-based literature regarding vitamin C supplementation after musculoskeletal injuries. The heterogeneity in vitamin C supplementation protocols, including the route of administration, dosage, frequency, and duration, limits direct comparisons when evaluating combined results. Furthermore, variations in the type of collagen (bone, tendon, ligament) studied and the treatment methods (conservative vs surgical) used limit the global interpretation of results on vitamin C supplementation. One clinical study evaluated the effects of only vitamin C supplementation, and 2 studies evaluated the effects of the combined supplementation of vitamin C and other antioxidants; this may affect the clinical findings and direct interpretation of vitamin C. In addition, no clinical studies evaluated the evidence for tendon healing after vitamin C supplementation. Also, there were differences in the injury location (ie, hand, wrist, knee), so the results may not be generalizable to other injury locations in the body (ie, shoulder, hip, ankle). In addition, because rats are capable of synthesizing vitamin C through a normal diet, a function that humans do not exhibit, this is a direct limitation of the preclinical studies. It is suggested that further preclinical studies be performed on animals that also do not contain this trait, including chickens, guinea pigs, and primates, which can isolate the effects of exogenous vitamin C.
Conclusion
Preclinical studies demonstrated that vitamin C has the potential to accelerate bone healing after fractures, increase type I collagen synthesis, and reduce oxidative stress parameters. No adverse effects were reported with vitamin C supplementation in either animal models or human participants; thus, oral vitamin C appears to be a safe supplement but lacks clinical evidence compared with controls. Because of the limited number of human studies, further clinical investigations are needed before the implementation of vitamin C as a postinjury supplement.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: R.F.L. is a consultant for and receives royalties from Arthrex, Ossur, and Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Badr G, Hozzein WN, Badr BM, Al Ghamdi A, Saad Eldien HM, Garraud O. Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor-3 (ATF-3) and inducible nitric oxide synthase (iNOS)-mediated oxidative stress and recruiting bone marrow-derived endothelial progenitor cells. J Cell Physiol. 2016;231(10):2159–2171. [DOI] [PubMed] [Google Scholar]
- 2. Barker T, Leonard SW, Hansen J, et al. Vitamin E and C supplementation does not ameliorate muscle dysfunction after anterior cruciate ligament surgery. Free Radic Biol Med. 2009;47(11):1611–1618. [DOI] [PubMed] [Google Scholar]
- 3. Chan EC, Jiang F, Peshavariya HM, Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther. 2009;122(2):97–108. [DOI] [PubMed] [Google Scholar]
- 4. Duconge J, Miranda-Massari JR, Gonzalez MJ, Jackson JA, Warnock W, Riordan NH. Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate. P R Health Sci J. 2008;27(1):7–19. [PubMed] [Google Scholar]
- 5. Duygulu F, Yakan B, Karaoglu S, Kutlubay R, Karahan OI, Ozturk A. The effect of zymosan and the protective effect of various antioxidants on fracture healing in rats. Arch Orthop Trauma Surg. 2007;127(7):493–501. [DOI] [PubMed] [Google Scholar]
- 6. Ekrol I, Duckworth AD, Ralston SH, Court-Brown CM, McQueen MM. The influence of vitamin C on the outcome of distal radial fractures: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2014;96(17):1451–1459. [DOI] [PubMed] [Google Scholar]
- 7. Fazzalari NL, Forwood MR, Smith K, Manthey BA, Herreen P. Assessment of cancellous bone quality in severe osteoarthrosis: bone mineral density, mechanics, and microdamage. Bone. 1998;22(4):381–388. [DOI] [PubMed] [Google Scholar]
- 8. Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994;9(6):843–854. [DOI] [PubMed] [Google Scholar]
- 9. Franceschi RT, Wilson JX, Dixon SJ. Requirement for Na(+)-dependent ascorbic acid transport in osteoblast function. Am J Physiol. 1995;268(6 pt 1):C1430–C1439. [DOI] [PubMed] [Google Scholar]
- 10. Fu SC, Cheng WH, Cheuk YC, et al. Development of vitamin C irrigation saline to promote graft healing in anterior cruciate ligament reconstruction. J Orthop Trans. 2013;1:67–77. [Google Scholar]
- 11. Ganta DR, McCarthy MB, Gronowicz GA. Ascorbic acid alters collagen integrins in bone culture. Endocrinology. 1997;138(9):3606–3612. [DOI] [PubMed] [Google Scholar]
- 12. Garnero P, Delmas PD. Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact. 2004;4(1):50–63. [PubMed] [Google Scholar]
- 13. Garnero P, Delmas PD. Noninvasive techniques for assessing skeletal changes in inflammatory arthritis: bone biomarkers. Curr Opin Rheumatol. 2004;16(4):428–434. [DOI] [PubMed] [Google Scholar]
- 14. Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85(3):632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giordano V, Albuquerque RP, do Amaral NP, Chame CC, de Souza F, Apfel MI. Supplementary vitamin C does not accelerate bone healing in a rat tibia fracture model. Acta Ortop Bras. 2012;20(1):10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gokturk E, Turgut A, Baycu C, Gunal I, Seber S, Gulbas Z. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66(5):473–475. [DOI] [PubMed] [Google Scholar]
- 17. Haigler HJ, Spring DD. Comparison of the analgesic effects of dimethyl sulfoxide and morphine. Ann N Y Acad Sci. 1983;411:19–27. [DOI] [PubMed] [Google Scholar]
- 18. Harada S, Matsumoto T, Ogata E. Role of ascorbic acid in the regulation of proliferation in osteoblast-like MC3T3-E1 cells. J Bone Miner Res. 1991;6(9):903–908. [DOI] [PubMed] [Google Scholar]
- 19. Hung LK, Fu SC, Lee YW, Mok TY, Chan KM. Local vitamin-C injection reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J Bone Joint Surg Am. 2013;95(7):e41. [DOI] [PubMed] [Google Scholar]
- 20. Kjaer M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19(4):500–510. [DOI] [PubMed] [Google Scholar]
- 21. Lee YW, Fu SC, Yeung MY, Lau CML, Chan KM, Hung LK. Effects of redox modulation on cell proliferation, viability, and migration in cultured rat and human tendon progenitor cells. Oxid Med Cell Longev. 2017;2017:8785042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27(5):469–474. [DOI] [PubMed] [Google Scholar]
- 23. Mimori K, Komaki M, Iwasaki K, Ishikawa I. Extracellular signal-regulated kinase 1/2 is involved in ascorbic acid-induced osteoblastic differentiation in periodontal ligament cells. J Periodontol. 2007;78(2):328–334. [DOI] [PubMed] [Google Scholar]
- 24. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Omeroglu S, Peker T, Turkozkan N, Omeroglu H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch Orthop Trauma Surg. 2009;129(2):281–286. [DOI] [PubMed] [Google Scholar]
- 26. Otsuka E, Yamaguchi A, Hirose S, Hagiwara H. Characterization of osteoblastic differentiation of stromal cell line ST2 that is induced by ascorbic acid. Am J Physiol. 1999;277(1 pt 1):C132–C138. [DOI] [PubMed] [Google Scholar]
- 27. Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–537. [DOI] [PubMed] [Google Scholar]
- 28. Phillips CL, Combs SB, Pinnell SR. Effects of ascorbic acid on proliferation and collagen synthesis in relation to the donor age of human dermal fibroblasts. J Invest Dermatol. 1994;103(2):228–232. [DOI] [PubMed] [Google Scholar]
- 29. Redondo P, Jimenez E, Perez A, Garcia-Foncillas J. N-acetylcysteine downregulates vascular endothelial growth factor production by human keratinocytes in vitro. Arch Dermatol Res. 2000;292(12):621–628. [DOI] [PubMed] [Google Scholar]
- 30. Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13(1):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell JE, Manske PR. Ascorbic acid requirement for optimal flexor tendon repair in vitro. J Orthop Res. 1991;9(5):714–719. [DOI] [PubMed] [Google Scholar]
- 32. Sandukji A, Al-Sawaf H, Mohamadin A, Alrashidi Y, Sheweita SA. Oxidative stress and bone markers in plasma of patients with long-bone fixative surgery: role of antioxidants. Hum Exp Toxicol. 2011;30(6):435–442. [DOI] [PubMed] [Google Scholar]
- 33. Sarisozen B, Durak K, Dincer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309–313. [DOI] [PubMed] [Google Scholar]
- 34. Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. 2017;105(1):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor AK, Lueken SA, Libanati C, Baylink DJ. Biochemical markers of bone turnover for the clinical assessment of bone metabolism. Rheum Dis Clin North Am. 1994;20(3):589–607. [PubMed] [Google Scholar]
- 36. Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009;47(1):32–40. [DOI] [PubMed] [Google Scholar]
- 37. Weber P. The role of vitamins in the prevention of osteoporosis: a brief status report. Int J Vitam Nutr Res. 1999;69(3):194–197. [DOI] [PubMed] [Google Scholar]
- 38. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85(1):1–3. [PubMed] [Google Scholar]
- 39. Wu X, Itoh N, Taniguchi T, et al. Zinc-induced sodium-dependent vitamin C transporter 2 expression: potent roles in osteoblast differentiation. Arch Biochem Biophys. 2003;420(1):114–120. [DOI] [PubMed] [Google Scholar]
- 40. Yasuda M, Ohzeki Y, Shimizu S, et al. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64(4):249–258. [DOI] [PubMed] [Google Scholar]
- 41. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426–428. [DOI] [PubMed] [Google Scholar]