Staphylococcus epidermidis is a leading cause of infections associated with indwelling medical devices, including prosthetic joint infection. While biofilm formation is assumed to be the main mechanism underlying the chronic infections S. epidermidis causes, we hypothesized that S. epidermidis also evades immune killing, contributing to its pathogenesis.
KEYWORDS: fibroblast, intracellular infection, osteoblast, Staphylococcus epidermidis
ABSTRACT
Staphylococcus epidermidis is a leading cause of infections associated with indwelling medical devices, including prosthetic joint infection. While biofilm formation is assumed to be the main mechanism underlying the chronic infections S. epidermidis causes, we hypothesized that S. epidermidis also evades immune killing, contributing to its pathogenesis. Here, we show that prosthetic joint-associated S. epidermidis isolates can persist intracellularly within human fibroblasts and inside human and mouse osteoblasts. We also show that the intracellularly persisting bacteria reside primarily within acidic phagolysosomes and that over the course of infection, small-colony variants are selected for. Moreover, upon eukaryotic cell death, these bacteria, which can outlive their host, can escape into the extracellular environment, providing them an opportunity to form biofilms on implant surfaces at delayed time points in implant-associated infection. In summary, the acidic phagolysosomes of fibroblasts and osteoblasts serve as reservoirs for chronic or delayed S. epidermidis infection.
INTRODUCTION
Staphylococcus epidermidis is a generally nonvirulent Gram-positive bacterium which is a member of the normal human skin microbiota. Similar to many gut bacteria, this commensal organism typically serves a beneficial role, educating the immune system and allowing immune cells to keep the potentially harmful microbiota in check while not mounting an inflammatory response (1–5). As an example, S. epidermidis produces phenol-soluble modulin (PSM) peptides, present on the surface of the human skin (1), which have antimicrobial activity against some pathogenic bacteria, including Staphylococcus aureus and Streptococcus pyogenes (6). In addition, S. epidermidis stimulates keratinocytes to produce antimicrobial peptides which inhibit the growth of S. aureus and S. pyogenes (2). Despite its normally constructive role, with the advent of implantation of medical devices, S. epidermidis has emerged as an important foreign body-associated pathogen and is now, for example, one of the leading causes of prosthetic joint infection (PJI) (7–11). Although S. epidermidis infection does not frequently lead to death, the chronic infection it causes contributes to a high economic burden and morbidity (8–12). In 2015, an estimated 1.3 million hip and knee replacement procedures were performed in the United States, with numbers estimated to double by 2020 (13). Infection rates have held steady at 2 to 2.4% and lead to an annual cost projected to reach 1.62 billion dollars by 2020 (13). Together, S. aureus and S. epidermidis are the causative agents for more than half of all PJI cases. Given the steadily increasing numbers of joint arthroplasty surgeries being performed, and accordingly the number of associated infections, it is important to define their pathogenesis.
Many specific mechanisms of immune evasion have been described for S. aureus, including the production of several factors that inhibit complement activation, compromise the activity of neutrophils and macrophages, lyse neutrophils, neutralize antimicrobial peptides (AMPs), and allow for both survival in and escape from phagosomes (12, 14–23). In contrast, there is less known about S. epidermidis pathogenicity. S. epidermidis does not express many of the virulence factors that S. aureus does, and biofilm formation has been traditionally considered its primary mechanism of pathogenesis when it establishes implant-associated infection (10, 24, 25). In addition to serving as a barrier, biofilm formation has also been shown to decrease deposition of C3b and IgG, both of which help facilitate phagocytosis and killing by polymorphonuclear leukocytes (PMNs), on bacterial surfaces (26). Moreover, polysaccharide intercellular adhesin (PIA), extracellular matrix-binding protein (Embp), and accumulation-associated protein (Aap), found on the surface of S. epidermidis bacteria, play roles in intercellular adhesion (27–29) and protect biofilms from macrophage phagocytosis (30).
Although existing in a biofilm may enable S. epidermidis to escape the host immune system and remain relatively undetected, this is unlikely to be the sole reason that this organism is such a prominent cause of implant-associated infections, because many bacteria are capable of biofilm formation. In addition, if biofilm formation were the only mechanism involved in infection, implant removal might be expected to be universally curative. In this regard, immune evasion may be operative. Factors found to play roles in immune evasion by S. epidermidis include its aps and sepA loci. The former senses the presence of human AMPs, while the latter destroys them (31). Further, the staphylococcal quorum sensing system, accessory gene regulator (agr), has been suggested to play a role in resistance to PMN killing and resistance to the PMN antimicrobial products (26, 32). agr regulates many colonization and virulence factors; one category of virulence factors upregulated by agr is PSMs. PSMs are amphipathic peptides produced by most staphylococci that have been demonstrated to play important roles in S. aureus infection (33) by lysing neutrophils, altering dendritic cell function, and skewing T cell priming (1, 14, 17, 31, 34, 35). Although S. epidermidis has the capacity to produce cytolytic PSMs, it produces such low levels of these peptides that it has little to no ability to lyse PMNs (31).
We hypothesized there to be an additional mechanism that S. epidermidis uses to cause chronic infection. Based on the demonstration that S. aureus can invade and persist in a myriad of cell types (36–47), we hypothesized that S. epidermidis also evades immune killing by persisting within nonimmune cells located around implants. Previously it has been reported that a reference strain not associated with human disease and two S. epidermidis strains isolated from peritonitis and osteomyelitis cases can be internalized in MG63 bone cells (48). In addition, we have shown that clinical S. epidermidis PJI isolates can be internalized by and persist within human fibroblasts (49).
The aim of this study was to further investigate intracellular persistence as another means, besides biofilm formation, that S. epidermidis may employ to escape innate immune killing and cause chronic infection. As several cell types, including fibroblasts and osteoblasts, can be exposed to pathogens in device-related infections, we studied the interactions of these cells with S. epidermidis. We evaluated the ability of clinical S. epidermidis PJI isolates to persist intracellularly and to escape host cells using in vitro cell culture infection models, flow cytometry, fluorescence microscopy, and spectrometry.
RESULTS
S. epidermidis invasion of and persistence in fibroblasts and osteoblasts in vitro.
Planktonic S. epidermidis clinical isolates are readily killed by human neutrophils (see Fig. S1 in the supplemental material), suggesting that some form of evasion of this killing is needed to allow for chronic infection. To test the hypothesis that S. epidermidis can invade and persist within nonprofessional phagocytic cells normally found at the site of joint arthroplasty, a lysostaphin/daptomycin protection assay was used to assay intracellular infection. Fibroblasts, commonly found at the site of implants due to their role in wound healing, and osteoblasts were examined. S. aureus strain 6850, previously shown to persist intracellularly, was used as a positive control. Two hours postinfection (p.i.), S. epidermidis strain RP62A and PJI isolates IDRL-8933 and IDRL-8864 were found to invade all host cell types tested (Fig. 1A). Differences were observed in the numbers of intracellular bacteria taken up by each cell type (Fig. 1A), with mouse osteoblasts taking up more S. aureus 6850, RP62A, and IDRL-8933 bacteria than the other cell types at 2 h p.i. There were no significant differences in the numbers of bacteria recovered for each strain at days 0 and 3 p.i. in human fibroblasts (Fig. 1A). Conversely, in primary human osteoblasts, the numbers of CFU recovered were lower for S. aureus 6850 and the two PJI isolates (IDRL-8933 and IDRL-8864) at day 3 day p.i. than at day 0 (P < 0.002, P < 0.0035, and P < 0.0023, respectively), suggesting microbicidal activity of human osteoblasts (Fig. 1A). Similarly, in mouse osteoblasts, the numbers of CFU recovered were lower for S. aureus 6850 and the two PJI isolates at day 3 day p.i. than at day 0 (P < 0.01, P < 0.0055, and P < 0.0022, respectively) (Fig. 1A).
FIG 1.

Intracellular persistence of S. epidermidis in nonprofessional phagocytes. S. aureus strain 6850, S. epidermidis strain RP62A, and two S. epidermidis prosthetic joint infection isolates, IDRL-8933 and IDRL-8864, were used to infect MRC5 (human fibroblasts), NHOst (normal human osteoblasts), and MC3T3 (murine osteoblasts) at a multiplicity of infection of 25 for 2 h, after which lysostaphin (25 μg/ml) or daptomycin (100 μg/ml) was added and intracellular bacterial growth was monitored at 0, 3, 5, 7, and 10 days postinfection (p.i.). (A) CFU recovered from each host cell type 2 h p.i. and 3 days p.i. *, P < 0.05, **, P < 0.01, ***, P < 0.001. (B to G) CFU recovered from infected human fibroblasts (B), human osteoblasts (C), and mouse osteoblasts (D) at indicated time points, and percentages of viable human fibroblasts (E), human osteoblasts (F), and mouse osteoblasts (G) over the course of infection. Data are mean values (±standard deviations of the means) from at least three independent experiments.
Next, the persistence of each strain in each of the cell types was examined. The two strains isolated from sepsis-related infections, S. aureus 6850 and S. epidermidis RP62A, were able to survive intracellularly for at least 10 days after infection (Fig. 1B to D). Although the two PJI isolates were also able to persist in human fibroblasts and mouse osteoblasts for the duration of the experiment, by day 5 p.i., no viable bacteria were detected by quantitative or qualitative culture in human osteoblasts. A similar but delayed trend was also seen during infection of mouse osteoblasts. To evaluate for differences in cytotoxicity among the strains tested, trypan blue exclusion was used to assess host cell death. At 2 h p.i., each strain tested induced less than 20% cell death (Fig. 1E to G), with at least 50% of the population persisting throughout the course of infection.
Neither agr nor PSMs are required for invasion or survival during intracellular infection.
To assess whether agr or PSMs are required for invasion and intracellular persistence, a PSMβ deletion mutant and an agr deletion mutant, and their corresponding wild-type (wt) strains, 1457 and Tü3298, respectively, were used to infect human fibroblasts (Fig. S2A). Each of the S. epidermidis strains was able to invade and persist for at least 7 days p.i., and there were no significant differences in the numbers of viable CFU recovered between the wt and deletion mutant pairs over the course of infection. There were also no differences in associated host cell death (Fig. S2B).
Lack of difference in uptake and intracellular persistence of S. aureus acute- and chronic-infection PJI isolates.
Resch et al. have shown that there are differences in gene expression between S. aureus planktonic and biofilm cells. They demonstrated upregulation of genes encoding toxins in planktonic cells and upregulation of genes associated with the stress response and cell surface proteins in biofilm cells (50). As it has been suggested that planktonic S. aureus cells are associated with acute infections, whereas biofilm cells are associated with chronic infections, we studied one S. aureus isolate each associated with chronic and acute PJI to examine for differences in invasiveness between the two isolates upon infection of mouse osteoblasts. IDRL-9072 (chronic PJI associated) and IDRL-11537 (acute PJI associated) invaded and persisted in osteoblasts for at least 7 days p.i., at similar levels as S. aureus 6850, inducing <40% cell host death over the course of infection (Fig. S2C and D).
Intracellular persistence is accompanied by increased numbers of SCVs.
Small-colony variants (SCVs) are slow-growing subpopulations that naturally arise in response to various environmental pressures (51–53). They normally comprise a minor fraction of the source population and are commonly described in chronic S. aureus infections (54–58). Some SCV observations have also been described for S. epidermidis, mostly in association with foreign-body-related infections, such as PJI (8, 59–64). Previously, we have shown that intracellular localization induces S. epidermidis SCV formation in human lung fibroblasts (49). To determine if these findings extend to intracellular persistence in other cell types, we assessed colony phenotypes of viable bacteria after an overnight incubation on sheep blood agar (SBA) at 37°C, followed by an additional incubation overnight at room temperature. SCVs were identified on the basis of their size (Fig. 2A). At 2 h p.i., an average of 3, 24, and 7% of all viable intracellularly localized bacteria had a SCV phenotype after infection in human fibroblasts, human osteoblasts, and mouse osteoblasts, respectively (Fig. 2B to D). While the number of viable intracellularly persisting bacteria remained fairly constant over the course of infection, the frequency of SCVs increased in the intracellular environment, reaching an average of 24% after 7 days in the human fibroblasts (Fig. 2B). Although the number of viable persisting bacteria declined over time in the human and mouse osteoblasts, the frequency of SCVs continued to increase over time, reaching an average of 19 and 25% after 10 days in the human osteoblasts and mouse osteoblasts, respectively (Fig. 2C and D). The drop observed in the average percentage of SCVs in the human osteoblasts was due to the death of the two PJI isolates.
FIG 2.

Small-colony variant (SCV) formation increases over time during intracellular persistence. Colony phenotypes of viable bacteria were assessed after an overnight incubation on sheep blood agar at 37°C, followed by an additional incubation overnight at room temperature. SCVs were identified on the basis of their size. (A) Representative images of colony phenotype of each strain; SCVs are denoted by arrowheads. (B to D) Percentages of viable intracellularly persisting bacteria with SCV phenotype in human fibroblasts (B), human osteoblasts (C), and mouse osteoblasts (D) over the course of infection. Data are mean values (±standard deviations of the means) from two independent experiments performed in triplicate.
Next, to assess whether SCVs have an advantage in intracellular invasiveness, we compared the numbers of CFU recovered after infection with two normal-colony-phenotype (NCP) isolates and their SCV counterparts recovered from chronic knee and shoulder PJI. There were no statistically significant differences in the numbers of recovered bacteria between the NCP and SCV pairs at 2 h p.i. (Fig. S2C), suggesting that SCVs do not have an increased ability to invade cells in comparison to NCP isolates, as previously shown for one S. aureus NCP and SCV pair (65).
Intracellularly persisting S. epidermidis bacteria colocalize with host cell phagolysosomes.
Previously, we have shown that S. epidermidis RP62A is located within the phagolysosomes of human fibroblasts (49). Here, we aimed to confirm that these findings are not limited to a single strain and to evaluate whether intracellularly persisting S. epidermidis bacteria are also located in phagolysosomes within human osteoblasts. CFSE (carboxyfluorescein succinimidyl ester)-labeled RP62A colocalized with LAMP-2+ vesicles in both fixed human fibroblasts and osteoblasts (Fig. 3A and B). Similarly, S. epidermidis 1457-green fluorescent protein (GFP) colocalized with phagolysosomes in live cells (Fig. 3C and D). Colocalizations were supported by analysis of Manders' coefficient. Manders' coefficient M1 denotes the fraction of phagolysosomes that colocalize with bacteria, whereas Manders' coefficient M2 denotes the fraction of bacteria that colocalize with phagolysosomes. Because there is an excess number of phagolysosomes compared to bacteria, Manders' coefficient M1 may be skewed, falsely indicating low colocalization. Thus, we ascribed increased significance to Manders' coefficient M2 in terms of quantifying colocalization. The average Manders' coefficient M2 values for the two S. epidermidis isolates with the two different phagolysosomal stains were all ≥0.9, suggesting that intracellularly persisting bacteria are located within phagolysosomes in both human fibroblasts and osteoblasts (Fig. 3E). These findings were similar for live cells infected with the USA300 strain LAC expressing GFP (LAC-GFP) 3 h p.i. (Fig. S3).
FIG 3.
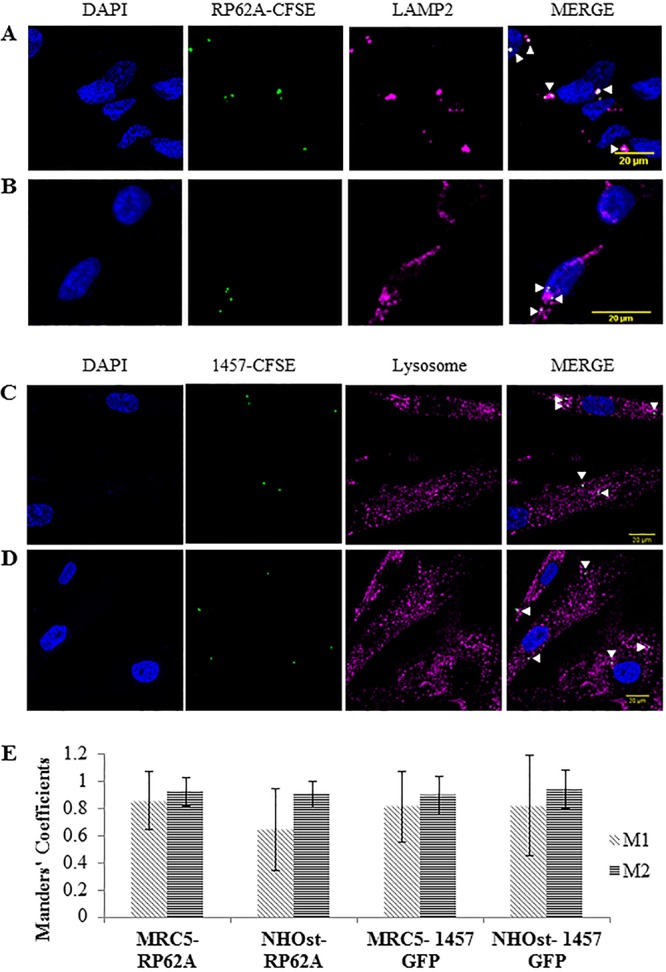
Intracellularly persisting S. epidermidis bacteria are located within phagolysosomes in both human fibroblasts and human osteoblasts. Host cells were infected with CFSE-labeled S. epidermidis RP62A or S. epidermidis 1457 expressing superfolder green fluorescent protein (sGFP) (multiplicity of infection of 50). Fixed cell phagolysosomes were visualized with LAMP-2 antibody (Alexa Fluor 594; red), and nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; blue). Live cell phagolysosomes were visualized using a CytoPainter lysosomal staining kit (ab112137; red), and Hoechst 33342 was used to stain nuclei. Images were acquired using confocal microscopy; arrowheads indicate examples of bacterial colocalization with phagolysosomes. (A and B) Representative images of fixed MRC5 (human fibroblast) (A) and NHOst (human osteoblast) (B) cells that were infected with S. epidermidis RP62A. (C and D) Representative images of live human fibroblasts (C) and human osteoblasts (D) that were infected with S. epidermidis 1457-GFP. (E) Quantification of the Manders' colocalization coefficient M1 (fraction of phagolysosomal marker in colocalization with bacteria) or M2 (fraction of bacteria in colocalization with phagolysosomal markers). Data are expressed as mean values (±standard deviations of the mean).
S. epidermidis RP62A and 1457 do not escape the phagolysosome of infected cells.
It has previously been shown that some S. aureus strains are capable of escaping from the phagosomal compartment to avoid phagolysosomal killing (15, 40, 44, 66–68). To address the question as to whether escape from the phagolysosome is a mechanism S. epidermidis uses to persist in nonprofessional phagocytes, we used a flow cytometry-based pH monitoring assay described by Lâm et al. (40). At 2 h p.i., all examined strains were located within an acidic environment (Fig. 4A to C). Differences in arbitrary fluorescent units (ΔAFU) at 6 h p.i. indicated translocation of the two S. aureus strains tested (Fig. 4A to C), previously shown to escape the phagosome in HeLa, 293, and THP-1 cells (40, 66). The escape of S. aureus strains 6850 and LAC-GFP was indicated by significant changes in ΔAFU between the 2- and 6-h time points (for 6850, P = 0.007, P = 0.006, P = 0.007; for LAC-GFP, P = 0.02, P = 0.005, P = 0.0007 [for MRC5 {human fibroblasts}, NHOst {normal human osteoblasts}, and MC3T3 {murine osteoblasts}, respectively]). However, both RP62A and 1457-GFP remained in the endosomal compartment (Fig. 4A to C). At 6 h p.i., the ΔAFU values were lower in the escaping strains than in the S. epidermidis strains RP62A (P < 0.001, P < 0.01, P < 0.01) and 1457 (P < 0.0001, P < 0.01, P < 0.01).
FIG 4.
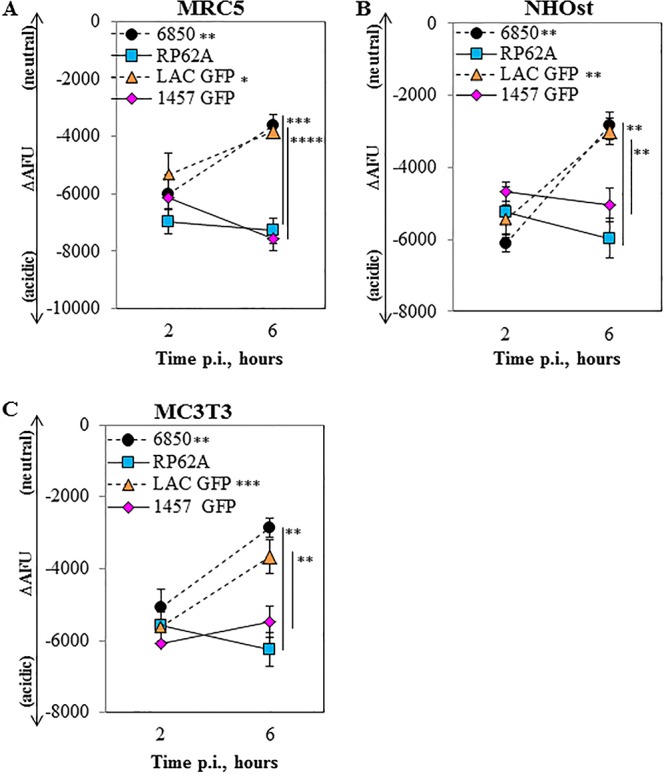
S. epidermidis does not escape the phagolysosome by 6 h. Host cells were infected with FITC-labeled S. aureus 6850, FITC-labeled S. epidermidis RP62A, S. aureus LAC-GFP, or S. epidermidis 1457-GFP at a multiplicity of infection of 50. The difference in arbitrary fluorescence units (ΔAFU) in the presence and absence of monensin, normalized to the invasion rate {ΔAFU = [(AFU−monensin − AFU+monensin)/AFU+monensin] × 105}, was used as the readout for pH. Larger negative values correspond to more-acidic (i.e., lower) pH values. Staphylococci localization in infected MRC5 (human fibroblast) (A), NHOst (human osteoblast) (B), and MC3T3 (mouse osteoblast) (C) cells is shown. Data are mean values (±standard deviations of the means) from two independent experiments done in triplicate. *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001. Significance indicated near strain designations was gathered from a comparison of results at 2 and 6 h p.i.; significance indicated near data points was gathered from a comparison of results at 6 h p.i. between the corresponding S. aureus and S. epidermidis strains.
Osteoblasts and fibroblasts have differential responses to intracellular infection.
To further confirm that S. epidermidis does not escape the phagolysosome, as suggested by the flow cytometry-based assay, we performed live time-lapse microscopy to monitor bacteria in real time in the intracellular milieu. From 4 to 7.5 h p.i., S. epidermidis 1457 remained in the lysosomal compartment of human fibroblasts (Video S1). Z-stack imaging and the Manders' coefficients supported this finding (Fig. 5). In human osteoblasts, time-lapse microscopy revealed these cells to be sensitive to bacterial infection, with cell death observed at less than 5 h p.i. (Video S2). Both nuclear fading and loss of lysosomal staining indicate apoptosis of the host cell at 5.5 h p.i. in the case of osteoblasts (Fig. 6A and B). The average colocalization coefficients of S. epidermidis 1457-GFP and the phagolysosome from 3.5 to 4 h p.i. provide support that the bacteria are located within the phagosome (Fig. 6C). There are no coefficients for 5 h p.i. due to the lack of colocalization found, but the continual GFP expression indicates that S. epidermidis 1457-GFP outlived its captor. Together, the time-lapse experiments provide evidence that S. epidermidis cannot actively escape the phagolysosome. However, we show that this organism can remain viable and be released upon death of the host cell.
FIG 5.

S. epidermidis bacteria do not escape phagolysosomal compartments. Live MRC5 (human fibroblast) cells were infected with S. epidermidis 1457-GFP at a multiplicity of infection of 50 and visualized by confocal microscopy. Nuclei, blue; 1457-GFP, green; lysosomes, magenta; cell membrane, cyan. (A) Image of human fibroblasts 7.5 h postinfection (p.i.). The dotted circle in the merged image represents the area analyzed for colocalization. (B) Quantification of the Manders' colocalization coefficient M1 (fraction of phagolysosomal marker in colocalization with bacteria) or M2 (fraction of bacteria in colocalization with phagolysosomal markers) at 7.5 h p.i.
FIG 6.

S. epidermidis escapes to the extracellular environment upon eukaryotic host cell death. Live NHOst (human osteoblast) cells were infected with S. epidermidis 1457-GFP at a multiplicity of infection of 50 and visualized by confocal microscopy. Nuclei, blue; 1457-GFP, green; lysosomes, magenta; cell membrane, cyan. (A and B) Images of infected human osteoblasts 3 h postinfection (p.i.) (A) and 5.5 h p.i. (B). The dotted circles represent the areas zoomed in for visualization of colocalization shown in the top right corners of the merged images. (C) Quantification of the Manders' colocalization coefficient M1 (fraction of phagolysosomal marker in colocalization with bacteria) or M2 (fraction of bacteria in colocalization with phagolysosomal markers) at 3 h p.i.
To compare the nature of infection between S. aureus and S. epidermidis, S. aureus LAC-GFP was also visualized during intracellular persistence in human fibroblasts and osteoblasts. LAC-GFP was found to escape lysosomal compartments in fibroblasts prior to 6 h p.i. and to replicate in the cytosol in a relatively short period of time (Fig. S4 and Video S3). This corroborates the previous findings of Grosz et al. that S. aureus replicates within the host cell cytoplasm (66). The high standard deviation (SD) seen in the M2 coefficient likely reflects the escape of some LAC-GFP bacteria and the replication of those bacteria that have escaped (Fig. S4D). Conversely, upon infection with osteoblasts, some LAC-GFP bacteria were killed and the death of the host cells was also visualized (Fig. S5A and B and Video S4). The decrease in and high SD of both the M1 and M2 coefficients from 4 to 8 h p.i. are indicative of both a decrease in GFP expression (death of bacteria) and a decrease in colocalization (death of host cell) (Fig. S5C). Together, the time-lapse experiments reveal a difference in sensitivity of fibroblasts and osteoblasts to bacterial infection in regard to microbicidal activity and death.
S. epidermidis persists during localization within acidic phagosomes.
Despite being unable to escape the phagolysosome, S. epidermidis is able to persist in host cells. We next assessed peribacterial pH to validate that bacteria are located within acidic vesicles and to determine whether S. epidermidis modulates the pH of phagolysosomes during intracellular persistence. We measured peribacterial pH during host cell infection with live or heat-killed (HK) bacteria labeled with Oregon Green 488, as described by Porte et al. (69). HK bacteria were used as a reference to confirm the pH within the phagolysosomes. By 2 h p.i., phagosomes containing live and HK bacteria were at an average pH of 4.8 for each of the host cells (Fig. 7). Treating host cells with monensin prior to infection resulted in phagosomes with an average pH of 6.6. There was little change in the peribacterial pH between 3 and 15 h p.i. for S. epidermidis RP62A and HK bacteria. S. aureus 6850 was the only bacterium tested that reached an average pH of 5.7, 5.9, and 5.3 for human fibroblasts and human and mouse osteoblasts, respectively. These findings suggest that during intracellular infection, S. epidermidis persists for at least 15 h in an acidic environment, whereas S. aureus 6850 either modulates the phagosomal pH, translocates into the cytosol, or both. In addition, these data suggest that a direct mechanism to escape the phagosome or modulate the acidic environment is not required to persist intracellularly.
FIG 7.

Intracellularly persisting S. epidermidis bacteria are located within an acidic environment. Peribacterial pH was evaluated in MRC5 (human fibroblast) (A), NHOst (human osteoblast) (B), and MC3T3 (mouse osteoblast) (C) cells treated with or without monensin and infected with unlabeled or Oregon Green 488-labeled bacteria (multiplicity of infection of 100). At 2 and 15 h postinfection (p.i.), fluorescence was measured with a spectrofluorometer with excitation at 498 nm and 450 nm and emission at 520 nm. Periphagosomal pH was calculated by comparing the 498 nm/450 nm ratio of each sample with a standard pH titration curve. Data are mean values (±standard deviations of the means) from two independent experiments. NT, not treated.
DISCUSSION
It is widely accepted that biofilm formation is a major reason for the establishment and persistence of S. epidermidis infections (10, 24, 25). We show here that intracellular persistence likely also plays a role in S. epidermidis pathogenesis. Multiple clinical S. epidermidis isolates were able to persist for several days in nonprofessional phagocytes found at the site of joint arthroplasty, localized in acidic phagolysosomes. We did not observe S. epidermidis escape the phagolysosome; however, it was able to persist in the phagolysosome, outlive its host, and later escape to the extracellular compartment upon host cell death. It is possible that this contributes to the typical delayed onset of symptoms associated with S. epidermidis infections such as PJI. In addition, we have previously shown and confirmed in this study that both acidic environments and intracellular persistence promote the formation of S. epidermidis SCVs, which may contribute to antibiotic tolerance and chronic infection. The results of our study suggest that fibroblasts, osteoblasts, and possibly other nonprofessional phagocytes constitute an intracellular niche potentially underlying some reported relapses of foreign-body-associated infections after foreign body removal and antibiotic therapy.
We have previously shown that S. epidermidis can be cultured from bone approximately 7 weeks after infection in rats (70) and rabbits (71), using a foreign body osteomyelitis model. In addition, Kwakman et al. (72) showed that S. epidermidis can persist in peri-implant tissue from an experimental catheter-associated infection model in mice. However, the method of culture of the tissues for each of these studies does not distinguish between intracellular and extracellular bacteria. Here, we found that S. epidermidis is capable of invading osteoblasts and fibroblasts in vitro. These findings corroborate previous reports which demonstrate that S. epidermidis can invade human bone cells after 2 h (48). We also demonstrated that S. epidermidis can persist intracellularly for longer than a week and that agr and PSM expression is not required for either invasion or persistence. Another difference between our studies and those previously performed is that we found S. epidermidis 1457 capable of invading and persisting in primary human osteoblasts, whereas Khalil et al. demonstrated this strain to be unable to invade the MG63 bone cell line (48). This difference may be due to a number of factors but is most likely due to a difference in cell type tested. This is supported by our findings (Fig. 1A) and those of others showing that invasion is strain and host cell type dependent (73). Previously, it has been shown that S. aureus invasion is dependent on the α5β1 integrin, whereas this receptor is not required for S. epidermidis strain 19 invasion of bone cells (48). The mechanism of invasion observed in osteoblasts and fibroblasts in this study is not currently known. Possibly, invasion can be accidental, where bacteria may be inadvertently phagocytosed during the wound healing process, or invasion can be accomplished via binding to a specific receptor, currently unknown, allowing for endocytosis. Future experiments are needed to understand how S. epidermidis is taken up into these cells. Our results also show little eukaryotic cell death over the course of infection, suggesting that human fibroblasts and osteoblasts (albeit to a slightly lower extent) are able to tolerate intracellular infection with S. epidermidis. We also show osteoblasts to have microbicidal activity. Differences in this activity between the human and mouse osteoblasts are likely due to species differences and perhaps a difference in function between primary cells and cell lines. In addition, our results obtained from intracellular infection in human and mouse osteoblasts reveal a different fate between isolates associated with sepsis or PJI, with sepsis-associated strains able to persist at stable levels. Together, these results show that the expression of virulence factors, such as agr and PSMs, is not required for invasion and intracellular persistence generally but perhaps plays a role in the ability to persist in microbicidal host cells. This hypothesis is supported by the work of Cheung et al., who previously showed that isogenic sepA and aps mutants of strain 1457 had a reduced ability to survive after phagocytic interaction with human neutrophils in comparison to the wild-type strain (31).
It has been reported that there are differences in gene expression between free-floating and adherent bacterial cells. For example, in S. aureus planktonic cells, agr and genes encoding toxins are upregulated, while no toxin gene was expressed at high levels in the S. aureus biofilm cells (50). In addition to changes in metabolism, biofilm cells also had an increase in the expression of ica genes in comparison to that of planktonic bacteria (50). Overall, it has been suggested that planktonic bacteria expressing toxins are generally more virulent and have a greater ability to cause acute infection than biofilm cells, whereas biofilm cells tend to employ defensive molecular mechanisms to promote their survival, at times resulting in chronic infection (50, 74, 75). Here we compared the invasion and persistence of one chronic-infection and one acute-infection S. aureus PJI isolate and showed that both isolates are successfully able to invade and persist within mouse osteoblasts. As seen for the S. epidermidis strains, the expression of virulence factors may not be required for invasion. However, further characterization of these two isolates (and other chronic and acute infection-associated isolates) and further time points are needed to distinguish any changes in the ability to persist past 7 days and to identify genes responsible for this persistence.
SCV formation is induced in response to diverse environmental pressures (51–53). SCVs typically comprise a minor proportion of the source population and are common in chronic infections (54–58). S. epidermidis SCVs are common in PJI (8). Here we showed there to be no difference in ability to invade cells between pairs of an isogenic SCV or closely related SCV and an NCP S. epidermidis isolate (see Fig. S2E and F in the supplemental material), as previously shown for S. aureus (65). In addition, we showed a positive correlation between SCV formation and time of intracellular persistence in human fibroblasts with multiple staphylococcal strains and extended these findings to infection in osteoblasts. Previously, we have shown that the number of SCVs can be reduced by treatment with lysosomotropic alkalinizing agents. For at least the first week, SCVs do not significantly contribute to survival, as there is no change in the total number of CFU recovered from cells treated with these drugs (49). Although the increasing numbers of SCVs suggest adaptation to the acidic environment, there may be another role this subpopulation plays during infection. Recently, two groups have implicated SCVs in proinflammatory cytokine suppression. Ou et al. showed that after 24 h, intracellular infection with NCP S. aureus in a human bronchial epithelial cell line induced a robust proinflammatory response evidenced by increased Toll-like receptor 2 (TLR2), interleukin 1 (IL-1), IL-6, and IL-12 expression, whereas SCV infection induced only TLR2 expression (65). Magryś et al. showed that 4 h after infection of THP-1 cells with S. epidermidis SCVs isolated from a hip PJI, there was an increase in AKT phosphorylation compared to that in NCP-infected and noninfected cells (76). This group did not show direct evidence for proinflammatory cytokine suppression, but because the phosphatidylinositol 3-kinase (PI3K)-AKT pathway has been shown to be involved with cytokine suppression, they suggested this role. Thus, together with our findings, this suggests that intracellular persistence and tolerance to this persistence are maintained by SCVs via mechanisms currently unknown, possibly by modulating signaling pathways.
Classified as nonprofessional phagocytes, both fibroblasts and osteoblasts use mechanisms similar to those used by macrophages to phagocytose and degrade the contents within their phagosomes (77–79). Using live time-lapse fluorescence microscopy, we showed that within 2 h of infection, bacteria are taken up by osteoblasts and fibroblasts into acidic phagosomes, where some but not all S. epidermidis cells are killed. In addition, our results indicate that S. epidermidis is unable to escape the phagosomes or modulate the acidic environment after 15 h. This is different from results generated with S. aureus, both in our study and in those by others, describing phagosomal escape and replication in the cytosol (15, 40, 66, 80). However, as not all S. aureus isolates are able to escape and some escape at later time points, our findings do not definitively prove that S. epidermidis cannot escape the phagolysosome. Because phagosomal escape for S. aureus is dependent upon PSMα expression (66), whose homologue is expressed in much lower concentrations by S. epidermidis (31), it is plausible that this organism simply requires more time for phagosomal escape than does S. aureus. Most importantly, we were able to show that viable S. epidermidis is capable of escaping into the extracellular environment upon host cell death. Together, the results suggest that S. epidermidis is able to tolerate the acidic phagosomal environment for several days.
In conclusion, our findings show that intracellular infection of osteoblasts, fibroblasts, and possibly other nonprofessional phagocytes comprise a second and equally important mechanism, beyond biofilm formation, enabling evasion from host immune defense and permitting bacteria to persist during infection and possibly treatment. Given that current treatments may not kill intracellularly persisting bacteria, targeting this population may contribute to increased treatment success. Additionally, SCV formation may contribute to the subtle and silent nature of infection through as-yet-undescribed processes.
MATERIALS AND METHODS
Microorganisms.
Bacterial strains used in this study are detailed in Table S1 in the supplemental material. Prior to assays, staphylococci were grown overnight in 10 ml of tryptic soy broth (TSB) at 37°C with rotation. Bacteria expressing superfolder green fluorescent protein (sGFP) were grown in 10 ml TSB supplemented with 10 μg/ml chloramphenicol. Bacteria were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), and resuspended in assay medium. Bacterial cell numbers were estimated spectrophotometrically at 600 nm and/or using McFarland standards. Heat-killed (HK) bacteria were prepared by heating the bacteria for 10 to 15 min at 65°C. Lack of viability was confirmed by no growth after plating (data not shown). Prior to experimentation, S. epidermidis and S. aureus stock cultures were confirmed for agr expression via the production of PSMγ and/or PSMβ2 via reverse transcription PCR or ELISA (data not shown). SCVs and NCP isolates from the same joint were previously assessed for relatedness by pulsed-field gel electrophoresis (PFGE), as described previously (81). IDRL-8933 and IDRL-8934 had a 3-band difference, whereas IDRL-8864 and IRL-8866 were indistinguishable (81). To confirm that both SCV and NCP isolates originated from the same clone, whole-genome sequencing (WGS) had been previously conducted at the Department of Infection Control Science at Juntendo University Graduate School of Medicine (Tokyo, Japan) (82). WGS results were insufficient to confirm that IDRL-8933 and IDRL-8934 were clonally related. However, WGS revealed approximately 10 mutations between IDRL-8866 and IDRL-8864, supporting the theory that these two strains are clonal (82).
Cell culture.
Human lung fibroblasts (MRC5 cells) were maintained in RPMI 1640, supplemented with 10% heat-inactivated fetal bovine serum (Sigma). Murine osteoblasts (MC3T3-E1) were maintained in minimum essential medium alpha (MEMα) supplemented with 10% heat-inactivated fetal bovine serum. Mouse osteoblast cell authentication was performed by IDEXX BioResearch using short tandem repeat (STR) DNA profiling. Primary normal human osteoblast (NHOst) cells were maintained in osteoblast basal medium supplemented with OGM SingleQuots (Lonza). Human osteoblast cell authentication was performed by the Mayo Clinic Genotyping Core using STR DNA profiling. Osteoblasts were also confirmed by alkaline phosphatase and bone mineralization staining (data not shown).
Intracellular persistence. (i) Lysostaphin-daptomycin protection assay.
Host cells were infected at a multiplicity of infection (MOI) of 25 for intracellular assay. Host cells were washed daily with PBS and lysostaphin (Sigma) or daptomycin (to kill extracellular bacteria) and provided fresh medium supplemented with 10% fetal bovine serum. At 0, 3, 5, and 7 days p.i., host cells were lysed and serial 10-fold dilutions of the cell lysates were plated onto sheep blood agar (SBA) to detect and quantitate intracellular bacteria. CFU were enumerated after overnight incubation at 37°C in air. The SCV phenotype was determined by size after a second overnight incubation at room temperature. Only intracellularly persisting bacteria were monitored over time, as no colonies were found in the washing medium after the lysostaphin/daptomycin treatment (data not shown).
(ii) Viability.
Host cells were detached from wells with trypsin-EDTA (0.25%) (Thermo Fisher), and a 100-μl aliquot was diluted 1:1 in 0.4% trypan blue solution. The percentage of live cells was calculated using a Countess II FL automated cell counter (Thermo Fisher); two independent samples were analyzed per time point.
Phagolysosomal localization. (i) Confocal microscopy of fixed cells.
S. epidermidis RP62A was grown and harvested as described above. Bacterial cells were washed and stained with 5 mM CFSE (eBioscience) in the dark for 10 min at room temperature. Host cells grown on glass coverslips were then infected at an MOI of 50 with the CFSE-labeled bacteria for 2 h at 37°C in 5% CO2. Following the 2-h incubation, host cells were washed and incubated with 20 μg/ml lysostaphin to kill extracellular adherent bacteria. Host cells were then further incubated for 1 h at 37°C in 5% CO2. The cells were washed, fixed, and permeabilized. After blocking, the cells were incubated with primary antibodies overnight at 4°C, followed by three washes with PBS and incubation with secondary antibodies for 1 h and then three more washes. Antibodies to human lysosomal-associated membrane protein-2 (LAMP-2) (H4B4; Abcam) were used at 1:100. LAMP-2 is abundantly and ubiquitously expressed on lysosomal membranes and is used to mark mature endosomes and lysosomes. The fluorophore-conjugated donkey anti-mouse secondary antibody was used at 1:500. Confocal microscopy was performed using an LSM 780 microscope (Carl Zeiss, Germany) with a 40× objective.
(ii) Confocal microscopy of live cells.
S. epidermidis 1457 expressing sGFP was grown and harvested as described above. Host cells were infected at an MOI of 50 for 1 h at 37°C in 5% CO2. Host cells were then washed as described above, and a CytoPainter lysosomal staining kit (Abcam) was used to stain for lysosomes. The lysotropic indicator from the kit is designed to selectively accumulate in the lysosomes of live cells. Cells were then incubated for 30 min at 37°C in 5% CO2, washed, and stained with NucBlue (Invitrogen) for 5 min, followed by staining with the CellMask deep red plasma membrane stain (Thermo Fisher) for 5 to 10 min, both at 37°C in 5% CO2. The cells were washed, and phenol-red free medium was added. Confocal microscopy was performed with an LSM 780 microscope (Carl Zeiss, Germany) using a 40× objective in a controlled chamber providing incubation at 37°C in 5% CO2.
(iii) Colocalization.
Colocalization of bacteria and phagolysosomes was determined by selecting at least 15 randomly chosen regions of interest (ROI) containing intracellular bacteria using ImageJ Coloc 2 software to determine the Manders' coefficients. There is one Manders' coefficient per channel, and each coefficient is proportional to the amount of fluorescence in that channel that colocalizes with the fluorescence in the other channel over its total intensity. The coefficient varies from 0 to 1, with 0 indicating no colocalization and 1 indicating perfect colocalization (83). The colocalization in live time-lapse images was determined by averaging the coefficients of each image within respective time frames.
(iv) Flow cytometry phagolysosome escape assay.
The flow cytometry phagolysosome escape assay, which monitors the pH of the environment of intracellular staphylococci, was performed as described by Lâm et al. (40), with slight modification. Bacterial cultures were grown and harvested as described above. Washed bacterial pellets were then resuspended in 0.1 M sodium bicarbonate buffer, and bacteria were labeled with fluorescein isothiocyanate (FITC) (Invitrogen) in the dark with rotation for 30 min at room temperature. Bacterial cells were washed with Hanks balanced salt solution (HBSS) to remove unbound dye and then resuspended in HBSS. Prior to infection, host cells were seeded at 3 × 105 cells per well and grown overnight in a 24-well culture disk to subconfluence. Host cells were placed on ice for 10 min to synchronize phagocytosis, and cells were then infected at an MOI of 50. Following incubation on ice for 15 min, the cells were moved to 37°C in 5% CO2 for 55 min. The cells were washed and incubated with medium supplemented with lysostaphin (20 μg/ml) at 37°C for 30 min. The cells were returned to the incubator for an additional 55 min or 4 h and 55 min, resulting in an overall infection time of 2 or 6 h, respectively. Cells were washed with PBS, trypsinized, and transferred to 5-ml round-bottom tubes. Samples were incubated with or without 50 μM monensin for 20 min at 37°C, followed by propidium iodide treatment for live/dead staining. Samples were analyzed using FACSCanto X. For each cell type, a preset fixed gating and a fixed amplification were used. The invasion rate with monensin (total internalized bacteria), expressed as numbers of arbitrary fluorescence units (AFU), was determined according to the formula AFU = [(mean fluorescence intensity of cells in M) × (percentage of cells in M)]/1,000 and normalized to the mean fluorescence intensity of each corresponding bacterial preparation. (The FITC-positive gate is denoted M.) The difference in AFU (ΔAFU) in the presence or absence of monensin, normalized to the invasion rate {ΔAFU = [(AFU−monensin − AFU+monensin)/AFU+monensin] × 105}, was used as the readout for pH. Larger negative values correspond to more-acidic (i.e., lower) pH values.
Measurement of peribacterial pH.
Live (overnight) or HK (prepared as described above) bacteria were labeled with the pH reporter dye Oregon Green 488 (OG-488) (Invitrogen), which can assess pH values between 3.5 and 7 (69). The experimental setup for this assay was as described by Tranchemontagne et al. (18). Live and HK cultures were washed with Dulbecco's phosphate-buffered saline (D-PBS), resuspended in OG-488 (10 mg/ml), and incubated for 30 min at 4°C in the dark. Labeled bacteria were centrifuged, and the labeling reaction was stopped by adding Tris-HCl (pH 8.3) to a final concentration of 100 mM. Bacteria were incubated at 4°C in the dark for 15 min, washed, and used for infection. Labeling with OG-488 did not affect the viability of live bacteria as measured by plating on bovine serum albumin (BSA) (data not shown).
To determine the pH of the environment surrounding S. epidermidis, host cells were grown in phenol red-free RPMI 1640 in tissue culture-treated, black-walled 24-well microplates (PerkinElmer). Cells were treated with or without 50 μM monensin and infected with Oregon Green 488-labeled or unlabeled bacteria at an MOI of 100, according to the procedure described above. At 2 and 15 h p.i., 0.1% trypan blue was added to infected cells for 1 min to quench the fluorescence of the remaining extracellular bacteria, and the cells were then washed three times with cell medium. With the emission wavelength at 520 nm, the ratio of fluorescence with excitation at 498 to that at 450 nm was measured using a Fluoroskan Ascent (Thermo Scientific). As described by Levitz et al. (84), a standard curve was generated for each condition by obtaining excitation ratios at 498 and 450 nm in infected cells where the phagosomal and extracellular pH values were equilibrated in nigericin buffers of defined pH (0.028 mM nigericin, 10 μM monensin, 150 mM sodium chloride, 80 mM potassium chloride, 1 mM magnesium chloride, 5.5 mM glucose, 2 mM calcium chloride, and 10 mM HEPES) (data not shown). Background values for excitation at 498 and 450 nm with emission at 520 nm for cells infected with unlabeled bacteria were subtracted from readings obtained with labeled bacteria. To calculate the average phagosomal pH of the samples, the ratios of excitation at 498 to that at 450 nm were compared with the standard pH titration curve generated from nigericin-permeabilized cells. HK bacteria were used as a reference to confirm the pH over time in the phagolysosomes, as dead bacteria would not actively modulate or escape the phagolysosome.
Statistical methods.
Statistical analysis was performed using a two-tailed t test. We used an alpha value of 0.05, so P values of <0.05 were considered significant (*, P < 0.05, **, P < 0.01, ***, P < 0.001, and ****, P < 0.0001). All error bars represent standard deviations (SD).
Supplementary Material
ACKNOWLEDGMENTS
S. aureus strain 6850 was kindly provided by Bettina Löffler (Institute of Medical Microbiology, Jena, Germany). S. epidermidis strains 1457, 1457ΔPSMβ, Tü3298, and TüF38 were kindly provided by Michael Otto (NIAID NIH, Bethesda, MD). S. epidermidis strain 1457 expressing GFP was kindly provided by Alexander Horswill (University of Colorado Department of Immunology and Microbiology, Denver, CO). MRC5 cells were kindly provided by Kelli Black (Mayo Clinic Virology/Parasitology Laboratory, Rochester, MN), and MC3T3 cells were kindly provided by Jennifer Westendorf (Mayo Clinic Department of Orthopedic Surgery, Rochester, MN). Primary normal human osteoblasts were kindly provided by Paul German Norambuena Morales (Mayo Clinic Department of Orthopedic Surgery, Rochester, MN).
This work was supported by NIH grant GM055252, a Ph.D. Training Grant in Basic Immunology (T32 AI07425-21), NIH grant R01 AR056647, and NIH grant R01 AI91594.
K.P. performed the experiments. R.P. supervised K.P. and helped edit and revise the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00237-18.
REFERENCES
- 1.Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, Lai Y, Kim JE, Nizet V, Gallo RL. 2010. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One 5:e8557. doi: 10.1371/journal.pone.0008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. 2010. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, Radek KA, Huang CM, Ryan AF, Gallo RL. 2009. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanke I, Steffen H, Christ C, Krismer B, Gotz F, Peschel A, Schaller M, Schittek B. 2011. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Invest Dermatol 131:382–390. doi: 10.1038/jid.2010.328. [DOI] [PubMed] [Google Scholar]
- 5.Scharschmidt TC, Vasquez KS, Truong H-A, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, Abbas AK, Fischbach MA, Rosenblum MD. 2015. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, Gallo RL. 2010. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol 130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamagni T. 2014. Epidemiology and burden of prosthetic joint infections. J Antimicrob Chemother 69(Suppl 1):i5–10. doi: 10.1093/jac/dku247. [DOI] [PubMed] [Google Scholar]
- 8.Tande AJ, Osmon DR, Greenwood-Quaintance KE, Mabry TM, Hanssen AD, Patel R. 2014. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio 5:e01910-14. doi: 10.1128/mBio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto M. 2009. Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat Rev Microbiol 7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuong C, Otto M. 2002. Staphylococcus epidermidis infections. Microb Infect 4:481–489. doi: 10.1016/S1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 12.Cheung GY, Joo HS, Chatterjee SS, Otto M. 2014. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol Rev 38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz SM, Ong KL, Lau E, Bozic KJ. 2014. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 96:624–630. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 14.Armbruster NS RJ, Schreiner J, Klenk J, Manina G, Kretschmer D, Poschel S, Schenke-Layland K, Kalbacher H, Clark K, Autnrieth SE. 2016. PSM peptides of Staphylococcus aureus activate the p38-CREB pathway in dendritic cells, thereby modulating cytokine production and T cell priming. J Immunol 196:1284–1292. doi: 10.4049/jimmunol.1502232. [DOI] [PubMed] [Google Scholar]
- 15.Blattner S, Das S, Paprotka K, Eilers U, Krischke M, Kretschmer D, Remmele CW, Dittrich M, Muller T, Schuelein-Voelk C, Hertlein T, Mueller MJ, Huettel B, Reinhardt R, Ohlsen K, Rudel T, Fraunholz MJ. 2016. Staphylococcus aureus exploits a non-ribosomal cyclic dipeptide to modulate survival within epithelial cells and phagocytes. PLoS Pathog 12:e1005857. doi: 10.1371/journal.ppat.1005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasigade JP, Trouillet-Assant S, Ferry T, Diep BA, Sapin A, Lhoste Y, Ranfaing J, Badiou C, Benito Y, Bes M, Couzon F, Tigaud S, Lina G, Etienne J, Vandenesch F, Laurent F. 2013. PSMs of hypervirulent Staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS One 8:e63176. doi: 10.1371/journal.pone.0063176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE. 2013. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol 190:3417–3426. doi: 10.4049/jimmunol.1202563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tranchemontagne ZR, Camire RB, O'Donnell VJ, Baugh J, Burkholder KM. 2016. Staphylococcus aureus strain USA300 perturbs acquisition of lysosomal enzymes and requires phagosomal acidification for survival inside macrophages. Infect Immun 84:241–253. doi: 10.1128/IAI.00704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. 2004. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol 172:1169–1176. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. 2012. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch TK, Reuter M, Barthel D, Bohm S, van den Elsen J, Kraiczy P, Zipfel PF, Skerka C. 2012. Staphylococcus aureus proteins Sbi and Efb recruit human plasmin to degrade complement C3 and C3b. PLoS One 7:e47638. doi: 10.1371/journal.pone.0047638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laarman AJ, Ruyken M, Malone CL, van Strijp JA, Horswill AR, Rooijakkers SH. 2011. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol 186:6445–6453. doi: 10.4049/jimmunol.1002948. [DOI] [PubMed] [Google Scholar]
- 23.McGuinness WA, Kobayashi SD, DeLeo FR. 2016. Evasion of neutrophil killing by Staphylococcus aureus. Pathogens 5:32. doi: 10.3390/pathogens5010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. 2004. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis 190:1498–1505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Sturdevant DE, Otto M. 2005. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis 191:289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]
- 26.Kristian SA, Birkenstock TA, Sauder U, Mack D, Gotz F, Landmann R. 2008. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis 197:1028–1035. doi: 10.1086/528992. [DOI] [PubMed] [Google Scholar]
- 27.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 28.Rohde H, Burdelski C, Bartscht K, Hussain M, Buck F, Horstkotte MA, Knobloch JK, Heilmann C, Herrmann M, Mack D. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol Microbiol 55:1883–1895. doi: 10.1111/j.1365-2958.2005.04515.x. [DOI] [PubMed] [Google Scholar]
- 29.Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P, Kroll G, Schulze C, Buck F, Mack D, Aepfelbacher M, Rohde H. 2010. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol Microbiol 75:187–207. doi: 10.1111/j.1365-2958.2009.06981.x. [DOI] [PubMed] [Google Scholar]
- 30.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. 2011. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun 79:2267–2276. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. 2010. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog 6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Y, Vuong C, Kocianova S, Villaruz AE, Lai Y, Sturdevant DE, Otto M. 2006. Characterization of the Staphylococcus epidermidis accessory-gene regulator response: quorum-sensing regulation of resistance to human innate host defense. J Infect Dis 193:841–848. doi: 10.1086/500246. [DOI] [PubMed] [Google Scholar]
- 33.Otto M. 2014. Phenol-soluble modulins. Int J Med Microbiol 304:164–169. doi: 10.1016/j.ijmm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laabei M, Jamieson WD, Yang Y, van den Elsen J, Jenkins AT. 2014. Investigating the lytic activity and structural properties of Staphylococcus aureus phenol soluble modulin (PSM) peptide toxins. Biochim Biophys Acta 1838:3153–3161. doi: 10.1016/j.bbamem.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 36.Fowler T, Wann ER, Joh D, Johansson S, Foster TJ, Hook M. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur J Cell Biol 79:672–679. doi: 10.1078/0171-9335-00104. [DOI] [PubMed] [Google Scholar]
- 37.Jevon M, Guo C, Ma B, Mordan N, Nair SP, Harris M, Henderson B, Bentley G, Meghji S. 1999. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun 67:2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziewanowska K, Patti JM, Deobald CF, Bayles KW, Trumble WR, Bohach GA. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun 67:4673–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalinka J, Hachmeister M, Geraci J, Sordelli D, Hansen U, Niemann S, Oetermann S, Peters G, Loffler B, Tuchscherr L. 2014. Staphylococcus aureus isolates from chronic osteomyelitis are characterized by high host cell invasion and intracellular adaptation, but still induce inflammation. Int J Med Microbiol 304:1038–1049. doi: 10.1016/j.ijmm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Lâm T-T, Giese B, Chikkaballi D, Kühn A, Wolber W, Pané-Farré J, Schäfer D, Engelmann S, Fraunholz M, Sinha B. 2010. Phagolysosomal integrity is generally maintained after Staphylococcus aureus invasion of nonprofessional phagocytes but is modulated by strain 6850. Infect Immun 78:3392–3403. doi: 10.1128/IAI.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed W, Sommer U, Sethi S, Domann E, Thormann U, Schutz I, Lips KS, Chakraborty T, Schnettler R, Alt V. 2014. Intracellular proliferation of S. aureus in osteoblasts and effects of rifampicin and gentamicin on S. aureus intracellular proliferation and survival. Eur Cell Mater 28:258–268. doi: 10.22203/eCM.v028a18. [DOI] [PubMed] [Google Scholar]
- 42.Hamill RJ, Vann JM, Proctor RA. 1986. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun 54:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clement S, Vaudaux P, Francois P, Schrenzel J, Huggler E, Kampf S, Chaponnier C, Lew D, Lacroix JS. 2005. Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis. J Infect Dis 192:1023–1028. doi: 10.1086/432735. [DOI] [PubMed] [Google Scholar]
- 44.Bayles KW, Wesson CA, Liou LE, Fox LK, Bohach GA, Trumble WR. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun 66:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci 79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 46.Hebert A, Sayasith K, Senechal S, Dubreuil P, Lagace J. 2000. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol Lett 193:57–62. doi: 10.1111/j.1574-6968.2000.tb09402.x. [DOI] [PubMed] [Google Scholar]
- 47.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, Hudson M. 2003. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br 85:918–921. doi: 10.1302/0301-620X.85B6.13509. [DOI] [PubMed] [Google Scholar]
- 48.Khalil H, Williams RJ, Stenbeck G, Henderson B, Meghji S, Nair SP. 2007. Invasion of bone cells by Staphylococcus epidermidis. Microb Infect 9:460–465. doi: 10.1016/j.micinf.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Perez K, Patel R. 2017. Staphylococcus epidermidis small-colony variants are induced by low pH and their frequency reduced by lysosomal alkalinization. J Infect Dis 215:488–490. doi: 10.1093/infdis/jiw503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Resch A, Rosenstein R, Nerz C, Gotz F. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71:2663–2676. doi: 10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bui LM, Turnidge JD, Kidd SP. 2015. The induction of Staphylococcus aureus biofilm formation or small colony variants is a strain-specific response to host-generated chemical stresses. Microbes Infect 17:77–82. doi: 10.1016/j.micinf.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Kahl BC. 2014. Small colony variants (SCVs) of Staphylococcus aureus—a bacterial survival strategy. Infect Genet Evol 21:515–522. doi: 10.1016/j.meegid.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Melter O, Radojevic B. 2010. Small colony variants of Staphylococcus aureus—review. Folia Microbiol (Praha) 55:548–558. doi: 10.1007/s12223-010-0089-3. [DOI] [PubMed] [Google Scholar]
- 54.Johns BE, Purdy KJ, Tucker NP, Maddocks SE. 2015. Phenotypic and genotypic characteristics of small colony variants and their role in chronic infection. Microbiol Insights 8:15–23. doi: 10.4137/MBI.S25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 56.Sendi P, Proctor RA. 2009. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol 17:54–58. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Tuchscherr L, Kreis CA, Hoerr V, Flint L, Hachmeister M, Geraci J, Bremer-Streck S, Kiehntopf M, Medina E, Kribus M, Raschke M, Pletz M, Peters G, Loffler B. 2016. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J Antimicrob Chemother 71:438–448. doi: 10.1093/jac/dkv371. [DOI] [PubMed] [Google Scholar]
- 58.von Eiff C, Peters G, Becker K. 2006. The small colony variant (SCV) concept—the role of staphylococcal SCVs in persistent infections. Injury 37(Suppl 2):S26–33. doi: 10.1016/j.injury.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Bogut A, Niedzwiadek J, Koziol-Montewka M, Strzelec-Nowak D, Blacha J, Mazurkiewicz T, Marczynski W, Plewik D. 2014. Characterization of Staphylococcus epidermidis and Staphyloccocus warneri small-colony variants associated with prosthetic-joint infections. J Med Microbiol 63:176–185. doi: 10.1099/jmm.0.066068-0. [DOI] [PubMed] [Google Scholar]
- 60.Sander G, Borner T, Kriegeskorte A, von Eiff C, Becker K, Mahabir E. 2012. Catheter colonization and abscess formation due to Staphylococcus epidermidis with normal and small-colony-variant phenotype is mouse strain dependent. PLoS One 7:e36602. doi: 10.1371/journal.pone.0036602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Eiff C, Proctor RA, Peters G. 2000. Small colony variants of staphylococci: a link to persistent infections. Berl Munch Tierarztl Wochenschr 113:321–325. [PubMed] [Google Scholar]
- 62.Baddour LM, Barker LP, Christensen GD, Parisi JT, Simpson WA. 1990. Phenotypic variation of Staphylococcus epidermidis in infection of transvenous endocardial pacemaker electrodes. J Clin Microbiol 28:676–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baddour LM, Simpson WA, Weems JJ Jr, Hill MM, Christensen GD. 1988. Phenotypic selection of small-colony variant forms of Staphylococcus epidermidis in the rat model of endocarditis. J Infect Dis 157:757–763. doi: 10.1093/infdis/157.4.757. [DOI] [PubMed] [Google Scholar]
- 64.Adler H, Widmer A, Frei R. 2003. Emergence of a teicoplanin-resistant small colony variant of Staphylococcus epidermidis during vancomycin therapy. Eur J Clin Microbiol Infect Dis 22:746–748. doi: 10.1007/s10096-003-1029-9. [DOI] [PubMed] [Google Scholar]
- 65.Ou JJJ, Drilling AJ, Cooksley C, Bassiouni A, Kidd SP, Psaltis AJ, Wormald PJ, Vreugde S. 2016. Reduced innate immune response to a Staphylococcus aureus small colony variant compared to its wild-type parent strain. Front Cell Infect Microbiol 6:187. doi: 10.3389/fcimb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grosz M, Kolter J, Paprotka K, Winkler AC, Schäfer D, Chatterjee SS, Geiger T, Wolz C, Ohlsen K, Otto M, Rudel T, Sinha B, Fraunholz M. 2014. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell Microbiol 16:451–465. doi: 10.1111/cmi.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giese B, Glowinski F, Paprotka K, Dittmann S, Steiner T, Sinha B, Fraunholz MJ. 2011. Expression of delta-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of beta-toxin. Cell Microbiol 13:316–329. doi: 10.1111/j.1462-5822.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- 68.Jarry TM, Cheung AL. 2006. Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect Immun 74:2568–2577. doi: 10.1128/IAI.74.5.2568-2577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Porte F, Liautard J-P, Köhler S. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun 67:4041–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park KH, Greenwood-Quaintance KE, Mandrekar J, Patel R. 2016. Activity of tedizolid in methicillin-resistant Staphylococcus aureus experimental foreign body-associated osteomyelitis. Antimicrob Agents Chemother 60:6568–6572. doi: 10.1128/AAC.01248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Del Pozo JL, Rouse MS, Euba G, Kang CI, Mandrekar JN, Steckelberg JM, Patel R. 2009. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother 53:4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwakman PH, te Velde AA, Vandenbroucke-Grauls CM, van Deventer SJ, Zaat SA. 2006. Treatment and prevention of Staphylococcus epidermidis experimental biomaterial-associated infection by bactericidal peptide 2. Antimicrob Agents Chemother 50:3977–3983. doi: 10.1128/AAC.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strobel M, Pförtner H, Tuchscherr L, Völker U, Schmidt F, Kramko N, Schnittler HJ, Fraunholz MJ, Löffler B, Peters G, Niemann S. 2016. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin Microbiol Infect 22:799–809. doi: 10.1016/j.cmi.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Phillips PL, Schultz GS. 2012. Molecular mechanisms of biofilm infection: biofilm virulence factors. Adv Wound Care (New Rochelle) 1:109–114. doi: 10.1089/wound.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Betancur JC, Goni-Moreno A, Horger T, Schott M, Sharan M, Eikmeier J, Wohlmuth B, Zernecke A, Ohlsen K, Kuttler C, Lopez D. 2017. Cell differentiation defines acute and chronic infection cell types in Staphylococcus aureus. Elife 6:e28023. doi: 10.7554/eLife.28023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Magryś A, Bogut A, Kiełbus M, Olender A. 2018. The role of the PI3K/mTOR signaling pathway in Staphylococcus epidermidis small colony variants intracellular survival. Immunol Invest 47:251–263. doi: 10.1080/08820139.20181423569:1-13. [DOI] [PubMed] [Google Scholar]
- 77.Rabinovitch M. 1995. Professional and non-professional phagocytes: an introduction. Trends Cell Biol 5:85–87. doi: 10.1016/S0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 78.Cerri PS. 2005. Osteoblasts engulf apoptotic bodies during alveolar bone formation in the rat maxilla. Anat Rec A Discov Mol Cell Evol Biol 286:833–840. doi: 10.1002/ar.a.20220. [DOI] [PubMed] [Google Scholar]
- 79.Arlein WJ, Shearer JD, Caldwell MD. 1998. Continuity between wound macrophage and fibroblast phenotype: analysis of wound fibroblast phagocytosis. Am J Physiol Regul Integr Comp Physiol 275:R1041–8. doi: 10.1152/ajpregu.1998.275.4.R1041. [DOI] [PubMed] [Google Scholar]
- 80.Rollin G, Tan X, Tros F, Dupuis M, Nassif X, Charbit A, Coureuil M. 2017. Intracellular survival of Staphylococcus aureus in endothelial ells: a matter of growth or persistence. Front Microbiol 8:1354. doi: 10.3389/fmicb.2017.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maduka-Ezeh AN, Greenwood-Quaintance KE, Karau MJ, Berbari EF, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. 2012. Antimicrobial susceptibility and biofilm formation of Staphylococcus epidermidis small colony variants associated with prosthetic joint infection. Diagn Microbiol Infect Dis 74:224–229. doi: 10.1016/j.diagmicrobio.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 82.Uehara Y, Sasaki T, Hiramatsu K, Tande A, Greenwood-Quaintance K, Perez K, Patel R. 2016. Identification of mutations in Staphylococcus epidermidis small-colony variants associated with prosthetic joint infection by direct whole genome sequencing from colonies. Open Forum Infect Dis 3:2215–2215. doi: 10.1093/ofid/ofw172.1763. [DOI] [Google Scholar]
- 83.Manders EMM, Verbeek FJ, Aten JA. 1993. Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 84.Levitz SM, Nong SH, Seetoo KF, Harrison TS, Speizer RA, Simons ER. 1999. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun 67:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


