Developing an effective and safe recombinant vaccine requires microbe-specific antigens combined with an adjuvant/delivery system to strengthen protective immunity. In this study, we designed and expressed a multivalent recombinant Coccidioides polypeptide antigen (rCpa1) that consists of three previously identified antigens (i.e., Ag2/Pra, Cs-Ag, and Pmp1) and five pathogen-derived peptides with high affinity for human major histocompatibility complex class II (MHC-II) molecules.
KEYWORDS: Coccidioides, coccidioidomycosis, HLA-DR transgenic mice, fungal vaccine, vaccine immunity, Th17 response and adaptive immunity, Th17
ABSTRACT
Developing an effective and safe recombinant vaccine requires microbe-specific antigens combined with an adjuvant/delivery system to strengthen protective immunity. In this study, we designed and expressed a multivalent recombinant Coccidioides polypeptide antigen (rCpa1) that consists of three previously identified antigens (i.e., Ag2/Pra, Cs-Ag, and Pmp1) and five pathogen-derived peptides with high affinity for human major histocompatibility complex class II (MHC-II) molecules. The purified rCpa1 was encapsulated into four types of yeast cell wall particles containing β-glucan, mannan, and chitin in various proportions or was mixed with an oligonucleotide (ODN) containing two methylated dinucleotide CpG motifs. This multivalent antigen encapsulated into glucan-chitin particles (GCP-rCpa1) showed significantly greater reduction of fungal burden for human HLA-DR4 transgenic mice than the other adjuvant-rCpa1 formulations tested. Among the adjuvants tested, both GCPs and β-glucan particles (GPs) were capable of stimulating a mixed Th1 and Th17 response. Mice vaccinated with GCP-rCpa1 showed higher levels of interleukin 17 (IL-17) production in T-cell recall assays and earlier lung infiltration by activated Th1 and Th17 cells than GP-rCpa1-vaccinated mice. Both C57BL/6 and HLA-DR4 transgenic mice that were vaccinated with the GCP-rCpa1 vaccine showed higher survival rates than mice that received GCPs alone. Concurrently, the GCP-rCpa1 vaccine stimulated greater infiltration of the injection sites by macrophages, which engulf and process the vaccine for antigen presentation, than the GP-rCpa1 vaccine. This is the first attempt to systematically characterize the presentation of a multivalent coccidioidomycosis vaccine encapsulated with selected adjuvants that enhance the protective cellular immune response to infection.
INTRODUCTION
Coccidioidomycosis, commonly known as San Joaquin Valley fever, affects residents in semiarid to arid regions of the southwestern United States, northern Mexico, and scattered areas of Central and South America. The incidence of reported coccidioidomycosis has increased substantially, from 5.3 to 42.6 per 100,000 population, in the southwestern United States from 1998 to 2011 (1). An estimated 150,000 people in the United States become infected with Coccidioides annually (2). In the areas of endemicity, 17 to 29% of community-acquired pneumonia cases are due to Coccidioides infection (3). Recent epidemiological studies show that the geographic range of coccidioidomycosis is expanding, since new cases have been identified in the state of Washington, well outside the historical areas of endemicity (4). Collectively, these statistics highlight the increasing health- and cost-related impacts of coccidioidomycosis as a major public health challenge. Thus, there is an urgent unmet need to develop a human vaccine against Coccidioides infection.
A number of experimental vaccines have been generated and evaluated previously in genetically susceptible murine models of coccidioidomycosis; these include formalin-killed spherules (FKS), live spores isolated from attenuated strains of this pathogen, chemical extracts of spherules, and recombinant antigens mixed with an adjuvant (5–7). Despite the apparent ability of live attenuated vaccines to elicit highly protective immunity in mice, they may not be safe for individuals with underlying conditions of compromised cell-mediated immune systems (7, 8). The use of recombinant antigens in vaccines is attractive due to their well-defined chemical composition and low risk for adverse effects. Several recombinant antigens of Coccidioides posadasii, including cell wall antigen 2 (Ag2/Pra), proline-rich protein 2 (Prp2), Coccidioides-specific antigen (Cs-Ag), proximal matrix protein 1 (Pmp1), urease (Ure), β-1,3-glucanosyltransferase (Gel1), aspartyl protease 1 (Pep1), α-mannosidase 1 (Amn1), and phospholipase B (Plb), have been evaluated using a murine model of coccidioidomycosis (9–11). Although each individual antigen has shown moderate but significant protective efficacy, a multivalent polypeptide antigen that can induce large repertoires of specific B-cell and T-cell responses might be more effective (11, 12).
Recombinant protein antigens elicit a relatively weak immune response and thus require the use of an adjuvant to optimize protective efficacy (13, 14). We have created a genetically engineered live attenuated vaccine (ΔT) with which to explore the nature of vaccine immunity in mice after intranasal challenge with a potentially lethal dose of Coccidioides spores (15). While mice lacking gamma interferon (IFN-γ) or interleukin 4 (IL-4) receptors could develop comparable vaccine immunity without loss of ΔT vaccine-induced resistance, deficiencies of IL-17A and the IL-17 receptor resulted in increased susceptibility to Coccidioides infection (16, 17). These data suggest that vaccine-induced CD4+ T cells, particularly Th1 and Th17 cells, are essential for vaccine immunity against Coccidioides infection (16–18).
Several types of purified porous yeast cell wall particles have been generated for vaccine development (19). Pure β-glucan particles (GPs) and glucan-mannan particles (GMPs) are derived from Saccharomyces cerevisiae, whereas glucan-chitin particles (GCPs) and glucan-chitin-mannan particles (GCMPs) are produced from Rhodotorula mucilaginosa, a nonpathogenic yeast (19). Notably, these particles have shown to be safe in both preclinical and human trials (20). GPs are phagocytosed via complement and Dectin-1 activation through interactions with β-glucan (21). Intranasal administration of GCPs and GMPs stimulates mice to produce significantly larger amounts of IL-6 and monocyte chemoattractant protein 1 (MCP-1; also called CCL2) in bronchoalveolar lavage specimens than administration of GPs (19), suggesting that GCPs and GMPs may augment Th17 immunity. In this study, we created a recombinant chimeric polypeptide antigen (rCpa1) that is designed to contain the most immunogenic fragment of Ag2/Pra, the full lengths of Cs-Ag and Pmp1, and five promiscuous, immunodominant T-cell epitopes derived from Coccidioides posadasii Pep1, Amn1, and Plb (6, 10–12, 22). We also assessed the protective efficacies and immunoreactivities of experimental vaccines consisting of rCpa1 encapsulated in four types of yeast cell wall particles (GPs, GCPs, GMPs, and GCMPs) or mixed with an oligonucleotide adjuvant containing 2 copies of the CpG motif (ODN) that has been shown to stimulate a predominantly Th1 response against Coccidioides infection (12, 23).
RESULTS
Generation of rCpa1.
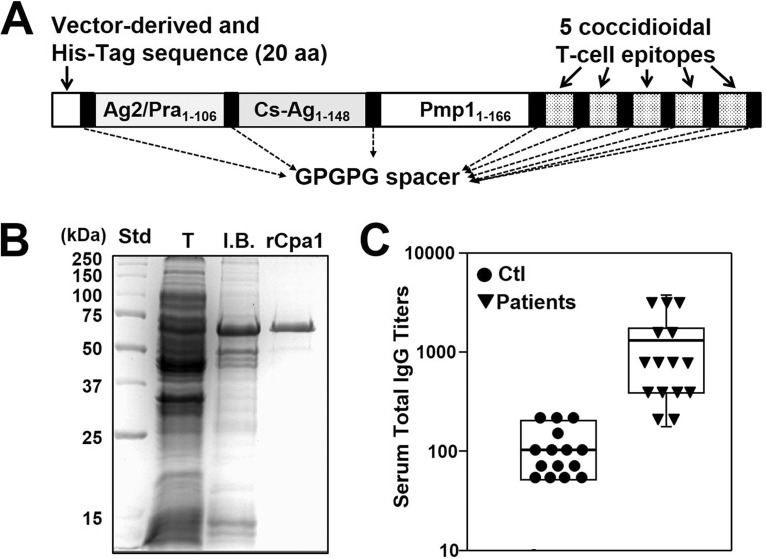
The translated amino acid sequence of the multivalent recombinant chimeric polypeptide antigen (rCpa1) was deposited in GenBank, and the accession number is given in Table 1. The C terminus of each antigenic peptide was flanked by a GPGPG spacer to avoid the processing of junctional epitopes, as shown in Fig. 1A (6, 24). The nucleotide sequence designed to encode rCpa1 was codon optimized for translation by Escherichia coli. Moderate amounts of rCpa1 were produced in the bacterial inclusion bodies, and the observed molecular mass of purified rCpa1 in the SDS-PAGE gel was 63 kDa (Fig. 1B). rCpa1 was purified to >95% homogeneity using nickel affinity chromatography, refolded, and solubilized in phosphate-buffered saline (PBS). The results of amino acid sequence analysis of rCpa1 were in agreement with the translated sequence of the rCpa1 protein. We performed enzyme-linked immunosorbent assays (ELISAs) to test the range of reactivities of Coccidioides patient sera with the purified rCpa1 protein (Fig. 1C). Sera from patients diagnosed with pulmonary Coccidioides infection exhibited significantly higher IgG reactivity with the recombinant protein than control sera (n = 15) (mean titers ± standard errors of the means [SEM], 1,160 ± 296 versus 103 ± 17; P, <0.001 by the Student t test).
TABLE 1.
NCBI accession numbers of rCpa1 and the three constituent antigens and amino acid sequences of the five human MHC-II-binding peptides derived from the Pep1, Amn1, and Plb antigens of Coccidioides posadasii isolate C735
| Antigen | Corresponding position in rCpa1 sequence (aa) | Length (aa) | NCBI accession no. or amino acid sequence of antigen |
|---|---|---|---|
| rCPA1 | 1–586 | KY883768 | |
| Ag2/Pra | 27–132 | 106 | XP_003069153 |
| Cs-Ag | 138–283 | 146 | XP_003065978 |
| Pmp | 289–454 | 166 | XP_003069274 |
| Pep1-P1 | 460–480 | 21 | MRNSILLAATVLLGCTSAKVH |
| Pep1-P2 | 486–506 | 21 | HVRALGQKYFGSLPSSQQQTV |
| Amn1-P9 | 512–531 | 20 | PAKVDVLLAQSLKLADVLKF |
| Amn1-P10 | 537–556 | 20 | NGLATTGTLVLEWTRLSDIT |
| Plb-P6 | 562–581 | 20 | TPLVVYIPNYPYTTWSNIST |
FIG 1.
Construction, expression, and antibody reactivity of the newly designed rCpa1 protein. Shown are a schematic design of the rCpa1 vaccine antigen (A) and its expression, purification (B), and antibody reactivity (C). (A) The three selected Coccidioides antigens (i.e., Ag2/Pra, Cs-Ag, and Pmp1) and five previously identified epitope peptides were linked with a glycine-proline spacer sequence (GPGPG) located at the C terminus of each peptide. The first 20-residue fragment at the N terminus of the rCpa1 protein was derived from the translated pET28b plasmid vector and includes a histidine motif for nickel affinity purification of the E. coli-expressed recombinant protein. (B) SDS-PAGE separation of the rCpa1 protein. Shown are the standard (Std) and total lysates (T) of E. coli transformed with the pET28b-CPA1 plasmid vector construct, a solubilized extract of bacterial inclusion bodies (I.B.), and nickel affinity-purified rCpa1. (C) Results of ELISAs of human control (Ctl) sera (n = 15) and sera from patients with confirmed Coccidioides infection (n = 15), each reacted with the purified rCpa1 protein. The y axis represents the levels of rCpa1-specific IgG, expressed as reactivity titers. Anti-rCpa1 serum titers are significantly different (P < 0.001) between patients with active coccidioidomycosis and controls (n = 15).
GCP-rCpa1 offered better protection of HLA-DR4 transgenic mice against coccidioidomycosis than the other vaccines tested.
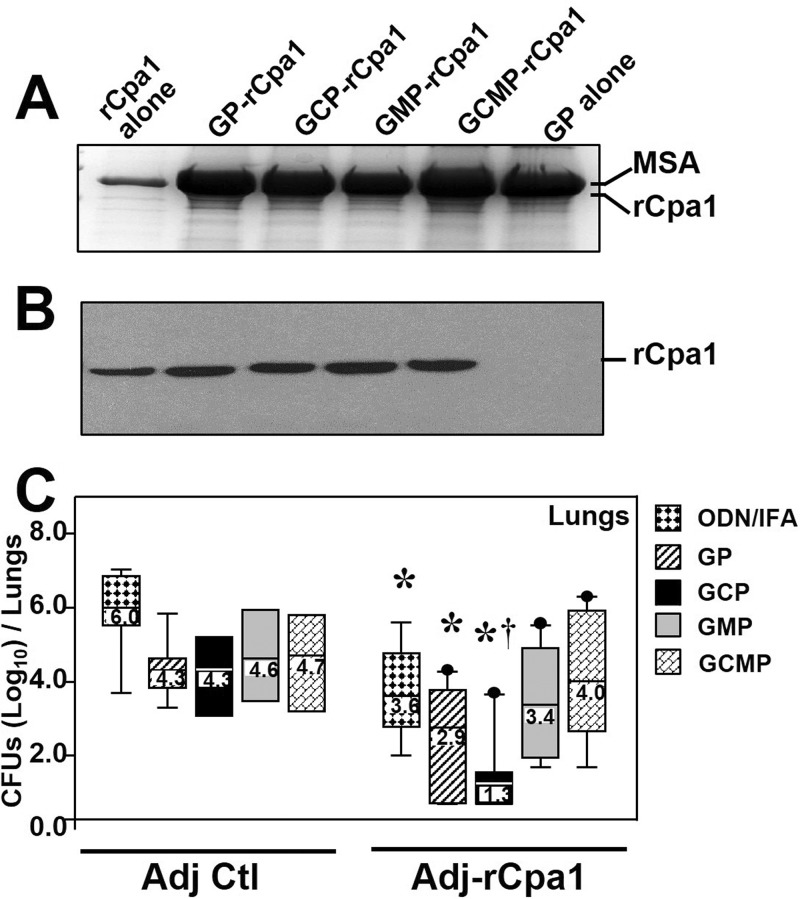
We sought to identify an effective adjuvant that could stimulate robust protective immunity against pulmonary coccidioidomycosis. We created five types of rCpa1-based vaccines and compared their protective efficacies against pulmonary coccidioidomycosis. Each dose of vaccine contained 10 μg rCpa1, 200 μg yeast tRNA, and 25 μg mouse serum albumin (MSA) loaded into 400 μg of GPs, GCPs, GMPs, or GCMPs. Yeast cell wall particles are porous and require trapping polymers (i.e., tRNA and MSA) to prevent premature leakage of the encapsulated vaccine antigen(s) before phagocytosis by antigen-presenting cells (APCs). Adjuvant controls were loaded with the same amounts of yeast tRNA and MSA. SDS-PAGE separation of the contents of the four types of vaccine particles revealed equal loading of rCpa1 and MSA, which have similar molecular weights (Fig. 2A). Western blot analysis showed that equal amounts of rCpa1 were loaded into each type of yeast cell wall particle (Fig. 2B). A vaccine containing 10 μg rCpa1 mixed with 10 μg ODN and 25 μg of MSA in 50% incomplete Freund's adjuvant (IFA) was included for comparison with the four types of vaccines containing yeast cell wall particles described above.
FIG 2.
Vaccination with GCP-rCpa1 offered the highest reduction of fungal burdens for HLA-DR4 transgenic mice. Purified rCpa1 was equally loaded into four types of yeast cell wall particles. The protein contents of GPs, GCPs, GMPs, and GCMPs loaded with rCpa1 protein (63 kDa) and murine serum albumin (MSA; 67 kDa) plus tRNA were revealed by a Coomassie brilliant blue-stained gel (A) and immunoblotting with an anti-His tag antibody (B). (A) The first gel lane contained 1 μg of the purified rCpa1 protein, and the second to fifth gel lanes were loaded with protein extracts from 10% of a dose of each vaccine preparation. A protein extract from GPs loaded only with MSA plus yeast tRNA was also analyzed (GP alone). (B) A duplicated gel was used for immunoblotting using an anti-His tag MAb. The optical densities of the first five gel lanes were comparable, indicating that equal amounts of rCpa1 protein were efficiently loaded into each type of yeast cell wall particle. (C) Coccidioides CFU counts were determined by dilution plate culture of lung homogenates obtained from mice vaccinated with rCpa1 formulated in one of the five selected adjuvants (i.e., ODN-IFA, GPs, GCPs, GMPs, or GCMPs). Control mice were immunized with each of the adjuvants alone (Adj Ctl). Lung CFU counts were determined at 14 dpc (n, 8 to 10 mice/group). An asterisk indicates a significant difference (P < 0.05) from the adjuvant control by the Mann-Whitney U test; a dagger indicates a significant difference (P < 0.05) between GCP-rCpa1 and the other four vaccines tested by the Kruskal-Wallis ranking test.
The protective efficacies of the coccidioidal vaccine candidates were evaluated using HLA-DR4 transgenic mice with antigen-binding specificity comparable to that of the HLA-DRB1*0401 haplotype. HLA-DR4 transgenic mice were vaccinated twice at a 2-week interval with one of the five rCpa1-based vaccines. Mice immunized with one of the four types of yeast cell wall particles or ODN-IFA without rCpa1 served as adjuvant controls. HLA-DR4 transgenic mice are highly susceptible to pulmonary Coccidioides infection (25). Aiming to have surviving mice for comparison of the fungal burden of all vaccinated and control mice at 14 days postchallenge (dpc), we challenged them with a low dose of Coccidioides spores (∼30 spores) that would cause a lethal infection around 18 to 20 dpc. The results revealed that GCP-rCpa1-vaccinated mice had significantly lower fungal burden in Coccidioides-infected lungs at 14 dpc than mice vaccinated with any of the other four rCpa1 vaccines tested (Fig. 2C). Mice vaccinated with GMP-rCpa1 or GCMP-rCpa1 showed a trend toward CFU counts lower than those in mice immunized with the corresponding adjuvant alone. However, the results were not statistically significant. At this time point, no dissemination of Coccidioides to extrapulmonary tissue (e.g., spleen) was detected for any of the 10 groups of mice that were challenged with a low dose of Coccidioides spores.
rCpa1 stimulated higher frequencies of Th17 cells in the spleen of both C57BL/6 and HLA-DR4 transgenic mice than each of the constituent peptides.
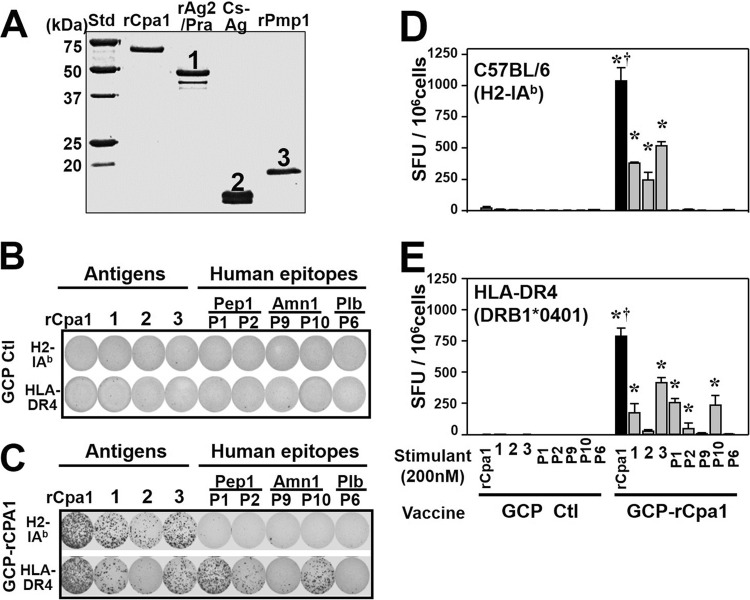
In order to further investigate which subunits of the rCpa1 protein could induce a CD4+ T-cell response, splenocytes from C57BL/6 (H2-IAb haplotype) and HLA-DR4 transgenic mice vaccinated with GCP-rCpa1 were isolated for enzyme-linked immunosorbent spot (ELISPOT) assays to measure the frequencies of IL-17A-producing cells as reported previously (26). The GCP adjuvant was selected for this assay, since it was the most effective of the adjuvants tested at reducing fungal burden, as shown in Fig. 2C. Purified rAg2/Pra and rPmp1 were obtained from transformed E. coli and S. cerevisiae expression systems, respectively, while Cs-Ag was purified from culture supernatants of Coccidioides posadasii as reported previously (10, 22, 27). The purities and concentrations of rCpa1, rAg2/Pra, Cs-Ag, and rPmp1 were confirmed by SDS-PAGE and amino acid sequence analysis (Fig. 3A). The purified rAg2/Pra showed two bands, as reported previously (22). The lower band was a C-terminally truncated form of rAg2/Pra that might occur during the expression and purification process. Purified native Cs-Ag from Coccidioides culture also showed two bands due to the difference in the truncation of the last 3 amino acids (aa) (unpublished data). The splenocytes were stimulated with 200 nM purified rCpa1 (12.2 μg/ml), described above, or each of the purified subunit antigens, which include rAg2/Pra (6.3 μg/ml), Cs-Ag (2.7 μg/ml), and rPmp1 (3.6 μg/ml), and the five synthetic peptides: Pep1-P1 (0.49 μg/ml), Pep1-P2 (0.47 μg/ml), Amn1-P9 (0.43 μg/ml), Amn1-P10 (0.43 μg/ml), and Plb-P6 (0.46 μg/ml). Splenocytes isolated from mice immunized with GCP alone did not respond to rCpa1 or any of the subunit peptides (Fig. 3B). Interestingly, rCpa1 elicited more IL-17 spot-forming units (SFU) for both C57BL/6 and HLA-DR4 transgenic mice than any of the constituent subunit peptides (Fig. 3C to E). Splenocytes of vaccinated C57BL/6 mice responded to stimulation with rAg2/Pra, Cs-Ag, and rPmp1, but not with the five synthetic peptides that contain human epitopes (Fig. 3C and D). In contrast, splenocytes isolated from vaccinated HLA-DR4 mice responded to the human epitopes P1, P2, and P10 in addition to rAg2/Pra and rPmp1 (Fig. 3C and E). These data indicate that rCpa1 could enable the induction of larger repertoires of CD4+ T cells than any of the subunit peptides.
FIG 3.
Purified rCpa1 protein stimulated a significantly higher frequency of IL-17A-producing CD4+ T cells than each of the antigen components. IL-17A-producing CD4+ T cells were assessed by ELISPOT assays. HLA-DR4 transgenic and C57BL/6 mice were vaccinated with either GCP-rCpa1 or GCP alone. Splenocytes were separately incubated with the purified rCpa1, rAg2/Pra, Cs-Ag, and rPmp1 proteins and the five synthetic peptides that contain human MHC-II binding epitopes at a concentration of 200 nM. (A) SDS-PAGE separation of 2 μg (each) of purified rCpa1, rAg2/Pra, Cs-Ag, and rPmp1, which migrated to the predicted corresponding sizes. The optical densities of the lanes were comparable, indicating that equal concentrations of the antigens could be calculated and achieved to stimulate CD4+ T cells. (B and C) Representative images of IL-17A ELISPOT wells for control (B) and vaccinated (C) C57BL/6 and HLA-DR4 transgenic mice expressing H2-IAb and HLA-DR4 haploid MHC-II molecules, respectively. (D and E) The frequencies (expressed as SFU per 106 splenocytes) of responders to individual peptides in C57BL/6 (D) and HLA-DR4 transgenic (E) mice were plotted. Asterisks indicate significant differences (P, <0.05 by the Student t test) between GCP-rCpa1 and the adjuvant control; daggers indicate significant differences (P, <0.05 by the Student-Newman-Keuls test) between rCpa1 and the eight constituent antigens tested. Data are means ± SEM (n, 4 mice/group).
Vaccination with GCP-rCpa1 stimulated an early mixed Th17 and Th1 response.
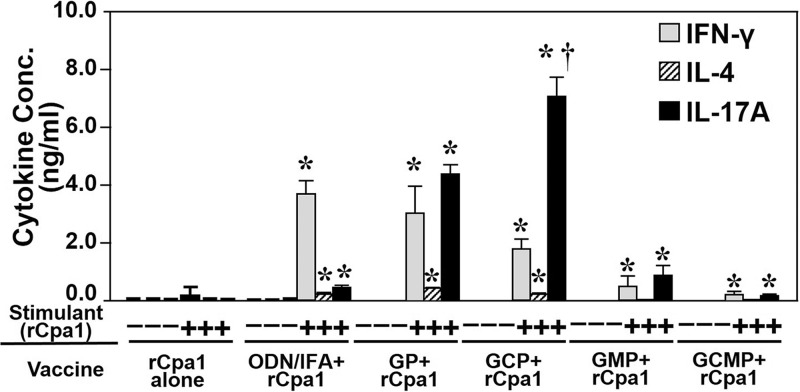
We employed a recall response assay to determine whether immune CD4+ T cells isolated from splenocytes obtained from HLA-DR4 mice vaccinated with rCpa1 plus each of the five adjuvants tested secreted greater amounts of IFN-γ, IL-4, and IL-17A than splenocytes isolated from control mice (Fig. 4). Control CD4+ T cells isolated from mice immunized with an adjuvant (data not shown) or rCpa1 alone secreted minimal amounts of these cytokines after rCpa1 stimulation (Fig. 4). In contrast, upon stimulation with rCpa1, CD4+ T cells isolated from mice vaccinated with rCpa1 formulated with ODN-IFA, GP, or GCP produced significant and comparable amounts of IFN-γ, while the GCP-rCpa1-primed CD4+ T cells secreted the largest amounts of IL-17A among these three vaccination groups (Fig. 4).
FIG 4.
GCP-rCpa1-primed splenocytes secreted significantly more IL-17A than splenocytes primed with any of the other four rCpa1-adjuvant formulations. Shown are concentrations of IFN-γ, IL-4, and IL-17A detected in culture supernatants of in vitro-stimulated immune splenocytes isolated from HLA-DR4 mice that had been subcutaneously vaccinated twice with the indicated vaccine. The stimulated cells were incubated with 10 μg/ml rCpa1 protein in the culture medium. Asterisks indicate significant differences (P, <0.001 by the Student t test) between data for stimulated (+) and nonstimulated (−) cells; the dagger indicates a significant difference (P, <0.05 by the Student-Newman-Keuls test) between GCP-rCpa1 and the other four antigen-adjuvant formulations.
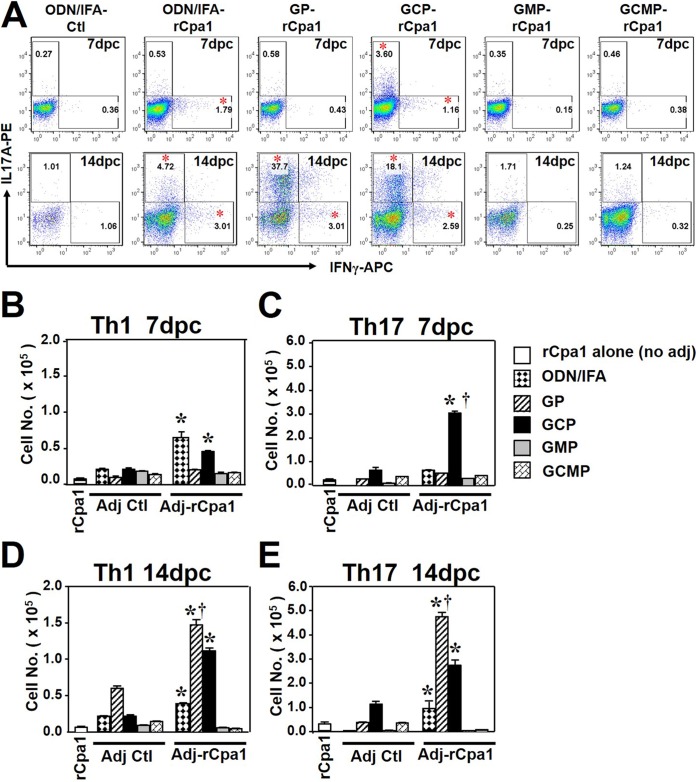
Next, we determined the numbers of IFN-γ- and IL-17A-producing CD4+ T cells that had infiltrated into the lungs of the vaccinated and control mice at 7 and 14 days postchallenge. The gating strategy for pulmonary Th1 and Th17 cells consisted of CD4+ CD8− IFN-γ+ and CD4+ CD8− IL-17A+ cells, respectively (17). At 7 dpc, the percentages and total numbers of Th1 cells in the lungs of mice vaccinated with GCP-rCpa1 or ODN-IFA-rCpa1 were significantly higher than those in the respective adjuvant controls (Fig. 5A, upper panels, and B). Interestingly, the percentages and total numbers of Th17 cells were significantly increased at 7 dpc only in the group of mice that had been vaccinated with GCP-rCpa1 (Fig. 5A, upper panels, and C). At 14 dpc, mice vaccinated with ODN-IFA-rCpa1, GP-rCpa1, or GCP-rCpa1 showed significant increases in Th1 and Th17 cells (Fig. 5A, lower panels, D, and E). Notably, mice vaccinated with GP-rCpa1 showed higher numbers of both Th1 and Th17 cells in the lungs at 14 dpc than the rest of the vaccination groups (Fig. 5D and E), indicating a delayed T-cell response that has been reported to be associated with chronic Coccidioides infection (25). In contrast, Th1 and Th17 cells were not recruited to the lungs of mice vaccinated with GMP-rCpa1 or GCMP-rCpa1. Concurrently, these two vaccines did not provide significant protection against pulmonary Coccidioides infection (Fig. 2). Collectively, these results indicated that GCP-rCpa1 stimulated an early mixed Th17 and Th1 response in the lungs of mice that had been subcutaneously (s.c.) vaccinated with GCP-rCpa1 and subjected to a pulmonary challenge with Coccidioides spores. This type of immune response was correlated with significant reductions in fungal burden at 14 days post-Coccidioides infection (Fig. 2C).
FIG 5.
HLA-DR4 transgenic mice vaccinated with GCP-rCpa1 acquired the highest numbers of Th17 cells in infected lungs at 7 dpc. (A) Flow cytometric analyses of IFN-γ- and IL-17A-expressing Th1 and Th17 cells, respectively, in the lungs of HLA-DR4 transgenic mice that had been vaccinated with rCpa1 encapsulated in each of the adjuvants tested. Mice immunized with an adjuvant alone served as controls. The percentages of gated, specific-cytokine-producing cells per lung (given in panel A) and the numbers of Th1 (CD4+ IFN-γ+) cells (B and D) and Th17 (CD4+ IL-17A+) cells (C and E) in Coccidioides-infected lungs at 7 (B and C) and 14 (D and E) dpc were determined by intracellular cytokine staining. Among the controls, representative plots are shown only for ODN-IFA (A), but the numbers of specific Th1 and Th17 cells were calculated for rCpa1 alone and for all five types of adjuvant controls (B to E). Asterisks indicate significant differences (P, <0.05 by the Student t test) from results for the adjuvant control; daggers indicate significant differences (P, <0.05 by the Student-Newman-Keuls test) between GCP-rCpa1 and the other four antigen-adjuvant formulations. Data are means ± SEM (n, 4 mice/group). The data presented are representative of two independent experiments.
The GCP-rCpa1 vaccine offered protection for both C57BL/6 and HLA-DR4 transgenic mice.
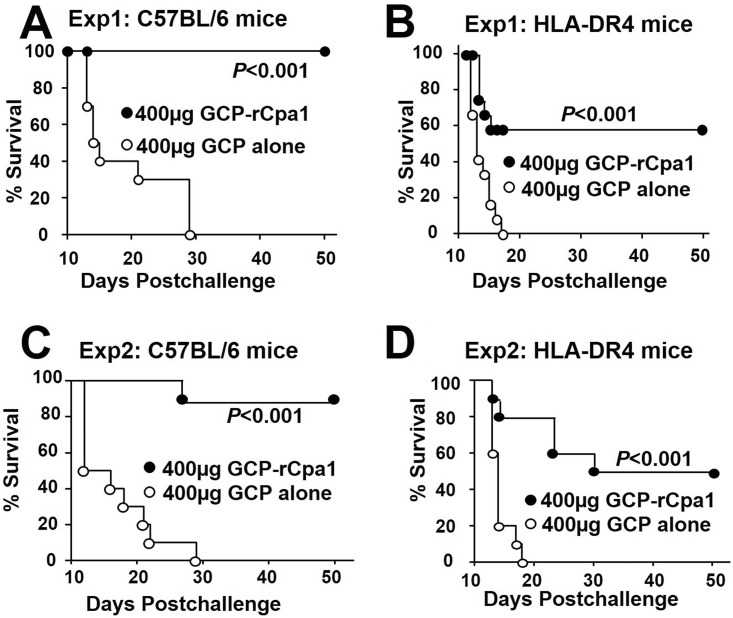
Both C57BL/6 and HLA-DR4 transgenic mice were vaccinated twice with GCP-rCpa1 via the subcutaneous route as described above and were evaluated for survival for 50 days after an intranasal challenge with a potentially lethal dose of Coccidioides spores (Fig. 6A to D). We observed that vaccination with rCpa1 alone, as with GCP alone, did not enable mice to mount a CD4+ T-cell response (Fig. 4), which was critical for protection against coccidioidomycosis; thus, only GCP was used as a negative control in this study. Two experiments showed that 90% and 100% of the GCP-rCpa1-vaccinated C57BL/6 mice survived for 50 days, while the GCP-alone control mice succumbed to coccidioidomycosis between 12 and 30 dpc (Fig. 6A and C). Similarly, 50 to 60% of vaccinated HLA-DR4 mice survived for 50 days, in contrast to no survival in the adjuvant control groups (Fig. 6B and D). The protective efficacies of the GCP-rCpa1 vaccine for C57BL/6 and HLA-DR4 mice were comparable to those of our previously reported ΔT live attenuated vaccine against pulmonary coccidioidomycosis (15, 25).
FIG 6.
The GCP-rCpa1 vaccine offered protection for both C57BL/6 and HLA-DR4 transgenic mice against pulmonary Coccidioides infection. Survival plots were determined for groups of 10 C57BL/6 (A and C) and HLA-DR4 transgenic (B and D) mice that had been vaccinated with the GCP-rCpa1 vaccine or GCP alone. Mice were challenged intranasally with 80 to 100 Coccidioides spores (P, <0.001 by Kaplan Meier survival analysis and the chi-square test). Exp, experiment.
The GCP adjuvant elicited more infiltration of the vaccination sites by Mϕs, which engulf and process the vaccine, than the GP adjuvant.
GCPs and GPs appear to be the two most effective adjuvants at stimulating a vaccine-induced Th17 response. We further compared the adjuvanticities of GCPs and GPs using comparative histopathology and imaging flow cytometry analysis of hypodermis tissue obtained from the s.c. injection sites. Mice vaccinated with GCP-rCpa1 or GP-rCpa1 showed minimal swelling and erythema at the injection sites at 2 days after injection. Coarsely visual examination revealed that the swelling could last for 3 to 6 days. Histopathological analysis of the skin biopsy specimens showed scattered aggregates of both types of vaccine particles that were visible in the centers of the hypodermis tissues and within a dense layer of inflammatory cells (Fig. 7A to H). Notably, the infiltrating inflammatory cells formed a denser layer surrounding GCP-rCpa1 than around GP-rCpa1 (Fig. 7A and C). Neutrophils (PMNs), macrophages (Mϕs), and dendritic cells (DCs) were visible in these vaccinated hypodermis areas (Fig. 7E and G).
FIG 7.
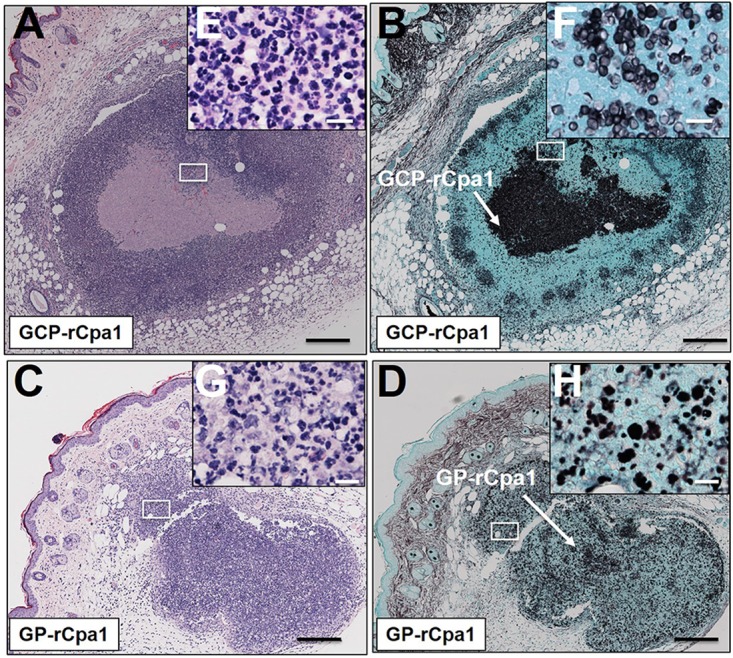
Comparison of reactogenicities of the subcutaneously administered GCP-rCpa1 and GP-rCpa1 vaccines at the injection sites of HLA-DR4 transgenic mice. (A to D) Paraffin sections of skin biopsy specimens from vaccination sites that were stained with hematoxylin and eosin (magnification, ×10) (A and C) or Gomori methenamine silver (B and D) showed moderate levels of inflammatory response at 2 days postvaccination. A denser layer of inflammatory cells surrounded GCPs than GPs, with scattered aggregates of vaccine particles (A, C, E, and G). Bars, 0.5 mm. (E to H) Higher magnifications (×40) of boxed areas in panels A to D, respectively. Bars, 200 μm. Both mononuclear monocytes and polymorphonuclear granulocytes are visible in hypodermis tissue injected with GCP-rCpa1 or GP-rCpa1 (E and G). White arrows in panels B and D indicate GCPs, GPs, and amorphous yeast cell wall materials.
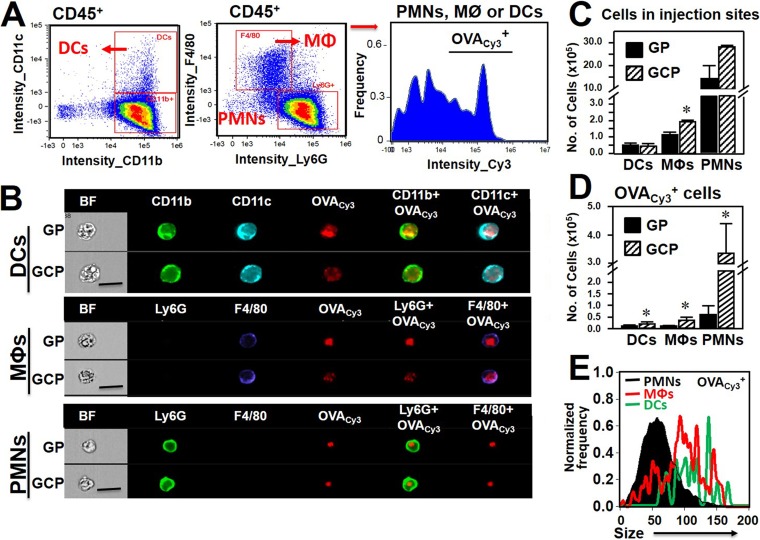
We further characterized infiltrating inflammatory cells in hypodermis tissue obtained at 2 days after injection with Cy3-labeled ovalbumin (OVACy3) encapsulated in GCPs and GPs by use of the gating strategy shown in Fig. 8A. Both GCPs and GPs were readily engulfed by PMNs, Mϕs, and DCs, which were the major infiltrating inflammatory cells in the injection sites (Fig. 8B and C). GCPs stimulated higher numbers of macrophages infiltrating the hypodermis tissue than GPs (Fig. 8C). The numbers of PMNs, Mϕs, and DCs positive for OVACy3 were significantly higher in sites injected with the antigen encapsulated in GCPs than in those injected with the antigen encapsulated in GPs (Fig. 8D). Subsequent proteolytic degradation of encapsulated antigens was evidenced by the increased sizes of OVACy3 areas in DCs and Mϕs, but not in PMNs (Fig. 8B and E). These results suggest that GCPs were better engulfed and processed by macrophages and dendritic cells than were GPs, potentially leading to an enhanced Th17 response.
FIG 8.
GCPs were better processed than GPs by macrophages and dendritic cells at the subcutaneous vaccination sites. Single-cell preparations from the excised hypodermis tissues of mice vaccinated with GCP-OVACy3 or GP-OVACy3 were analyzed using an Amnis ImageStream Mark II cytometer. (A) Numbers of DCs, Mϕs, and PMNs were determined for CD11b+ CD11c+, F4/80+ Ly6G−, and F4/80− Ly6G+ cells in the gated, live CD45+ leukocyte population. (B) Representative bright-field (BF) images, images in each fluorochrome channel, and overlaid images (CD11b + OVACy3, CD11c + OVACy3, Ly6G + OVACy3, and F4/80 + OVACy3) of DCs, Mϕs, and PMNs that were positive for OVACy3. (C) PMNs, Mϕs, and DCs were the most abundant phagocytes that infiltrated the vaccination sites. Vaccine particles were engulfed by all three types of phagocytes. GCPs induced significantly greater recruitment of Mϕs into the vaccinated sites than GPs. (D) Numbers of DCs, Mϕs, and PMNs that were positive for OVACy3 were also significantly higher in sites injected with GCP-OVACy3 than in those injected with GP-OVACy3. (E) The vaccine antigen was processed only in Mϕs and DCs, as evidenced by the fact that the OVACy3-positive areas were increased significantly in these two cell populations. The data presented are representative of three independent experiments. Asterisks indicate significant differences (P < 0.05) by the Student t test.
DISCUSSION
We describe a newly created Coccidioides vaccine consisting of a recombinant multivalent antigen (rCpa1) that is loaded into yeast glucan-chitin particles (GCPs) to enhance Th17 immunity. The results of vaccination reveal that augmented Th17 immunity is associated with improved protective efficacy for mice. The GCP-rCpa1 vaccine offers 100% and 60% survival over 50 days for C57BL/6 mice and highly susceptible HLA-DR4 transgenic mice, respectively. The protective efficacy of the GCP-rCpa1 vaccine is comparable to that of the live attenuated (ΔT) vaccine that we have reported previously (15, 25). Efforts to develop a vaccine against coccidioidomycosis started in the 1960s, when the lead vaccine candidate was formalin-killed spherules (FKS) (8, 28). The FKS vaccine conferred protection for genetically susceptible C57BL/6 and BALB/c mice and monkeys against a potentially lethal infection with Coccidioides (28, 29). However, a double-blinded clinical phase III study conducted in 1980–1983 revealed that the FKS vaccine provided only a slight, statistically insignificant reduction of coccidioidomycosis incidence in the vaccinated group from that in the placebo population (30). FKS injection caused significant local irritation in 75% of recipients and flu-like symptoms in 12% of participants, which together may have contributed to failure of the FKS clinical trial and potential use of FKS as a human vaccine (30). Subsequently, several live attenuated vaccines have been created for preventive measures against pulmonary coccidioidomycosis. These cellular vaccines include a temperature-sensitive mutant created by UV irradiation, two genetically engineered mutants lacking the expression of two chitinases (ΔT) and a mutant deficient in the expression of an acyl coenzyme A (acyl-CoA) ligase-like protein (ΔCps1) (5, 15, 31). Despite the apparent ability of live attenuated vaccines to elicit highly protective immunity in mice, they may not be safe for individuals with underlying conditions of compromised cell-mediated immune systems (7, 8).
The generation of recombinant antigens is an alternative strategy for the design of a clinically acceptable Coccidioides vaccine. The protective antigens characterized to date include Ag2/Pra, Pra2, Cs-Ag, Ure, Gel1, Pmp1, Pep1, Amn1, and Plb, which are expressed in Coccidioides parasitic cells (9–12, 22, 23, 32). Additionally, potential antigenic epitopes can be identified in silico, since the genome sequences of both Coccidioides posadasii and Coccidioides immitis are available (33). The newly created antigen rCpa1 contains the most immunogenic fragment of Ag2/Pra, Cs-Ag, Pmp1, and five peptides derived from Pep1, Amn1, and Plb that contain human epitopes (11, 18, 24). These antigens were selected because they are expressed at maximum levels during different stages of the parasitic cycle. In vivo, Coccidioides spores grow isotropically and develop into large, multinucleate parasitic cells (spherules; diameter, >80 μm). The latter undergo a process of segmentation of their cytoplasm, followed by differentiation of a multitude of endospores (approximately 200 to 300), which are still contained within the intact spherule wall. Endospores (diameter, 2 to 10 μm) are finally released when they enlarge and cause the spherule wall to rupture. Endospore release is essential for lymphogenous or hematogenous dissemination of the pathogen within the tissues of the host. The constituent antigens, Ag2/Pra, Cs-Ag, and Pmp1, are differentially upregulated during the parasitic life cycle (10, 22). Ag2/Pra is most abundant on mature spherules (34), while Cs-Ag is highly expressed on endospores (unpublished data). PLB and AMN1 transcription occurs during the initiation of the parasitic cycle, while the PEP1 gene is expressed constitutively (11). We include vaccine antigens expressed during initial spherule formation to ensure activation of the host immune response to coccidioidal infection in vaccinated mice prior to metastasis (endosporulation) of the pathogen. The inclusion of additional immunogenic antigens, which are produced during spherule rupture and endospore release, may further enhance protection. The five peptides containing human epitopes were selected for incorporation into the multivalent rCpa1 antigen on the basis of the following criteria: (i) computational prediction of promiscuous binding to the human major histocompatibility complex class II (MHC-II) receptor, (ii) demonstration of the ability to be processed by antigen-presenting cells and presented to CD4+ T cells, and (iii) the ability to bind with high affinity to human MHC-II molecules (6). We suggest that rCpa1 is a multivalent antigen capable of eliciting a broad spectrum of CD4+ T cells that recognize antigens expressed on different developmental stages of Coccidioides parasitic cells.
Indeed, we have confirmed that multivalent Coccidioides antigens can stimulate higher frequencies of Th17 cells than single recombinant protein vaccines (Fig. 3). Compelling evidence suggests that multivalent vaccines are more potent against pulmonary Coccidioides infection than single peptide vaccines (11, 12, 22). An experimental vaccine consisting of a recombinant epitope-based vaccine (rEBV) that contains only the five previously identified human epitopes mixed with various adjuvants, including GPs and ODN-IFA, singly or in combination, provided short-term protection (2 days) for C57BL/6 mice against pulmonary Coccidioides infection (6). Although the protective efficacy of rCpa1 has not been directly compared to that of Ag2/Pra, Cs-Ag, and Pmp1, our data suggest that the GCP-rCpa1 vaccine is superior to rEBV based upon its ability to prolong survival of both HLA-DR4 transgenic and C57BL/6 mice to 50 days postchallenge (60% and 100%, respectively). This protective efficacy is comparable to that of FKS and the attenuated ΔT vaccine that we created previously (15, 25).
We found that enhancement of Th17-mediated immunity by glucan-chitin particles is associated with improved vaccine protection against pulmonary Coccidioides infection. Upon in vitro stimulation with the autologous antigen, immune T cells isolated from mice vaccinated with GCP-rCpa1 secrete IFN-γ levels comparable to those from GP-rCpa1-primed T cells, but much higher IL-17A amounts. Similarly, mice vaccinated with GCP-rCpa1 recruit higher levels of Th17 cells during the first 7 days postchallenge, and contain lower fungal burden in Coccidioides-infected lungs, than mice vaccinated with GP-rCpa1. These data suggest that GCPs can stimulate mixed Th17 and Th1 immunity, which contributes to protective efficacy. Chitin is covalently bound to β-(1,3)-glucans in the yeast cell wall. GPs contain 80 to 85% β-glucan, 2 to 4% chitin, and <1% mannan, while GCPs are composed of ∼50 to 60% glucan, 20 to 30% chitin, and <1% mannan (19). Chitin is the major polysaccharide component that is different between GPs and GCPs (19, 35). While β-glucan is known as the ligand for Dectin-1 (36), recent studies propose several potential chitin recognition receptors of mice, including Toll-like receptor 2 (TLR2), fibrinogen C domain-containing protein 1 (FIBCD1), and mannose receptor, which are activated by chitin fragments isolated from shrimp shells, Aspergillus fumigatus, and Candida albicans, respectively (37–39). Shrimp chitin is reported to have proinflammatory properties by inducing IL-17 via the TLR2 signaling in murine cells (37). A recent study reveals the recognition of Aspergillus chitin via the mannose receptor on the surface of phagocytes and by nucleotide-binding oligomerization domain 2 (NOD2) and TLR9 receptors in the cytoplasm, leading to the induction of the anti-inflammatory cytokine IL-10 in mouse macrophages (39). It is speculated that differences in the chitin content or polymer length may account for the observed divergence in immune stimulation properties, driving the induction of anti- and proinflammatory cytokines. We suggest that GCPs may interact with Dectin-1 and other pattern recognition receptors that function synergistically to activate the protective T-cell response. Studies are under way to investigate the mechanisms of Th1 and Th17 stimulation associated with the GCP adjuvant/delivery system.
MATERIALS AND METHODS
Fungal strain, growth conditions, and spore preparation.
Coccidioides posadasii (C735), a virulent clinical isolate, was used in this study. The saprobic phase was grown on GYE agar (1% glucose, 0.5% yeast extract, 1.5% agar) to produce spores as reported previously (6). All culturing and preparatory procedures, which involved live C. posadasii cells, were conducted in a biosafety level 3 (BSL3) laboratory located at the University of Texas at San Antonio (UTSA).
Mouse strains.
Eight- to 10-week-old C57BL/6 mice were purchased from Charles River Laboratories. A breeding colony of HLA-DR4 (DRB1*0401) transgenic mice that express human MHC class II molecules (40) was also used in this study. The HLA-DR4 mice were genetically engineered from a C57BL/6 background and were backcrossed to MHC class II-deficient mice lacking IA and IE alleles in order to eliminate the production of endogenous murine MHC class II molecules (40). All mice were housed in a specific-pathogen-free animal facility at UTSA and were handled according to the guidelines of the Institutional Animal Care and Use Committee. Mice were transported to the animal BLS3 (ABSL3) laboratory before challenge with live Coccidioides spores.
Design, expression, and purification of the rCpa1 protein.
A single bacterially expressed recombinant Coccidioides polypeptide (rCpa1) was designed to contain the most immunogenic fragment of Ag2/Pra (aa 1 to 106; NCBI Reference Sequence Database accession no. XM_003069107), the full lengths of Cs-Ag (XM_003056932) and Pmp1 (XM_003069228), and the five most promiscuous immunodominant T-cell epitopes derived from Pep1, Amn1, and Plb (6, 11, 24). The N terminus of each antigen and epitope was flanked by a GPGPG spacer to avoid the processing of junctional epitopes (6). The upstream 20-residue segment of the protein construct is a component of the translated plasmid expression vector (pET28b; Novagen) and includes 6 histidine residues used for nickel affinity chromatographic purification of rCpa1 (6). The nucleotide sequence designed to encode rCpa1 was codon optimized for translation by Escherichia coli and was synthesized by Biomatik Corporation. The synthetic 1,701-bp sequence included a stop codon and two restriction sites (NdeI and XhoI) engineered to permit the gene to be ligated into the E. coli expression vector in the correct translation frame. rCpa1 was purified under denaturing conditions as described previously (22). To minimize endotoxin contamination, the purified rCpa1 was passed through an ActiClean Etox affinity column (Sterogene Bioseparations). The amount of residual endotoxin was determined using a Limulus amebocyte lyase kit with Pyrochrome reagent (Associates of Cape Cod). Stock solutions of rCpa1 contained approximately 0.005 endotoxin unit/μg of purified protein. Confirmation of the correct amino acid sequence of rCpa1 was performed by the Biomarker core facility at UTSA. ELISAs of the purified rCpa1 protein were conducted as described previously (41). Sera from patients with coccidioidomycosis and from healthy controls were tested for total IgG antibody reactivity with the purified rCpa1 protein. Patient sera (n = 15) were kindly provided by Craig Rundbaken (Valley Fever Clinic, Phoenix, AZ). Control sera (n = 15) were obtained from Innovative Research, Inc. Serum samples were tested in triplicate and were serially diluted 1:2 in 1% bovine serum albumin (BSA)-PBS. The wells incubated with the secondary antibody alone were used to establish the baseline. The inverse titers were determined as the fold dilution that gave an optical density significantly higher than that for the baseline.
Vaccine preparations.
Synthesized ODN (10 μg; Integrated DNA Technologies, Inc.) was solubilized in 100 μl PBS together with 10 μg rCpa1 and 25 μg mouse serum albumin (MSA) and was mixed with 100 μl of incomplete Freund's adjuvant (Sigma) for each immunization as described previously (6, 11, 23). Yeast cell well particles derived from Saccharomyces cerevisiae (GPs and GMPs) or Rhodotorula mucilaginosa (GCPs and GCMPs) were prepared as reported previously (6, 19). Each dose of vaccine contained 10 μg rCpa1, 200 μg yeast tRNA, and 25 μg MSA as a trapping matrix loaded into 400 μg of GPs, GCPs, GMPs, or GCMPs. The successful incorporation of rCpa1 into the various types of yeast cell wall particles was confirmed by SDS-PAGE followed by Western blotting with an anti-His tag antibody.
Vaccination protocol, animal challenge, and evaluation of protection.
HLA-DR4 transgenic and C57BL/6 mice were subcutaneously immunized twice in the abdominal region at a 2-week interval. Each dose of vaccine was equally divided and was administered at two sites. Mice were challenged intranasally with a suspension of 30 to 100 viable spores of C. posadasii in 35 μl of PBS 4 weeks after completion of the immunization protocol, as reported previously (6). Mice that received a corresponding adjuvant containing 200 μg yeast tRNA and 25 μg MSA were used as nonvaccinated controls. Mice were sacrificed at 14 days postchallenge for determination of the fungal burden in their lungs and spleen as described previously (6, 15, 25). Survival studies of vaccinated versus nonvaccinated mice were conducted over 50 days postchallenge as reported previously (15).
IL-17A ELISPOT assays and cytokine ELISAs.
T-cell reactivity was evaluated by IL-17A ELISPOT assays essentially as reported elsewhere (26). At 14 days after the second vaccination, spleens of vaccinated or control mice were separately harvested, pooled, and macerated for isolation of immune splenocytes and CD4+ T cells as reported previously (6). Both C57BL/6 (H2-IAb haplotype) and HLA-DR4 mice were used for these assays. Bacterially expressed rAg2/Pra, native CS-Ag, and rPmp1 were purified as described previously (10, 22, 27). The purities and concentrations of all three antigens described above, along with purified rCpa1, were analyzed by SDS-PAGE. Endotoxin was removed as described above, and the stock protein solutions containing 0.001 to 0.005 endotoxin unit/μg were used for all vaccination and immune response studies. Theses concentrations did not trigger a nonspecific response, since our assay background was lower than 10 SFU per 106 splenocytes (Fig. 3C). T lymphocytes were tested for an in vitro recall response to the four purified antigens and five synthetic peptides at a concentration of 200 nM. Cytokine assays were conducted by incubation of the immune and control splenocytes with 10 μg/ml rCpa1 for 48 h at 37°C in the presence of 5% CO2. Splenocytes incubated in growth medium alone served as negative controls. After incubation, a cocktail of protease inhibitors (EDTA free; Roche) was added to each well, and culture supernatants were collected from the centrifuged (at 11,950 × g for 10 min at 4°C) samples. The concentration of selected cytokines was determined using a Bio-Plex suspension array system (Bio-Rad) according to the manufacturer's instructions.
Fluorescence-activated cell sorting (FACS) analysis.
Total pulmonary leukocytes were isolated from vaccinated and control HLA-DR4 mice at 7 and 14 days postchallenge (4 mice per group) as reported previously (17). A standard flow cytometry methodology was employed for direct monoclonal antibody (MAb) labeling and enumeration of selected pulmonary immune T-cell phenotypes using a FACSCalibur cytometer as described previously (17). Permeabilized leukocytes were stained with a cocktail of fluorochrome-conjugated antibodies for IFN-γ, IL-17A, CD4, and CD8 molecules. Data were analyzed using FlowJo software, version 10.
Histopathology.
Mice were vaccinated with one dose of GCP-rCpa1 or GP-rCpa1 at two subcutaneous sites in their abdominal regions. Mice vaccinated with 100 μl of IFA plus 10 μg rCpa1 were used as a control. Comparative histopathology analysis was conducted with excised tissue from the subcutaneous vaccination sites at 2 days postinfection as reported previously (42). Tissue sections were stained with hematoxylin and eosin (H&E) and Grocott-Gomori's methenamine silver stain (GMS) by standard procedures. Paraffin sections were examined using a Leica DMI6000 microscope equipped with an automated Turboscan stage (Objective Imaging, Ltd.). Microscope images of tissue were acquired and were analyzed using Surveyor software (Objective Imaging) as reported previously (42).
Imaging flow cytometry analysis.
Mice were subcutaneously vaccinated with a total of 400 μg of GCPs or GPs containing Cy3-labeled ovalbumin (OVACy3), 200 μg yeast tRNA, and 25 μg MSA at two sites in their abdominal regions. Mice vaccinated with IFA plus OVACy3 were used as a control. Hypodermis tissue was excised at 2 days postvaccination, and single cells were prepared and stained with fluorochrome-labeled antibodies against F4/80, CD11b, CD11c, and Ly6G as described previously (17). Following staining, cells were analyzed with an ImageStreamX Mark II cytometer (Millipore) with a 60× objective lens at a low flow rate and high sensitivity using INSPIRE software. Immune phenotyping and phagocytosis of vaccine particles were analyzed using IDEAS software, version 6.2 (Millipore).
Statistical analyses.
A Student t test was used to analyze the differences between two treatment groups for cytokine ELISAs, ELISPOT assays, cytokine concentrations, calculations of numbers of lung-infiltrating immune cells, and percentages of specific-cytokine-producing T cells, while the Student-Newman-Keuls test, a type of analysis of variance (ANOVA) for multiple comparisons of three or more independently treated groups, was used as reported previously (43). The differences in fungal burden (expressed as CFU) between two groups were analyzed by the Mann-Whitney U test for ranking of the data (17). For the comparison of fungal burden among three or more groups of mice, the Kruskal-Wallis test, a nonparametric ranking method, was used as reported previously (17). Survival data were examined by the Kaplan-Meier test using log rank analysis to compare survival plots as reported previously (6). A P value of ≤0.05 was considered statistically significant.
Accession number(s).
The translated amino acid sequence of the multivalent recombinant chimeric polypeptide antigen (rCpa1) has been deposited in GenBank under accession number KY883768.
ACKNOWLEDGMENTS
This work was supported by a research grant from the National Institutes of Health, NIAID (R21 AI114762). Additional support was provided by Coccidioides research funds donated by the Valley Fever of the Americas Foundation and community supporters.
The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
We declare that no competing interests exist.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb Mortal Wkly Rep 62:217–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, Johnson RH, Kusne S, Lisse J, MacDonald JD, Meyerson SL, Raksin PB, Siever J, Stevens DA, Sunenshine R, Theodore N. 2016. Executive summary: 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 63:e112–e146. doi: 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 3.Thompson GR., III 2011. Pulmonary coccidioidomycosis. Semin Respir Crit Care Med 32:754–763. doi: 10.1055/s-0031-1295723. [DOI] [PubMed] [Google Scholar]
- 4.Litvintseva AP, Marsden-Haug N, Hurst S, Hill H, Gade L, Driebe EM, Ralston C, Roe C, Barker BM, Goldoft M, Keim P, Wohrle R, Thompson GR III, Engelthaler DM, Brandt ME, Chiller T. 2015. Valley fever: finding new places for an old disease: Coccidioides immitis found in Washington state soil associated with recent human infection. Clin Infect Dis 60:e1–e3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narra HP, Shubitz LF, Mandel MA, Trinh HT, Griffin K, Buntzman AS, Frelinger JA, Galgiani JN, Orbach MJ. 2016. A Coccidioides posadasii CPS1 deletion mutant is avirulent and protects mice from lethal infection. Infect Immun 84:3007–3016. doi: 10.1128/IAI.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. 2012. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect Immun 80:3960–3974. doi: 10.1128/IAI.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole GT, Hurtgen BJ, Hung CY. 2012. Progress toward a human vaccine against coccidioidomycosis. Curr Fungal Infect Rep 6:235–244. doi: 10.1007/s12281-012-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkland TN. 2016. The quest for a vaccine against coccidioidomycosis: a neglected disease of the Americas. J Fungi (Basel) 2:E34. doi: 10.3390/jof2040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, Herr RA, Yu JJ, Hung CY. 2004. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol 42:189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 10.Orsborn KI, Shubitz LF, Peng T, Kellner EM, Orbach MJ, Haynes PA, Galgiani JN. 2006. Protein expression profiling of Coccidioides posadasii by two-dimensional differential in-gel electrophoresis and evaluation of a newly recognized peroxisomal matrix protein as a recombinant vaccine candidate. Infect Immun 74:1865–1872. doi: 10.1128/IAI.74.3.1865-1872.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarcha EJ, Basrur V, Hung CY, Gardner MJ, Cole GT. 2006. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect Immun 74:5802–5813. doi: 10.1128/IAI.00961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shubitz LF, Yu JJ, Hung CY, Kirkland TN, Peng T, Perrill R, Simons J, Xue J, Herr RA, Cole GT, Galgiani JN. 2006. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine 24:5904–5911. doi: 10.1016/j.vaccine.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Azmi F, Ahmad Fuaad AA, Skwarczynski M, Toth I. 2014. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccin Immunother 10:778–796. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitz SM, Golenbock DT. 2012. Beyond empiricism: informing vaccine development through innate immunity research. Cell 148:1284–1292. doi: 10.1016/j.cell.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun 77:3196–3208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wüthrich M, Hung CY, Gern BH, Pick-Jacobs JC, Galles KJ, Filutowicz HI, Cole GT, Klein BS. 2011. A TCR transgenic mouse reactive with multiple systemic dimorphic fungi. J Immunol 187:1421–1431. doi: 10.4049/jimmunol.1100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung CY, Gonzalez A, Wüthrich M, Klein BS, Cole GT. 2011. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect Immun 79:4511–4522. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung CY, Hurtgen BJ, Bellecourt M, Sanderson SD, Morgan EL, Cole GT. 2012. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine 30:4681–4690. doi: 10.1016/j.vaccine.2012.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young SH, Ostroff GR, Zeidler-Erdely PC, Roberts JR, Antonini JM, Castranova V. 2007. A comparison of the pulmonary inflammatory potential of different components of yeast cell wall. J Toxicol Environ Health A 70:1116–11124. doi: 10.1080/15287390701212224. [DOI] [PubMed] [Google Scholar]
- 20.Weitberg AB. 2008. A phase I/II trial of β-(1,3)/(1,6) d-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J Exp Clin Cancer Res 27:40. doi: 10.1186/1756-9966-27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, Specht CA, Levitz SM. 2012. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J Immunol 189:312–317. doi: 10.4049/jimmunol.1200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herr RA, Hung CY, Cole GT. 2007. Evaluation of two homologous proline-rich proteins of Coccidioides posadasii as candidate vaccines against coccidioidomycosis. Infect Immun 75:5777–5787. doi: 10.1128/IAI.00807-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K, Yu JJ, Hung CY, Lehmann PF, Cole GT. 2001. Recombinant urease and urease DNA of Coccidioides immitis elicit an immunoprotective response against coccidioidomycosis in mice. Infect Immun 69:2878–2887. doi: 10.1128/IAI.69.5.2878-2887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurtgen BJ, Hung CY. 2017. Rational design of T lymphocyte epitope-based vaccines against Coccidioides infection. Methods Mol Biol 1625:45–64. doi: 10.1007/978-1-4939-7104-6_4. [DOI] [PubMed] [Google Scholar]
- 25.Hurtgen BJ, Castro-Lopez N, Jimenez-Alzate MDP, Cole GT, Hung CY. 2016. Preclinical identification of vaccine induced protective correlates in human leukocyte antigen expressing transgenic mice infected with Coccidioides posadasii. Vaccine 34:5336–5343. doi: 10.1016/j.vaccine.2016.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji N, Kovalovsky A, Fingerle-Rowson G, Guentzel MN, Forsthuber TG. 2015. Macrophage migration inhibitory factor promotes resistance to glucocorticoid treatment in EAE. Neurol Neuroimmunol Neuroinflamm 2:e139. doi: 10.1212/NXI.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan S, Cole GT. 1995. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect Immun 63:3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappagianis D. 1967. Histopathologic response of mice to killed vaccines of Coccidioides immitis. J Invest Dermatol 49:71–77. doi: 10.1038/jid.1967.105. [DOI] [PubMed] [Google Scholar]
- 29.Levine HB, Pappagianis D, Cobb JM. 1970. Development of vaccines for coccidioidomycosis. Mycopathol Mycol Appl 41:177–185. doi: 10.1007/BF02051493. [DOI] [PubMed] [Google Scholar]
- 30.Pappagianis D. 1993. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis 148:656–660. doi: 10.1164/ajrccm/148.3.656. [DOI] [PubMed] [Google Scholar]
- 31.Kirkland TN, Fierer J. 1983. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun 40:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abuodeh RO, Shubitz LF, Siegel E, Snyder S, Peng T, Orsborn KI, Brummer E, Stevens DA, Galgiani JN. 1999. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect Immun 67:2935–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neafsey DE, Barker BM, Sharpton TJ, Stajich JE, Park DJ, Whiston E, Hung CY, McMahan C, White J, Sykes S, Heiman D, Young S, Zeng Q, Abouelleil A, Aftuck L, Bessette D, Brown A, FitzGerald M, Lui A, Macdonald JP, Priest M, Orbach MJ, Galgiani JN, Kirkland TN, Cole GT, Birren BW, Henn MR, Taylor JW, Rounsley SD. 2010. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res 20:938–946. doi: 10.1101/gr.103911.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galgiani JN, Sun SH, Dugger KO, Ampel NM, Grace GG, Harrison J, Wieden MA. 1992. An arthroconidial-spherule antigen of Coccidioides immitis: differential expression during in vitro fungal development and evidence for humoral response in humans after infection or vaccination. Infect Immun. 60:2627–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gow NAR, Latge JP, Munro CA. 2017. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 5(3). doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. 2008. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol 181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlosser A, Thomsen T, Moeller JB, Nielsen O, Tornøe I, Mollenhauer J, Moestrup SK, Holmskov U. 2009. Characterization of FIBCD1 as an acetyl group-binding receptor that binds chitin. J Immunol 183:3800–3809. doi: 10.4049/jimmunol.0901526. [DOI] [PubMed] [Google Scholar]
- 39.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. 2014. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog 10:e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, Falcioni F, Vidovic D, Hammer J, Nagy ZA. 1996. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med 183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue J, Hung CY, Yu JJ, Cole GT. 2005. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine 23:3535–3544. doi: 10.1016/j.vaccine.2005.01.147. [DOI] [PubMed] [Google Scholar]
- 42.Hung CY, Castro-Lopez N, Cole GT. 2016. Card9- and MyD88-mediated gamma interferon and nitric oxide production is essential for resistance to subcutaneous Coccidioides posadasii infection. Infect Immun 84:1166–1175. doi: 10.1128/IAI.01066-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hung CY, del Pilar Jiménez-Alzate M, Gonzalez A, Wüthrich M, Klein BS, Cole GT. 2014. Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect Immun 82:2106–2114. doi: 10.1128/IAI.01579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]