Serotype M28 group A streptococcus (GAS) is a common cause of infections such as pharyngitis (“strep throat”) and necrotizing fasciitis (“flesh-eating” disease). Relatively little is known about the molecular mechanisms underpinning M28 GAS pathogenesis.
KEYWORDS: RocA, group A streptococcus, molecular pathogenesis, virulence
ABSTRACT
Serotype M28 group A streptococcus (GAS) is a common cause of infections such as pharyngitis (“strep throat”) and necrotizing fasciitis (“flesh-eating” disease). Relatively little is known about the molecular mechanisms underpinning M28 GAS pathogenesis. Whole-genome sequencing studies of M28 GAS strains recovered from patients with invasive infections found an unexpectedly high number of missense (amino acid-changing) and nonsense (protein-truncating) polymorphisms in rocA (regulator of Cov), leading us to hypothesize that altered RocA activity contributes to M28 GAS molecular pathogenesis. To test this hypothesis, an isogenic rocA deletion mutant strain was created. Transcriptome sequencing (RNA-seq) analysis revealed that RocA inactivation significantly alters the level of transcripts for 427 and 323 genes at mid-exponential and early stationary growth phases, respectively, including genes for 41 transcription regulators and 21 virulence factors. In contrast, RocA transcriptomes from other GAS M protein serotypes are much smaller and include fewer transcription regulators. The rocA mutant strain had significantly increased secreted activity of multiple virulence factors and grew to significantly higher colony counts under acid stress in vitro. RocA inactivation also significantly increased GAS virulence in a mouse model of necrotizing myositis. Our results demonstrate that RocA is an important regulator of transcription regulators and virulence factors in M28 GAS and raise the possibility that naturally occurring polymorphisms in rocA in some fashion contribute to human invasive infections caused by M28 GAS strains.
INTRODUCTION
Group A streptococcus (GAS) is a human-specific pathogen that causes diseases ranging in severity from relatively innocuous pharyngitis (“strep throat”) to life-threatening necrotizing fasciitis (“flesh-eating” disease) (1, 2). In addition, GAS is responsible for postinfectious immune sequelae such as rheumatic heart disease and poststreptococcal glomerulonephritis (3). The global disease burden and economic impact of GAS disease are immense. The World Health Organization estimates that GAS causes over 700 million superficial infections, 1.78 million invasive infections, and 512,000 deaths annually (4). Despite decades of research, there is no commercially available vaccine to prevent GAS infections.
GAS strains are commonly classified by sequence variation in the emm gene, which encodes the highly polymorphic M protein virulence factor (3). Serotype M28 strains are among the more common causes of GAS pharyngitis and invasive infections in the United States and other countries (1, 5–11). Of note, serotype M28 GAS strains are strongly associated with puerperal sepsis (7–9, 12–14). Despite the importance of M28 strains in human disease, relatively little is known about the molecular pathogenesis of M28 GAS (1, 5, 12, 13). Historically, GAS pathogenesis research has focused on strains of other numerically important serotypes, such as M1 and M3 (2, 15–21).
One gene of increasingly recognized importance to GAS pathogenesis is rocA (regulator of Cov), encoding the RocA protein (22). RocA was initially identified as a positive regulator of the CovRS (control of virulence) two-component system, which is a negative regulator of virulence (22–24). Further study of rocA in multiple GAS serotypes identified a naturally occurring nonsense mutation in all serotype M18 strains that results in hyperencapsulation and increases carriage longevity in the mouse nasopharynx (25). Similarly, RocA is inactivated in all serotype M3 strains by a single nucleotide deletion that introduces a frameshift mutation, and restoration of RocA by introduction of the serotype M1 wild-type rocA allele decreases M3 GAS virulence in a mouse model of bacteremia (26–28). Deletion of rocA in serotype M1, M3, M6, M14, M18, and M89 GAS results in increased expression of virulence factors known to be regulated by the CovRS system (26–32). Although the molecular mechanism for RocA function has not been determined, RocA increases phosphorylation of the DNA binding response regulator CovR in the presence of its cognate sensor histidine kinase CovS, resulting in CovR activation (28, 30). The N-terminal transmembrane domains of RocA are crucial for the regulatory activity of RocA, suggesting that RocA functions as an accessory protein to the CovRS system (32). However, the role, if any, of RocA in pathogenesis has not been studied in M28 GAS.
Whole-genome sequencing studies of M28 GAS strains recovered from human invasive infections found an unexpectedly high number of missense (amino acid-altering) and nonsense (protein-truncating) polymorphisms in rocA (GenBank accession no. MH884522 to MH884551) (Fig. 1). Previous studies of rocA in strains of other GAS M protein serotypes identified several nonsense mutations and many frameshifting insertions and deletions (indels) that result in protein truncation (25–28, 30–32, 34–36). However, very few rocA missense mutations have been reported in other GAS serotypes, and none have been studied previously (25–28, 30–32, 34–36). The striking increase in missense mutation frequency in M28 strains led us to speculate that RocA has serotype-specific functions in M28 GAS strains. We hypothesized that RocA inactivation significantly contributes to the molecular pathogenesis of invasive infections caused by serotype M28 GAS. To test this hypothesis, we created an isogenic rocA deletion mutant strain and discovered that RocA regulates a substantial portion of the M28 GAS transcriptome, including many genes encoding transcription regulators and virulence factors. Consistent with the transcriptome data, in vitro assays showed that RocA inactivation significantly increases the secreted activity of multiple virulence factors and increases CFU under acid stress. The RocA-inactivated strain was also significantly more virulent in a mouse model of necrotizing myositis.
FIG 1.
rocA is unusually polymorphic in serotype M28 GAS strains. The affected codon and amino acid change conferred by each polymorphism are shown. For polymorphisms due to nucleotide deletion, the affected nucleotide is identified. Alleles identified in multiple isolates are indicated. Polymorphisms that result in RocA protein truncation or loss of rocA mRNA translation are shown below the protein schematic, and polymorphisms that result in amino acid changes are shown above the protein schematic. Missense polymorphisms in the predicted domains sufficient for regulatory activity (32) are colored red. Predicted domains of the RocA protein using Phyre2 are indicated (TM, transmembrane domain; HATPase, histidine kinase ATPase domain) (107). Predicted functional domains of the potential histidine kinase domain (H box, N box, F box, and G box) are identified (22). #, one strain has two polymorphisms in rocA.
RESULTS
rocA is unusually polymorphic in serotype M28 GAS.
Recent whole-genome sequencing studies of serotype M28 GAS strains recovered from human invasive infections revealed an unexpectedly high number of missense and nonsense polymorphisms in rocA (GenBank accession no. MH884522 to MH884551) (Fig. 1). We identified 29 unique polymorphisms (25 single nucleotide polymorphisms [SNPs]) and 4 insertions/deletions [indels]) among 2,101 M28 GAS strains. The frequency of polymorphisms in rocA is significantly greater than expected by chance alone (P < 0.01, Fisher's exact test). The 29 unique polymorphisms included 17 missense mutations, 11 nonsense mutations, and one six-nucleotide deletion in the upstream noncoding region that affects the presumed ribosomal binding site (30) (Fig. 1). Of note, 13/17 (76.5%) of the missense mutations occur in the 5′ end of rocA, resulting in amino acid changes in the N terminus of RocA that may be crucial for its function as an accessory protein to the CovRS system (32) (Fig. 1).
The abundance of rocA polymorphisms found in the M28 GAS strains prompted a reevaluation of data from our previously published whole-genome sequencing studies of large, comprehensive, population-based collections of other GAS M protein serotypes (15, 37, 38). We discovered that M1 and M59 GAS strains had a much lower frequency of rocA polymorphisms (see Fig. S1 in the supplemental material) (15, 37, 38). Although 29 unique rocA polymorphisms were found among 2,101 M28 GAS strains (13.8 rocA alleles per 1,000 M28 strains), only 16 unique rocA polymorphisms were identified among 3,443 M1 GAS strains (4.6 alleles per 1,000 strains) (15), and two unique rocA polymorphisms were identified among 310 M59 GAS strains (6.5 alleles per 1,000 strains) (38). In contrast, 18 unique rocA polymorphisms were found among 1,193 M89 GAS strains (15.1 alleles per 1,000 strains) (37).
The very high number of variants identified in serotype M28 GAS strains suggests that the rocA polymorphisms are selected for during human invasive infection to alter the regulatory activity of RocA. We hypothesize that altered RocA activity contributes to the molecular pathogenesis of M28 GAS.
Creation of an isogenic rocA deletion mutant strain.
As a first step toward investigating the role of RocA in serotype M28 GAS molecular pathogenesis, we created an isogenic rocA deletion mutant strain using allelic exchange (39). Wild-type (WT) strain MGAS28426 was chosen as the parental strain because it is genetically representative of serotype M28 GAS strains that commonly cause human infections, and it has a wild-type allele for all major global transcription regulatory genes, including covRS, ropB, mga, ccpA, and rocA. Whole-genome sequencing of the isogenic rocA deletion (ΔrocA) mutant strain confirmed the absence of spurious mutations. To determine if rocA deletion alters the growth phenotype of M28 GAS, the parental WT and isogenic ΔrocA mutant strains were grown in Todd-Hewitt broth supplemented with yeast extract (THY), a nutrient-rich medium. No significant difference in growth was observed (Fig. 2A) (P = not significant [NS], two-way analysis of variance [ANOVA]).
FIG 2.

Deletion of rocA significantly alters the GAS transcriptome. (A) No significant growth difference in nutrient-rich liquid medium was observed between the parental wild-type (WT) and isogenic ΔrocA mutant strains. Dashed lines represent the OD600 of mid-exponential (ME) and early stationary (ES) growth phases for cultures that were collected for RNA-seq analysis. (B) Principal-component analysis of the WT and ΔrocA strain transcriptomes at the ME and ES growth phases. (C) Numbers of genes with significantly altered transcript levels at the ME and ES growth phases (108).
Deletion of rocA in M28 GAS strain MGAS28426 results in a substantial transcriptome change.
To test the hypothesis that rocA deletion results in altered global gene transcript levels in M28 GAS, transcriptome sequencing (RNA-seq) analysis was performed using strains grown to mid-exponential (ME) (optical density at 600 nm [OD600] = 0.5) and early stationary (ES) (OD600 = 1.65) growth phases (Fig. 2A). Principal-component analysis showed that rocA deletion markedly alters the global transcriptome of serotype M28 GAS at both growth phases (Fig. 2B). In total, 427 (25.8%) and 323 (19.5%) genes had significantly altered transcript levels at ME and ES growth phases, respectively (absolute transcript fold change, ≥1.5; P < 0.05 after Baggerly's test with Bonferroni's correction for multiple comparisons) (Fig. 2C). Of these genes, 109 were common to both growth phases (Fig. 2C). Many of the significantly differentially expressed genes encode transcription regulators and proven virulence factors (see below). A complete list of genes with significantly altered transcript levels is provided in Tables S1 and S2 in the supplemental material.
RocA directly or indirectly regulates transcription regulators involved in virulence in serotype M28 GAS.
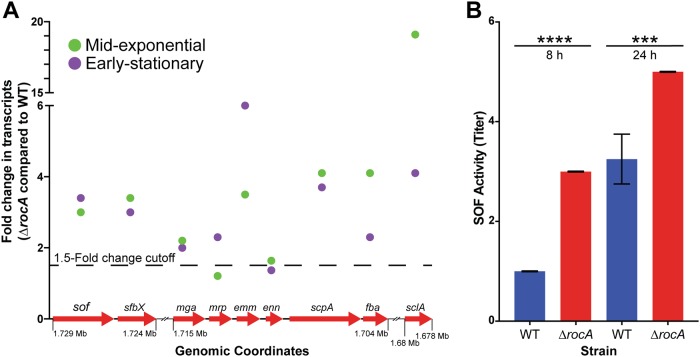
The RNA-seq data demonstrated that RocA inactivation significantly altered the transcript levels of 41 transcription regulators in serotype M28 strain MGAS28426 (Table 1) (40–61). Of the 41 transcription regulators that are directly or indirectly regulated by RocA in M28 GAS, 22 have been previously studied in GAS, 11 have inferred function by homology with transcription regulators in other Streptococcus species, and 8 are of unknown function (Table 1) (40–61). One particularly interesting regulator whose expression is significantly altered by RocA inactivation in M28 GAS is mga (multiple virulence gene regulator of GAS) (57). Mga regulates the expression of multiple genes encoding proven virulence factors, including sclA (encoding a collagen binding protein) (62), fba (encoding a fibronectin binding protein) (63), scpA (encoding C5a peptidase) (64), enn (encoding IgA binding protein) (65), emm (encoding antiphagocytic M protein) (66), mrp (encoding M-related protein) (67), sfbX (encoding a fibronectin binding protein) (68), and sof (encoding serum opacity factor [SOF]) (69). Compared to the parental WT strain, the isogenic ΔrocA deletion mutant strain had significantly increased transcript levels for each gene in the Mga regulon at one or both time points (Fig. 3A and Tables S1 and S2).
TABLE 1.
GAS transcription regulator genes (proven and inferred) directly or indirectly regulated by RocA at mid-exponential and early stationary growth phases
| Locus taga | Gene | Known or putative function (reference)b | Fold change relative to WTc |
|
|---|---|---|---|---|
| ME | ES | |||
| M28_Spy0034 | comRd,f | Competence (40) | −1.7 | |
| M28_Spy0104 | rofAd,e,f | Regulator of fibronectin binding protein (41) | −1.9 | |
| M28_Spy0153 | sgaR | Ascorbate utilization (42) | −1.5 | |
| M28_Spy0184 | rivRd,e | Negative regulator of GRAB (43) | 2.4 | |
| M28_Spy0189 | yjdR | Multidrug resistance transporters (42) | −2.3 | |
| M28_Spy0276 | nrdRd | Ribonucleotide metabolism (44) | 1.8 | |
| M28_Spy0522 | agaR2 | Carbohydrate metabolism (42) | −1.9 | |
| M28_Spy0538 | ralp3d | RofA-like transcription regulator (45) | −2.3 | −1.8 |
| M28_Spy0681 | cpsYd,e,f | Resistance to opsonophagocytic killing (46) | 1.6 | |
| M28_Spy0780 | srtKd | Lantibiotic biosynthesis(47) | 1.7 | |
| M28_Spy0872 | M28_Spy0872f | GntR family transcription regulator (42) | −1.8 | |
| M28_Spy0889 | nagR | N-Acetylglucosamine utilization (42) | 2.8 | |
| M28_Spy0896 | pdxR | Pyridoxin metabolism (42) | 1.8 | |
| M28_Spy0919 | ciaHd | Acid and oxidative stress (48) | 2.1 | |
| M28_Spy0920 | ciaRe | Acid and oxidative stress (48) | 2.0 | |
| M28_Spy0963 | M28_Spy0963f | Transport (42) | 2.0 | −1.7 |
| M28_Spy1346 | trxRd,e | Two-component system (49) | −1.9 | |
| M28_Spy1347 | trxSd,e | Two-component system (49) | −2.0 | |
| M28_Spy1373 | liaRd,e | Regulator of pilus proteins (50) | −1.7 | |
| M28_Spy1384 | atoRd,e,f | Short-chain fatty acid metabolism (51) | 1.7 | |
| M28_Spy1420 | M28_Spy1420 | Mga family transcription regulator (42) | −2.0 | |
| M28_Spy1445 | lacR.1d | Galactose metabolism (52) | −2.3 | |
| M28_Spy1449 | copYd,f | Copper toxicity (53) | −2.4 | |
| M28_Spy1501 | codYd | Pleiotropic transcription regulator (54) | 1.5 | |
| M28_Spy1531 | scrR | Sucrose utilization (42) | −1.6 | |
| M28_Spy1545 | M28_Spy1545f | XRE family transcription regulator (42) | 1.7 | |
| M28_Spy1546 | M28_Spy1546f | XRE family transcription regulator (42) | 1.5 | |
| M28_Spy1564 | srvd,e | Streptococcal regulator of virulence (55) | 3.1 | |
| M28_Spy1566 | M28_Spy1566f | XRE family transcription regulator (42) | −2.5 | |
| M28_Spy1569 | M28_Spy1569 | MerR family transcription regulator (42) | −1.6 | |
| M28_Spy1615 | salRd | Lantibiotic biosynthesis (56) | 1.8 | |
| M28_Spy1636 | M28_Spy1636 | XRE family transcription regulator (42) | −1.9 | |
| M28_Spy1704 | mgad,e | Multiple gene regulator (57) | 2.2 | 2.0 |
| M28_Spy1708 | ihkd,e,f | Polymorphonuclear leukocyte evasion (58) | 1.5 | |
| M28_Spy1724 | ropBd,e | Regulator of SpeB (59) | −2.3 | |
| M28_Spy1750 | ctsRf | Stress and heat shock response (60) | 2.7 | |
| M28_Spy1763 | M28_Spy1763f | LuxR family transcription regulator (42) | −1.6 | |
| M28_Spy1769 | treRf | Trehalose utilization (42) | −1.5 | |
| M28_Spy1782 | spxA2d,e | Stress resistance, regulator of SpeB (61) | 4.0 | |
| M28_Spy1835 | ywzGf | Transport (42) | −1.5 | −2.0 |
| M28_Spy1839 | pipR | Phage infection protein (42) | 1.8 | |
Locus tag identified in the serotype M28 reference genome MGAS6180.
Known or putative function based on known role in GAS or inferred homology.
ME, mid-exponential growth phase; ES, early stationary growth phase. Empty (blank) cell, the gene does not satisfy the P value and/or fold change requirement.
Transcription regulator that has been previously studied in GAS.
Transcription regulator with a proven role in GAS virulence.
FIG 3.
Deletion of rocA significantly increases the transcript levels of genes in the Mga regulon. (A) The transcript levels of mga and eight Mga-regulated genes were significantly increased in the ΔrocA mutant strain compared to the WT strain. Genomic coordinates and fold change in transcripts are shown for each gene at mid-exponential and early stationary growth phases (P < 0.05, Baggerly test with Bonferroni correction for multiple comparisons). Points below the 1.5-fold-change cutoff did not reach statistical significance and are included for completeness. (B) Serum opacity factor (SOF) activity assay results. Data are shown as mean ± standard deviation. ***, P < 0.001; ****, P < 0.0001 (Student's t test).
To determine if the observed difference in transcript levels of genes in the Mga regulon results in an altered phenotype, SOF activity was assayed in vitro. Consistent with the RNA-seq data, the isogenic ΔrocA deletion mutant strain had significantly increased SOF activity compared to the parental WT strain (Fig. 3B).
RocA directly or indirectly regulates transcription regulators and virulence factors involved in the stress response in serotype M28 GAS.
During infection, GAS cells are exposed to oxidative and acid stress in purulent lesions (70–72). Among the 41 transcription regulators that are directly or indirectly regulated by RocA in M28 GAS, 4 are implicated in oxidative and acidic stress responses, including the CiaHR two-component system, NrdR, and SpxA2 (Table 1) (44, 48, 61). Additionally, the arcABCD operon had significantly increased transcript levels in the ΔrocA mutant strain (Fig. 4A and Table S1). The arcABCD operon encodes the ArcABCD proteins of the arginine deiminase pathway, which are also involved in the GAS response to acidic environments (73–75).
FIG 4.
Deletion of rocA significantly increases the transcript levels of genes encoding transcription regulators and proteins involved in the stress response. (A) The transcript levels of arcABCD and spxA2 were significantly increased in the ΔrocA mutant strain compared to the WT strain at mid-exponential growth phase. M28_Spy1209 encodes a putative dipeptidase, and M28_Spy1212 encodes a putative N-acetyltransferase. Genomic coordinates and fold change in transcripts are shown for each gene (P < 0.05, Baggerly test with Bonferroni correction for multiple comparisons). (B) The transcript levels of nrdR and ciaHR were significantly increased in the ΔrocA mutant strain compared to the WT strain at early stationary growth phase. Genomic coordinates and fold change in transcripts are shown for each gene (P < 0.05, Baggerly test with Bonferroni correction for multiple comparisons). (C) Growth of strains in THY buffered with HEPES (pH 7.5). (D) Growth of strains in THY buffered with 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.0). (E) CFU counts of strains grown in THY buffered with MES (pH 6.0) at 3 h. Data are shown as mean ± standard error of the mean (SEM). *, P < 0.05 (Mann-Whitney test).
Because ciaHR, nrdR, spxA2, and arcABCD had significantly increased transcript levels in the isogenic ΔrocA deletion mutant strain compared to the parental WT strain (Fig. 4A and B), we hypothesized that the isogenic ΔrocA deletion mutant strain is significantly more resistant to acidic stress. To test this hypothesis, the isogenic ΔrocA deletion mutant and parental WT strains were grown in THY alone, THY buffered to neutral conditions using HEPES (pH 7.5), and THY buffered to acidic conditions using 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.0) (29). Under neutral conditions (THY alone and HEPES, pH 7.5), the growth curves of the isogenic ΔrocA mutant and parental WT strains were nearly superimposable (Fig. 2A and 4C). Consistent with our hypothesis, when grown under acidic conditions (MES, pH 6.0), the isogenic ΔrocA deletion mutant strain had a shortened lag phase and increased slope of the exponential phase compared to the parental WT strain (Fig. 4D). After 3 h of growth under acidic conditions, significantly more CFU were present in cultures of the isogenic ΔrocA deletion mutant strain than in those of the parental WT strain (Fig. 4E).
Deletion of rocA results in differential transcript levels of multiple GAS virulence factors.
Several proven and putative virulence factors had significantly altered transcript levels in the isogenic ΔrocA deletion mutant strain compared to the parental WT strain (Table 2). Many of the differentially expressed virulence factors are known to be regulated by the CovRS two-component system, as well as RocA, in other GAS serotypes (24, 28, 32). Selected virulence factor genes with increased transcript levels in the isogenic ΔrocA mutant strain include nga (encoding NAD+-glycohydrolase [SPN]) (39), slo (encoding streptolysin O [SLO]) (39), spyCEP (encoding interleukin-8 [IL-8] protease) (76), mac (encoding an IgG endopeptidase and an inhibitor of reactive oxygen species generation) (77–79), and sse (encoding streptococcal secreted esterase [SSE]) (80). Selected virulence factor genes with decreased transcript levels in the isogenic ΔrocA strain include M28_Spy0109 (encoding pilin protein) (50, 81), the sag operon (carrying streptolysin S biosynthesis genes) (82), grab (encoding protein-G-related α2-macroglobulin binding protein) (83), ska (encoding streptokinase [SKA]) (84), and speB (encoding streptococcal cysteine protease B) (85).
TABLE 2.
Selected proven and putative virulence factors of GAS regulated by rocA at mid-exponential and early stationary growth phases
| Locus taga | Gene | Function | Fold change relative to WTb |
|
|---|---|---|---|---|
| ME | ES | |||
| M28_Spy0109 | M28_Spy0109 | Pilin protein | −2.8 | |
| M28_Spy0137 | nga | NAD+-glycohydrolase | 6.3 | 7.6 |
| M28_Spy0139 | slo | Streptolysin O | 6.0 | 7.2 |
| M28_Spy0329 | spyCEP | IL-8 protease | 38.0 | |
| M28_Spy0540 | sagA | Streptolysin S precursor | −2.7 | |
| M28_Spy0541 | sagB | Streptolysin S biosynthesis protein | −3.4 | |
| M28_Spy0542 | sagC | Streptolysin S biosynthesis protein | −3.2 | |
| M28_Spy0543 | sagD | Streptolysin S biosynthesis protein | −3.5 | |
| M28_Spy0544 | sagE | Streptolysin S self-immunity protein | −3.0 | |
| M28_Spy0545 | sagF | Streptolysin S biosynthesis protein | −2.5 | |
| M28_Spy0546 | sagG | Streptolysin S export ATP binding protein | −3.1 | |
| M28_Spy0547 | sagH | Streptolysin S export transmembrane protein | −2.9 | |
| M28_Spy0548 | sagI | Streptolysin S export transmembrane protein | −2.7 | |
| M28_Spy0649 | mac | IgG endopeptidase and inhibitor of reactive oxygen species generation | 38.1 | |
| M28_Spy1098 | grab | Protein G-related α2-macroglobulin binding protein | −5.5 | −8.7 |
| M28_Spy1450 | sse | Streptococcal secreted esterase | 3.6 | 1.7 |
| M28_Spy1672 | ska | Streptokinase | −2.2 | −7.4 |
| M28_Spy1675 | sclA | Collagen binding protein | 19.1 | 4.1 |
| M28_Spy1699 | fba | Fibronectin binding protein | 4.1 | 2.3 |
| M28_Spy1700 | scpA | C5a peptidase | 4.1 | 3.7 |
| M28_Spy1701 | enn | IgA binding protein | 1.6 | |
| M28_Spy1702 | emm | Antiphagocytic M protein | 3.5 | 6.0 |
| M28_Spy1703 | mrp | M-related protein | 2.3 | |
| M28_Spy1715 | sfbX | Fibronectin binding protein | 3.4 | 3.0 |
| M28_Spy1716 | sof | Serum opacity factor | 3.0 | 3.4 |
| M28_Spy1721 | speB | Streptococcal cysteine protease B | −3.2 | |
| M28_Spy1884 | hasA | Hyaluronan synthase | 17.2 | |
| M28_Spy1885 | hasB | UDP-glucose 6-dehdrogenase | 18.8 | |
| M28_Spy1886 | hasC | UTP-glucose-1-phospate uridylyltransferase | 17.7 | |
Locus tag identified in the serotype M28 reference genome MGAS6180.
ME, mid-exponential growth phase; ES, early stationary growth phase. Empty (blank) cells, the gene does not satisfy the P value and/or fold change requirement.
To assess the phenotypic effect of the differential transcript levels for selected virulence factors, a series of in vitro assays was performed. Compared to the parental WT strain, the isogenic ΔrocA deletion mutant strain expressed increased amounts of immunoreactive SPN and SLO proteins (Fig. 5A). Additionally, compared to the parental WT strain, the isogenic ΔrocA mutant deletion strain had significantly increased SOF, SPN, SPN, SLO, and SSE secreted activity (Fig. 3B and 5B to D) and significantly decreased SKA secreted activity (Fig. 5E). The results are consistent with the RNA-seq data and raise the possibility that deletion of rocA in M28 GAS increases virulence.
FIG 5.
Deletion of rocA significantly increases GAS virulence factor levels and activity in the culture supernatant. (A) Western immunoblot analysis of NAD+-glycohydrolase (SPN) and streptolysin O (SLO). (B) SPN activity. (C) SLO activity. (D) Platelet activating factor (PAF) acetylhydrolase activity. (E) SKA activity. Data are shown as mean ± standard deviation. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (Student's t test).
Deletion of rocA results in increased virulence in a mouse model of necrotizing myositis.
Next, we hypothesized that deletion of rocA significantly increases M28 GAS virulence. To test this hypothesis, the virulences of the isogenic ΔrocA deletion mutant and parental WT strains were compared using a well-established mouse model of necrotizing myositis (37, 86, 87). Compared to the parental WT strain, the isogenic ΔrocA deletion mutant strain caused significantly more mortality (Fig. 6A) and larger lesions with more tissue destruction (Fig. 6B). Also, compared to infection with the parental WT strain, significantly more CFU were recovered from mouse limbs infected with the isogenic ΔrocA deletion mutant strain (Fig. 6C). Together, these data demonstrate that deletion of rocA in serotype M28 GAS significantly increases virulence.
FIG 6.
Deletion of rocA increases GAS virulence in a mouse model of necrotizing myositis. (A) Kaplan-Meier survival curve (n = 40 mice/strain). *, P < 0.05 (log rank test). (B) Representative microscopic lesions from the inoculation site of the right lower hindlimb of mice infected with the WT or ΔrocA mutant strain on day 1 postinoculation. The necrotic lesions are encompassed by black ovals. Original magnification, ×4. (C) CFU/gram of tissue recovered on day 3 postinoculation (n = 20 mice/strain). Data are shown as mean ± SEM. *, P < 0.05 (Mann-Whitney test).
DISCUSSION
Serotype M28 GAS strains are a common cause of pharyngeal and invasive infections globally (1, 5–11). However, relatively little is known about the molecular pathogenesis of M28 strains. GAS molecular pathogenesis studies have historically used other numerically important serotypes such as M1 and M3 strains as model organisms (2). Recent whole-genome sequence analysis of 2,101 serotype M28 GAS strains recovered from large population-based studies of patients with invasive infections revealed an unexpectedly high number of missense and nonsense polymorphisms in rocA (GenBank accession no. MH884522 to MH884551) (Fig. 1), a finding that was not observed in our previous studies with M1, M59, and M89 GAS (15, 37, 38). Inactivation of rocA in a genetically representative serotype M28 GAS strain resulted in a substantial transcriptome change (38.8% of all GAS genes) (Fig. 2), including many genes encoding transcription regulators and proven or putative virulence factors. In vitro assays confirmed the RNA-seq results (Fig. 3 to 5), and the M28 RocA-inactivated strain was significantly more virulent in a mouse model of necrotizing myositis (Fig. 6). Taken together, these data suggest that RocA plays a key role in M28 GAS molecular pathogenesis in human invasive infections (Fig. 7).
FIG 7.
Model of RocA contribution to the molecular pathogenesis of serotype M28 GAS. GAS strains with a wild-type rocA gene (such as MGAS28426, left panel) have a basal level of rocA expression, RocA-regulated genes, and a wild-type virulence phenotype. GAS strains with rocA mutations (such as ΔrocA, right panel) have a substantially altered transcriptome that significantly increase virulence factor gene expression, stress response, and virulence.
We found that deletion of rocA in a genetically representative serotype M28 GAS strain resulted in a very substantial transcriptome change (Fig. 2; see Tables S1 and S2 in the supplemental material). Previous research on RocA has led to the publication of RocA transcriptomes from serotype M1 and M3 GAS strains (28, 32). The time point for collecting GAS cells, the culture media used, and the process of making RNA-seq libraries for the published M1 and M3 studies were very similar to those for our M28 RocA RNA-seq experiment (28, 32). However, the bioinformatic processes used for analysis in each study differed. Thus, to compare the three transcriptomes, we reanalyzed the publicly available M1 (accession number GSE97325) (32) and M3 (accession number GSE68277) (28) RocA RNA-seq data using a bioinformatics process identical to that for our M28 ME RocA RNA-seq data (see Materials and Methods). In contrast to the M1 and M3 RocA transcriptomes, we discovered a much higher number of genes directly or indirectly regulated by RocA in M28 GAS (see Table S3 and Fig. S2 in the supplemental material). At ME growth, 427 genes have significantly altered transcript levels in the serotype M28 isogenic ΔrocA deletion mutant strain, whereas only 357 and 224 genes have significantly altered transcript levels in the rocA deletion M1 and M3 strains, respectively (Table S3 and Fig. S2). The substantially increased size of the M28 RocA regulon compared to those of the M1 and M3 RocA regulons may be due, in part, to the number of genes encoding transcription regulators that were differentially expressed (Fig. S2). Of the 41 transcription regulators directly or indirectly regulated by RocA in serotype M28 GAS (Table 1), 26 had altered transcript levels at mid-exponential growth. In comparison, RocA altered the expression of only 24 and 15 transcription regulators in the M1 and M3 strains, respectively. That is, RocA may regulate the expression of more genes in M28 GAS due to its effect on many different transcription regulators. Another possible explanation for the much smaller RocA transcriptome in M3 GAS is the experimental strategy used. The M1 and M28 RocA transcriptomes were determined by creating isogenic deletion mutant strains lacking the rocA gene (32). In contrast, the M3 study compared an M3 GAS strain containing the naturally occurring rocA mutation to an isogenic strain containing the serotype M1 wild-type rocA allele (28). The naturally occurring RocA mutant protein in M3 GAS has partial RocA function when overexpressed, suggesting that M3 strains may natively retain some very limited amount of RocA activity (28, 32).
The RocA transcriptome has not been previously studied at early stationary growth phase (28, 32). In most GAS RNA-seq studies, regulators typically alter the expression of more genes at early stationary growth than at mid-exponential growth (24, 88–91). However, in M28 GAS, we discovered that rocA deletion significantly altered the transcript levels of fewer genes at early stationary growth phase (323 compared to 427) (Fig. 2C). Analysis of rocA expression at both growth phases identified significantly more transcripts at mid-exponential growth phase (see Fig. S3 in the supplemental material), suggesting that RocA is expressed at a higher level during early growth. Additionally, more genes encoding transcription regulators had significantly altered transcript levels at mid-exponential growth (26 at mid-exponential growth phase compared to 19 at early stationary growth phase). The two findings may, in part, explain the higher number of genes directly or indirectly regulated by RocA at mid-exponential growth phase compared to early stationary growth phase.
One possible molecular mechanism for RocA inactivation to increase M28 GAS virulence is by increasing CovR phosphorylation via the CovRS two-component regulatory system. Spontaneous mutations in covR and covS have been identified in GAS strains recovered from animals with experimental infections and humans with invasive infections (23, 92). In general, mutations in covR or covS substantially alter the GAS transcriptome and increase strain virulence (23, 24, 93). That is, covRS mutations may provide a fitness advantage in some host environments. We speculate that rocA mutations may also be selected in vivo after the initial infection is established. Although covR and covS expression was not significantly altered in the M28 isogenic rocA deletion mutant strain (Tables S1 and S2), RocA increases CovR phosphorylation, which is a key step in CovRS activation (28, 30). In support, approximately 15% of the genes that are differentially expressed in the ΔrocA M28 strain are known to be regulated by CovRS in other GAS serotypes (Tables S1 and S2) (24). For example, RocA inactivation significantly increased nga and slo expression (Fig. 5). CovRS is known to regulate nga and slo (23, 24). Possibly important to the role of RocA inactivation in M28 GAS virulence, increased expression and activity of SPN and SLO was recently implicated as the central factor underlying the emergence and global spread of epidemic clade 3 M89 strains (37, 94).
Another possible molecular mechanism for RocA inactivation to increase M28 GAS virulence is by significantly altering gene expression independently of CovRS (Table 2). That is, RocA may directly or indirectly regulate or act as an accessory protein to additional regulatory systems in GAS. For example, the isogenic ΔrocA deletion mutant strain had significantly increased transcript levels of mga and Mga-regulated genes (Fig. 3). Sanson et al. demonstrated that a naturally occurring mutation in mga increased the expression of mga and Mga-regulated genes in M59 GAS to significantly increase virulence in mice, nonhuman primates, and humans (95–97). In the rocA-inactivated serotype M1 and M3 GAS strains, mga and some genes in the Mga regulon also had increased expression (Table S3). Possibly important to the serotype-specific effect of RocA on mga expression, the genes comprising the Mga regulon differ in each of the three serotypes (57). Similarly, the stress response genes ciaHR, nrdR, and spxA2 and the arginine deiminase pathway genes arcABCD had significantly increased transcript levels in the ΔrocA M28 mutant strain (Fig. 4) (44, 48, 61, 74, 75). Consistent with the RNA-seq data, the isogenic ΔrocA strain had increased resistance to acidic stress, a condition encountered in purulent lesions (70–72). The expression of spxA2 was increased in the rocA-inactivated serotype M1 and M3 GAS strains (Table S3). In comparison, the arcABCD genes had significantly decreased expression in the rocA-inactivated serotype M1 GAS strain, whereas the arcABCD genes had significantly increased expression in the rocA-inactivated serotype M3 and M28 GAS strains (Table S3). The molecular basis for the serotype-specific regulatory activity of RocA on arcABCD is unknown.
Unexpectedly, the M28 ΔrocA isogenic deletion mutant strain had significantly decreased transcript levels of ska (Tables S1 and S2). The ska gene encodes streptokinase (SKA), an important virulence factor that disrupts the host fibrinolytic system (84, 98). SKA also leads to degradation of host extracellular matrix and basement membrane proteins. Whereas ska has significantly decreased transcript levels in the M28 ΔrocA strain, it had significantly increased transcript levels in the M1 and M3 RocA-inactivated strains (Table S3) (28, 32). Compared to that in the WT strain, SKA activity was significantly decreased in the M28 ΔrocA strain (Fig. 5E). The reason for the observed serotype-specific differences in regulation of ska is uncertain. To date, ska is known to be regulated by two different systems, FasBCAX and CovRS (43, 99–101). We detected no significant difference in expression of fasBCA or covRS in the M28 isogenic ΔrocA deletion mutant strain under the conditions studied. Our RNA-seq protocol does not capture small RNA transcripts, so fasX, a small RNA, could not be measured. One possible explanation for the serotype-specific regulation of ska is that one of the 41 differentially expressed transcription regulators, including the 14 regulators that are uniquely regulated by RocA in M28 GAS (Table 1 and Fig. S2), directly or indirectly regulates ska expression. Further studies will be needed to better understand the molecular mechanisms underlying altered expression of ska.
The difference in rocA allele frequency between M28 and other M protein serotype strains may be due to inherent genetic differences that favor a RocA-inactivated phenotype. For example, most serotype M1 and M59 GAS strains are encapsulated (15, 38), but virtually all M28 and epidemic clade 3 M89 GAS are capsule deficient (94, 102). Epidemic clade 3 M89 GAS strains lack the hasABC locus, which is required for hyaluronic acid capsule production (94). M28 GAS strains have a one-nucleotide frameshifting insertion in hasA that results in a truncated HasA protein without enzymatic activity (GenBank accession no. MH884522 to MH884551) (102). Thus, although the M28 rocA isogenic deletion mutant strain demonstrated significantly increased hasABC transcript levels (Table 2), virtually all M28 strains are incapable of capsule biosynthesis (see Fig. S4 in the supplemental material). Additionally, virtually all serotype M28 GAS strains have a missense mutation in mac, the gene encoding Mac, an IgG endopeptidase and inhibitor of reactive oxygen species generation (77–79). The missense mutation results in a loss of Mac IgG endopeptidase activity (78). The two phenotypic characteristics, possibly in combination with other, yet-unrecognized genomic factors, may predispose serotype M28, and also M89, GAS strains to select for rocA polymorphisms that increase fitness in the invasive infection environment. Additionally, the preponderance of missense mutations in the transmembrane domains of RocA in serotype M28 GAS compared to the other serotypes is of particular interest (Fig. S1). As the transmembrane domains are crucial for the regulatory activity of RocA, detailed analyses of the missense mutations located in the transmembrane domains are warranted.
In summary, our study shows that in serotype M28 GAS, RocA directly or indirectly regulates a substantial portion of the GAS transcriptome (38.8% of all GAS genes), including many transcription regulators and proven or putative virulence factors. The number of genes and transcription regulators directly or indirectly regulated by RocA in serotype M28 GAS is greater than that observed in RocA studies performed with serotype M1 and M3 GAS. The M28 RocA-inactivated strain was significantly more virulent in a mouse model of necrotizing myositis. Taken together, these data suggest that RocA plays a key role in M28 GAS molecular pathogenesis and thus may contribute to the high number of naturally occurring polymorphisms found in M28 strains recovered from human invasive infections. Our findings underscore the critical need for molecular pathogenesis research efforts to study many different microbial strains from many different genetic backgrounds (i.e., within the same serotype and across different serotypes).
MATERIALS AND METHODS
Determination of SNPs in rocA in serotype M28 GAS strains.
Whole-genome sequence analysis of the 2,101 serotype M28 GAS strains was previously performed (GenBank accession no. MH884522 to MH884551). Six strains are distant outliers and were excluded from further analysis. Among the remaining 2,095 M28 strains, 20,135 single nucleotide polymorphism (SNP) sites were identified across the core genome that spans 1,735,432 bp. Assuming that SNP sites are randomly distributed across the core genome, 1 SNP site is expected to occur every 86 bp. Given a random distribution, approximately 16 SNP sites are expected to occur in the rocA coding and upstream regulatory region that spans 1,374 bp. As previously described, Fisher's exact test was used to demonstrate that significantly more SNP sites were identified in rocA than would be expected to occur by random chance (17, 37, 59).
Construction of an isogenic rocA deletion strain.
Strain MGAS28426 was selected as the serotype M28 parental wild-type (WT) strain because it is genetically representative of serotype M28 GAS strains and has a wild-type allele for all major global transcription regulators. The isogenic rocA deletion (ΔrocA) mutant strain was constructed by deleting the entire open reading frame of rocA, as previously described (39). Sequences for primers used are listed in Table S4 in the supplemental material. Whole-genome sequence analysis of the isogenic ΔrocA mutant strain confirmed that no spurious mutations were introduced during strain construction.
RNA-seq analysis.
The WT and isogenic ΔrocA mutant strains were grown in triplicate in THY with 5% CO2 at 37°C to mid-exponential (ME) (OD600 = 0.5) and early stationary (ES) (OD600 = 1.65) growth phases as previously described (95, 96). RNAprotect Bacteria Reagent (Qiagen Inc., Germantown, MD) was added (2:1), and cells were lysed by ballistic disintegration (FastPrep-96 instrument and lysing matrix B [MP Biomedicals, Santa Ana, CA]). RNA was extracted using standard methods (RNeasy kit [Qiagen Inc., Germantown, MD]), and RNA quality and quantity were assessed (Agilent 2100 Bioanalyzer [Agilent Technologies, Santa Clara, CA] and Qubit [Invitrogen, Carlsbad, CA]). RNA-seq libraries were prepared using standard methods (ScriptSeq Complete kit [Epicentre, Madison, WI]) and sequenced with an Illumina NextSeq instrument (Illumina, San Diego, CA) using the default settings.
On average, we obtained 39.5 million reads/sample for the 12 samples (WT and isogenic ΔrocA mutant strains grown in triplicate and collected at ME and ES growth phases). Reads were mapped to the genome of serotype M28 GAS reference strain MGAS6180 (12), and differential transcript analysis was performed with CLC Genomics Workbench 10.5 (Qiagen Inc., Germantown, MD) using the default settings. Genes encoding rRNA, tRNA, phage, and mobile genetic elements were excluded from analysis, as there are limitations in read mapping to repetitive sequences found within the aforementioned elements. Genes with an absolute transcript change of ≥1.5-fold and a P value of <0.05 after Baggerly's test with Bonferroni's correction for multiple comparisons were considered to be significantly differentially expressed.
RocA transcriptomes from M1 and M3 GAS strains have been published (28, 32). The time point for collecting the GAS cells at ME growth phase, the culture media used, and the process of making RNA-seq libraries in the published M1 and M3 studies were very similar to those used in our M28 RocA RNA-seq experiment conducted at ME growth phase. However, the bioinformatics processes used for analysis in each study differed. To compare the M1, M3, and M28 RocA transcriptomes, publicly available M1 and M3 RNA-seq sequencing data (28, 32) were reanalyzed with a bioinformatics process identical to that described above. That is, to compare the RocA transcriptomes of the M1, M3, and M28 GAS strains, we used a common bioinformatics process to analyze the three RNA-seq data sets. Briefly, the publicly available RNA-seq reads for the M1 (accession number GSE97325) (32) and M3 (accession number GSE68277) (28) GAS strains were downloaded and mapped to the serotype M1 MGAS5005 (103) and M3 MGAS315 (104) reference genomes, respectively. Differential transcript analysis was performed with CLC Genomics Workbench 10.5 (Qiagen Inc., Germantown, MD) using the default settings. Genes encoding rRNA, tRNA, phage, and mobile genetic elements were excluded, as there are limitations in the read mapping to repetitive sequences found within the aforementioned elements. Genes not present in the M28 reference strain also were excluded from analysis. Genes with an absolute transcript change of ≥1.5-fold and a P value of <0.05 after Baggerly's test with Bonferroni's correction for multiple comparisons were considered to be significantly differentially expressed. The differentially expressed genes were then compared across the three strains.
SOF activity assay.
Serum opacity factor (SOF) activity in the culture supernatants was assayed as previously described (69), with the modification that samples were serially diluted 2-fold in phosphate-buffered saline (PBS) with 1% sodium dodecyl sulfate (SDS) before incubation with horse serum (1:10 sample-to-horse-serum volume). PBS with 1% SDS was used as a negative control. Dilutions were determined to be positive for serum opacity factor activity at an absorbance at 405 nm of greater than 0.8 (105). Mean titers of four biological replicates were compared using Student's t test (Prism 7 [GraphPad, La Jolla, CA]), with a P value of <0.05 considered to be statistically significant.
Growth under acidic conditions.
For growth under acidic conditions, THY supplemented with 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.0) (Sigma-Aldrich, St. Louis, MO) was used. THY supplemented with 0.1 M HEPES (pH 7.5) (Sigma-Aldrich, St. Louis, MO) was used to determine the baseline effects of a buffered medium on GAS strain growth (29). For CFU, cultures grown in quadruplicate were harvested at 3 h, serially diluted 10-fold, and plated onto THY agar supplemented with 5% sheep blood. Colonies were counted after incubation overnight. The mean CFU of four biological replicates were compared using the Mann-Whitney test (Prism 7 [GraphPad, La Jolla, CA]), with a P value of <0.05 considered to be statistically significant.
Western immunoblot analysis of SPN and SLO in culture supernatant.
GAS strains were grown to mid-exponential growth phase and pelleted by centrifugation, and supernatants were assessed for the presence of immunoreactive NAD+-glycohydrolase (SPN) and streptolysin O (SLO) as described previously (39).
SPN and SLO activity assay.
GAS strains were grown to mid-exponential growth phase and pelleted by centrifugation, and supernatants were assayed for NAD+-glycohydrolase (SPN) and streptolysin O (SLO) activity as previously described (86). The mean titers (SPN) and activities (SLO) of three biological replicates were compared using Student's t test (Prism 7 [GraphPad, La Jolla, CA]), with P value of <0.05 considered to be statistically significant.
PAF acetylhydrolase activity assay.
Streptococcal secreted esterase (SSE) is a known secreted GAS virulence factor that hydrolyzes platelet-activating factor (PAF) (80). Thus, SSE activity in culture supernatants collected at mid-exponential growth phase was assayed with the PAF acetylhydrolase assay kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer's instructions with minor modifications (31). GAS strains were grown in triplicate in THY to mid-exponential growth phase. Supernatants were collected by centrifugation at 4,000 rpm for 10 min. The supernatant (10 μl) was added to assay buffer 2 (5 μl) and incubated with 2-thio-PAF (200 μl) at room temperature for 30 min. 5,5-Dithio-bis(2-nitrobenzoic acid) (10 μl) was added to each sample, mixed, and incubated for 1 min at room temperature. The absorbance of each sample at 412 nm was measured and used to calculate the PAF acetylhydrolase activity. Fresh THY was used as a negative control. The mean activities of three biological replicates were compared using Student's t test (Prism 7 [GraphPad, La Jolla, CA]), with a P value of <0.05 considered to be statistically significant.
SKA activity assay.
GAS strains were grown to mid-exponential and early stationary growth phases and pelleted by centrifugation, and cell-free supernatants were assayed for streptokinase (SKA) activity as previously described (100). Activity was determined as the change in absorbance over time from the initial time point to maximum absorbance at 405 nm. The mean relative activities of three biological replicates were compared using Student's t test (Prism 7 [GraphPad, La Jolla, CA]), with P value of <0.05 considered to be statistically significant.
Mouse model of necrotizing myositis.
Mouse necrotizing myositis studies were performed as previously described (37, 86, 87). Immunocompetent 4-week-old female CD1 mice (Envigo Laboratories, Houston, TX) were randomly assigned to treatment groups and inoculated in the right lower hindlimb with 5 × 108 CFU of each bacterial strain in 100 μl PBS (n = 40 mice/strain). Mice were monitored at least once daily, and mortality was determined using internationally recognized criteria (106). Survival was compared using the log rank test (Prism 7 [GraphPad, La Jolla, CA]), with a P value of <0.05 considered statistically significant. For gross and histopathological evaluation, mice were sacrificed on day 1 postinoculation, and limbs were processed by standard methods (87). For quantitative culture (n = 20 mice/strain), mice were sacrificed on day 3 postinoculation. Infected limbs were amputated, placed in tared tubes containing sterile PBS, weighed, and homogenized. Tenfold serial dilutions were grown overnight on THY agar supplemented with 5% sheep blood, and CFU were counted. Mean CFU were compared by the Mann-Whitney test (Prism 7 [GraphPad, La Jolla, CA]), with a P value of <0.05 considered statistically significant. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Houston Methodist Research Institute. Sample sizes for each experiment were determined using a power calculation.
Hyaluronic acid capsule assay.
GAS strains were grown in triplicate in THY to mid-exponential growth phase. Cells were collected from 10 ml of culture by centrifugation at 4,000 rpm for 10 min, and the supernatant was discarded. The cells were resuspended in a 1:1 (vol/vol) mixture of water and chloroform (1 ml) and vortexed for 30 s. The mixture was centrifuged (13,200 rpm, 10 min), and the resulting aqueous phase was used to assay for the presence of hyaluronic acid capsule using the hyaluronic acid quantitative test kit (Corgenix, Broomfield, CO) according to the manufacturer's instructions. MGAS2221, a serotype M1 GAS strain whose capsule production is well documented (39), was used as a positive control.
Accession number(s).
The serotype M28 RNA-seq sequence data have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject no. PRJNA470894.
Supplementary Material
ACKNOWLEDGMENTS
We thank Matthew Ojeda Saavedra, Sarah Linson, and Concepcion Cantu for assistance with the mouse experiments and Kathryn Stockbauer for assistance in preparing the manuscript.
This study was supported by funds from the Fondren Foundation (to P.E.B. and J.M.M.).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00467-18.
REFERENCES
- 1.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen RJ, Musser JM. 2010. Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol Mech Dis 5:1–31. doi: 10.1146/annurev-pathol-121808-102135. [DOI] [PubMed] [Google Scholar]
- 3.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. 2014. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev 27:264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 5.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. 2009. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis 9:611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 6.Chochua S, Metcalf BJ, Li Z, Rivers J, Mathis S, Jackson D, Gertz RE Jr, Srinivasan V, Lynfield R, Van Beneden C, McGee L, Beall B. 2017. Population and whole genome sequence based characterization of invasive group A streptococci recovered in the United States during 2015. mBio 8:e01422-17. doi: 10.1128/mBio.01422-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colman G, Tanna A, Efstratiou A, Gaworzewska E. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J Med Microbiol 39:165–178. doi: 10.1099/00222615-39-3-165. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson BKG, Norgen M, McGregor K, Spratt BG, Henriques-Normark B. 2003. Group A streptococcal infections in Sweden: a comparative study of invasive and nonivasive infections and analysis of dominant T28 emm28 isolates. Clin Infect Dis 37:1189–1193. doi: 10.1086/379013. [DOI] [PubMed] [Google Scholar]
- 9.Gaworzewska E, Colman G. 1988. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol Infect 100:257–269. doi: 10.1017/S095026880006739X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyrrell GJ, Lovgren M, Forwick B, Hoe NP, Musser JM, Talbot JA. 2002. M types of group A streptococcal isolates submitted to the National Centre for Streptococcus (Canada) from 1993 to 1999. J Clin Microbiol 40:4466–4471. doi: 10.1128/JCM.40.12.4466-4471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlaminckx B, van Pelt W, Schouls L, van Silfhout A, Elzenaar C, Mascini E, Verhoef J, Schellekens J. 2004. Epidemiological features of invasive and noninvasive group A streptococcal disease in the Netherlands, 1992-1996. Eur J Clin Microbiol Infect Dis 23:434–444. doi: 10.1007/s10096-004-1147-z. [DOI] [PubMed] [Google Scholar]
- 12.Green NM, Zhang S, Porcella SF, Nagiec MJ, Barbian KD, Beres SB, LeFebvre RB, Musser JM. 2005. Genome sequence of a serotype M28 strain of group A Streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J Infect Dis 192:760–770. doi: 10.1086/430618. [DOI] [PubMed] [Google Scholar]
- 13.Green NM, Beres SB, Graviss EA, Allison JE, McGeer AJ, Vuopio-Varkila J, LeFebvre RB, Musser JM. 2005. Genetic diversity among type emm28 group A Streptococcus strains causing invasive infections and pharyngitis. J Clin Microbiol 43:4083–4091. doi: 10.1128/JCM.43.8.4083-4091.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang I, Van Beneden C, Beall B, Schuchat A. 2002. Population-based surveillance for postpartum invasive group A streptococcus infection, 1995-2000. Clin Infect Dis 35:665–670. doi: 10.1086/342062. [DOI] [PubMed] [Google Scholar]
- 15.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, Kristinsson KG, Gottfredsson M, Vuopio J, Raisanen K, Caugant DA, Steinbakk M, Low DE, McGeer A, Darenberg J, Henriques-Normark B, Van Beneden CA, Hoffmann S, Musser JM. 2014. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci U S A 111:E1768–E1776. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, Ricklefs SM, Babar I, Parkins LD, Romero RA, Corn GJ, Gardner DJ, Bailey JR, Parnell MJ, Musser JM. 2005. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc Natl Acad Sci U S A 102:9014–9019. doi: 10.1073/pnas.0503671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beres SB, Carroll RK, Shea PR, Sitkiewicz I, Martinez-Gutierrez JC, Low DE, McGeer A, Willey BM, Green K, Tyrrell GJ, Goldman TD, Feldgarden M, Birren BW, Fofanov Y, Boos J, Wheaton WD, Honisch C, Musser JM. 2010. Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc Natl Acad Sci U S A 107:4371–4376. doi: 10.1073/pnas.0911295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beres SB, Sylva GL, Sturdevant DE, Granville CN, Liu M, Ricklefs SM, Whitney AR, Parkins LD, Hoe NP, Adams GJ, Low DE, DeLeo FR, McGeer A, Musser JM. 2004. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc Natl Acad Sci U S A 101:11833–11838. doi: 10.1073/pnas.0404163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Ortega MJ, Norais N, Bensi G, Liberatori S, Capo S, Mora M, Scarselli M, Doro F, Ferrari G, Garaguso I, Maggi T, Neumann A, Covre A, Telford JL, Grandi G. 2006. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol 24:191–197. doi: 10.1038/nbt1179. [DOI] [PubMed] [Google Scholar]
- 20.McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh J. 2008. Coiled-coil irregularities and instabilities in group A Streptococcus are required for virulence. Science 319:1405–1408. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macheboeuf P, Buffalo C, Fu C, Zinkernagel AS, Cole JN, Johnson JE, Nizet V, Ghosh P. 2011. Streptococcal M1 protein constructs a pathological host fibrinogen network. Nature 472:64–68. doi: 10.1038/nature09967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas I, Scott JR. 2003. Identification of rocA, a positive regulator of covR expression in the group A streptococcus. J Bacteriol 185:3081–3090. doi: 10.1128/JB.185.10.3081-3090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog 2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelburne SA, Olsen RJ, Suber B, Sahasrabhojane P, Sumby P, Brennan RG, Musser JM. 2010. A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog 6:e1000817. doi: 10.1371/journal.ppat.1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynskey NN, Goulding D, Gierula M, Turner CE, Dougan G, Edwards RJ, Sriskandan S. 2013. RocA truncation underpins hyper-encapsulation, carriage longevity and transmissibility of serotype M18 group A streptococci. PLoS Pathog 9:e1003842. doi: 10.1371/journal.ppat.1003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynskey NN, Turner CE, Heng LS, Sriskandan S. 2015. A truncation in the regulator RocA underlies heightened capsule expression in serotype M3 group A streptococci. Infect Immun 83:1732–1733. doi: 10.1128/IAI.02892-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller EW, Pflughoeft KJ, Sumby P. 2015. Reply to “A truncation in the regulator RocA underlies heightened capsule expression in serotype M3 group A streptococci.” Infect Immun 83:1734. doi: 10.1128/IAI.03162-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EW, Danger JL, Ramalinga AB, Horstmann N, Shelburne SA, Sumby P. 2015. Regulatory rewiring confers serotype-specific hyper-virulence in the human pathogen group A Streptococcus. Mol Microbiol 98:473–489. doi: 10.1111/mmi.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paluscio E. 2015. Adaptive mechanisms to niche remodeling in Streptococcus pyogenes. Ph.D.thesis, Washington University in St. Louis, St. Louis, MO. [Google Scholar]
- 30.Zhu L, Olsen RJ, Horstmann N, Shelburne SA, Fan J, Hu Y, Musser JM. 2016. Intergenic variable-number tandem-repeat polymorphism upstream of rocA alters toxin production and enhances virulence in Streptococcus pyogenes. Infect Immun 84:2086–2093. doi: 10.1128/IAI.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng W, Minor D, Liu M, Li J, Ishaq SL, Yeoman C, Lei B. 2017. Null mutations of group A Streptococcus orphan kinase RocA: selection in mouse infection and comparison with CovS mutations in alteration of in vitro and in vivo protease SpeB expression and virulence. Infect Immun 85:e00790-16. doi: 10.1128/IAI.00790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain I, Miller EW, Danger JL, Pflughoeft KJ, Sumby P. 2017. RocA is an accessory protein to the virulence-regulating CovRS two-component system in group A Streptococcus. Infect Immun 85:e00274-17. doi: 10.1128/IAI.00274-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Yoshida H, Ishigaki Y, Takizawa A, Moro K, Kishi Y, Takahashi T, Matsui H. 2015. Comparative genomics of the mucoid and nonmucoid strains of Streptococcus pyogenes, isolated from the same patient with streptococcal meningitis. Genome Announc 3:e00221-15. doi: 10.1128/genomeA.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikebe T, Matsumura T, Nihonmatsu H, Ohya H, Okuno R, Mitsui C, Kawahara R, Kameyama M, Sasaki M, Shimada N, Ato M, Ohnishi M. 2016. Spontaneous mutations in Streptococcus pyogenes isolates from streptococcal toxic shock syndrome patients play roles in virulence. Sci Rep 6:28761. doi: 10.1038/srep28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar P, Sumby P. 2017. Regulatory gene mutation: a driving force behind group A Streptococcus strain- and serotype-specific variation. Mol Microbiol 103:576–589. doi: 10.1111/mmi.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beres SB, Kachroo P, Nasser W, Olsen RJ, Zhu L, Flores AR, de la Riva I, Paez-Mayorga J, Jimenez FE, Cantu C, Vuopio J, Jalava J, Kristinsson KG, Gottfredsson M, Corander J, Fittipaldi N, Di Luca MC, Petrelli D, Vitali LA, Raiford A, Jenkins L, Musser JM. 2016. Transcriptome remodeling contributes to epidemic disease caused by the human pathogen Streptococcus pyogenes. mBio 7:e00403-16. doi: 10.1128/mBio.00403-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fittipaldi N, Beres SB, Olsen RJ, Kapur V, Shea PR, Watkins ME, Cantu CC, Laucirica DR, Jenkins L, Flores AR, Lovgren M, Ardanuy C, Linares J, Low DE, Tyrrell GJ, Musser JM. 2012. Full-genome dissection of an epidemic of severe invasive disease caused by a hypervirulent, recently emerged clone of group A Streptococcus. Am J Pathol 180:1522–1534. doi: 10.1016/j.ajpath.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Olsen RJ, Nasser W, Beres SB, Vuopio J, Kristinsson KG, Gottfredsson M, Porter AR, DeLeo FR, Musser JM. 2015. A molecular trigger for intercontinental epidemics of group A Streptococcus. J Clin Invest 125:3545–3559. doi: 10.1172/JCI82478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashburn-Warren L, Morrison DA, Federle MJ. 2012. The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J Bacteriol 194:4589–4600. doi: 10.1128/JB.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogg GC, Caparon MG. 1997. Constitutive expression of fibronectin binding in Streptococcus pyogenes as a result of anaerobic activation of rofA. J Bacteriol 179:6172–6180. doi: 10.1128/jb.179.19.6172-6180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravcheev DA, Best AA, Sernova NV, Kazanov MD, Novichkov PS, Rodionov DA. 2013. Genomic reconstruction of transcriptional regulatory networks in lactic acid bacteria. BMC Genomics 14:94. doi: 10.1186/1471-2164-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao TN, Liu Z, Cao TH, Pflughoeft KJ, Trevino J, Danger JL, Beres SB, Musser JM, Sumby P. 2014. Natural disruption of two regulatory networks in serotype M3 group A Streptococcus isolates contributes to the virulence factor profile of this hypervirulent serotype. Infect Immun 82:1744–1754. doi: 10.1128/IAI.01639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Okada R, Isaka M, Tatsuno I, Isobe K-I, Hasegawa T. 2015. Analysis of the roles of NrdR and DnaB from Streptococcus pyogenes in response to host defense. APMIS 123:252–259. doi: 10.1111/apm.12340. [DOI] [PubMed] [Google Scholar]
- 45.Siemens N, Fiedler T, Normann J, Klein J, Munch R, Patenge N, Kreikemeyer B. 2012. Effects of the ERES pathogenicity region regulator Ralp3 on Streptococcus pyogenes serotype M49 virulence factor expression. J Bacteriol 194:3618–3626. doi: 10.1128/JB.00227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vega LA, Valdes KM, Sundar GS, Belew AT, Islam E, Berge J, Curry P, Chen S, El-Sayed NM, Le Breton Y, McIver KS. 2017. The transcriptional regulator CpsY is important for innate immune evasion in Streptococcus pyogenes. Infect Immun 85:e00925-16. doi: 10.1128/IAI.00925-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wescombe PA, Tagg JR. 2003. Purification and characterization of streptin, a type A1 lantibiotic produced by Streptococcus pyogenes. Appl Environ Microbiol 69:2737–2747. doi: 10.1128/AEM.69.5.2737-2747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatsuno I, Isaka M, Okada R, Zhang Y, Hasegawa T. 2014. Relevance of the two-component sensor protein CiaH to acid and oxidative stress responses in Streptococcus pyogenes. BMC Res Notes 7:189. doi: 10.1186/1756-0500-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leday TV, Gold KM, Kinkel TL, Roberts SA, Scott JR, McIver KS. 2008. TrxR, a new CovR-repressed response regulator that activates the Mga virulence regulon in group A streptococcus. Infect Immun 76:4659–4668. doi: 10.1128/IAI.00597-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flores AR, Olsen RJ, Cantu C, Pallister KB, Guerra FE, Voyich JM, Musser JM. 2017. Increased pilus production conferred by a naturally occurring mutation alters host-pathogen interaction in favor of carriage in Streptococcus pyogenes. Infect Immun 85:e00949-16. doi: 10.1128/IAI.00949-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sitkiewicz I, Musser JM. 2017. Deletion of atoR from Streptococcus pyogenes results in hypervirulence in a mouse model of sepsis and is LuxS independent. Pol J Microbiol 66:17–24. doi: 10.5604/17331331.1234989. [DOI] [PubMed] [Google Scholar]
- 52.Loughman JA, Caparon MG. 2007. Comparative functional analysis of the lac operons in Streptococcus pyogenes. Mol Microbiol 64:269–280. doi: 10.1111/j.1365-2958.2007.05663.x. [DOI] [PubMed] [Google Scholar]
- 53.Young CA, Gordon LD, Fang Z, Holder RC, Reid SD. 2015. Copper tolerance and characterization of a copper-responsive operon, copYAZ, in an M1T1 clinical strain of Streptococcus pyogenes. J Bacteriol 197:2580–2592. doi: 10.1128/JB.00127-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malke H, Steiner K, McShan WM, Ferretti JJ. 2006. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol 296:259–275. doi: 10.1016/j.ijmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Reid SD, Chaussee MS, Doern CD, Chaussee MA, Montgomery AG, Sturdevant DE, Musser JM. 2006. Inactivation of the group A Streptococcus regulator srv results in chromosome wide reduction of transcript levels, and changes in extracellular levels of Sic and SpeB. FEMS Immunol Med Microbiol 48:283–292. doi: 10.1111/j.1574-695X.2006.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Namprachan-Frantz P, Rowe HM, Runft DL, Neely MN. 2014. Transcriptional analysis of the Streptococcus pyogenes salivaricin locus. J Bacteriol 196:604–613. doi: 10.1128/JB.01009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribardo DA, McIver KS. 2006. Defining the Mga regulon: comparative transcriptome analysis reveals both direct and indirect regulation by Mga in the group A streptococcus. Mol Microbiol 62:491–508. doi: 10.1111/j.1365-2958.2006.05381.x. [DOI] [PubMed] [Google Scholar]
- 58.Voyich JM, Sturdevant DE, Braughton KR, Kobayashi SD, Lei B, Virtaneva K, Dorward DW, Musser JM, DeLeo FR. 2003. Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A 100:1996–2001. doi: 10.1073/pnas.0337370100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olsen RJ, Laucirica DR, Watkins ME, Feske ML, Garcia-Bustillos JR, Vu C, Cantu C, Shelburne SA III, Fittipaldi N, Kumaraswami M, Shea PR, Flores AR, Beres SB, Lovgren M, Tyrrell GJ, Efstratiou A, Low DE, Van Beneden CA, Musser JM. 2012. Polymorphisms in regulator of protease B (RopB) alter disease phenotype and strain virulence of serotype M3 group A Streptococcus. J Infect Dis 204:1719–1729. doi: 10.1093/infdis/jir825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derre I, Rapoport G, Msadek T. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and moelcular chaperone gene expression in Gram-positive bacteria. Mol Microbiol 31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 61.Port GC, Cusumano ZT, Tumminello PR, Caparon MG. 2017. SpxA1 and SpxA2 act coordinately to fine-tune stress responses and virulence in Streptococcus pyogenes. mBio 8:e00288-17. doi: 10.1128/mBio.00288-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Ireland RM, Reid SD, Adams GG, Musser JM. 2000. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect Immun 68:6542–6553. doi: 10.1128/IAI.68.12.6542-6553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol Microbiol 42:75–86. doi: 10.1046/j.1365-2958.2001.02579.x. [DOI] [PubMed] [Google Scholar]
- 64.Wexler DE, Chenoweth DE, Cleary PP. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci U S A 82:8144–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenberg L, O'Toole P, Lindahl G. 1992. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol Microbiol 6:1185–1194. doi: 10.1111/j.1365-2958.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 66.Smeesters PR, McMillan DJ, Sriprakash KS. 2010. The streptococcal M protein: a highly versatile molecule. Trends Microbiol 18:275–282. doi: 10.1016/j.tim.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Courtney HS, Hasty DL, Dale JB. 2006. Anti-phagocytic mechanisms of Streptococcus pyogenes: binding of fibrinogen to M-related protein. Mol Microbiol 59:936–947. doi: 10.1111/j.1365-2958.2005.04977.x. [DOI] [PubMed] [Google Scholar]
- 68.Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. 2003. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J Bacteriol 185:1208–1217. doi: 10.1128/JB.185.4.1208-1217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L, Olsen RJ, Musser JM. 2017. Opacification domain of serum opacity factor inhibits beta-hemolysis and contributes to virulence of Streptococcus pyogenes. mSphere 2:e00147-17. doi: 10.1128/mSphereDirect.00147-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nekoofar MH, Namazikhah MS, Sheykhrezae MS, Mohammadi MM, Kazemi A, Aseeley Z, Dummer PMH. 2009. pH of pus collected from periapical abscesses. Int Endod J 42:534–538. doi: 10.1111/j.1365-2591.2009.01550.x. [DOI] [PubMed] [Google Scholar]
- 71.Bryant RE, Mazza JA. 1989. Effect of the abscess environment on the antimicrobial activity of ciprofloxacin. Am J Med 87:23S–27S. [DOI] [PubMed] [Google Scholar]
- 72.Wiese KG. 1994. Electrolyte concentration, real and osmotic pressure in abscesses. Zentralbl Chir 119:54–59. [PubMed] [Google Scholar]
- 73.Degnan BA, Fontaine MC, Doebereiner AH, Lee JJ, Mastroeni P, Dougan G, Goodacre JA, Kehoe MA. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect Immun 68:2441–2448. doi: 10.1128/IAI.68.5.2441-2448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cusumano ZT, Watson ME Jr, Caparon MG. 2014. Streptococcus pyogenes arginine and citrulline catabolism promotes infection and modulates innate immunity. Infect Immun 82:233–242. doi: 10.1128/IAI.00916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cusumano ZT, Caparon MG. 2015. Citrulline protects Streptococcus pyogenes from acid stress using the arginine deiminase pathway and the F1Fo-ATPase. J Bacteriol 197:1288–1296. doi: 10.1128/JB.02517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards RJ, Taylor GW, Ferguson M, Murray S, Rendell N, Wrigley A, Bai Z, Boyle J, Finney SJ, Jones A, Russell HH, Turner C, Cohen J, Faulkner L, Sriskandan S. 2005. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis 192:783–790. doi: 10.1086/432485. [DOI] [PubMed] [Google Scholar]
- 77.von Pawel-Rammingen U, Johansson BP, Bjorck L. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J 21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soderberg JJ, Engstrom P, von Pawel-Rammingen U. 2008. The intrinsic immunoglobulin G endopeptidase activity of streptococcal Mac-2 proteins implies a unique role for the enzymatically impaired Mac-2 protein of M28 serotype strains. Infect Immun 76:2183–2188. doi: 10.1128/IAI.01422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lei B, DeLeo FR, Reid SD, Voyich JM, Magoun L, Liu M, Braughton KR, Ricklefs S, Hoe NP, Cole RL, Leong JM, Musser JM. 2002. Opsonophagocytosis-inhibiting Mac protein of group A Streptococcus: identification and characterisitcs of two gene complexes. Infect Immun 70:6880–6890. doi: 10.1128/IAI.70.12.6880-6890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu M, Zhu H, Li J, Garcia CC, Feng W, Kirpotina LN, Hilmer J, Tavares LP, Layton AW, Quinn MT, Bothner B, Teixeira MM, Lei B. 2012. Group A Streptococcus secreted esterase hydrolyzes platelet-activating factor to impede neutrophil recruitment and facilitate innate immune evasion. PLoS Pathog 8:e1002624. doi: 10.1371/journal.ppat.1002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calfee G, Danger JL, Jain I, Miller EW, Sarkar P, Tjaden B, Kreikemeyer B, Sumby P. 2018. Identification and characterization of serotype-specific variation in group A Streptococcus pilus expression. Infect Immun 86:e00792-17. doi: 10.1128/IAI.00792-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nizet V. 2002. Streptococcal beta-hemolysins: genetics and role in disease pathogenesis. Trends Microbiol 10:575–580. doi: 10.1016/S0966-842X(02)02473-3. [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen M, Muller H-P, Bjorck L. 1999. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding alpha2-macroglobulin. J Biol Chem 274:15336–15344. doi: 10.1074/jbc.274.22.15336. [DOI] [PubMed] [Google Scholar]
- 84.Huang T-T, Malke H, Ferretti JJ. 1989. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun 57:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olsen RJ, Raghuram A, Cantu C, Hartman MH, Jimenez FE, Lee S, Ngo A, Rice KA, Saddington D, Spillman H, Valson C, Flores AR, Beres SB, Long SW, Nasser W, Musser JM. 2015. The majority of 9,729 group A streptococcus strains causing disease secrete SpeB cysteine protease: pathogenesis implications. Infect Immun 83:4750–4758. doi: 10.1128/IAI.00989-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu L, Olsen RJ, Lee JD, Porter AR, DeLeo FR, Musser JM. 2017. Contribution of secreted NADase and streptolysin O to the pathogenesis of epidemic serotype M1 Streptococcus pyogenes infections. Am J Pathol 187:605–613. doi: 10.1016/j.ajpath.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, Beres SB, Green NM, Lei B, Humbird T, Greaver J, Chang E, Ragasa WP, Montgomery CA, Cartwright J Jr, McGeer A, Low DE, Whitney AR, Cagle PT, Blasdel TL, DeLeo FR, Musser JM. 2010. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A 107:888–893. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eraso JM, Olsen RJ, Beres SB, Kachroo P, Porter AR, Nasser W, Bernard PE, DeLeo FR, Musser JM. 2016. Genomic landscape of intrahost variation in group A Streptococcus: repeated and abundant mutational inactivation of the fabT gene encoding a regulator of fatty acid synthesis. Infect Immun 84:3268–3281. doi: 10.1128/IAI.00608-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flores AR, Jewell BE, Yelamanchili D, Olsen RJ, Musser JM. 2015. A single amino acid replacement in the sensor kinase LiaS contributes to a carrier phenotype in group A Streptococcus. Infect Immun 83:4237–4246. doi: 10.1128/IAI.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graham MR, Smoot LM, Lux Migiliaccio CA, Virtaneva K, Sturdevant DE, Porcella SF, Federle MJ, Adams GJ, Scott JR, Musser JM. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc Natl Acad Sci U S A 99:13855–13860. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voyich JM, Braughton KR, Sturdevant DE, Vuong C, Kobayashi SD, Porcella SF, Otto M, Musser JM, DeLeo FR. 2004. Engagement of the pathogen survival response used by group A Streptococcus to avert destruction by innate host defense. J Immunol 173:1194–1201. doi: 10.4049/jimmunol.173.2.1194. [DOI] [PubMed] [Google Scholar]
- 92.Li J, Liu G, Feng W, Zhou Y, Liu M, Wiley JA, Lei B. 2014. Neutrophils select hypervirulent CovRS mutants of M1T1 group A Streptococcus during subcutaneous infection of mice. Infect Immun 82:1579–1590. doi: 10.1128/IAI.01458-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horstmann N, Saldana M, Sahasrabhojane P, Yao H, Su X, Thompson E, Koller A, Shelburne SA III. 2014. Dual-site phosphorylation of the control of virulence regulator impacts group a streptococcal global gene expression and pathogenesis. PLoS Pathog 10:e1004088. doi: 10.1371/journal.ppat.1004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu L, Olsen RJ, Nasser W, de la Riva Morales I, Musser JM. 2015. Trading capsule for increased cytotoxin production: contribution to virulence of a newly emerged clade of emm89 Streptococcus pyogenes. mBio 6:e01378-15. doi: 10.1128/mBio.01378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanson M, O'Neill BE, Kachroo P, Anderson JR, Flores AR, Valson C, Cantu CC, Makthal N, Karmonik C, Fittipaldi N, Kumaraswami M, Musser JM, Olsen RJ. 2015. A naturally occurring single amino acid replacement in multiple gene regulator of group A streptococcus significantly increases virulence. Am J Pathol 185:462–471. doi: 10.1016/j.ajpath.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanson M, Makthal N, Gavagan M, Cantu C, Olsen RJ, Musser JM, Kumaraswami M. 2015. Phosphorylation events in the multiple gene regulator of group A Streptococcus significantly influence global gene expression and virulence. Infect Immun 83:2382–2395. doi: 10.1128/IAI.03023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fittipaldi N, Tyrrell GJ, Low DE, Martin I, Lin D, Hari KL, Musser JM. 2013. Integrated whole-genome sequencing and temporospatial analysis of a continuing group A Streptococcus epidemic. Emerg Microbes Infect 2:e13. doi: 10.1038/emi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun H, Xu Y, Sitkiewicz I, Ma Y, Wang X, Yestrepsky BD, Huang Y, Lapadatescu MC, Larsen MJ, Larsen SD, Musser JM, Ginsbutg D. 2012. Inhibitor of streptokinase gene expression improves survival after group A streptococcus infection in mice. Proc Natl Acad Sci U S A 109:3469–3474. doi: 10.1073/pnas.1201031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Churchward G, Bates C, Gusa AA, Stringer V, Scott JR. 2009. Regulation of streptokinase expression by CovR/S in Streptococcus pyogenes: CovR acts through a single high-affinity binding site. Microbiology 155:566–575. doi: 10.1099/mic.0.024620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramirez-Pena E, Trevino J, Liu Z, Perez N, Sumby P. 2010. The group A Streptococcus small regulatory RNA FasX enhances streptokinase activity by increasing the stability of the ska mRNA transcript. Mol Microbiol 78:1332–1347. doi: 10.1111/j.1365-2958.2010.07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Danger JL, Cao TN, Cao TH, Sarkar P, Trevino J, Pflughoeft KJ, Sumby P. 2015. The small regulatory RNA FasX enhances group A Streptococcus virulence and inhibits pilus expression via serotype-specific targets. Mol Microbiol 96:249–262. doi: 10.1111/mmi.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tagini F, Aubert B, Troillet N, Pillonel T, Praz G, Crisinel PA, Prod'hom G, Asner S, Greub G. 2017. Importance of whole genome sequencing for the assessment of outbreaks in diagnostic laboratories: analysis of a case series of invasive Streptococcus pyogenes infections. Eur J Clin Microbiol Infect Dis 36:1173–1180. doi: 10.1007/s10096-017-2905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, Sturdevant DE, Graham MR, Vuopio-Varkila J, Hoe NP, Musser JM. 2005. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis 192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 104.Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, Liu M-Y, Smoot JC, Porcella SF, Parkins LD, Campbell DS, Smith TM, McCormick JK, Leung DYM, Schlievert PM, Musser JM. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci U S A 99:10078–10083. doi: 10.1073/pnas.152298499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Courtney HS, Zhang Y-M, Frank MW, Rock CO. 2006. Serum opacity factor, a streptococcal virulence factor that binds to apolipoproteins A-I and A-II and disrupts high density lipoprotein structure. J Biol Chem 281:5515–5521. doi: 10.1074/jbc.M512538200. [DOI] [PubMed] [Google Scholar]
- 106.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 107.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H, Boutros PC. 2011. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.