Pharyngeal tonsillitis is one of the most common upper respiratory tract infections, and group A streptococcus is the most important bacterial pathogen causing it. While most patients experience tonsillitis only rarely, a subset of patients suffers from recurrent or chronic tonsillitis or pharyngitis.
KEYWORDS: group A streptococcus, psoriasis, tonsillitis
ABSTRACT
Pharyngeal tonsillitis is one of the most common upper respiratory tract infections, and group A streptococcus is the most important bacterial pathogen causing it. While most patients experience tonsillitis only rarely, a subset of patients suffers from recurrent or chronic tonsillitis or pharyngitis. The predisposing factors for recurring or chronic forms of this disease are not yet fully understood, but genetic predisposition has been suggested. A genetic association study using Illumina's Immunochip single-nucleotide polymorphism (SNP) array was performed to search for new genetic biomarkers in pharyngeal tonsillitis. More than 100,000 SNPs relevant to immune-mediated diseases were analyzed in a cohort of 95 patients subjected to tonsillectomy due to recurrent/chronic tonsillitis and 504 controls. Genetic association between the cases and controls showed strongest association with two peaks in the HLA locus (odds ratio [OR], 3.7 to 4.7; P = 4.9 × 10−6 to 5.7 × 10−6). Further analysis with imputed classical HLA alleles suggested the known psoriasis risk allele HLA-C*06:02 as a risk factor for tonsillitis (P = 4.8 × 10−4; OR, 2.3). In addition, the imputed HLA haplotype HLA-C*06:02/HLA-B*57:01, a reported risk haplotype in psoriasis, had the strongest risk for tonsillitis (P = 3.2 × 10−4; OR, 6.5). These findings further support the previously reported link between streptococcal throat infections and psoriasis.
INTRODUCTION
Pharyngeal tonsillitis is a common disease characterized by inflammation of the posterior pharynx and tonsils. It can be caused by several bacterial species and viruses. Bacterial tonsillitis is most often caused by Streptococcus pyogenes, also called group A streptococcus (GAS), and in a number of cases by group G or C streptococci. GAS is the clinically most important bacterial pathogen in pharyngeal tonsillitis, not only due to the high number of cases but also because of its important link to postinfectious sequelae, such as poststreptococcal glomerulonephritis and acute rheumatic fever (1). To avoid these complications, tonsillitis is considered to require relatively rapid diagnosis and treatment with antibiotics. Rapid antigen detection tests or bacterial culture is used to detect GAS from throat swab (2). According to population studies, approximately 2.5% of asymptomatic individuals may be chronic carriers of GAS in the pharynx (3). In acute tonsillitis, penicillin is the primary treatment of choice. Tonsillectomy, the operative removal of pharyngeal tonsils, is used to remove an existing bacterial reservoir and the site of infection from patients with tonsillar hypertrophy or recurrent/chronic tonsillitis (4, 5).
The reasons why some individuals are more susceptible to recurrent or chronic tonsillitis are unclear. To date, environmental factors, such as recurrent exposure to the pathogen, carrier state, and differences in host immune functions, have been suggested (6). In addition, it is not known why some individuals are prone to be asymptomatic carriers (7, 8). Susceptibility to tonsillitis has been suggested to have a genetic component due to reported evidence of a substantial genetic predisposition for this disease (9). There is also evidence that polymorphism in two host immunity-associated genes, TLR4A and CFH, is associated with GAS tonsillitis (10, 11).
Several reports have shown that streptococcal throat infections may precede exacerbation of chronic plaque psoriasis as well as acute guttate psoriasis. In severe psoriasis cases, patients benefit from early antimicrobial treatment of streptococcal throat infections or tonsillectomy, suggesting a causal link (12–14). The major histocompatibility complex (MHC) region contains a risk allele(s) for psoriasis, with HLA-C*06:02 being the most widely replicated association (15–17). The prevalence of a pharyngeal GAS culture positivity has been shown to be higher among the HLA-C*06:02-positive than the HLA-C*06:02-negative psoriasis patients (18).
To explore the role of genetic variation in susceptibility of human host to tonsillitis, we performed a case-control association study using Illumina's Immunochip single-nucleotide polymorphism (SNP) array. We used clinically well-characterized cases with chronic or recurrent tonsillitis and observed that the HLA-C*06:02 allele was more common in the tonsillitis group than the control group, with an odds ratio (OR) of 2.3 (P = 4.8 × 10−4). This indicates that tonsillitis and psoriasis share the same risk allele and may partially explain why patients with psoriasis develop tonsillitis much more frequently than nonpsoriatic individuals (12).
RESULTS
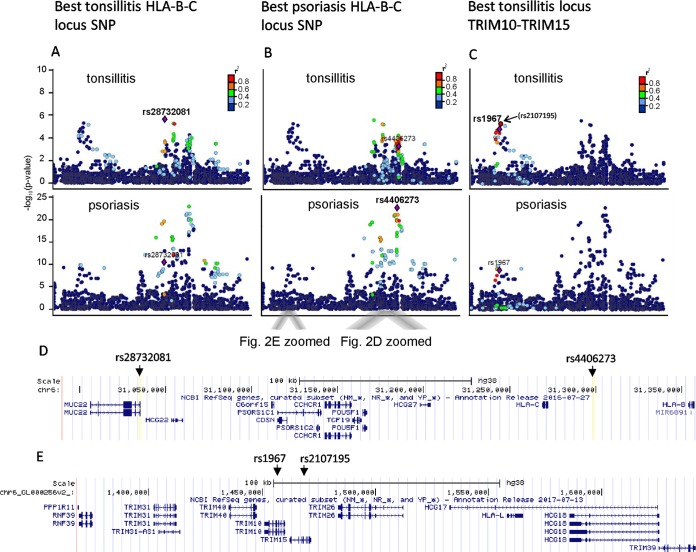
Immunochip genotyping and association analysis among 95 Finnish tonsillitis patients and 504 controls showed strongest association on chromosome 6 at the MHC locus at two separate regions, one near HLA-C and HLA-B at 31 to 31.5 Mb (rs28732081; OR, 4.7; P = 5.7 × 10−6) and the other near genes TRIM10 and TRIM15 at 30.2 Mb (rs2107195; OR, 3.7; P = 4.9 × 10−6) (Fig. 1).
FIG 1.
Immunochip genotyping and association analysis. (A) Manhattan plot of the GWAS. Negative log10 P values for tonsillitis association across all autosomes. The strongest association signals were identified at 31 to 31.5 Mb on chromosome 6 (rs28732081; OR, 4.7; P = 5.7 × 10−6). (B) Manhattan plot of the negative log10 P values on chromosome 6. The major histocompatibility complex locus at 29 to 32 Mb on chromosome 6 is highlighted in green. Blue line, suggestive association; red line, genome-wide association.
Association analysis of the imputed HLA alleles suggested HLA-B*57:01 (P = 2.5 × 10−5; OR, 6.2) and HLA-C*06:02 (P = 4.8 × 10−4; OR, 2.3) as risk factors for tonsillitis (Table 1). Haplotype analysis pointed toward HLA-C*06:02/HLA-B*57:01 as the haplotype conferring the strongest risk for tonsillitis (P = 3.2 × 10−4; OR, 6.5) (Table 2).
TABLE 1.
Tonsillitis association results of imputed classical HLA alleles with minor allele frequency of >0.01 in cases and P < 0.05a
| HLA allele | F_A | F_U | P value | OR (95% CI) |
|---|---|---|---|---|
| B*57:01 | 0.047 | 0.008 (0.013) | 2.49E−05 | 6.2 (2.4–16.3) |
| C*06:02 | 0.142 | 0.067 (0.062) | 4.78E−04 | 2.3 (1.4–3.7) |
| B*14:02 | 0.011 | 0.000 (0.005) | 1.11E−03 | NA |
| C*08:02 | 0.011 | 0.000 (0.006) | 1.11E−03 | NA |
| DRB1*01:02 | 0.011 | 0.000 (0.004) | 1.11E−03 | NA |
| A*30:01 | 0.016 | 0.002 (0.003) | 6.78E−03 | 8.1 (1.3–48.6) |
| DRB1*04:05 | 0.016 | 0.002 (0.001) | 6.78E−03 | 8.1 (1.3–48.6) |
| B*37:01 | 0.032 | 0.009 (0.008) | 0.01 | 3.6 (1.3–10.3) |
| DPB1*19:01 | 0.016 | 0.003 (0.004) | 0.02 | 5.4 (1.1–26.8) |
| DRB1*10:01 | 0.026 | 0.008 (0.010) | 0.02 | 3.4 (1.1–10.4) |
| DQA1*01:01 | 0.263 | 0.194 (0.177) | 0.03 | 1.5 (1.0–2.1) |
F_A, allele frequency in cases; F_U, allele frequency in controls (values are from reference 42); OR, odds ratio; CI, confidence interval.
TABLE 2.
Haplotype association analysis of the HLA*C-06:02|HLA*B-57:01 haplotype in tonsillitis and psoriasisa
| Haplotype and condition | F_A | F_U | OR (95% CI) | P value |
|---|---|---|---|---|
| HLA*C-06:02|HLA*B-57:01 | ||||
| Tonsillitis | 0.04737 | 0.0079 | 6.488 (2.44; 17.3) | 3.25E−04 |
| Psoriasis | 0.07362 | 10.71 (4.71; 24.4) | 5.72E−10 | |
| HLA*C-06:02|HLA*B-13:02 | ||||
| Tonsillitis | 0.04737 | 0.0387 | 1.248 (0.583; 2.67) | 0.576 |
| Psoriasis | 0.1319 | 4.123 (2.56; 6.63) | 6.5E−09 |
F_A, allele frequency in cases; F_U, allele frequency in controls; OR, odds ratio; CI, confidence interval.
As HLA-C*06:02 is a known risk allele also for psoriasis, an autoimmune condition associated with tonsillitis, we next reanalyzed a data set of 163 Finnish psoriasis patients from whom both Immunochip data and HLA-C genotype data, determined with traditional methods, were available (19). Imputed HLA alleles matched with classical typing, which confirmed the imputation accuracy for the HLA-C locus. Comparison of the tonsillitis and psoriasis association data at the HLA locus showed that the same two association peaks at ∼30 to 31.5 Mb were associated with both diseases, although with slightly different leading SNPs (Fig. 2). Association analysis of the imputed HLA alleles suggested that the same HLA-B and -C alleles and haplotypes increase the risk of both diseases (Table 2).
FIG 2.
Regional association plots of the major histocompatibility complex (MHC) region on chromosome 6 in tonsillitis and psoriasis. The association P values are plotted against their physical position. The SNPs are colored according to their level of linkage disequilibrium (LD), with the index SNP indicated with a purple diamond. The LD measure r2 is calculated from the Hg19/1000 Genomes Nov 2014 EUR data (http://www.internationalgenome.org/1000-genomes-browsers). The genes contained in this locus are shown in the lower images. (A) SNPs in LD with the rs28732081 variant associated with tonsillitis mapping to HLA-B and HLA-C region are indicated in both diseases. (B) The same region for the SNP rs4406273 showing strongest association with psoriasis. (C) SNPs in LD within the second association, with peak mapping to the TRIM10-TRIM15 gene locus, are shown. The best SNP in tonsillitis rs2107195 (in brackets) in this region was not present in psoriasis data for comparison, and thus the next best rs1967 present in both data sets is shown as the leading SNP. (D and E) Gene-level zoom-ins of positions of these 4 SNPs.
DISCUSSION
Various theories have been presented to explain the recurrence and microbiological treatment failures in tonsillitis. One important finding is the correlation between recurrent tonsillitis and the presence of the bacterial reservoir in tonsillar tissue. GAS, the most important bacterial pathogen causing tonsillitis, has been reported to be able to invade epithelial cells (20). The intracellular survival of the bacterium and poor penetration of penicillin, the primary drug of choice, into the epithelial cells may explain treatment failures and the recurrence of tonsillitis at least in some patients. GAS is also a common finding in throat cultures of children with tonsillar hypertrophy, indicating that chronic enlargement of tonsils promotes a pathogenic bacterial reservoir (5).
Tonsillar hyperplasia is also a frequent finding among patients with recurrent tonsillitis. A clear correlation observed between tonsillar hyperplasia and increased bacterial load or increased B- and T-lymphocyte proliferation suggests that GAS is able to induce proliferation of immune cells, thereby explaining the mechanism for tonsillar hypertrophy in children (20). Therefore, the abnormal immune response in the tonsils caused by the bacterium might explain the increase in tonsillar size and persistence of the infection. This is concordant with a recent study that shows induction of epidermal hyperplasia by streptococcal extracts via activation of skin-associated memory T cells (21). In that work the hyperplasia-associated T cells expressed cutaneous lymphocyte-associated antigen (CLA), and such cells have also been shown to be overrepresented in tonsils of psoriasis patients (22). This indicates that effector T cells generated in the tonsils could migrate through circulation to the skin and thereby be involved in the pathogenesis of psoriasis. The same study also shows that psoriasis tonsils have unique histological characteristics that distinguish them from other tonsils. These characteristics include smaller lymphoid follicles, lower ratios of germinal center to marginal-zone area, and fewer tingible body macrophages per unit area than tonsils from individuals without psoriasis. These histological and immunological similarities between the two diseases might explain why streptococcal infections are more common in psoriatic than healthy individuals.
The molecular mechanisms by which streptococcal tonsillitis can trigger the onset and exacerbation of psoriasis have been explained by the molecular mimicry between bacterial and human antigens. The streptococcal M protein is structurally related to epithelial keratins and shares extensive amino acid homology with keratins 16 and 17, which are not present in normal epidermis but are markedly upregulated in psoriatic lesions (23, 24). As a consequence, the M-protein-primed T cells might recognize the atypical K16 and K17 keratin epitopes via molecular mimicry (25).
The previous studies, however, do not fully explain the possible heritability of recurrent tonsillitis. Recently, common variants in the HORMAD2 gene on chromosome 22 were associated with tonsillectomy in a study of 1,464 patients who had a tonsillectomy for any clinical reason (26). This gene also showed some weak evidence of association in our study population (P = 0.007), where the patient selection criteria were based only on infection-related tonsillectomy. The data presented here show that the HLA locus is significantly associated with tonsillitis, with two independent association peaks. The strongest SNP association (rs2873201) was at 31.0 Mb in the intron of the MUC22 gene close to PSORS1 and HLA-C and -B loci, with several other SNPs in linkage disequilibrium (LD) within this locus. A second association peak with only weak LD with the MUC22 locus was seen within the TRIM10-TRIM15 locus at 20.1 Mb, having the strongest association with rs2107195. TRIM family proteins are known to be involved in the regulation of inflammatory and innate immune signaling (27), and a nearby locus with the TRIM39 gene has been associated with Behcet's disease (28), an autoimmune disease also known to be triggered by streptococcal infections (29).
As classical HLA genes are associated with the risk of numerous inflammatory or autoimmune diseases, we analyzed the association of imputed classical HLA class I and II genes with tonsillitis. The results suggested that HLA-C*06:02 is a risk allele for susceptibility to chronic/recurrent tonsillitis, with an OR of 2.3 (P = 4.8 × 10−4). The frequency of HLA-C*06:02 was 14% in the tonsillitis cases and 6.7% in the controls. Haplotype analysis pointed toward HLA-C*06:02/HLA-B*57:01 as the haplotype conferring the strongest risk for tonsillitis, with an OR of 6.5. This haplotype was present in 4.7% of the case chromosomes and only 0.8% of the controls. These data indicate that the presence of the HLA-C*06:02 allele increases the risk of the individual for tonsillitis, and the risk is particularly high with the specific haplotype HLA-C*06:02/HLA-B*57:01. Comparison of the imputed HLA data in tonsillitis and a set of Finnish psoriasis patients showed that the same alleles and haplotypes were associated with both diseases. Reanalysis of the MHC region SNPs also showed that the two independent association peaks in these two conditions overlapped. Because of the small patient sample size, it is possible that the results do not represent distribution of the whole population, and a larger sample size would increase the significance of the study. The revealed tonsillitis loci, however, are also in line with other psoriasis reports on various independent signals in the MHC region (30, 31). As shown by others, the presence of the HLA-C*06:02 allele not only affects the frequency of the bacterial load in asymptomatic individuals but also, as seen in our clinical study population (Table 3), correlates with a clinical tonsillar disease (18). A prospective study of 28 tonsillectomy patients also points to association of HLA-C*06:02 with streptococcal throat infections and suggests that the degree of clinical improvement after tonsillectomy is associated with HLA-C*06:02 homozygocity (32). The finding that psoriasis and streptococcal tonsillitis share the same risk allele is not unexpected, because streptococcal M protein is known to induce activation of the skin-homing CLA+ CD8+ T cells that are found in psoriasis skin lesions at high frequencies (25). These cells express the polymorphic HLA class I antigen HLA-C, where the psoriasis-associated HLA-C*06:02 allele seems to affect the phenotype of the disease (33). In conclusion, our study suggests a shared HLA association between streptococcal tonsillitis and psoriasis. This may point to a role of HLA-C in pathogenesis of streptococcal tonsillitis on the molecular level and might, to some extent, explain why tonsillectomy can have a beneficial effect in both recurrent tonsillitis and psoriasis. These results outline the need for molecular-level studies on HLA-C in the pathogenesis of streptococcal tonsillitis.
TABLE 3.
Streptococcal culture results at the time of patient recruitment (admission due to tonsillectomy)
| Microbea (n = 95) | No. of culturesb taken from patients with: |
||
|---|---|---|---|
| Hyperplasia (n = 35) | Chronic tonsillitis (n = 45) | Recurrent tonsillitis (n = 15) | |
| GAS (n = 25) | 16 (3) | 5 (2) | 4 (1) |
| GBS (n = 2) | 2 | 0 | 0 |
| GCS (n = 3) | 1 | 2 (1) | 0 |
| GGS (n = 4) | 3 (1) | 1 | 0 |
| Strep− (n = 61) | 13 (3) | 37 (14) | 11 (2) |
GAS, GBS, GCS, and GGS, group A, B, C, and G streptococci, respectively; Strep−, streptococcal culture negative.
Values in parentheses indicate the numbers of HLA-C*06:02-positive results.
MATERIALS AND METHODS
Study populations.
Study populations consisted of 95 Finnish tonsillitis patients and 504 control subjects. The tonsillitis cases were pediatric patients referred to Helsinki University Central Hospital for scheduled tonsillectomy due to chronic or recurrent tonsillitis and/or pharyngitis. From the total of 214 tonsillectomy patients, we included only patients with recurrent tonsillitis (at least six episodes/year or three episodes/year for two consecutive years, with at least one positive culture for GAS), prolonged tonsillar infection refractory to antimicrobial therapy, or symptomatic tonsillar hyperplasia (34). At the time of recruitment, i.e., the admission due to tonsillectomy, a total of 24 out of 95 patients had GAS in the streptococcal throat swab culture (Table 3). The study on the tonsillitis patients was approved by the Ethical Review Board of the Hospital District of Helsinki and Uusimaa (decision 647/E9/01). Written informed consent was provided by the study participants and/or their legal guardians. Immunochip genotype data from 504 Finnish controls from a DILGOM cohort of over 5,000 adult Finns were available for the study as unselected random population controls. The genotype data have been deposited at the European Genome-Phenome Archive (EGA), which is hosted by the EBI and the CRG, under accession number EGAS00001003069. The control data can be found from the Finnish National Institute for Health and Welfare biobank upon request.
Genotyping and genetic analysis.
Blood samples were drawn from each patient at the time of the tonsillectomy at the Clinic of Otolaryngology, Head and Neck Surgery, Helsinki University Hospital, Helsinki, Finland. Genomic DNA was isolated from blood leukocytes using a QIAamp blood kit (Qiagen, Austria) and Puregene kit (Gentra Systems, MN, USA). The samples were genotyped at the Department of Medical Sciences, Molecular Medicine, Uppsala University Hospital, Uppsala, Sweden, with an Illumina Infinium Immunochip (Illumina, Inc., San Diego, CA) according to Illumina's protocols. Immunochip is a high-density SNP genotyping array that consists of 196,524 variants selected from previous findings from genome-wide association studies (GWAS) of immunological diseases (35). A total of 504 control samples from the DILGOM/FINRISKI 2007 cohort were previously genotyped with Immunochip (36).
The quality control and association analyses of the Immunochip data were performed with PLINK v1.07 (37). The tonsillitis data were combined with the control data, and only autosomes were included. The following quality control filters were applied: minor allele frequency (MAF) of >0.01 in the cases and controls, SNP call rate of >0.95 in the cases and controls, and Hardy-Weinberg equilibrium (HWE) of >0.05 in controls. The total genotyping call rate of the individuals was 0.998. A total of 113,424 variants passed the quality control and were included in the subsequent analyses. Genome-wide pairwise identity-by-state (IBS) values were calculated to control relatedness and duplicate samples, which were not observed. Multidimensional scaling plots based on the first two components of the IBS distances and a quantile-quantile plot of observed versus expected distribution of association P values were produced to detect population outliers and stratification (Fig. 3). The allelic association P values were adjusted for a genomic inflation factor of 1.08187 based on median chi-squared test. Five single SNPs (rs10435889 upstream of C5, rs72664814 intronic to PPP2R3C, rs1950122 intronic to LRRTM4, rs56359726 upstream of C5orf47, and rs9261313 upstream of RNF39) on different chromosomes showed the lowest association P values (<3.9 × 10−7). They were removed as likely genotyping artifacts, as no other nearby SNPs in LD with them were associated, and they also showed potentially false association with two other patient cohorts with other conditions genotyped in the same round (conditions not reported in this paper). The quantile-quantile plot and the Manhattan plots were created using the qqman package (38) in R, v.3.0.2. The regional association plots were generated with LocusZoom (39).
FIG 3.
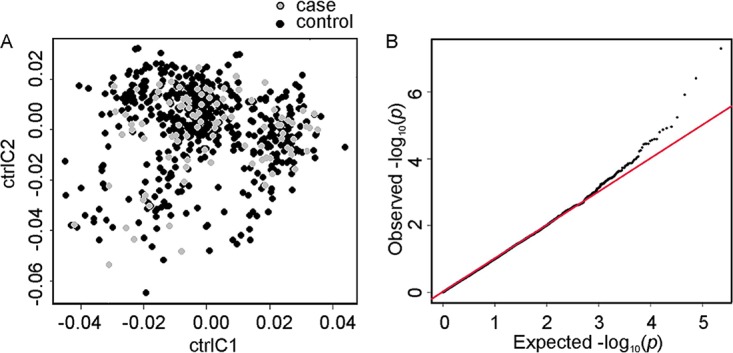
Statistical analysis. (A) Multidimensional scaling plot based on the first two components, C1 and C2, of identity by state values, indicating that the case and control groups are ethnically matched. (B) Quantile-quantile plot of observed versus expected distribution of association P values.
The HLA genotypes were imputed from the Immunochip data with SNP2HLA V1.0.3 using the T1DGC reference panel (40). Classical alleles and amino acid polymorphisms were imputed for HLA-A, -B, -C, DPA1, DPB1, DQA1, DQB1, and DRB1.
Previously determined Immunochip genotypes of 163 Finnish patients with psoriasis (19) were available for the comparison of HLA haplotypes carried by patients with tonsillitis and psoriasis. As the psoriasis patient samples were also previously genotyped for HLA-C by traditional methods (41), this data set was used to validate the SNP2HLA imputation accuracy. All HLA-C*06:02 alleles were correctly detected by imputation. In order to compare the association at the MHC locus in tonsillitis and psoriasis, the psoriasis Immunochip data were passed through a quality control procedure similar to that for the tonsillitis data and analyzed for association at the MHC locus using the DILGOM/FINRISKI controls with SNP2HLA-imputed HLA alleles. The strand assignment was assessed when combining the data sets and flipped when required. Totals of 14,915/128,923 A/T and C/G SNPs were removed from the data.
The haplotype association analyses were performed using a likelihood ratio test, and specific haplotypes were tested against all others using the −chap option in PLINK v1.07 (37).
Accession number(s).
The genotype data have been deposited at the European Genome-Phenome Archive (EGA), which is hosted by the EBI and the CRG, under accession number EGAS00001003069.
ACKNOWLEDGMENTS
This study makes use of data generated by the DILGOM. We thank the late Ulpu Saarialho-Kere for leading the Finnish psoriasis patient collection.
This work was financially supported by Academy of Finland grants 255985, 255922, 255636, 128646, and 259793, Finnish Cultural Foundation grants 00131060 and 00142390, and grants from the Sigrid Jusélius Foundation.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisno AL. 1996. Acute pharyngitis: etiology and diagnosis. Pediatrics 97:949–954. [PubMed] [Google Scholar]
- 3.Pichichero ME, Marsocci SM, Murphy ML, Hoeger W, Green JL, Sorrento A. 1999. Incidence of streptococcal carriers in private pediatric practice. Arch Pediatr Adolesc Med 153:624–628. doi: 10.1001/archpedi.153.6.624. [DOI] [PubMed] [Google Scholar]
- 4.Brook I, Shah K. 2001. Bacteriology of adenoids and tonsils in children with recurrent adenotonsillitis. Ann Otol Rhinol Laryngol 110:844–848. doi: 10.1177/000348940111000908. [DOI] [PubMed] [Google Scholar]
- 5.Roberts AL, Connolly KL, Kirse DJ, Evans AK, Poehling KA, Peters TR, Reid SD. 2012. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr 12:3. doi: 10.1186/1471-2431-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichichero ME, Casey JR. 2007. Systematic review of factors contributing to penicillin treatment failure in Streptococcus pyogenes pharyngitis. Otolaryngol Head Neck Surg 137:851–857. doi: 10.1016/j.otohns.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Courtney HS, Hasty DL, Dale JB. 2002. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann Med 34:77–87. doi: 10.1080/07853890252953464. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh N, Leonard E, Martin JM. 2010. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126:e557–e564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 9.Kvestad E, Kvaerner KJ, Roysamb E, Tambs K, Harris JR, Magnus P. 2005. Heritability of recurrent tonsillitis. Arch Otolaryngol Head Neck Surg 131:383–387. doi: 10.1001/archotol.131.5.383. [DOI] [PubMed] [Google Scholar]
- 10.Haapasalo K, Vuopio J, Syrjanen J, Suvilehto J, Massinen S, Karppelin M, Jarvela I, Meri S, Kere J, Jokiranta TS. 2012. Acquisition of complement factor H is important for pathogenesis of Streptococcus pyogenes infections: evidence from bacterial in vitro survival and human genetic association. J Immunol 188:426–435. doi: 10.4049/jimmunol.1102545. [DOI] [PubMed] [Google Scholar]
- 11.Liadaki K, Petinaki E, Skoulakis C, Tsirevelou P, Klapsa D, Germenis AE, Speletas M. 2011. Toll-like receptor 4 gene (TLR4), but not TLR2, polymorphisms modify the risk of tonsillar disease due to Streptococcus pyogenes and Haemophilus influenzae. Clin Vaccine Immunol 18:217–222. doi: 10.1128/CVI.00460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. 2003. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol 149:530–534. doi: 10.1046/j.1365-2133.2003.05552.x. [DOI] [PubMed] [Google Scholar]
- 13.Wardrop P, Weller R, Marais J, Kavanagh G. 1998. Tonsillitis and chronic psoriasis. Clin Otolaryngol Allied Sci 23:67–68. doi: 10.1046/j.1365-2273.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 14.Telfer NR, Chalmers RJ, Whale K, Colman G. 1992. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol 128:39–42. [PubMed] [Google Scholar]
- 15.Russell TJ, Schultes LM, Kuban DJ. 1972. Histocompatibility (HL-A) antigens associated with psoriasis. N Engl J Med 287:738–740. doi: 10.1056/NEJM197210122871503. [DOI] [PubMed] [Google Scholar]
- 16.Tiilikainen A, Lassus A, Karvonen J, Vartiainen P, Julin M. 1980. Psoriasis and HLA-Cw6. Br J Dermatol 102:179–184. doi: 10.1111/j.1365-2133.1980.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 17.Mallon E, Newson R, Bunker CB. 1999. HLA-Cw6 and the genetic predisposition to psoriasis: a meta-analysis of published serologic studies. J Investig Dermatol 113:693–695. doi: 10.1046/j.1523-1747.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 18.Mallbris L, Wolk K, Sanchez F, Stahle M. 2009. HLA-Cw*0602 associates with a twofold higher prevalence of positive streptococcal throat swab at the onset of psoriasis: a case control study. BMC Dermatol 9:5. doi: 10.1186/1471-5945-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, Kang HM, Allen MH, McManus R, Novelli G, Samuelsson L, Schalkwijk J, Stahle M, Burden AD, Smith CH, Cork MJ, Estivill X, Bowcock AM, Krueger GG, Weger W, Worthington J, Tazi-Ahnini R, Nestle FO, Hayday A, Hoffmann P, Winkelmann J, Wijmenga C, Langford C, Edkins S, Andrews R, Blackburn H, Strange A, Band G, Pearson RD, Vukcevic D, Spencer CC, Deloukas P, Mrowietz U, Schreiber S, Weidinger S, Koks S, Kingo K, Esko T, Metspalu A, Lim HW, Voorhees JJ, Weichenthal M, Wichmann HE, Chandran V, Rosen CF, Rahman P, Gladman DD, Griffiths CE, Reis A, Kere J, Collaborative Association Study of Psoriasis (CASP), Genetic Analysis of Psoriasis Consortium, Psoriasis Association Genetics Extension, Wellcome Trust Case Control Consortium 2, Nair RP, Franke A, Barker JN, Abecasis GR, Elder JT, Trembath RC. 2012. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterlund A, Popa R, Nikkila T, Scheynius A, Engstrand L. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107:640–647. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Ferran M, Galvan AB, Rincon C, Romeu ER, Sacrista M, Barboza E, Gimenez-Arnau A, Celada A, Pujol RM, Santamaria-Babi LF. 2013. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Investig Dermatol 133:999–1007. doi: 10.1038/jid.2012.418. [DOI] [PubMed] [Google Scholar]
- 22.Sigurdardottir SL, Thorleifsdottir RH, Valdimarsson H, Johnston A. 2013. The association of sore throat and psoriasis might be explained by histologically distinctive tonsils and increased expression of skin-homing molecules by tonsil T cells. Clin Exp Immunol 174:139–151. doi: 10.1111/cei.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. 1995. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol 133:501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 24.Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L. 1995. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol Today 16:145–149. doi: 10.1016/0167-5699(95)80132-4. [DOI] [PubMed] [Google Scholar]
- 25.Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. 2004. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin Exp Immunol 138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feenstra B, Bager P, Liu X, Hjalgrim H, Nohr EA, Hougaard DM, Geller F, Melbye M. 2017. Genome-wide association study identifies variants in HORMAD2 associated with tonsillectomy. J Med Genet 54:358–364. doi: 10.1136/jmedgenet-2016-104304. [DOI] [PubMed] [Google Scholar]
- 27.Uchil PD, Hinz A, Siegel S, Coenen-Stass A, Pertel T, Luban J, Mothes W. 2013. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J Virol 87:257–272. doi: 10.1128/JVI.01804-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurata R, Nakaoka H, Tajima A, Hosomichi K, Shiina T, Meguro A, Mizuki N, Ohono S, Inoue I, Inoko H. 2010. TRIM39 and RNF39 are associated with Behcet's disease independently of HLA-B *51 and -A *26. Biochem Biophys Res Commun 401:533–537. doi: 10.1016/j.bbrc.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 29.Galeone M, Colucci R, D'Erme AM, Moretti S, Lotti T. 2012. Potential infectious etiology of Behcet's disease. Patholog Res Int 2012:595380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight J, Spain SL, Capon F, Hayday A, Nestle FO, Clop A, Wellcome Trust Case Control Consortium, Genetic Analysis of Psoriasis Consortium, I-chip for Psoriasis Consortium, Barker JN, Weale ME, Trembath RC. 2012. Conditional analysis identifies three novel major histocompatibility complex loci associated with psoriasis. Hum Mol Genet 21:5185–5192. doi: 10.1093/hmg/dds344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setsirichok D, Tienboon P, Jaroonruang N, Kittichaijaroen S, Wongseree W, Piroonratana T, Usavanarong T, Limwongse C, Aporntewan C, Phadoongsidhi M, Chaiyaratana N. 2013. An omnibus permutation test on ensembles of two-locus analyses can detect pure epistasis and genetic heterogeneity in genome-wide association studies. Springerplus 2:230. doi: 10.1186/2193-1801-2-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Petersen H, Sigurdsson MI, Gudjonsson JE, Johnston A, Valdimarsson H. 2016. HLA-Cw6 homozygosity in plaque psoriasis is associated with streptococcal throat infections and pronounced improvement after tonsillectomy: a prospective case series. J Am Acad Dermatol 75:889–896. doi: 10.1016/j.jaad.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gudjonsson JE, Karason A, Runarsdottir EH, Antonsdottir AA, Hauksson VB, Jonsson HH, Gulcher J, Stefansson K, Valdimarsson H. 2006. Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients–an analysis of 1019 HLA-C- and HLA-B-typed patients. J Investig Dermatol 126:740–745. doi: 10.1038/sj.jid.5700118. [DOI] [PubMed] [Google Scholar]
- 34.Suvilehto J, Jarva H, Seppanen M, Siljander T, Vuopio-Varkila J, Meri S. 2008. Binding of complement regulators factor H and C4b binding protein to group A streptococcal strains isolated from tonsillar tissue and blood. Microbes Infect 10:757–763. doi: 10.1016/j.micinf.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Trynka G, Hunt KA, Bockett NA, Romanos J, Mistry V, Szperl A, Bakker SF, Bardella MT, Bhaw-Rosun L, Castillejo G, de la Concha EG, de Almeida RC, Dias KR, van Diemen CC, Dubois PC, Duerr RH, Edkins S, Franke L, Fransen K, Gutierrez J, Heap GA, Hrdlickova B, Hunt S, Plaza Izurieta L, Izzo V, Joosten LA, Langford C, Mazzilli MC, Mein CA, Midah V, Mitrovic M, Mora B, Morelli M, Nutland S, Nunez C, Onengut-Gumuscu S, Pearce K, Platteel M, Polanco I, Potter S, Ribes-Koninckx C, Ricano-Ponce I, Rich SS, Rybak A, Santiago JL, Senapati S, Sood A, Szajewska H, Troncone R, Varade J, Wallace C, Wolters VM, Zhernakova A, Spanish Consortium on the Genetics of Coeliac D, Prevent CDSG, Wellcome Trust Case Control C, Thelma BK, Cukrowska B, Urcelay E, Bilbao JR, Mearin ML, Barisani D, Barrett JC, Plagnol V, Deloukas P, Wijmenga C, van Heel DA. 2011. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet 43:1193–1201. doi: 10.1038/ng.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inouye M, Silander K, Hamalainen E, Salomaa V, Harald K, Jousilahti P, Mannisto S, Eriksson JG, Saarela J, Ripatti S, Perola M, van Ommen GJ, Taskinen MR, Palotie A, Dermitzakis ET, Peltonen L. 2010. An immune response network associated with blood lipid levels. PLoS Genet 6:e1001113. doi: 10.1371/journal.pgen.1001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner SD. 2014. qqman: an R package for visualizing GWAS results using Q-Q and Manhattan plots. bioRxiv doi: 10.1101/005165. [DOI]
- 39.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. 2010. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia X, Han B, Onengut-Gumuscu S, Chen WM, Concannon PJ, Rich SS, Raychaudhuri S, de Bakker PI. 2013. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One 8:e64683. doi: 10.1371/journal.pone.0064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suomela S, Kainu K, Onkamo P, Tiala I, Himberg J, Koskinen L, Snellman E, Karvonen SL, Karvonen J, Uurasmaa T, Reunala T, Kivikas K, Jansen CT, Holopainen P, Elomaa O, Kere J, Saarialho-Kere U. 2007. Clinical associations of the risk alleles of HLA-Cw6 and CCHCR1*WWCC in psoriasis. Acta Derm Venereol 87:127–134. doi: 10.2340/00015555-0184. [DOI] [PubMed] [Google Scholar]
- 42.Haimila K, Perasaari J, Linjama T, Koskela S, Saarinenl T, Lauronen J, Auvinen MK, Jaatinen T. 2013. HLA antigen, allele and haplotype frequencies and their use in virtual panel reactive antigen calculations in the Finnish population. Tissue Antigens 81:35–43. doi: 10.1111/tan.12036. [DOI] [PubMed] [Google Scholar]