CASE
A previously healthy 23-year-old male student presented with a painless ulcer (diameter, 5 cm; depth, 0.5 cm) with irregular boundaries, slightly undermined edges, and adjacent small nodules on his dorsal right upper arm (Fig. 1A). Physical examination revealed an enlargement of the epitrochlear and axillary lymph nodes, but no fever or other systemic signs and symptoms. Differential blood count, C-reactive protein, aminotransferase, and creatinine levels were within normal ranges. The patient had stayed in his home country, Peru, for 4 weeks, mostly in Lima but also in rural areas around Cuzco. While staying with his family, he had contact with cats and worked in the family's vegetable garden. He did not visit any caves or mines or recall any (even superficial) injury to his arm, but during hiking on narrow trails he might have contracted minor scratches from bushes. Two weeks after returning to Germany, he noticed a papule, which then developed into an ulcer within the following week.
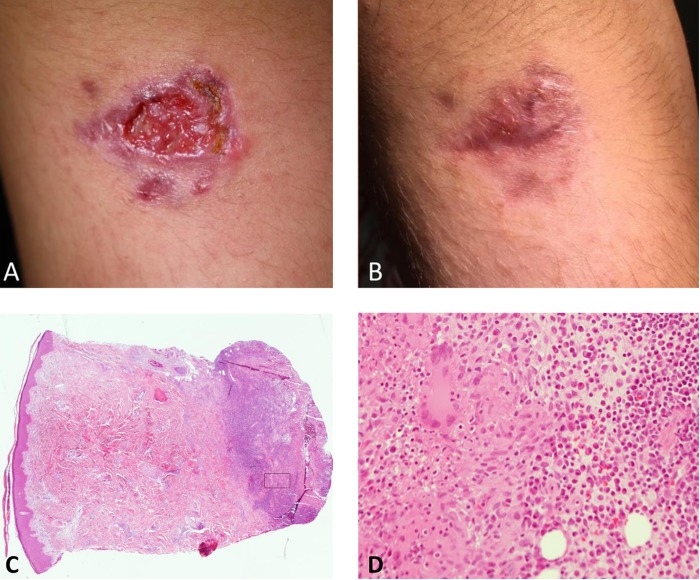
FIG 1.
(A) Ulcerative lesion on the right upper arm at first presentation. (B) Epithelialized lesion after a total of 11 weeks of therapy with posaconazole. (C and D) Histopathology of the skin biopsy specimen prior to treatment (hematoxylin-eosin staining; magnification, ×40 [C] and 200× [D]; the frame in [C] depicts the magnified area shown in [D]). A dense, granulomatous infiltrate of epithelioid histiocytes surrounded by lymphocytes, plasma cells, and some neutrophils is seen in the deep dermis.
Cutaneous leishmaniasis was suspected. Skin slit smears, scrapings, or swabs were repeatedly negative for bacteria (by Gram stain and culture), Mycobacterium ulcerans (PCR) (1), Leishmania (by Giemsa staining and genus-specific PCR [2]), and fungi (by culture). Subsequently, two lesional biopsy specimens were taken. Histopathology revealed perivascular infiltrates of lymphocytes and histiocytes in the upper and middle layer of the dermis. The deep dermis contained a dense, granulomatous, noncaseous infiltrate of epithelioid histiocytes surrounded by lymphocytes, plasma cells, and some neutrophils; a few multinucleated foreign body giant cells were also present. These findings were suggestive of a mycobacterial infection, but Ziehl-Neelsen staining did not reveal acid-fast rods, and PCR remained negative for mycobacteria (by unpublished in-house PCR targeting part of the 16S rRNA gene). Repeatedly performed layered and serial sections (n = 14) that were subjected to Grocott's methenamine silver (GMS) and periodic acid-Schiff (PAS) staining and analyzed by several pathologists did not reveal any fungal elements. Giemsa-stained sections, Leishmania genus-specific 18S rRNA-PCR (3), and cultures in modified Schneider's Drosophila medium (SDM) (4) all failed to detect Leishmania. However, after 7 days at 28°C, fungal elements were observed in the SDM culture. Subcultures on Sabouraud dextrose agar with chloramphenicol yielded white colonies without (36°C) or with (28°C) short marginal extensions after 48 h. Microscopy of colonies revealed pleomorphic yeast-like cells (36°C) or hyphae with teardrop-like microconidia (28°C) (Fig. 2). The organisms were identified as Sporothrix schenckii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Biotyper software version 3.1; Bruker Daltonik GmbH, Bremen, Germany) using the MALDI Biotyper reference library MBT 6903 MSP (April 2016). As the score of 1.85 did not allow firm conclusions on the species, sequencing of the D1/D2 region of the fungal 26S rRNA gene (by in-house PCR) (5) was performed. The amplified 548-bp fragment was 100% identical to S. schenckii ATCC 26327 (GenBank accession number KP780463). Diagnosis was confirmed by the National Reference Center for Invasive Fungal Infections (Jena, Germany).
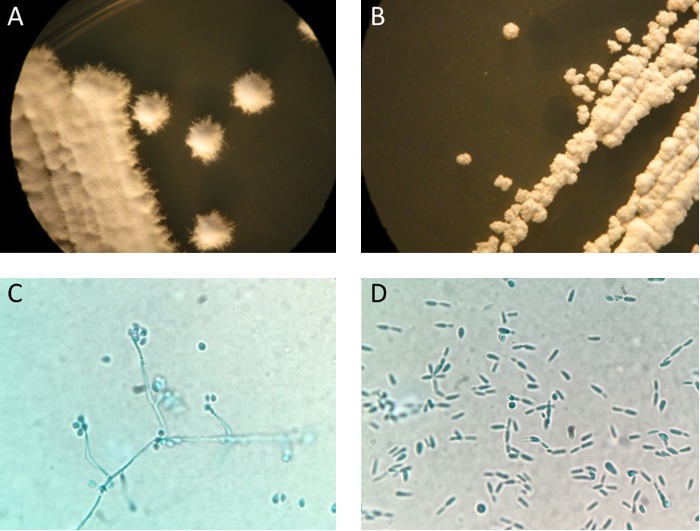
FIG 2.
Colonies of S. schenckii after 7 days of incubation on Sabouraud dextrose agar at 28°C (A) and 36°C (B). Lactophenol cotton blue wet mount preparation of S. schenckii after 7 days of growth at 28°C (C) and at 36°C (D).
Empirical therapy was initiated with oral itraconazole (1 × 200 mg/day). Subsequent susceptibility testing of the S. schenckii isolate (by microdilution method according to EUCAST) revealed MICs of >8 μg/ml for itraconazole, voriconazole, fluconazole, isavuconazole, and anidulafungin, suggesting resistance, and MICs of 0.5 and 1 μg/ml for posaconazole and amphotericin B, respectively. Therefore, after 11 days without obvious improvement of the skin lesion, itraconazole was replaced by posaconazole (100 mg three times a day [TID]), although the duration of itraconazole therapy was certainly too short to imply clinical treatment failure. Both itraconazole and posaconazole were well tolerated, and neither itraconazole nor posaconazole serum levels were determined. The patient performed daily wound care with povidone-iodine ointment. Sporadically, he also applied moderate heat to the lesion for 5 to 10 min using a hot water bottle. Under this regimen, the lesion was epithelialized after 6 weeks. Treatment was continued for 10 more days. Two weeks later, a small new encrusted lesion was noted at the former lesion site. Therapy with posaconazole was resumed, clinical healing was achieved after 3 weeks, and treatment was continued for another 2 weeks (Fig. 1B). No further relapse occurred during the follow-up of 3 months.
DISCUSSION
Sporothrix schenckii is a dimorphic fungus and was first isolated in 1896 by medical student Benjamin Schenck at John-Hopkins Hospital in Baltimore from skin lesions of a 36-year-old patient (6). S. schenckii sensu lato is a complex of phylogenetically distinct species that differ in geographical distribution and biochemical, phenotypic, and genetic characteristics (6, 7). Members of the complex are found worldwide, especially in cellulose-rich soils of tropical and subtropical areas with temperatures around 30°C and high humidity (6, 7). S. schenckii has the ability to switch between filamentous (mold) and yeast forms in response to thermal stimuli and other environmental cues. In nature, the fungus grows in its mycelial form, producing abundant infectious conidia (7, 8). After transmission to humans or other mammalian hosts, the fungus converts to the yeast form, which can cause serious chronic infections (8).
Infection usually occurs through traumatic inoculation of organic matter contaminated with fungal conidia (6). Risk factors are farming (e.g., baling of hay), gardening (e.g., pruning of plants), forest work, mining, and recreational outdoor activities. In some regions, the infection is acquired after contact with sick or carrier animals like cats, dogs, or armadillos (7, 8).
Sporotrichosis can be classified into fixed cutaneous, lymphocutaneous, disseminated cutaneous, and extracutaneous or systemic forms (8, 9). Clinical presentation of sporotrichosis depends on the host immune response, the fungal inoculum, and the virulence of the Sporothrix strain. The most common manifestation is the lymphocutaneous form. It is characterized by a primary nodular or ulcerative lesion (usually at the extremities) with seropurulent secretion, followed by secondary lesions along the draining lymphatics that lead to the typical “sporotrichoid” appearance (6, 9). In the fixed cutaneous form of sporotrichosis, the yeast stage of the fungus does not spread but causes a single skin lesion that frequently ulcerates with erythematous edges; papulopustular, nodular, verrucous, or plaque-like lesions have also been described (6, 9). In disseminated cutaneous sporotrichosis, hematogenous dissemination or multiple inoculations of the fungus cause skin lesions at different anatomical sites, but extracutaneous or visceral organs are not affected (6, 8, 9). Systemic sporotrichosis is rare and only occurs in immunocompromised individuals. The most common systemic entity is the osteoarticular form (6, 9). Primary pulmonary sporotrichosis, resulting from inhalation of fungal conidia, is seen in patients with chronic obstructive pulmonary disease and resembles pulmonary tuberculosis (6, 7, 9). Chronic meningitis due to S. schenckii is very rare and usually requires a severe cellular immune defect (6).
Sporotrichosis needs to be distinguished from infections with intracellular bacteria (e.g., ubiquitous Mycobacteria spp., Nocardia spp., or Francisella tularensis) or parasites (Leishmania spp.) (6). Apart from clinical suspicion and histological and microbiological analyses, knowledge of the epidemiology of the pathogen and of the patient's history (e.g., travel, vocational, and outdoor activities) is crucial to reach the correct diagnosis. Our patient had visited his home country, Peru, where he stayed in Lima, but also in Pisac (30 km northeast of Cuzco), close to the areas in the Peruvian Andes where sporotrichosis is hyperendemic (10).
The diagnostic gold standard is the isolation and identification of S. schenckii sensu lato by culture of skin biopsy specimens or other clinical specimens, followed by MALDI-TOF MS (6, 9). Fungal cultures should be handled with gloves and in a class II biological safety cabinet (11). Growth of S. schenckii can be observed after 2 to 5 days, but may take up to 2 weeks (9). Incubation at 25 to 28°C will yield filamentous hyaline, initially cream-colored colonies that may develop a brownish/black color after some time (6, 9). Subculture on enriched medium like brain heart infusion agar at 35 to 37°C allows conversion to the yeast stage (6, 9). The dimorphic yeast-to-mycelium transition seems to be dependent on calcium uptake and a Ca2+/calmodulin-dependent protein kinase. Inhibition of this kinase pathway prevented transformation of yeast cells into hyphae (8). The dark pigment of S. schenckii results from the production of melanin that protects the fungus from phagocytosis, oxidative killing by phagocytic cells, and extracellular proteinases, and reduces susceptibilities to different antifungal drugs (8, 9). The temperature optimum for S. schenckii is around 30 to 37°C, whereas temperatures above 40°C prevent its growth (6). Strains that grew at 35°C but not at 37°C were incapable of causing lymphatic sporotrichosis and therefore led to fixed cutaneous lesions (6). In contrast, strains isolated from lymphatic and extracutaneous lesions showed growth at 37°C (6). Thus, the thermotolerance of S. schenckii, which is variable among different geographic isolates (6), appears to be a virulence factor.
Identification of species within the S. schenckii sensu lato complex can be achieved by sequencing of the calmodulin gene, whereas morphological and biochemical features are insufficient (8).
If attempts to culture the organism are not successful, PCR or serological testing using enzyme-linked immunosorbent assay (ELISA) can be of diagnostic value (6, 9). Because of the paucity of fungal cells in the tissue, direct smear examination of pus or biopsy specimens may be negative for the typical cigar-shaped yeast cells of S. schenckii (9). Histopathology, in contrast, can aid the diagnosis, as it is frequently indicative of granulomatous disease and might reveal a few characteristic yeast cells, provided that thorough microscopy of GMS- or PAS-stained tissue sections is performed (6, 9).
Before the introduction of azoles in the 1990s, oral application of a saturated solution of potassium iodide was the standard treatment protocol, and it is still used in developing countries (6, 9). Today, the antifungal compound of choice is itraconazole, based on case reports, retrospective reviews, and nonrandomized trials. It has been used effectively, with low toxicity in most cases (6, 9). However, the in vitro and in vivo efficacy of itraconazole is variable, as S. schenckii sensu lato comprises a complex of different species (6, 9). Thus, an antifungal susceptibility profile of cultured S. schenckii isolates should always be obtained. Because most reported cases were treated with itraconazole, very few data on the therapeutic use of other agents in sporotrichosis exist. In addition, there is a lack of standard breakpoints and correlation between Etest and microdilution susceptibility testing (6), and epidemiological cutoff values only begin to emerge (12), which makes in vitro testing, interpretation of results, and therapy even more difficult (9).
Several small case series using the allylamine terbinafine demonstrated good efficacy in cutaneous sporotrichosis, but consensus on the dose and duration of the therapy is still missing (6, 9). Clinical data on treatment with posaconazole alone have not been published. Our case demonstrates that oral posaconazole can be effective in cutaneous sporotrichosis and therefore might be an excellent option for the treatment of infections with S. schenckii strains showing an elevated MIC for itraconazole. Knowing that the growth of all strains is prevented at 40°C (6, 9) and that hyperthermia is thought to enhance the intracellular killing capacity of neutrophils (9), the daily application of local heat (42 to 43°C) to the lesion in addition to antifungal drugs may improve the clinical outcome (9). To prevent a relapse like that which occurred in our case, prolonged therapy for at least 4 to 6 weeks after full clinical remission is recommended (9).
SELF-ASSESSMENT QUESTIONS
- Which of the following growth characteristics for S. schenckii is correct?
- Filamentous form at 37°C and yeast form at 25°C
- Yeast form at 25° and 37°C
- Filamentous form at 25° and 37°C
- Filamentous form at 25°C and yeast form at 37°C
- Which of the following environmental exposures is not a risk factor for acquiring sporotrichosis?
- Gardening
- Working in a mine
- Contact to cats
- Swimming
- Which of the following diseases would be the most common differential diagnoses of cutaneous sporotrichosis in an immunocompetent patient who spent his vacation in a rural and mountainous area of Peru?
- Eumycetoma and actinomycosis
- Mycobacteriosis and leishmaniasis
- Bartonellosis and rickettsiosis
- Dracunculiasis and cryptococcosis
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.01961-17 in this issue.
ACKNOWLEDGMENTS
We are grateful to the personnel at the National Reference Center for Invasive Fungal Infections (Jena, Germany) for confirming the identity of our S. schenckii isolate and for additional susceptibility testing.
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We declare no conflict of interest.
D.W. performed the mycological analyses, gave advice on treatment and wrote the first version of the manuscript. T.F. examined and treated the patient and took his history. J.R. performed the histopathological analyses. H.S. performed the parasitological analysis and detected the fungal growth. C.B. supervised the parasitological analyses and initiated the mycological analyses, gave advice on differential diagnoses, and wrote the manuscript. All authors read, corrected, and approved the final version of the manuscript.
REFERENCES
- 1.Beissner M, Symank D, Phillips RO, Amoako YA, Awua-Boateng NY, Sarfo FS, Jansson M, Huber KL, Herbinger KH, Battke F, Loscher T, Adjei O, Bretzel G. 2012. Detection of viable Mycobacterium ulcerans in clinical samples by a novel combined 16S rRNA reverse transcriptase/IS2404 real-time qPCR assay. PLoS Negl Trop Dis 6:e1756. doi: 10.1371/journal.pntd.0001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schonian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, Jaffe CL. 2003. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis 47:349–358. doi: 10.1016/S0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 3.Wortmann G, Sweeney C, Houng HS, Aronson N, Stiteler J, Jackson J, Ockenhouse C. 2001. Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. Am J Trop Med Hyg 65:583–587. doi: 10.4269/ajtmh.2001.65.583. [DOI] [PubMed] [Google Scholar]
- 4.Leitherer S, Clos J, Liebler-Tenorio EM, Schleicher U, Bogdan C, Soulat D. 2017. Characterization of the protein tyrosine phosphatase LmPRL-1 secreted by Leishmania major via the exosome pathway. Infect Immun 85:e00084-. doi: 10.1128/IAI.00084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros MB, de Almeida Paes R, Schubach AO. 2011. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev 24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. 2015. Global epidemiology of sporotrichosis. Med Mycol 53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 8.Tellez MD, Batista-Duharte A, Portuondo D, Quinello C, Bonne-Hernandez R, Carlos IZ. 2014. Sporothrix schenckii complex biology: environment and fungal pathogenicity. Microbiology 160:2352–2365. doi: 10.1099/mic.0.081794-0. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan VK. 2014. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract 2014:272376. doi: 10.1155/2014/272376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez Soto MC. 2015. Sporotrichosis: the story of an endemic region in Peru over 28 years (1985 to 2012). PLoS One 10:e0127924. doi: 10.1371/journal.pone.0127924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choosewood LC, Wilson DE. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/biosafety/publications/bmbl5/. [Google Scholar]
- 12.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Cordoba S, Gonzalez GM, Govender NP, Guarro J, Johnson EM, Kidd SE, Pereira SA, Rodrigues AM, Rozental S, Szeszs MW, Balleste Alaniz R, Bonifaz A, Bonfietti LX, Borba-Santos LP, Capilla J, Colombo AL, Dolande M, Isla MG, Melhem MSC, Mesa-Arango AC, Oliveira MME, Panizo MM, Pires de Camargo Z, Zancope-Oliveira RM, Meis JF, Turnidge J. 2017. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother 61:e01057-17. doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]