Diagnostic testing for Lyme disease (LD) remains dependent on detection of antibodies to LD Borrelia using serologic assays, in adherence to the standard two-tiered testing (STTT) algorithm. We present the first analytic evaluation of the automated Borrelia B31 ViraChip IgM and IgG microarray immunoblot (MIB) assays (Viramed Biotech AG, Planegg, Germany) in comparison to two different, semiautomated blot assays for LD, including the Borrelia B31 ViraStripe IgM and IgG line immunoassays (LIAs) (Viramed) and the MarDx Borrelia burgdorferi IgM and IgG Western blot (WB) assays (Trinity Biotech, Carlsbad, CA), using prospectively collected sera (n = 411) and archived, clinically characterized samples (n = 91).
KEYWORDS: Lyme disease, microarray immunoblot
ABSTRACT
Diagnostic testing for Lyme disease (LD) remains dependent on detection of antibodies to LD Borrelia using serologic assays, in adherence to the standard two-tiered testing (STTT) algorithm. We present the first analytic evaluation of the automated Borrelia B31 ViraChip IgM and IgG microarray immunoblot (MIB) assays (Viramed Biotech AG, Planegg, Germany) in comparison to two different, semiautomated blot assays for LD, including the Borrelia B31 ViraStripe IgM and IgG line immunoassays (LIAs) (Viramed) and the MarDx Borrelia burgdorferi IgM and IgG Western blot (WB) assays (Trinity Biotech, Carlsbad, CA), using prospectively collected sera (n = 411) and archived, clinically characterized samples (n = 91). We show comparable overall agreement (>84%) of the ViraChip MIB assays against the two aforementioned LD blot methods. The ViraChip MIB assays were also compared to a consensus standard, whereby samples were classified as positive or negative for IgM or IgG to B. burgdorferi if the analyte-matched ViraStripe LIA or MarDx WB assay were positive or negative, respectively. The ViraChip IgM and IgG MIB assays showed >93% positive, negative, and overall agreement versus these consensus criteria. The ViraChip MIB assays were associated with a time savings of 28 min to process one full batch of samples compared to the time required for the ViraStripe LIAs. The ViraChip MIB assays can be programmed and performed on an open-system, automated enzyme-linked immunosorbent assay (ELISA) processor, negating the need for assay-specific equipment and enabling laboratories to consolidate LD testing onto a single platform. We conclude that the ViraChip IgM and IgG MIB assays may be added to the repertoire of supplemental, second-tier blot testing systems for diagnosis of LD.
INTRODUCTION
Lyme disease (LD), caused by infection with pathogenic members of the Borrelia burgdorferi sensu lato complex and transmitted by Ixodes species ticks, is the most common tick-borne infection in both North America and Europe, with up to 400,000 infections estimated to occur annually in the United States alone (1–3). Within the B. burgdorferi sensu lato complex, B. burgdorferi sensu stricto (here referred to as B. burgdorferi) is the predominant genospecies circulating in North America and is associated with nearly all cases of LD (4). Following infection, LD may progress through multiple different stages, including early localized disease, which classically presents with an erythema migrans (EM) lesion (5). In the absence of treatment, LD can progress to systemic illness, including symptoms of myalgia, arthralgia, fatigue, and fever; more severe sequelae, including neuroinvasive disease and cardiac involvement (e.g., artrioventricular heart block) are also well described in the literature (6).
For patients without EM but who are symptomatic and have had tick exposure in an area of LD endemicity, serologic testing for the presence of antibodies to B. burgdorferi remains the main diagnostic method for LD (7). Currently, the Centers for Disease Control and Prevention (CDC) recommends that serologic testing for LD be performed using the standard two-tiered testing (STTT) algorithm (8). Briefly, the STTT begins with an initial screen using an enzyme-linked immunosorbent assay (ELISA) or immunofluorescence assay for detection of anti-B. burgdorferi IgM- and IgG-class antibodies, with positive or equivocal samples requiring supplemental blotting (e.g., Western blotting [WB] or immunoblotting) for detection of discrete IgM and/or IgG antibodies to the spirochete. Currently, a positive anti-B. burgdorferi IgM or IgG blot is defined as the presence of host antibodies to at least 2 out of 3 or at least 5 out of 10 B. burgdorferi proteins, respectively (8, 9). Importantly, due to the seropersistence of IgM antibodies to B. burgdorferi and the documented high rate of false-positive B. burgdorferi IgM blots, results from IgM immunoblotting should be considered only in patients with 30 days of symptoms or less in order to minimize the risk of erroneously misdiagnosing a patient with recent LD (8, 10).
Although screening ELISAs for LD are highly sensitive for detection of anti-B. burgdorferi antibodies, their specificity varies depending on the type of antigen used (e.g., whole-cell sonicate [WCS] or purified or recombinant antigens) and remains imperfect; this limitation is resolved by supplemental blot testing of ELISA-reactive samples (9). Despite the presence of shared, cross-reactive epitopes for a number of the targeted B. burgdorferi proteins (e.g., p41, p66, etc.) in other bacteria, blot testing for detection of antibodies to B. burgdorferi provides a small yet statistically significant improvement in specificity versus testing for LD by an ELISA alone (11–13). This increase in specificity translates to approximately 37,000 fewer false-positive LD test results in the United States, where nearly 3.4 million serologic tests for LD are performed annually (1, 14, 15). Supplemental LD blot testing, however, is associated with a number of interpretive and analytic challenges. First, the presence or absence of antibody bands on LD blots is determined by comparing the intensity of the band in the patient sample to the intensity of a control band. Visual examination of these blots, a subjective and technologist-dependent process, can lead to over- or undercalling band presence, resulting in low test accuracy and reproducibility (16). To avoid the challenges of visual LD blot interpretation, many testing centers rely on reference laboratories to perform second-tier blot testing; this practice of sending samples out for additional testing, however, is associated with a delay in turnaround time to final results (14). Alternatively, an increasing number of laboratories have transitioned to assessing band intensities using blot scanners and band densitometry measurements, a method which provides more objective interpretation of blot results than visual inspection (17). However, differences in software settings and platforms may still lead to result disparity across laboratories (D. Granger and E. Theel, unpublished data). The variability associated with blot interpretation is most concerning for the anti-B. burgdorferi IgM blots, which have been associated with a high rate of false positivity (10, 14, 18). Finally, when performed manually, blot testing requires significant technologist hands-on time, largely dedicated to pipetting of samples and processing. While automated blot processors are available, there are few stand-alone platforms that incorporate both automated specimen pipetting and blot processing. Although there are instruments capable of performing these two functions, they are typically dedicated to blot testing only and are unable to be used for completion of alternative immunoassays (e.g., ELISAs).
In this study, we present the first evaluation of the recently FDA-cleared Borrelia B31 ViraChip IgM and IgG microarray immunoblot assays (ViraChip MIB assays; Viramed Biotech AG, Planegg, Germany), which were entirely automated on a Gemini Compact Microplate Processor (Stratec Biomedical AG, Birkenfeld, Germany). Results of the ViraChip MIB assays were compared to those of the Borrelia B31 ViraStripe IgM and IgG line immunoassays (LIAs) (Viramed Biotech AG, Planegg, Germany) and the MarDx B. burgdorferi IgM and IgG WB assays (Trinity Biotech, Carlsbad, CA), both performed in a semiautomated manner using a BeeBlot strip processor (Bee Robotics, Gwynedd, Wales, UK).
MATERIALS AND METHODS
Study design.
Specimens evaluated in this study were split into two arms. The first arm included 411 prospectively collected, residual sera from unique patients submitted to Mayo Medical Laboratories (MML; Rochester, MN) for clinician-ordered serologic evaluation of LD using the STTT algorithm. Due to receipt of these samples through our MML reference laboratory practice, clinical information on these patients, including exposure history, symptoms, and treatment regimens, was not available. Inclusion criteria for these sera included being either positive (n = 366) or equivocal (n = 45) by the first-tier C6 Lyme ELISA (Oxford Immunotec, Marlborough, MA) and having sufficient residual specimen volume for testing by six different B. burgdorferi blot assays (described below). Prospective samples meeting these criteria were consecutively collected over a 30-day period between August and September 2017, deidentified, and stored frozen at −20°C until testing. All included samples were tested within one freeze-thaw cycle by the following second-tier supplemental assays: the Borrelia B31 ViraChip IgM and IgG microarray immunoblots (ViraChip MIB assays; Viramed Biotech AG, Planegg, Germany), the Borrelia B31 ViraStripe IgM and IgG LIAs (Viramed Biotech AG, Planegg, Germany), and the MarDx B. burgdorferi IgM and IgG WB assays (Trinity Biotech, Carlsbad, CA). This arm of the study was exempt from review by the Mayo Clinic Institutional Review Board (IRB).
The second study arm included 91 sera from 20 patients with confirmed LD and 71 control sera collected from unique, healthy individuals without a history of LD and residing in an area of LD endemicity (here, endemic control samples). A total of 79 of these 91 sera were received from the Bay Area Lyme Foundation (Portola Valley, CA) National Lyme Disease Biobank (LDB) (https://www.bayarealyme.org/our-research/biobank/). Among these 79 patients, 8 were classified by the LDB as confirmed early LD based on the presence of a documented EM lesion and exposure in a state with a high incidence of LD (Wisconsin), in accordance with the LD CDC case definitions (19). Convalescent, posttreatment serum samples from these eight patients, collected 1 to 2 months post-symptom onset by the LDB, were used for this study. The remaining 71 serum samples from the LDB were collected from asymptomatic individuals living in an area of LD endemicity (Wisconsin) (20, 21). An additional 12 serum samples collected 1 to 2 months post-symptom onset from 12 unique Mayo Clinic patients with confirmed LD between 2016 and 2018 were classified using the 2017 CDC LD case definitions and were also included in this arm following approval by the Mayo Clinic IRB (19). Among these 12 patients, 2 had Lyme carditis with atrioventricular conduction block, 3 had arthralgia attributed to LD, and 7 had Lyme neuroborreliosis, confirmed by the presence of lymphocytic pleocytosis in cerebrospinal fluid and an elevated Lyme antibody index. All samples were tested within one freeze-thaw cycle by the IgM and IgG Borrelia B31 ViraChip MIB assays and ViraStripe LIAs.
Borrelia B31 ViraChip MIB assays.
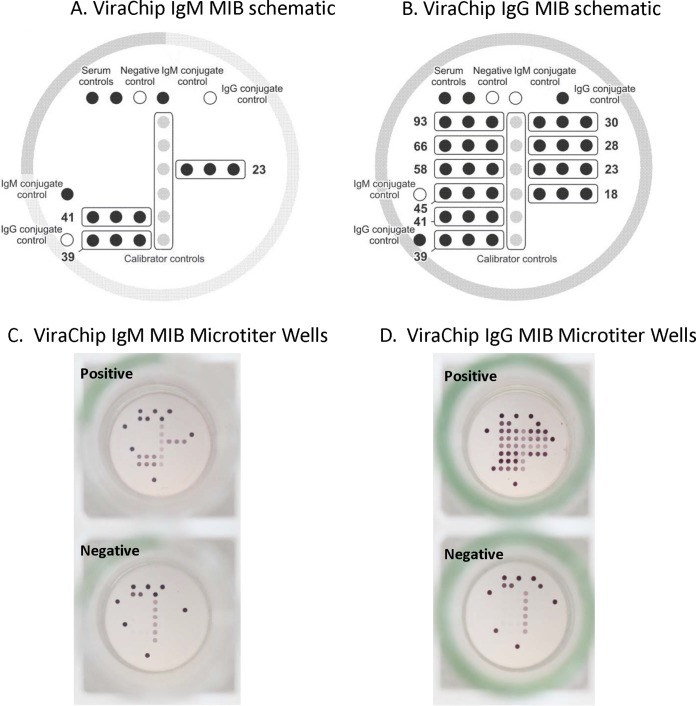
The Borrelia B31 ViraChip IgM and IgG assays are both novel microarray immunoblot (MIB) assays which received FDA clearance in 2017. The ViraChip MIB assays are designed using a 96-well microtiter plate format, and to the bottom of each well is adhered a nitrocellulose membrane onto which either recombinant or highly purified B. burgdorferi B31 antigens are immobilized in triplicate at defined positions, or spots; the IgM and IgG ViraChip MIB assays are adhered in individual wells (Fig. 1). The B. burgdorferi B31 antigens used in the ViraChip MIB assays are identical to those recommended by the CDC for second-tier blot testing and include the p23, p39, and p41 antigens for the ViraChip IgM MIB assay and the p18, p23, p28, p30, p39, p41, p45, p58, p66, and p93 antigens for the ViraChip IgG MIB assay. Additionally, each IgM and IgG ViraChip MIB plate has spots for one negative control, two serum controls, two IgM or two IgG conjugate controls, and a calibrator control spotted in sextuplet. Sample pipetting and ViraChip MIB processing were performed using a Gemini automated microplate processor (Stratec Biomedical AG, Birkenfeld, Germany) with strict adherence to the manufacturer's instructions. Briefly, each patient serum was diluted at 1:76 in sample diluent prior to addition into the IgM and IgG ViraChip MIB wells. Samples were incubated for 30 min at room temperature (RT), followed by removal of serum and three wash steps to eliminate unbound antibodies. Alkaline-phosphatase anti-human IgM or IgG conjugate, which binds to the respective immobilized anti-B. burgdorferi antibody (if present), was subsequently added. Following a 30-min incubation at RT, unbound conjugate was aspirated, wells were washed three times, and chromogen/substrate solution was added. Plates were incubated for 15 min at RT, and if conjugate-antibody complexes were present, the substrate precipitated, leading to a color change. The substrate was subsequently removed, and wells were washed three times and allowed to dry under continuous airflow for 20 min prior to colorimetric imaging using a high-sensitivity charge-coupled-device (CCD) camera on a CLAIR reader (Sensovation, Stockach, Germany).
FIG 1.
(A and B) Schematic representation and arrangement of control and B. burgdorferi antigens for the ViraChip IgM and IgG microarray immunoblot (MIB) assays. (C and D) Representative images of positive and negative results, as indicated. (Images adapted and used with permission from Viramed Biotech AG.)
Evaluation of the colorimetric intensity for each triplicate B. burgdorferi antigen was calculated in relation to the calibrator control, lot-specific correction factor, and individual antigen-specific correction factors using customized ViraChip software (version 1.10_1056). Briefly, the mean colorimetric intensity of the replicate calibrator controls is multiplied by the lot-specific factor for each antigen to establish the antigen-specific cutoff value. The mean intensity for each triplicate B. burgdorferi antigen is divided by the cutoff value to determine a signal-to-cutoff (S/CO) ratio, which is multiplied by 100. Antibodies to each B. burgdorferi antigen are considered present if the S/CO ratio is equal to or greater than 100. S/CO values of 19 or less are reported as 0 by the ViraChip software. Both the CLAIR reader and ViraChip software are FDA cleared for use with the ViraChip MIB assays. Manual (visual) interpretation of results from the ViraChip MIB assays is not possible due to antigen-specific cutoff intensity values and the compact size of the microarray. Qualitative interpretation of the ViraChip IgM and IgG MIB assays adhered to current CDC guidelines as described above (8). Per the manufacturer's instructions, ViraChip IgM and IgG MIB assays for which the ViraChip software could not assess the serum, conjugate, or calibrator controls were interpreted as invalid.
Borrelia B31 ViraStripe LIAs.
The Borrelia B31 ViraStripe IgM and IgG line immunoblot assays (LIAs) (Viramed Biotech AG, Planegg, Germany) are FDA-cleared assays, utilizing highly purified B. burgdorferi B31 antigens which are immobilized onto nitrocellulose membranes at defined positions. Each IgM and IgG LIA strip has an integrated control system, including a cutoff calibrator, serum control, and IgM or IgG conjugate control bands. The assays were performed according to the manufacturer's instructions for use as described previously (17). Briefly, samples and controls were manually pipetted, and strips were processed using a BeeBlot semiautomated blot strip processor (Bee Robotics, Gwynedd, Wales, UK). The LIAs were objectively read using a Viramed ViraCam reader and ViraScan software (version 2.10 B.0022). The ViraScan software locates each B. burgdorferi antigen and calibrator control band and measures the intensity by densitometric analysis. The background signal intensity level of each LIA is also measured and subtracted from that of the calibrator control, the cutoff value, and each B. burgdorferi antigen band. The resulting normalized B. burgdorferi band signal intensities are divided by the cutoff signal intensity, resulting in an S/CO ratio for each band. An IgM or IgG response to each B. burgdorferi protein is considered present if the S/CO is 80 or higher. S/CO levels of 19 or less are reported as 0 by the ViraScan software. Qualitative, anti-B. burgdorferi IgM and IgG LIA interpretations were determined in accordance with current CDC guidelines (8). ViraStripe IgM and IgG LIAs for which the ViraScan software was unable to identify the cutoff calibrator or serum control bands were considered uninterpretable, as per the manufacturer's instructions.
MarDx B. burgdorferi IgM and IgG Marblot WB assays.
The MarDx B. burgdorferi IgM and IgG Western blot (WB) assays are FDA cleared and are based on separation of B. burgdorferi B31 antigens via polyacrylamide gel electrophoresis followed by transfer of the proteins onto a nitrocellulose membrane. Processing of the MarDx IgM and IgG WBs was performed in accordance with the manufacturer's instructions. Briefly, following manual pipetting of the samples, controls, and a serum band locator onto the MarDx IgM and IgG WB strips, a BeeBlot semiautomated processor was used to complete all WB incubation and wash steps as described previously (17). The WBs were read manually by a single technologist using results from the serum band locator strip to identify the required B. burgdorferi IgM and IgG bands; scoring of the bands (i.e., present or absent) was based on visual comparison of the intensity of each band to the cutoff calibrator band located on the weakly reactive control strip. WBs were considered uninterpretable when bands were completely or partially masked due to heavy background or blotching. Qualitative interpretations of the B. burgdorferi IgM and IgG WBs were determined in accordance with current CDC guidelines (8).
Statistics.
MeasuringU software (MeasuringU, Denver, CO) was used to calculate positive, negative and overall percent agreement and 95% confidence intervals (CIs). Weighted kappa was calculated using MedCalc statistical software (22). Kappa values of <0.20, 0.21 to 0.40, 0.41 to 0.60, 0.61 to 0.80, and 0.81 to 1.00 were interpreted as poor, fair, moderate, good, and very good interrater agreement, respectively (23). Microsoft Excel 2010 with the Data Analysis ToolPak (version 14.0) was used to create correlation plots and to determine Pearson's coefficient (R) and P values. Pearson's correlation coefficient values of <0.3, 0.3 to 0.49, and >0.5 were interpreted as indicative of little, medium to moderate, and strong correlations, respectively (24).
RESULTS
Agreement between the B. burgdorferi ViraChip MIB assays, ViraStripe LIAs, and MarDx WB assays.
Using the prospectively collected samples (n = 411), the ViraChip IgM and IgG MIB assays showed positive, negative, and overall percent agreement values of 75.5% and 93.1%, 99.0% and 90.4%, and 85.6% and 91.2%, respectively, compared to results with the ViraStripe IgM/IgG LIAs (Table 1). Cohen's kappa values were 0.71 and 0.81, indicating good and very good interrater agreement between the ViraStripe LIA and ViraChip MIB IgM and IgG assays, respectively. Analysis of ViraChip IgM and IgG MIB results compared to those with the corresponding MarDx WB assays exhibited positive, negative, and overall percent agreement values of 85.4% and 80.4%, 84.5% and 94.8%, and 84.7 and 86.4%, respectively, with Cohen's kappa values of 0.68 and 0.72, both indicative of good interrater agreement between the assays (Table 2).
TABLE 1.
Comparison of the ViraChip MIB assays and ViraStripe LIA for detection of anti-B. burgdorferi IgM and IgG antibodies in serum
| ViraChip assay and resulta | ViraStripe assay and no. to tests with indicated resultb |
% agreement (95% CI)c |
Kappa value (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Uninterpretable | Positive | Negative | Overall | ||
| IgM MIB | IgM LIA (n = 411) | ||||||
| Positive | 160 | 1 | 1 | ||||
| Negative | 49d | 191 | 4 | 75.5 (69.2–80.8) | 99.0 (96.1–99.9) | 85.6 (81.9–88.7) | 0.71 (0.65–0.78) |
| Invalid | 3 | 1 | 1 | ||||
| IgG MIB | IgG LIA (n = 411) | ||||||
| Positive | 122 | 25 | 0 | ||||
| Negative | 9 | 253 | 0 | 93.1 (87.3–96.5) | 90.4 (86.3–93.3) | 91.2 (88.1–93.6) | 0.81 (0.75–0.87) |
| Invalid | 0 | 2 | 0 | ||||
MIB, microarray immunoblot; LIA, line immunoassay.
The ViraChip IgM MIB assay detected antibodies to only p41 in 37/49 samples and to only p23 in only 1 sample. The ViraChip IgM MIB assay did not detect antibodies to p39 in any of the 49 discordant samples.
CI, confidence interval.
Eleven samples had 0 bands, and 38 samples had only 1 band detected by the ViraChip IgM MIB assay.
TABLE 2.
Comparison of the ViraChip MIB assay and MarDx WB assay for detection of anti-B. burgdorferi IgM and IgG antibodies in serum
| ViraChip assay and resulta | MarDx WB assay and no. of tests with indicated resultb |
% agreement (95% CI)c |
Kappa (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Uninterpretable | Positive | Negative | Overall | ||
| IgM MIB | IgM WB (n = 411) | ||||||
| Positive | 123 | 39 | 0 | ||||
| Negative | 20 | 223 | 1 | 85.4 (78.7–90.3) | 84.5 (79.6–88.4) | 84.7 (80.9–87.9) | 0.68 (0.61–0.75) |
| Invalid | 1 | 2 | 2 | ||||
| IgG MIB | IgG WB (n = 411) | ||||||
| Positive | 135 | 10 | 2 | ||||
| Negative | 33 | 220 | 9 | 80.4 (73.7–85.7) | 94.8 (91.1–97.1) | 86.4 (82.7–89.4) | 0.72 (0.66–0.79) |
| Invalid | 0 | 2 | 0 | ||||
MIB, microarray immunoblot.
WB, Western blot.
CI, confidence interval.
Performance of the ViraChip IgM and IgG MIB assays was also compared to a consensus standard, whereby a sample was classified as positive for anti-B. burgdorferi IgM if both of the ViraStripe IgM LIA and MarDx IgM WB assay were positive. Similar criteria were used to define a sample as positive for IgG to B. burgdorferi, while a negative result by both the ViraStripe and MarDx blot assays was used as the consensus definition for a negative anti-B. burgdorferi IgM or IgG sample. Samples discordant by the matched ViraStripe LIA and MarDx WB assays were excluded from this subset analysis. Using these criteria, of the 411 sera evaluated, 311 and 350 samples had consensus results for IgM and IgG to B. burgdorferi, respectively, by the ViraStripe LIA and MarDx WB assays. Compared to this consensus standard, the ViraChip IgM and IgG MIB assays exhibited greater than 93% positive, negative, and overall agreement values, with kappa values of 0.92 and 0.91, respectively, indicating very good interrater agreement (Table 3).
TABLE 3.
Comparison of the ViraChip IgM and IgG MIB assays to a consensus standard for the presence or absence of IgM and IgG antibodies to B. burgdorferi
| ViraChip assay and resulta | Consensus standard and no. of tests with the indicated result |
% agreement (95% CI)d |
Kappa value (95% CI) | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Overall | ||
| IgM MIB | IgM (n = 311)b | |||||
| Positive | 122 | 1 | ||||
| Negative | 8 | 178 | 93.1% (87.3–96.5) | 98.9% (95.8–99.9) | 96.5% (93.7–98.1) | 0.92 (0.88–0.97) |
| Invalid | 1 | 1 | ||||
| IgG MIB | IgG (n = 350)c | |||||
| Positive | 118 | 8 | ||||
| Negative | 5 | 217 | 96.0% (90.6–98.5) | 95.6% (92.0–97.7) | 95.7% (93.0–97.4) | 0.91 (0.86–0.95) |
| Invalid | 0 | 2 | ||||
MIB, microarray immunoblot.
The consensus standard for an anti-B. burgdorferi IgM-positive sample was defined as positivity by both the ViraStripe IgM LIA and the MarDx IgM WB assay; the consensus standard for a negative IgM sample was defined as a negative result by both assays.
The consensus standard for an anti-B. burgdorferi IgG-positive sample was defined as positivity by both the ViraStripe IgG LIA and the MarDx IgG WB assay; the consensus standard for a negative IgG sample was defined as a negative result by both assays.
CI, confidence interval.
Comparison of the B. burgdorferi antigens detected by the ViraChip MIB assays and ViraStripe LIAs.
Correlation between the ViraChip and ViraStripe IgM and IgG assays was evaluated by comparing the S/CO ratios and qualitative results from both assays for each individual B. burgdorferi target protein. The ViraChip IgM MIB assay and ViraStripe IgM LIA showed strong and statistically significant (P < 0.001) qualitative correlations for all three B. burgdorferi antigens (p41, p39 and p23) with Pearson's coefficient (R) values of 0.57, 0.65, and 0. 67, respectively (see Fig. S1 in the supplemental material). Qualitative correlation between the ViraChip IgG MIB assay and ViraStripe IgG LIA was strong for 4 of the 10 relevant B. burgdorferi proteins, including p66 (R = 0.50), p41 (R = 0.65), p28 (R = 0.77), and p18 (R = 0.72) (Fig. S2). Correlation of the two IgG immunoblot assays for the remaining six proteins ranged from little to moderate (R = 0.23 to 0.48), with the lowest correlation exhibited for p23.
Performance of the ViraChip MIB assays and ViraStripe LIAs using clinical characterized patient samples.
Sensitivity levels of the ViraChip IgM and IgG MIB assays were 65% (13/20) and 45% (9/20), respectively, among 20 convalescent-phase serum samples collected posttreatment from patients with confirmed LD. In comparison, sensitivities of the ViraStripe IgM assay and IgG LIAs among this group were slightly higher at 75% (15/20) and 50% (10/20), respectively (Table 4). The low positivity rates observed for these samples may be attributed to the use of sera collected posttreatment of LD for these patients. The ViraChip IgM MIB assay was positive for 2.8% (2/71) of endemic control samples, compared to 5.6% (4/71) for the ViraStripe IgM LIA. Both the ViraChip IgG MIB assay and ViraStripe IgG LIA were negative for all 71 endemic control samples (Table 4).
TABLE 4.
Performance of the ViraStripe LIA and ViraChip MIB assay in clinically characterized sera
| Serum type (n = 96)a | No. of days post-symptom onsetb | No. of samples | No. (%) of samples positive by: |
|||
|---|---|---|---|---|---|---|
| ViraChip MIB assay |
ViraStripe LIA |
|||||
| IgM | IgG | IgM | IgG | |||
| Confirmed LD | 30–60 | 20 | 13 (65)e | 9 (45) | 15 (75)e | 10 (50) |
| Endemic control | N/A | 71 | 2 (2.8)c | 0 (0) | 4 (5.6)d | 0 (0) |
Confirmed LD cases were defined by the LDB using the CDC case criteria, including the presence of an EM rash in a patient with tick exposure in a high-incidence state or using the CDC laboratory criteria for diagnosis, including a positive standard two-tiered test or a positive nucleic acid amplification test for B. burgdorferi. Endemic control serum samples were from patients living in areas of endemicity for LD who were seronegative for antibodies to B. burgdorferi using standard two-tiered testing.
NA, not applicable.
One of the two samples was negative by the screening C6 Lyme ELISA.
Three of the four samples were negative by the screening C6 Lyme ELISA.
Testing of samples for IgM-class antibodies to B. burgdorferi by immunoblotting is not recommended for patients with more than 30 days of symptoms. These data were included for comparison purposes only.
Workflow analysis between the ViraChip MIB assays and ViraStripe LIAs.
The total hands-on and processing times for the ViraChip MIB assays and the ViraStripe LIAs were evaluated for one full batch of patients (n = 46) and appropriate controls (n = 2) per assay. Full-batch testing consisted of processing one 96-well plate, including 48 IgM and 48 IgG MIB wells for the ViraChip assay using a Gemini instrument, and 96 ViraStripe LIAs, including 48 IgM and 48 IgG LIAs, performed on two separate BeeBlot instruments. Total technologist hands-on time was 31 min to process samples for the ViraStripe IgM and IgG LIAs, which included 26 min for manual pipetting of serum samples and controls onto the LIA strips (Table 5). Comparatively, the ViraChip MIB assays did not require any time devoted to manual pipetting as this function was entirely automated; 15 min was required for preanalytical assay preparation. Assay run times for the ViraChip MIB assay and ViraStripe LIA were 165 min and 175 min, respectively; total times per batch for these two assays were 180 min and 208 min, respectively, a difference of 28 min.
TABLE 5.
Total processing times for the ViraChip MIB and ViraStripe LIA for a full batch of patient samples and controls per assay
| Assaya | Technologist hands-on time (min) for: |
Assay run time (min) | Total time/batch (min) | |
|---|---|---|---|---|
| Manual pipetting | Preanalytical assay preparation | |||
| ViraStripe IgM and IgG LIA | 26 | 7 | 175 | 208 |
| ViraChip IgM and IgG MIB | 0 | 15 | 165 | 180 |
For each assay, 46 patient samples and 2 control samples were processed. LIA, line immunoassay; MIB, microarray immunoblot.
DISCUSSION
We present the first analytic evaluation of the recently FDA-cleared Borrelia B31 ViraChip IgM and IgG MIB assays in comparison to two different second-tier blot assays for LD, including the Borrelia B31 ViraStripe IgM and IgG LIAs and the MarDx B. burgdorferi IgM and IgG WB assays, both also FDA cleared. Our findings indicate substantial overall agreement (>84%) between the ViraChip IgM and IgG MIB assays and the respective IgM and IgG LIA and WB assays in both prospectively collected sera and in banked samples collected from both control individuals and patients with confirmed LD. Additionally, we show a significant reduction in technologist time and, importantly, risk for ergonomic injury in performance of the ViraChip IgM and IgG MIB assays, which are entirely automated, compared to levels with the semiautomated blot processing method currently used in our laboratory.
A number of specific observations from this comparison deserve discussion. First, while the ViraChip IgG MIB assay showed over 90% negative and positive agreement values with the ViraStripe IgG LIA, the ViraChip IgM MIB assay showed 99% negative agreement and only 75.5% positive agreement with the ViraStripe IgM LIA. The lower positive agreement of the ViraChip IgM MIB assay was due to the lack of any antibodies detected in 11 of the 49 ViraChip-negative/ViraStripe-positive samples and detection of antibodies to only one B. burgdorferi protein (p41) in 37 of the remaining discrepant specimens; antibodies to p23 were detected in only one sample, and antibodies to p39 were not identified in any of the 49 discordant samples by the ViraChip IgM MIB assay. This finding is consistent with the S/CO scatter plot analysis of the ViraChip IgM MIB assay and ViraStripe IgM LIA results for the p23 and p39 proteins, which suggests that although there is a strong qualitative result correlation between the two assays, the ViraChip IgM MIB assay shows lower S/CO ratios for these two antigens than the predicate ViraStripe IgM LIA. Additionally, we observed only a moderate to low correlation between the ViraStripe LIA and ViraChip MIB assay for 6 of the 10 B. burgdorferi proteins; this is surprising given that these assays are produced by the same manufacturer (Viramed). Although we cannot identify a specific cause for the differences in levels of agreement or correlation between the Viramed MIB assay and LIA, we speculate that this may be the result of multiple factors. First, while the targeted B. burgdorferi proteins are equivalent between the two methods, five of the proteins used in the ViraChip IgG MIB assay are produced using recombinant methods, whereas the remaining 5 proteins and all 10 proteins printed on the ViraStripe IgG LIA are highly purified extracts from B. burgdorferi cell culture (M. Kintrup, Viramed, personal communication). The precise identification of which antigens are recombinant in the ViraChip IgG MIB assays is considered proprietary by the manufacturer; however, this difference in antigen preparation may account for the difference in correlations observed for multiple proteins between the ViraChip IgG MIB assay and ViraStripe IgG LIA. Second, the method by which the presence or absence of antibodies to B. burgdorferi proteins is established differs between the two assays: signal intensity at each individual protein band on the LIAs is determined using densitometry; whereas for the MIB assays, signal intensity is determined using colorimetric imaging at three replicate protein spots, which are averaged, and the signal is adjusted using both lot- and protein-specific correction factors. Third, the amount of purified antigen printed on the LIA and MIB membranes may differ, impacting assay performance, and, finally, the nitrocellulose membranes used for the MIB assay and LIA, including the method of antigen application and adherence, differ (M. Kintrup, Viramed, personal communication) and may lead to different surface chemistry reactions impacting reactivity. Although our limited evaluation of the ViraChip assays in sera from clinically characterized patients and healthy controls suggests that performance is similar to that of the ViraStripe LIAs, further assessment of the ViraChip MIB assays is necessary, using a larger cohort of patients presenting at different stages of LD, to determine the clinical accuracy of the ViraChip IgM and IgG MIB assays.
Notably, while the ViraChip IgM MIB assay showed higher positive agreement with the MarDx IgM WB assay (85%) than the ViraStripe IgM LIA (75.5%), the overall concordance between the ViraChip MIB assays and corresponding MarDx WB assay was lower than that observed between the ViraChip and ViraStripe assays. This finding is likely attributable to both the subjectivity associated with visual evaluation of the MarDx WBs and the use of different protein preparations between the MIB (recombinant or highly purified proteins) and WB (WCS material) assays. A key limitation associated with WCS-based blot assays is the higher rate of cross-reactivity, in part due to the presence of antigens recognized by antibodies induced by non-B. burgdorferi bacteria and due to the comigration of multiple B. burgdorferi proteins with similar molecular weights to the same region of the blot as the targeted B. burgdorferi protein (10, 25). Overall, while the ViraChip MIB assay results generally suggest lower analytic sensitivity than other blot methods, there is no accepted reference comparator method for second-tier LD blot evaluation. Therefore, we also compared performance of the ViraChip MIB assays to a consensus standard, whereby a sample was defined as positive or negative for anti-B. burgdorferi IgM or IgG only if both the ViraStripe LIA and MarDx WB assay results matched. Compared to this consensus definition, positive, negative, and overall agreement values of the ViraChip IgM and IgG MIB assays were above 93% for all parameters, with kappa values above 0.90 for both IgM- and IgG-class antibodies. This indicates that for samples with concordant anti-B. burgdorferi IgM and/or IgG results across blot methods, the ViraChip MIB assays perform equivalently.
The 96-well microtiter plate format of the ViraChip IgM and IgG MIB assays allows for these assays to be entirely automated, from sample pipetting to processing, on an open-system microplate ELISA analyzer. We compared the time required to process a full batch of 48 samples by the ViraChip IgM and IgG MIB assays using a Gemini microplate processor and ViraStripe IgM and IgG LIAs, which in our laboratory require manual pipetting of samples onto the LIA strips prior to automated processing on the BeeBlot instruments. We determined that the use of the ViraChip MIB assays is associated with a time savings of 28 min for one full-batch run compared to time required for the ViraStripe LIAs. For laboratories which perform high-volume testing during the season for tick-borne diseases in the United States (approximately June through October), this time savings can be significant. For example, our laboratory tests approximately 300 patient samples per day during LD season by the ViraStripe IgM and IgG LIAs. Based on the timings indicated above, running the ViraChip IgM and IgG MIB assays will decrease the processing time for second-tier LD testing by approximately 3 h daily and will significantly decrease the risk of ergonomic injury by eliminating the repetitive motion associated with manual pipetting. Notably, while manual pipetting for any blot assay may be obviated via a liquid handler, this would be an added expense to the laboratory and would require dedicated instrument space. Also, while alternative, entirely automated platforms for sample pipetting and blot processing are currently available (e.g., EUROBlotOne from Euroimmun AG, Luebeck, Germany), these instruments are restricted to blot testing. In contrast, the ViraChip MIB assays can be programmed on a variety of different open-system, automated ELISA processors, negating the need to buy dedicated equipment for this assay and enabling laboratories to consolidate LD testing assays on a single, existing platform.
A number of limitations deserve mention. First, a limited number of the samples evaluated in this study were well characterized for the presence or absence of LD, and therefore firm conclusions cannot be made with respect to the clinical sensitivity and specificity of the ViraChip MIB assays. This is particularly relevant for the ViraChip IgM MIB assay as the duration of disease prior to sample collection was unknown, and second-tier testing may not have been indicated for certain samples. Although we assessed performance of the ViraChip MIB assays compared to a consensus standard in an effort to identify a true positive or negative result, this approach remains imperfect. Despite this, however, we provide an in-depth evaluation of the analytic performance of the ViraChip MIB assays against two different FDA-cleared blot methods and were able to determine differences in antibody detection trends against each B. burgdorferi antigen between the ViraChip MIB assays and ViraStripe LIAs. Second, sample inclusion criteria for the prospective arm required a positive or equivocal result by the C6 Lyme ELISA, which has imperfect specificity and may have led to evaluation of samples that would have tested negative by alternative first-tier screening assays (15, 26). Finally, we did not compare the performance of this assay to that of an alternative, entirely automated platform for second-tier LD blot testing and therefore cannot comment on differences in time saved between similar systems.
In summary, we present the first evaluation of the Viramed ViraChip MIB assays for detection of IgM- and IgG-class antibodies to B. burgdorferi. We show comparable performance characteristics of these assays when they are evaluated against two commonly used LD blot methods and note that when a consensus result between the ViraStripe LIAs and MarDx WB assays is used to define samples as positive or negative for the presence or absence of anti-B. burgdorferi antibodies, the overall percent agreement of the ViraChip IgM and IgG MIB assays increases to over 95%. Additionally, we show that performance of the ViraChip MIB assays on an open-system, automated ELISA analyzer is associated with a significant time savings, particularly for high-throughput laboratories, and decreases the risk of ergonomic injury due to repetitive motions associated with manual specimen pipetting. Finally, it deserves mention that there is increasing data to support amendment of the current STTT algorithm for LD and, specifically, to replace the supplemental blot testing with a second-tier B. burgdorferi-specific ELISA (14, 27, 28). This modified two-tiered testing (MTTT) algorithm would obviate many of the aforementioned limitations associated with blot testing, significantly improving both the sensitivity and accuracy of LD testing overall. Until the MTTT algorithm is officially adopted, however, second-tier blot testing as part of the STTT algorithm will remain necessary, and as our study suggests, the ViraChip IgM and IgG MIB assays may be added to the repertoire of supplemental blot testing systems for diagnosis of LD.
Supplementary Material
ACKNOWLEDGMENTS
We thank the technologists in the Infectious Diseases Serology laboratory for their assistance with this study. Additionally, we acknowledge Viramed Biotech AG for their provision of the ViraChip IgM and IgG MIB assays and for providing the raw output S/CO intensity values (which are masked to the end user) for the ViraChip MIB assays.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00992-18.
REFERENCES
- 1.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. 2015. Information materials about borreliosis. European Centre for Disease Prevention and Control, Solna, Sweden: https://ecdc.europa.eu/en/borreliosis/facts/information-materials. [Google Scholar]
- 4.Stanek G, Reiter M. 2011. The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin Microbiol Infect 17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 5.Schutzer SE, Berger BW, Krueger JG, Eshoo MW, Ecker DJ, Aucott JN. 2013. Atypical erythema migrans in patients with PCR-positive Lyme disease. Emerg Infect Dis 19:815–817. doi: 10.3201/eid1905.120796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 7.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 9.Theel ES. 2016. The past, present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol 54:1191–1196. doi: 10.1128/JCM.03394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. 2012. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 18:1236–1240. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 11.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol 37:3990–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnaboldi PM, Dattwyler RJ. 2015. Cross-reactive epitopes in Borrelia burgdorferi p66. Clin Vaccine Immunol 22:840–843. doi: 10.1128/CVI.00217-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma B, Christen B, Leung D, Vigo-Pelfrey C. 1992. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol 30:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. 2011. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 53:541–547. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 15.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, Nowakowski J, Marques A, Johnson BJ, Dumler JS. 2013. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 75:9–15. doi: 10.1016/j.diagmicrobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. 2018. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 66:1133–1139. doi: 10.1093/cid/cix943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binnicker MJ, Jespersen DJ, Harring JA, Rollins LO, Bryant SC, Beito EM. 2008. Evaluation of two commercial systems for automated processing, reading, and interpretation of Lyme borreliosis Western blots. J Clin Microbiol 46:2216–2221. doi: 10.1128/JCM.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, Johnson BJ, Wormser GP, Steere AC. 2010. 2-Tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin Infect Dis 50:20–26. doi: 10.1086/648674. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. 2017. Lyme disease (Borrelia burgdorferi) 2017 case definition. Centers for Disease Control and Prevention, Atlanta, GA: https://wwwn.cdc.gov/nndss/conditions/lyme-disease/case-definition/2017/. [Google Scholar]
- 20.Horn EJ, Dempsy G, Schotthoefer A, Prisco UL, Djavaherian C, Umamoto S, Golightly M, Luca CD, Evans M, Pritt B, Theel ES, Iyer R, Liveris D, Wang G, Schwartz I. 2017. Bay Area Lyme Foundation's Biorepository, the Lyme Disease Biobank—a resource to advance our understanding of Lyme disease and other tick-borne infections. Characterization of samples collected from 2014–2016, poster 7. 2nd Annu Lyme Dis Era Precis Med Conf, New York, NY, 3 October 2017. [Google Scholar]
- 21.Horn EJ, Dempsy G, Prisco UL, Golightly M, Iyer R, Evans M, Schwartz I. 2016. Lyme Disease Biobank: a resource to advance our understanding of Lyme disease and other tick-borne infections, poster 9. Gordon Res Conf Biol Spirochetes, Ventura, CA, 10 to 15 January 2016. [Google Scholar]
- 22.MedCalc Software. 2016. MedCalc, version 15.4.3. MedCalc Software, Ostend, Belgium: https://www.medcalc.org. [Google Scholar]
- 23.Landis JR, Koch GG. 1977. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33:363–374. doi: 10.2307/2529786. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd ed Lawrence Erlbaum Associates, Mahwah, NJ. [Google Scholar]
- 25.Nowalk AJ, Gilmore RD Jr, Carroll JA. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun 74:3864–3873. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledue TB, Collins MF, Young J, Schriefer ME. 2008. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 15:1796–1804. doi: 10.1128/CVI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molins CR, Delorey MJ, Sexton C, Schriefer ME. 2016. Lyme borreliosis serology: performance of several commonly used laboratory diagnostic tests and a large resource panel of well-characterized patient samples. J Clin Microbiol 54:2726–2734. doi: 10.1128/JCM.00874-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pegalajar-Jurado A, Schriefer ME, Welch RJ, Couturier MR, MacKenzie T, Clark RJ, Ashton LV, Delorey MJ, Molins CR. 2018. Evaluation of modified two-tiered testing algorithms for Lyme disease laboratory diagnosis using Well-characterized serum samples. J Clin Microbiol 56:e01943-17. doi: 10.1128/JCM.01943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.