QuantiFERON-TB Gold Plus (QFT-Plus) is a new-generation QuantiFERON-TB Gold In-Tube (QFT-GIT) assay which has two antigen-coated tubes called TB1, which contains long peptides derived from ESAT-6 and CFP-10, and TB2, which contains the same components as TB1 and additional short peptides which potentially stimulate CD8+ T cells through the presentation of major histocompatibility complex class I. This is the first study to compare QFT-Plus and QFT-GIT for use in the diagnosis of latent tuberculosis infection (LTBI) among immunocompromised patients in the Republic of Korea.
KEYWORDS: IGRA, LTBI, assay comparison, immunocompromised hosts
ABSTRACT
QuantiFERON-TB Gold Plus (QFT-Plus) is a new-generation QuantiFERON-TB Gold In-Tube (QFT-GIT) assay which has two antigen-coated tubes called TB1, which contains long peptides derived from ESAT-6 and CFP-10, and TB2, which contains the same components as TB1 and additional short peptides which potentially stimulate CD8+ T cells through the presentation of major histocompatibility complex class I. This is the first study to compare QFT-Plus and QFT-GIT for use in the diagnosis of latent tuberculosis infection (LTBI) among immunocompromised patients in the Republic of Korea. Among 317 consecutive patients who underwent screening for LTBI before solid organ or hematopoietic stem cell transplantation and tumor necrosis factor alpha inhibitor treatment, LTBI was identified in 92 (29.0%) and 88 (27.8%) patients by QFT-GIT and QFT-Plus, respectively. The rate of concordance between QFT-GIT and QFT-Plus was 93.7% (κ value, 0.860), and the indeterminate rate (3.2%) was similar between QFT-GIT and QFT-Plus. Of 20 (6.3%) samples with discordant results, 11 (55.0%) and 7 (35.0%) were positive by QFT-GIT alone and QFT-Plus alone, respectively, and 2 (15.0%) were indeterminate by each assay. The interferon gamma level in samples with discordant results ranged from 0.39 to 1.10 IU/ml, except for one sample, in which the gamma interferon level was 2.97 IU/ml only in TB2. Conclusively, there was a high degree of agreement between the results of QFT-GIT and QFT-Plus for the screening of immunocompromised patients for LTBI. The reactivity in TB2 contributed substantially to the difference between QFT-GIT and QFT-Plus, particularly in solid organ transplant candidates. The significance of the discrete responses in TB1 and TB2 of QFT-Plus needs to be explored further by means of an immunological and clinical approach in different patient groups and clinical settings.
INTRODUCTION
Latent tuberculosis infection (LTBI) is a state of Mycobacterium tuberculosis infection without evidence of clinical symptoms and signs of active tuberculosis and is nontransmissible. Individuals with LTBI show a positive tuberculin skin test (TST) or interferon gamma (IFN-γ) release assay (IGRA) result but have negative findings on M. tuberculosis culture and sputum smear. However, it has been reported that about 5 to 15% of individuals with LBTI eventually develop active tuberculosis (1, 2). Moreover, immunocompromised patients and those receiving immunosuppressive drugs for the treatment of various diseases are at an increased risk of opportunistic infections, such as M. tuberculosis infections, which contribute to the high rates of M. tuberculosis prevalence (3). The incidence of M. tuberculosis infection is shown to be 20 to 74 times higher in solid organ transplant (SOT) recipients and 2 times higher in hematopoietic stem cell transplant (HSCT) recipients than in the general population (4). SOT recipients living in areas with lower levels of endemicity had an M. tuberculosis infection prevalence of 0.5 to 6.4%, while those living in areas with higher levels of endemicity (1 to 16%) had a prevalence of 15.2% (4, 5). Mortality rates among SOT recipients ranged from 19% to 40%, which is 10-fold higher than the overall mortality rate from M. tuberculosis infection (4–6), while the mortality rate after HSCT ranged from 0% to 50% (4, 7). Several studies have confirmed the increased risk of infections among patients with rheumatoid arthritis. Kourbeti et al. reported the importance of M. tuberculosis as an opportunistic infection in patients receiving biologic agents with an odds ratio of 3.73 (95% confidence interval [CI], 1.72 to 8.13; I2 = 0) (8). Thus, before the initiation of tumor necrosis factor alpha (TNF-α) inhibitor treatment, screening for LTBI and prophylaxis in patients have become standardized guidelines (9–11).
Nowadays, IGRAs performed using an enzyme-linked immunosorbent assay (ELISA), such as the QuantiFERON-TB Gold In-Tube (QFT-GIT) assay (Qiagen, Hilden, Germany), or an enzyme-linked immunosorbent spot (ELISPOT) assay, such as the T-SPOT.TB assay (Oxford Immunotec, Abingdon, UK), are commonly used to identify LTBI. IGRAs do not yield false-positive results in M. bovis bacillus Calmette-Guérin (BCG)-vaccinated individuals due to the absence of cross-reactivity with the BCG strain and do not require a second visit, as is needed for TST. Moreover, the IGRA is more objective in terms of the procedure and interpretation of the results (12). However, there are limitations, such as an inability to distinguish between active tuberculosis and LTBI (13, 14), a poor correlation between LTBI detection and the risk of developing active disease (13, 15–17), and a relatively poor sensitivity of LTBI detection in children and immunocompromised individuals (13, 18–21). In addition, indeterminate results (neither positive nor negative results) are more frequent in immunocompromised patients than in immunocompetent patients (22). Hence, there is a need to improve the diagnosis of LTBI among immunocompromised patients, including clinical risk groups, such as patients starting TNF-α inhibitor therapy and patients preparing for SOT and HSCT.
QuantiFERON-TB Gold Plus (QFT-Plus) is a new-generation QFT-GIT which has been widely used for the diagnosis of LTBI. The major difference between QFT-Plus and QFT-GIT is that QFT-Plus has two M. tuberculosis-specific antigen-coated tubes, called TB1 and TB2. TB1 contains the long peptides of ESAT-6 and CFP-10 but not TB7.7, while TB2 contains six short peptides, in addition to same components in TB1, thus inducing both CD4+ and CD8+ T-cell immune responses (23). There have been recent reports on the performance of the QFT-Plus assay in the diagnosis of active tuberculosis in high-risk patients and LTBI in low-risk populations (24–26); however, the performance evaluation of the QFT-Plus assay in immunocompromised patients has rarely been reported. The Republic of Korea is a country with an intermediate M. tuberculosis burden, and BCG vaccination is mandatory at birth. The estimated LTBI prevalence in the Republic of Korea, based on TST, is about 33.2% according to the 2016 Korean National Tuberculosis Association annual report (27). So far, the application of QFT-Plus in the clinical setting has just started, and no systematic data about the performance of QFT-Plus in the population of the Republic of Korea have yet been published.
This is the first study that aimed to compare QFT-Plus and QFT-GIT for the diagnosis of LTBI in patients who underwent screening for LTBI before therapeutic interventions which increase the risk of LTBI in the Republic of Korea, a country with an intermediate M. tuberculosis burden.
MATERIALS AND METHODS
Study population.
We prospectively included 317 consecutive patients referred for screening for LTBI in high-risk clinical settings from February to August 2017 at the Samsung Medical Center (Seoul, Republic of Korea), a university-affiliated tertiary care referral hospital. All patients were considered to have a high risk of LTBI reactivation on the basis of the underlying disease and scheduled therapy: 169 patients who had primary organ failure and who were awaiting SOT (the SOT group), 105 patients who had received chemotherapy for an underlying malignancy and who were going to have HSCT (the HSCT group), and 43 patients who were scheduled to undergo TNF-α inhibitor therapy for systemic autoimmune diseases, including rheumatoid arthritis, inflammatory bowel diseases, psoriatic arthritis, and ankylosing spondylitis (the TNF-α inhibitor therapy group). We reviewed the patients' medical records, including for previous antituberculosis medication, microbiological and radiological studies, and concomitant medication history. At our institution, we made a diagnosis of LTBI primarily by IGRA using QFT-GIT, which is more advantageous than TST in a country with an intermediate M. tuberculosis burden and a mandatory BCG vaccination program (9, 28). TST was performed when the IGRA showed indeterminate results according to the discretion of the physician. It was considered positive if the diameter of induration was ≥10 mm at 48 to 72 h. Written informed consent was obtained from the patients. The study protocol was approved by the Samsung Medical Center Ethics Review Committee (institutional review board no. 2016-04-076).
QFT-GIT and QFT-Plus assay.
The QFT-GIT and QFT-Plus assays (Qiagen, Hilden, Germany) were performed according to the manufacturer's instructions. Within 4 h of whole-blood collection in lithium heparin tubes, each milliliter of whole blood was transferred to an M. tuberculosis antigen tube (TB) for QFT-GIT, M. tuberculosis antigen tubes (TB1 and TB2) for QFT-Plus, and nil and mitogen tubes. The five tubes were immediately incubated at 37°C for 20 h. ELISA for quantitation of interferon gamma (IFN-γ) was performed simultaneously for both tests using a DS-2 automated ELISA processor (Dynex, Chantilly, VA). The results were interpreted as positive when the IFN-γ concentration in the M. tuberculosis antigen tube (TB for QFT-GT and either TB1 or TB2 for QFT-Plus) minus the IFN-γ concentration in the nil tube was ≥0.35 IU/ml and ≥25% of the nil tube value. Test results with a nil tube IFN-γ concentration of greater than 8.0 IU/ml or a mitogen tube IFN-γ concentration of less than 0.5 IU/ml were considered indeterminate.
Statistical analysis.
Data were expressed as the number (percentage) or median, range, and/or interquartile range and were analyzed using IBM SPSS Statistics (version 23.0) software (IBM Corporation, Armonk, NY, USA). Categorical variables were analyzed using Pearson's χ2 test or Fisher's exact test; continuous variables were analyzed using the Kruskal-Wallis test for comparisons among several groups and the Mann-Whitney U test for pairwise comparisons. The concordance between the QFT-GIT and QFT-Plus assay results was assessed using κ coefficients and was interpreted according to the Landis and Koch classification: the concordance for a κ value of ≤0.20 was considered slight, that for 0.20 < κ value ≤0.40 was considered fair, that for 0.40 < κ value ≤0.60 was considered moderate, that for 0.60 < κ value ≤ 0.80 was considered substantial, and that for 0.80 < κ value ≤1.00 was considered almost perfect (29). All reported P values were two-tailed and calculated with statistical significance set at a P value of less than 0.05.
RESULTS
Baseline clinical characteristics.
The baseline characteristics of 317 patients who had undergone QFT-GIT and QFT-Plus assays for screening for LTBI are shown in Table 1. Among the SOT, HSCT, and TNF-α inhibitor therapy groups, there was no difference in previous antituberculosis medication, and none tested positive by staining for acid-fast bacilli and M. tuberculosis culture. However, the age distribution and the proportion of males were significantly different between the groups. In particular, the proportion of patients on concomitant steroid and/or immunosuppressant therapy at the time of the tests was significantly higher in the TNF-α inhibitor therapy group (P < 0.001).
TABLE 1.
Characteristics of patient groupsc
| Characteristic | Values for the following patient groups: |
P | |||
|---|---|---|---|---|---|
| Total (n = 317) | SOT (n = 169) | HSCT (n = 105) | TNF-α inhibitor therapy (n = 43) | ||
| Median (range) age (yr) | 53 (16–78) | 54 (22–76) | 53 (23–70) | 45 (16–78) | 0.003a |
| No. (%) of male patients | 205 (64.7) | 123 (72.8) | 60 (57.1) | 22 (51.2) | 0.004b |
| No. (%) of patients who previously received antituberculosis medication | 19 (6.0) | 11 (6.5) | 8 (7.6) | 0 (0.0) | 0.158b |
| No. (%) of patients with the following AFB staining result: | |||||
| Not done | 246 (77.6) | 125 (74.0) | 34 (79.1) | 87 (82.9) | |
| Positive | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Negative | 71 (22.4) | 44 (26.0) | 9 (20.9) | 18 (17.1) | |
| No. (%) of patients with the following M. tuberculosis culture result: | |||||
| Not done | 246 (77.6) | 125 (74.0) | 34 (32.4) | 87 (49.4) | |
| Positive | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Negative | 71 (22.4) | 44 (26.0) | 9 (8.6) | 18 (41.9) | |
| No. (%) of patients with concomitant steroid and/or immunosuppressant treatment | 61 (19.2) | 17 (10.1) | 22 (21.0) | 22 (51.2) | <0.001b |
Determined by the Kruskal-Wallis test.
Determined by the χ2 test.
Abbreviations: AFB, acid-fast bacillus; SOT, solid organ transplantation; HSCT, hematopoietic stem cell transplantation; TNF-α, tumor necrosis factor alpha.
Detection of LTBI using QFT-GIT and QFT-Plus.
Overall, 92 patients (29.0%) and 88 patients (27.8%) were diagnosed with LTBI on the basis of the results of QFT-GIT and QFT-Plus, respectively. Indeterminate results were observed in 10 patients (3.2%) by both QFT-GIT and QFT-Plus. For each of TB1 and TB2 of QFT-Plus, 79 (24.9%) and 86 (27.1%) patients tested positive, respectively. The SOT group had a higher prevalence of LTBI than the HSCT group and the TNF-α inhibitor therapy group (P = 0.001 and < 0.001 for QFT-GIT and QFT-Plus, respectively). The responses to both tests by the different patient groups are shown in Table 2. Among 88 patients with positive results by QFT-Plus, 77 patients (87.5%) showed positive results in both TB1 and TB2 (TB1+ TB2+), but 2 and 9 patients showed positive results only in TB1 (TB1+ TB2−; 2.3%) and TB2 (TB1− TB2+; 10.2%), respectively. Nine out of 11 samples with discordant results (81.8%) between TB1 and TB2 belonged to the SOT group. For the indeterminate results, one patient from the SOT group and one patient from the TNF-α inhibitor therapy group had an indeterminate result by QFT-Plus but tested negative by QFT-Plus and vice versa.
TABLE 2.
Detection of LTBI using QFT-GIT and QFT-Plus in the different groups of immunocompromised patientsb
| Assay and result | No. (%) of patients in the following groups: |
Pa | |||
|---|---|---|---|---|---|
| Total (n = 317) | SOT (n = 169) | HSCT (n = 105) | TNF-α inhibitor therapy (n = 43) | ||
| QFT-GIT | |||||
| TB+ | 92 (29.0) | 64 (37.9) | 18 (17.1) | 10 (23.3) | 0.001 |
| Indeterminate | 10 (3.2) | 3 (1.8) | 6 (5.7) | 1 (2.3) | 0.153 |
| QFT-Plus | |||||
| TB1+ or TB2+ | 88 (27.8) | 63 (37.3) | 16 (15.2) | 9 (20.9) | <0.001 |
| TB1+ | 79 (24.9) | 55 (32.5) | 15 (14.3) | 9 (20.9) | 0.003 |
| TB2+ | 86 (27.1) | 62 (36.7) | 16 (15.2) | 8 (18.6) | <0.001 |
| TB1+ and TB2+ | 77 (24.3) | 54 (32.0) | 15 (14.3) | 8 (18.6) | 0.003 |
| Indeterminate | 10 (3.2) | 4 (2.4) | 5 (4.8) | 1 (2.3) | 0.495 |
Determined by the χ2 test.
Abbreviations: QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT-Plus, QuantiFERON-TB Gold Plus; SOT, solid organ transplantation; HSCT, hematopoietic stem cell transplantation; TNF-α, tumor necrosis factor alpha.
Concordance between QFT-GIT and QFT-Plus.
The degrees of concordance between QFT-GIT and QFT-Plus are shown in Tables 3 and 4. The agreement rate between the QFT-GIT and QFT-Plus assays was 93.7% (κ value = 0.860). Of 20 samples with discordant results (6.3%), 11 (55.0%) and 7 (35.0%) were positive by QFT-GIT alone (G+ P−) and QFT-Plus alone (G− P+), respectively, and 2 (15.0%) were indeterminate by each assay. All 7 samples with G− P+ results were from the SOT group. Among the 11 samples with G+ P− results, 8 from the SOT group, 2 from the HSCT group, and 1 from the TNF-α inhibitor therapy group were observed. The correlations between the IFN-γ responses of QFT-Plus TB1 or TB2 and QFT-GIT TB were relatively poorer in the SOT group than in the HSCT and TNF-α inhibitor therapy groups, but those between TB1 and TB2 were not (Table 4; see also Fig. S1 in the supplemental material).
TABLE 3.
Qualitative comparisons between QFT-GIT and QFT-Plusa
| QFT-GIT result | No. (%) of patients with the following QFT-Plus result: |
|||
|---|---|---|---|---|
| Positive | Negative | Indeterminate | Total | |
| Positive | 81 (25.6) | 11 (3.5) | 0 (0.0) | 92 (29.0) |
| Negative | 7 (2.2) | 207 (65.3) | 1 (0.3) | 215 (67.8) |
| Indeterminate | 0 (0.0) | 1 (0.3) | 9 (2.8) | 10 (3.2) |
| Total | 88 (27.8) | 219 (69.1) | 10 (3.2) | 317 (100.0) |
Abbreviations: QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT-Plus, QuantiFERON-TB Gold Plus.
TABLE 4.
Concordance between QFT-GIT and QFT-Plusa
| Patient group and assays compared | No. of patients for whom the assays showed agreement/total no. of patients (%) | κ value |
|---|---|---|
| All patients (n = 317) | ||
| QFT-GIT vs QFT-Plus | 297/317 (93.7) | 0.860 |
| QFT-GIT vs QFT-Plus TB1 | 296/317 (93.4) | 0.849 |
| QFT-GIT vs QFT-Plus TB2 | 295/317 (93.1) | 0.845 |
| QFT-Plus TB1 vs QFT-Plus TB2 | 306/317 (96.5) | 0.919 |
| SOT (n = 169) | ||
| QFT-GIT vs QFT-Plus | 153/169 (90.5) | 0.808 |
| QFT-GIT vs QFT-Plus TB1 | 152/169 (90.5) | 0.804 |
| QFT-GIT vs QFT-Plus TB2 | 153/169 (89.9) | 0.796 |
| QFT-Plus TB1 vs QFT-Plus TB2 | 160/169 (94.7) | 0.890 |
| HSCT (n = 105) | ||
| QFT-GIT vs QFT-Plus | 102/105 (97.1) | 0.919 |
| QFT-GIT vs QFT-Plus TB1 | 101/105 (96.2) | 0.891 |
| QFT-GIT vs QFT-Plus TB2 | 102/105 (97.1) | 0.919 |
| QFT-Plus TB1 vs QFT-Plus TB2 | 104/105 (99.0) | 0.971 |
| TNF-α inhibitor therapy (n = 43) | ||
| QFT-GIT vs QFT-Plus | 42/43 (97.7) | 0.939 |
| QFT-GIT vs QFT-Plus TB1 | 42/43 (97.7) | 0.939 |
| QFT-GIT vs QFT-Plus TB2 | 41/43 (95.3) | 0.874 |
| QFT-Plus TB1 vs QFT-Plus TB2 | 42/43 (97.7) | 0.934 |
Abbreviations: QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT-Plus, QuantiFERON-TB Gold Plus; SOT, solid organ transplantation; HSCT, hematopoietic stem cell transplantation; TNF-α, tumor necrosis factor-alpha.
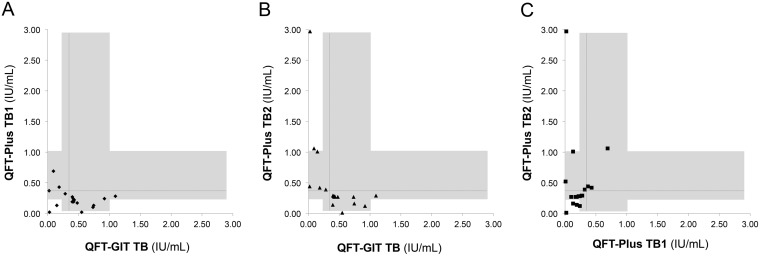
As shown in Tables 5 and S1, the IFN-γ responses in the 11 samples with G+ P− results ranged from 0.40 to 1.10 IU/ml (median, 0.48 IU/ml), and those in the 7 samples with G− P+ results ranged from 0.01 to 0.69 IU/ml (median, 0.32 IU/ml) and from 0.39 to 2.97 IU/ml (median, 0.52 IU/ml) for TB1 and TB2, respectively. About 77.8% of the discordant results except the indeterminate results occurred within the IFN-γ response of between 0.30 and 1.00 IU/ml (10/11 samples with G+ P− results and 4/7 samples with G− P+ results), and all 7 samples with G− P+ results were reactive in TB2 (3 samples with TB1+ TB2+ results and 4 samples with TB1− TB2+ results) but none were reactive in TB1 alone (TB1+ TB2−). Also, the IFN-γ responses in 8 out of 11 cases (72.7%) which had a discrepancy between TB1 and TB2 of QFT-Plus, regardless of agreement with QFT-GIT, ranged from 0.30 to 1.00 IU/ml. Figure 1 shows the correlations between the IFN-γ responses of the different antigen tubes for the 20 discordant cases.
TABLE 5.
Distribution of IFN-γ values in discordant results between QFT-GIT and QFT-Plusa
| QFT-GIT/QFT-Plus result | No. (%) of cases | Concn of IFN-γ (IU/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| QFT-GIT (concn in TB minus concn in nil tube) |
QFT-Plus (concn in TB1 minus concn in nil tube) |
QFT-Plus (concn in TB2 minus concn in nil tube) |
||||||||
| Range | IQR | Median | Range | IQR | Median | Range | IQR | Median | ||
| R/NR | 11 (55.0) | 0.40–1.10 | 0.41–0.74 | 0.48 | 0.02–0.28 | 0.14–0.24 | 0.19 | 0.01–0.29 | 0.15–0.28 | 0.27 |
| NR/R | 7 (35.0) | 0.00–0.28 | 0.02–0.18 | 0.09 | 0.01–0.69 | 0.05–0.42 | 0.32 | 0.39–2.97 | 0.43–1.05 | 0.52 |
| I/NRb | 1 (5.0) | |||||||||
| NR/Ic | 1 (5.0) | |||||||||
Data are for 20 samples with discordant results. Abbreviation: QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT-Plus, QuantiFERON-TB Gold Plus; IFN-γ, interferon gamma; R, reactive; NR, nonreactive; I, indeterminate; IQR, interquartile range. Positive results are in boldface.
The concentration of IFN-γ in the mitogen tube minus that in the nil tube was 0.49 IU/ml.
The concentration of IFN-γ in the mitogen tube minus that in the nil tube was 0.17 IU/ml.
FIG 1.
Discordant results in 20 cases. The figure shows quantitative results for QFT-GIT TB versus QFT-Plus TB1 (A), QFT-GIT TB versus QFT-Plus TB2 (B), and QFT-Plus TB1 versus QFT-Plus TB2 (C) for the 20 cases with discordant results. The horizontal and vertical dashed lines indicate the cutoff value (0.35 IU/ml), and the shaded areas indicate the range of 0.3 to 1.0 IU/ml.
Variability in the results of QFT-GIT and QFT-Plus.
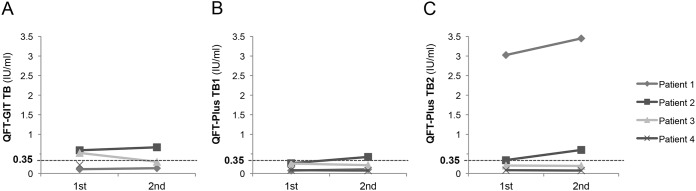
We observed the variability of the two tests in two aspects, one with a repeated IFN-γ ELISA with the same samples on the same day (repeatability) and the other with a short-term retest with newly collected samples (reproducibility). The repeated IFN-γ ELISA was performed on four samples, including two with results initially discordant between QFT-GIT and QFT-Plus. One became G− P− from G+ P− and the other became G+ P+ from G+ P−, which eventually resulted in agreement between QFT-GIT and QFT-Plus (Fig. 2). The repeated QFT-GIT and QFT-Plus assays with newly collected samples were performed for 37 patients who provided informed consent prior to undergoing significant therapeutic interventions (range of the time interval to repeat tests, 3 to 161 days; median, 23 days) (Table S2). Of the 37 patients, 28 belonged to the SOT group, 8 belonged to the HSCT group, and 1 belonged to the TNF-α inhibitor therapy group. Two patients (5.4%) each for QFT-GIT and QFT-Plus showed altered IFN-γ responses between the first and second tests. In case 7, QFT-GIT was initially nonreactive but became reactive 92 days later. Although QFT-Plus was persistently nonreactive, the IFN-γ responses of TB1 and TB2 of the second test were higher than those of the first test. In case 28, both QFT-GIT and QFT-Plus were initially nonreactive but became reactive in both tests after 23 days. Case 13 was persistently reactive in QFT-GIT but changed from nonreactive to reactive in QFT-Plus after 10 days. None was exposed to conditions which increased the tuberculosis infection risk. Instead, case 28 received granulocyte colony-stimulating factor (G-CSF) to collect autologous peripheral blood stem cells at the first test but not at the second test, although his absolute lymphocyte counts were the same (320/μl).
FIG 2.
Repeatability of QFT-GIT and QFT-Plus. This figure shows the distribution of the IFN-γ concentration from the first ELISA and the repeat ELISA results for four patients for QFT-GIT TB (A), QFT-Plus TB1 (B), and QFT-Plus TB2 (C). Horizontal dashed lines indicate the cutoff value (0.35 IU/ml). Abbreviations: QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT-Plus, QuantiFERON-TB Gold Plus.
DISCUSSION
In this study, we aimed to show the comparability of the fourth-generation QFT-Plus assay with the QFT-GIT assay for the diagnosis of LTBI in immunocompromised patients who were to undergo therapeutic interventions, such as SOT, HSCT, and TNF-α inhibitor therapy, in the setting of a country with an intermediate M. tuberculosis burden. The detection rate was comparable between the two assays, but the qualitative agreements between different antigen stimulations were not the same in different patient groups. Given the assumption that QFT-Plus with two antigen tubes might contribute to a higher sensitivity and fewer indeterminate results, QFT-Plus did not demonstrate performance superior to that of QFT-GIT in this patient population.
Considering the prevalence of LTBI in the Republic of Korea and the importance of screening for LTBI in immunocompromised patients, who have an increased risk of M. tuberculosis reactivation after therapeutic interventions, the diagnostic performance of the screening methods is paramount. The overall sensitivity of QFT-GIT for the diagnosis of active tuberculosis infection in the Republic of Korean population has been reported to be 80.2% by Bae et al. (30). However, the sensitivity or specificity of IGRAs for predicting the development of active tuberculosis is rather limited due to the need for long-term follow-up in the absence of a new exposure episode. So far, we have observed that 1 out of 103 (1.0%) QFT-GIT-positive systemic autoimmune disease patients who underwent LTBI treatment developed active tuberculosis at 20.5 months after the initiation of TNF-α inhibitor therapy, while 4 out of 239 (1.7%) QFT-GIT-negative patients who did not receive LTBI treatment developed active tuberculosis at 7.2, 20.8, 22.0, and 22.7 months after the initiation of TNF-α inhibitor therapy (31).
Compared to the previous changes in the QuantiFERON-TB series, the addition of a new antigen tube (TB2) containing new short peptides that stimulate IFN-γ production by CD8+ T cells in QFT-Plus has drawn much attention not only for performance but also for the potential new implications of this assay compared to QFT-GIT. Overall, we found a high degree of agreement (93.7%; κ value, 0.860) between QFT-GIT and QFT-Plus. However, among the three patient groups, the SOT group showed the lowest degree of agreement between the two assays (90.5%; κ value, 0.808), followed by the HSCT group (97.1%; κ value, 0.919) and the TNF-α inhibitor therapy group (97.7%; κ value, 0.939). In recent comparative studies, Moon et al. reported that low-risk health care workers in the United States showed 2.1% and 3.0% positivity rates by QFT-GIT and QFT-Plus, respectively (24). Hoffman et al. reported 94.5% agreement between the two assays within various populations, including health care workers and patients with suspicion of active tuberculosis in a German hospital (25). Yi et al. reported sensitivities of 98.6% and 97.6% for QFT-GIT and QFT-Plus, respectively, for the diagnosis of active tuberculosis in a Japanese population (26). In particular, the higher positivity rate with QFT-Plus in the study conducted by Moon et al. was due to more frequent positive results with TB2 than TB1 (24). In this study, we observed a higher positivity rate with QFT-GIT (29.0%) than with QFT-Plus (27.8%), although we observed more TB1− TB2+ results with QFT-Plus. Thus, the discrepancy between QFT-GIT and QFT-Plus might be affected not only by the differences in the antigenic stimulants (the absence of TB7.7 in the TB1 tube and addition of the TB2 tube) but also by the populations examined and the underlying conditions of the patients as well. However, as observed in previous IGRA studies (9, 24, 25, 32), most of the discordant results (77.8%) were scattered within IFN-γ levels of 0.30 to 1.00 IU/ml, which cross the assay cutoff (Fig. 1). Moon et al. found that the majority of discordant results between QFT-GIT and QFT-Plus TB1 (84.8%) and QFT-GIT and QFT-Plus TB2 (88.6%) existed within the range of 0.2 to 0.7 IU/ml (24).
Among the 317 patients evaluated in this study, the concordance between QFT-GIT and QFT-Plus was the lowest for those in the SOT group, which could be explained by the greater frequency of positive cases with TB2 alone in the SOT group than in the other groups in this study. Thus, it seems to be evident that the patients in the SOT group had a different immunologic challenge to LTBI than those in the other groups. We do not have a clear explanation for the reason why the SOT group had more frequent responses in TB2 alone; however, in part, it is speculated that the transplant candidates in the SOT group had relatively preserved CD8+ T cells responding to antigen stimulation compared to patients in the HSCT and TNF-α inhibitor therapy groups, who are likely to be pan-immunosuppressed by previous or ongoing toxic chemotherapy or steroid treatment. Several studies have been performed to elucidate the meaning of discrete responses in TB1 and TB2 of QFT-Plus. Petruccioli et al. (13) showed that the TB2-specific response elicited by CD8+ T cells was primarily associated with active tuberculosis and, consequently, with severe M. tuberculosis disease, which was consistent with the important contribution of CD8+ T cells to the host defense against M. tuberculosis by both cytokine secretion and cytotoxic activity (33–36). Barcellini et al. also reported a greater TB2 antigen response (TB2-TB1 difference, >0.6 IU · ml−1) in a subgroup of latently infected contacts with a higher antigenic burden (33). The rates of indeterminate results were the same (3.2%, 10 of 317 patients) for both QFT-GIT and QFT-Plus, although in one case in this study the indeterminate results occurred in different patients. Reactivity in the TB2 tube contributed substantially to the difference between QFT-GIT and QFT-Plus, which may provide a better understanding of the tuberculosis infection status and further diagnostic utility in the diagnosis of active tuberculosis.
Indeterminate results were observed most frequently in the HSCT group for both assays. The overall rate of indeterminate results in immunocompromised patients has been reported to range from 7% to 20% (17, 37). Although the recipients of SOT and HSCT with severe CD4+ lymphopenia had the highest rate of indeterminate results (38), the immunocompromised patients with organ failure, postchemotherapeutic hematological malignancy, and systemic autoimmune diseases taking steroids included in this study were consistently susceptible to indeterminate results due to underlying anemia, lymphopenia, hypoproteinemia, and hypoalbuminemia (22, 37).
In this study, we did not find a substantial improvement in the repeatability and reproducibility of QFT-Plus compared with those of QFT-GIT, possibly due to the limited number of observations. However, although the number of samples used in the assessment of repeatability was small, the assessment certainly showed a variation of IFN-γ detection by ELISA near the cutoff in both QFT-GIT and QFT-Plus. Also, in the evaluation of reproducibility with 37 samples newly collected within a relatively short term from the time of collection of the initial samples, both QFT-GIT and QFT-Plus showed a similar variability (2/37, 5.4%). Except for one patient who showed conversion in both tests, probably due to the effect of G-CSF in the first test (39), there were no significant factors affecting the M. tuberculosis risk identified in the other two patients, who showed conversion in each QFT-GIT and QFT-Plus assay. Knierer et al. confirmed the occurrence of conversions and reversions for QFT-Plus in serial testing of a high-risk cohort in a low-incidence setting (40). They suggested that the conversion and reversion rates for QFT-Plus were slightly higher than those for QFT-GIT, but the increase in variability was not significant due to the high rate of agreement between QFT-GIT and QFT-Plus (40). Based on the results reported by Knierer et al. (40) and our studies, the assay variability of QFT-Plus near the cutoff level remained the same as that with QFT-GIT. Currently, the QFT-Plus results are interpreted by applying the same cutoff applied to QFT-GIT and are based on the positivity of either TB1 or TB2; however, more data are needed to validate the interpretation criteria in different cohorts with different risks of M. tuberculosis infection or different clinical conditions.
As a limitation of this study, we aimed to show the comparability of QFT-Plus to QFT-GIT for the diagnosis of LTBI in immunocompromised patients; therefore, we did not address the sensitivity and specificity of QFT-Plus, which could be evaluated by the inclusion of patients with active tuberculosis and a population at low risk for LTBI. These aspects need to be investigated in further separate studies in the Republic of Korea. Also, there is no gold standard test for the diagnosis of LTBI, so the definite performance of QFT-Plus in this study can be drawn from long-term follow-up data. The other limitation is that the number of repeatedly collected samples having discordance between QFT-GIT and QFT-Plus was too small to evaluate the differences in variability.
In conclusion, there was a high degree of agreement between QFT-GIT and QFT-Plus results in the screening of immunocompromised patients for LTBI. The IFN-γ responses in the TB2 tube of the QFT-Plus assay mainly contributed to the difference between QFT-GIT and QFT-Plus, particularly in SOT candidates. The significance of discrete responses in TB1 and TB2 of QFT-Plus needs to be further explored immunologically and clinically in different patient groups and clinical settings.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jang Seop Kim and Jinyoung Lee for their excellent technical support in performing the assay and Hae Eun Kim and Mi Jeong Jeong for data management.
This study was supported by Qiagen, Hilden, Germany.
The sponsor had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00438-18.
REFERENCES
- 1.Dheda K, Barry CE III, Maartens G. 2016. Tuberculosis. Lancet 387:1211–1226. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. 2016. Tuberculosis. Nat Rev Dis Primers 2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh AM, Marjani M, Moniri A, Baghaei P, Jabbehdari S, Javanmard P, Tabarsi P. 2016. Tuberculosis in solid organ transplantation. Tanaffos 15:124–127. [PMC free article] [PubMed] [Google Scholar]
- 4.Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, Jones CE, Kampmann B, Kotton CN, Lange C, Ljungman P, Milburn H, Morris MI, Muller E, Munoz P, Nellore A, Rieder HL, Sester U, Theodoropoulos N, Wagner D, Sester M. 2012. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J 40:990–1013. doi: 10.1183/09031936.00000712. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Paterson DL. 1998. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis 27:1266–1277. doi: 10.1086/514993. [DOI] [PubMed] [Google Scholar]
- 6.Aguado JM, Herrero JA, Gavalda J, Torre-Cisneros J, Blanes M, Rufi G, Moreno A, Gurgui M, Hayek M, Lumbreras C, Cantarell C. 1997. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation 63:1278–1286. [DOI] [PubMed] [Google Scholar]
- 7.Russo RL, Dulley FL, Suganuma L, Franca IL, Yasuda MA, Costa SF. 2010. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int J Infect Dis 14(Suppl 3):e187–e191. doi: 10.1016/j.ijid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Kourbeti IS, Ziakas PD, Mylonakis E. 2014. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 58:1649–1657. doi: 10.1093/cid/ciu185. [DOI] [PubMed] [Google Scholar]
- 9.Jung YJ, Woo HI, Jeon K, Koh WJ, Jang DK, Cha HS, Koh EM, Lee NY, Kang ES. 2015. The significance of sensitive interferon gamma release assays for diagnosis of latent tuberculosis infection in patients receiving tumor necrosis factor-alpha antagonist therapy. PLoS One 10:e0141033. doi: 10.1371/journal.pone.0141033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, Wallis RS, Sotgiu G, Scholvinck EH, Goletti D, Zellweger JP, Diel R, Carmona L, Bartalesi F, Ravn P, Bossink A, Duarte R, Erkens C, Clark J, Migliori GB, Lange C. 2010. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 11.Furst DE, Keystone EC, So AK, Braun J, Breedveld FC, Burmester GR, De Benedetti F, Dorner T, Emery P, Fleischmann R, Gibofsky A, Kalden JR, Kavanaugh A, Kirkham B, Mease P, Rubbert-Roth A, Sieper J, Singer NG, Smolen JS, Van Riel PL, Weisman MH, Winthrop KL. 2013. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Ann Rheum Dis 72(Suppl 2):ii2–ii34. doi: 10.1136/annrheumdis-2013-203348. [DOI] [PubMed] [Google Scholar]
- 12.Horvat RT. 2015. Gamma interferon assays used in the diagnosis of tuberculosis. Clin Vaccine Immunol 22:845–849. doi: 10.1128/CVI.00199-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Goletti D, Sanduzzi A, Delogu G. 2014. Performance of the tuberculin skin test and interferon-gamma release assays: an update on the accuracy, cutoff stratification, and new potential immune-based approaches. J Rheumatol Suppl 91:24–31. doi: 10.3899/jrheum.140099. [DOI] [PubMed] [Google Scholar]
- 15.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, Fielding K, Wilkinson RJ, Pai M. 2012. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Huitric E, Sandgren A, Manissero D. 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 37:100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 17.Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, Dominguez J, Duarte R, Ernst M, Eyuboglu FO, Gerogianni I, Girardi E, Goletti D, Janssens JP, Julander I, Lange B, Latorre I, Losi M, Markova R, Matteelli A, Milburn H, Ravn P, Scholman T, Soccal PM, Straub M, Wagner D, Wolf T, Yalcin A, Lange C. 2014. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med 190:1168–1176. doi: 10.1164/rccm.201405-0967OC. [DOI] [PubMed] [Google Scholar]
- 18.Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, Den Boon S, Borroto Gutierrez SM, Bruchfeld J, Burhan E, Cavalcante S, Cedillos R, Chaisson R, Chee CB, Chesire L, Corbett E, Dara M, Denholm J, de Vries G, Falzon D, Ford N, Gale-Rowe M, Gilpin C, Girardi E, Go UY, Govindasamy D, Grant AD, Grzemska M, Harris R, Horsburgh CR Jr, Ismayilov A, Jaramillo E, Kik S, Kranzer K, Lienhardt C, LoBue P, Lonnroth K, Marks G, Menzies D, Migliori GB, Mosca D, Mukadi YD, Mwinga A, Nelson L, Nishikiori N, Oordt-Speets A, Rangaka MX, Reis A, Rotz L, Sandgren A. 2015. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 46:1563–1576. doi: 10.1183/13993003.01245-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santin M, Munoz L, Rigau D. 2012. Interferon-gamma release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One 7:e32482. doi: 10.1371/journal.pone.0032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincenti D, Carrara S, Butera O, Bizzoni F, Casetti R, Girardi E, Goletti D. 2007. Response to region of difference 1 (RD1) epitopes in human immunodeficiency virus (HIV)-infected individuals enrolled with suspected active tuberculosis: a pilot study. Clin Exp Immunol 150:91–98. doi: 10.1111/j.1365-2249.2007.03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SH, Gao Q, Tsoi KK, Wu WK, Tam LS, Lee N, Chan FK, Wu JC, Sung JJ, Ng SC. 2016. Effect of immunosuppressive therapy on interferon gamma release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax 71:64–72. doi: 10.1136/thoraxjnl-2015-207811. [DOI] [PubMed] [Google Scholar]
- 22.Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, Park BH, Moon JW, Park MS, Kim YS, Kim SK, Chang J, Kang YA. 2009. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis 9:207. doi: 10.1186/1471-2334-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiagen. 2015. QuantiFERON®-TB Gold Plus (QFT-Plus) ELISA package insert 02/2015. Qiagen, Hilden, Germany. [Google Scholar]
- 24.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. 2017. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55:1650–1657. doi: 10.1128/JCM.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann H, Avsar K, Gores R, Mavi SC, Hofmann-Thiel S. 2016. Equal sensitivity of the new generation QuantiFERON-TB Gold Plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 22:701–703. doi: 10.1016/j.cmi.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Yi L, Sasaki Y, Nagai H, Ishikawa S, Takamori M, Sakashita K, Saito T, Fukushima K, Igarashi Y, Aono A, Chikamatsu K, Yamada H, Takaki A, Mori T, Mitarai S. 2016. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 6:30617. doi: 10.1038/srep30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Health and Welfare, Korean National Tuberculosis Association. 2016. 2016 Korean National Tuberculosis Association annual report: tuberculin skin test based on the Korea National Health and Nutrition Examination Survey. KNTA, Seoul, Republic of Korea: https://www.knta.or.kr/reference/promotion/promotionView.asp?idx=1446&search_type=&search_text=&page=1. [Google Scholar]
- 28.Chang B, Park HY, Jeon K, Ahn JK, Cha HS, Koh EM, Kang ES, Koh WJ. 2011. Interferon-gamma release assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Clin Rheumatol 30:1535–1541. doi: 10.1007/s10067-011-1771-9. [DOI] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 30.Bae W, Park KU, Song EY, Kim SJ, Lee YJ, Park JS, Cho YJ, Yoon HI, Yim JJ, Lee CT, Lee JH. 2016. Comparison of the sensitivity of QuantiFERON-TB Gold In-Tube and T-SPOT.TB according to patient age. PLoS One 11:e0156917. doi: 10.1371/journal.pone.0156917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Park HY, Jeon K, Jeong B-H, Hwang J-W, Lee J, Cha H-S, Koh E-M, Kang E-S, Koh W-J. 2015. QuantiFERON-TB Gold In-Tube assay for screening arthritis patients for latent tuberculosis infection before starting anti-tumor necrosis factor treatment. PLoS One 10:e0119260. doi: 10.1371/journal.pone.0119260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banaei N, Gaur RL, Pai M. 2016. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol 54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF, Codecasa LR, Cugnata F, Di Serio C, Ferrarese M, Goletti D, Lipman M, Rancoita PM, Russo G, Tadolini M, Vanino E, Cirillo DM. 2016. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 48:1411–1419. doi: 10.1183/13993003.00510-2016. [DOI] [PubMed] [Google Scholar]
- 34.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. 2003. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med 168:1346–1352. doi: 10.1164/rccm.200306-837OC. [DOI] [PubMed] [Google Scholar]
- 35.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, Taskov H, Saltini C, Amicosante M. 2013. Antigen-specific CD4− and CD8− positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 75:277–281. doi: 10.1016/j.diagmicrobio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Richeldi L, Losi M, D'Amico R, Luppi M, Ferrari A, Mussini C, Codeluppi M, Cocchi S, Prati F, Paci V, Meacci M, Meccugni B, Rumpianesi F, Roversi P, Cerri S, Luppi F, Ferrara G, Latorre I, Gerunda GE, Torelli G, Esposito R, Fabbri LM. 2009. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest 136:198–204. doi: 10.1378/chest.08-2575. [DOI] [PubMed] [Google Scholar]
- 38.Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. 2007. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J 30:945–950. doi: 10.1183/09031936.00040007. [DOI] [PubMed] [Google Scholar]
- 39.Xiao BG, Lu CZ, Link H. 2007. Cell biology and clinical promise of G-CSF: immunomodulation and neuroprotection. J Cell Mol Med 11:1272–1290. doi: 10.1111/j.1582-4934.2007.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knierer J, Gallegos Morales EN, Schablon A, Nienhaus A, Kersten JF. 2017. QFT-Plus: a plus in variability?—evaluation of new generation IGRA in serial testing of students with a migration background in Germany. J Occup Med Toxicol 12:1. doi: 10.1186/s12995-016-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.