Bordetella pertussis is the primary causative agent of pertussis (whooping cough), which is a respiratory infection that leads to a violent cough and can be fatal in infants. There is a need to develop more effective vaccines because of the resurgence of cases of pertussis in the United States since the switch from the whole-cell pertussis vaccines (wP) to the acellular pertussis vaccines (aP; diphtheria-tetanus-acellular-pertussis vaccine/tetanus-diphtheria-pertussis vaccine).
KEYWORDS: Bordetella pertussis, DTaP, acellular pertussis vaccines, adenylate cyclase toxin, pertussis, vaccines, whooping cough
ABSTRACT
Bordetella pertussis is the primary causative agent of pertussis (whooping cough), which is a respiratory infection that leads to a violent cough and can be fatal in infants. There is a need to develop more effective vaccines because of the resurgence of cases of pertussis in the United States since the switch from the whole-cell pertussis vaccines (wP) to the acellular pertussis vaccines (aP; diphtheria-tetanus-acellular-pertussis vaccine/tetanus-diphtheria-pertussis vaccine). Adenylate cyclase toxin (ACT) is a major virulence factor of B. pertussis that is (i) required for establishment of infection, (ii) an effective immunogen, and (iii) a protective antigen. The C-terminal repeats-in-toxin domain (RTX) of ACT is sufficient to induce production of toxin-neutralizing antibodies. In this study, we characterized the effectiveness of vaccines containing the RTX antigen against experimental murine infection with B. pertussis. RTX was not protective as a single-antigen vaccine against B. pertussis challenge, and adding RTX to 1/5 human dose of aP did not enhance protection. Since the doses of aP used in murine studies are not proportionate to mouse/human body masses, we titrated the aP from 1/20 to 1/160 of the human dose. Mice receiving 1/80 human aP dose had bacterial burden comparable to those of naive controls. Adding RTX antigen to the 1/80 aP base resulted in enhanced bacterial clearance. Inclusion of RTX induced production of antibodies recognizing RTX, enhanced production of anti-pertussis toxin, decreased secretion of proinflammatory cytokines, such as interleukin-6, and decreased recruitment of total macrophages in the lung. This study shows that adding RTX antigen to an appropriate dose of aP can enhance protection against B. pertussis challenge in mice.
INTRODUCTION
Whooping cough is a disease caused by an upper respiratory tract infection that is primarily caused by the Gram-negative pathogen Bordetella pertussis and several other Bordetellae. These infections, commonly referred to as pertussis, can be fatal in infants but can also cause significant suffering in children, adolescents, and adults. Fortunately, two generations of vaccines have been developed and have greatly reduced the incidence of pertussis in the United States and worldwide. Killed whole-cell pertussis vaccines (wPs) were first reported in 1933 (1). With the development of the Kendrick test in 1947, wPs were standardized, leading to an increase in vaccine efficacy, such that 80% of those immunized developed long-term immunity to pertussis (2). Use of the wP resulted in drastic reduction of the incidence of whooping cough in the second half of the twentieth century. However, in the 1990s, due to reactogenicity and public concern, wPs were replaced with acellular pertussis vaccines (aPs; diphtheria-tetanus-acellular-pertussis vaccine [DTaP]; tetanus-diphtheria-pertussis vaccine [Tdap]) containing 3 to 5 pertussis protein antigens and alum adjuvant (3). Since the introduction of aPs, increases in pertussis cases throughout the United States and Europe have been observed, and a number of reasons have been postulated to explain the lack of complete efficacy of the aPs (4).
One of the major issues with the aPs is that their efficacy wanes each year after administration (5, 6). Recent data from the nonhuman primate model of pertussis demonstrate that while the aPs prevent the paroxysmal whooping cough, they do not protect from colonization and transmission (7). In light of the increased incidence and our appreciation of the lack of efficacy of the aP, there is a need to develop another generation of vaccines with improved efficacy and duration of protection. Various approaches have been considered for the development of the new generation of pertussis vaccines, including (i) addition of new antigens to the current aPs, (ii) repolarization of the host Th2 response triggered by alum-adjuvanted aPs to a Th1/Th17 response by changing the adjuvant and adding new antigens, and (iii) reintroduction of wPs (live attenuated or killed) either as primary immunization or as a repolarizing booster.
B. pertussis expresses pertussis toxin (PT) and adenylate cyclase toxin (ACT) to facilitate infection and impair the host immune response in a variety of ways. Detoxified PT antigens are included in all commercial aPs. When the aP was developed, ACT was recognized as an essential virulence factor, but as of the late 1980s, ACT had not been extensively evaluated as a protective antigen and was not available in purified form. ACT was therefore not included in aP formulations. The cyaA gene encodes ACT, a soluble toxin of 1,706 amino acids (8), comprised of an adenylate cyclase (AC) domain and a repeats-in-toxin (RTX) domain (9). The RTX domain is responsible for the hemolytic activity of ACT (10). The cyclase domain can be delivered to eukaryotic cells, where it catalyzes ATP into supraphysiological concentrations of cyclic AMP (cAMP), which impairs many cell functions and leads to cell death (9). B. pertussis requires ACT to establish lethal infection in neonatal mice (11), where the toxin targets macrophages, neutrophils, dendritic cells, and other integrin αMβ2-expressing cells (9). ACT has both adenylate cyclase and hemolytic activities, and its hemolytic activity is required for B. pertussis penetration into the lung parenchyma (12). Underscoring its role in disease, AC enzyme activity has been detected in airway samples from a nonhuman primate model of pertussis infection (13), and humans and convalescent humans have antibodies against ACT (13–15). Furthermore, adenylate cyclase activity can be detected in wPs (16), and in our own mass spectral analysis we have confirmed the presence of ACT in wPs (unpublished).
In light of the role of ACT in virulence and the fact that B. pertussis infection induces production of anti-ACT antibodies, ACT has been evaluated as a protective antigen in preclinical murine immunization/challenge models. Before aPs replaced wPs in the United States and Europe, Guiso et al. demonstrated that a polypeptide consisting of the AC domain purified from B. pertussis was a protective antigen in mice (17). The same group demonstrated that full-length toxin purified from B. pertussis was protective (18). Subsequent studies showed that recombinant ACT was also a protective antigen (19) and an adjuvant (20). While immunization with ACT results in toxin-neutralizing antibodies, the presence of these antibodies does not correlate directly with protection. However, antibodies that recognize the C-terminal domain (RTX) are protective (19). These studies were further supported by evidence that the hemolytic domain-only antigen (RTX) was protective in mice against sublethal and lethal challenge with B. pertussis (19). In all of the aforementioned studies, ACT antigens were evaluated as single-antigen formulations without any of the aP antigens. Cheung et al. observed that inclusion of enzymatically inactive recombinant ACT enhanced protection of the aP (21) and that, unlike results of previous single-antigen studies, neither active nor enzymatically inactive ACT provided protection on its own (21). Cheung et al. also showed that inclusion of ACT in the aP skewed the T-helper cell responses from a humoral Th2 response to a more cell-mediated Th1 response (21).
Multiple studies have demonstrated the protective capacity of ACT (8). In order to determine the appropriate form of ACT for use in a human vaccine, it is necessary to identify a protective toxoid form that would be readily purified for mass production. Genetic mutation of the ACT coding sequence results in an enzymatically inactive form/molecule that is protective in mice (21). However, the use of a full-length ACT would be problematic for use in human vaccines due to the susceptibility of the toxin to proteolytic degradation (22) and because overexpression of ACT leads to formation of inclusion bodies in the Escherichia coli cytoplasm (23–25). A recent study confirmed that ACT is prone to aggregation and proteolysis (9). Wang et al. characterized a recombinant RTX antigen that (i) was readily purified, (ii) was immunodominant, and (iii) elicited neutralizing antibody production (9). While these results are very promising, it is essential to determine the efficacy of recombinant RTX antigen in preclinical models of pertussis, both alone and in combination with aP. It is likely that a new aP would still include PT, FHA, and FIM antigens. Our objective was to investigate the RTX antigen with the standard aP antigens as a multivalent formulation. In the present study, inclusion of the RTX antigen in an aP formulation was evaluated with a murine immunization and challenge model. We observed that immunization with RTX alone was not protective, and when either RTX or full-length ACT was added to the 1/5 human dose, no significant enhancement of protection occurred. The use of large amounts of the human aP in mice (such as 1/4, 1/5, or 1/10 human dose) relates to doses intended for commercial vaccine lot validation. It is known that as little as 1/50 human dose of aP is protective in mice (26). We hypothesized that identification of the minimal protective and maximal nonprotective aP doses would allow us to investigate enhancement of protection by inclusion of new antigens, thereby refining this evaluation platform. By performing a vaccine titration study using 2-fold dilutions, we were able to identify 1/80 human dose of aP as a minimal protective dose. When the RTX antigen was added to the 1/80 human dose, improved bacterial clearance was observed. Inclusion of RTX antigen also enhanced antibody production to PT, demonstrating the adjuvant capacity of RTX. The enhanced protection correlated with (i) decreased proinflammatory cytokine production, (ii) decreased blood and lung neutrophils, and (iii) decreased macrophages in the lung. This study begins to redefine murine immunization and B. pertussis challenge models for evaluation of pertussis vaccines and sheds light on the positive effects of including RTX as an antigen in the acellular vaccines.
RESULTS
Immunization with RTX alone does not enhance clearance of B. pertussis in CD-1 mice.
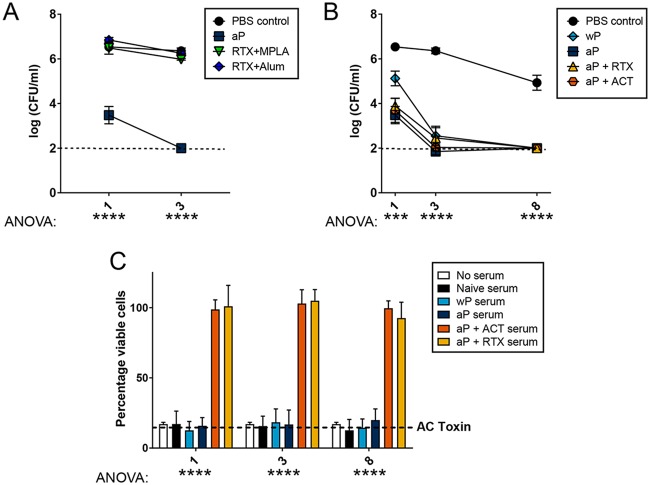
ACT plays a key role in the pathogenesis of B. pertussis, and many studies have shown that both native and recombinant ACTs can be adjuvants and/or antigens for protection against pertussis in murine challenge studies (17–20, 27–31). In this study, we aimed to systematically evaluate the RTX antigen (toxoid version of ACT, residues 751 to 1706) for inclusion into the acellular pertussis vaccine. Wang et al. previously described that recombinant RTX antigen was immunogenic and readily purified, making it an attractive candidate for further evaluation (9). Here, we immunized outbred CD-1 mice with the RTX antigen (see Table 1) formulated with alum, which is a Th2 adjuvant, or monophosphoryl lipid A (MPLA), which is a Toll-like receptor 4 (TLR4) agonist that induces Th1 responses. CD-1 mice were immunized intraperitoneally with phosphate-buffered saline (PBS) vehicle control (naive) or a 1/5 human dose of aP as a positive control for protection. Twenty-one days later, the mice were boosted with the same vaccines. Two weeks after boost, each of these groups of mice were challenged with 2 × 107 CFU of B. pertussis strain UT25Sm1 by intranasal administration. When this challenge dose of B. pertussis is intranasally administered to anesthetized mice, 26% of the initial dose is recoverable from the lung at 30 min postchallenge and only 5% (106 CFU) is recoverable at 4 h postchallenge (data not shown). These data suggest that many of the B. pertussis cells that were instilled by intranasal administration are killed by innate immune responses during the early stages of infection. However, this dose is sufficient to establish respiratory infection, as 4 × 106 CFU can be recovered in the airways 24 h postchallenge in naive (PBS control immunized) CD-1 mice (Fig. 1A).
TABLE 1.
Compositions of vaccines used for Fig. 1
| Vaccine component | Value for vaccine groupa: |
|||||
|---|---|---|---|---|---|---|
| RTX + alum | RTX + MPLA | aP (1/5 human dose) | aP + ACT | aP + RTX | wPb | |
| Pertussis toxoidc | 0 | 0 | 5 | 5 | 5 | 0.4 |
| Filamentous hemagglutinin | 0 | 0 | 5 | 5 | 5 | 3.5 |
| Pertactin | 0 | 0 | 1.6 | 1.6 | 1.6 | 0.3 |
| Adenylate cyclase toxin or RTX | 5.6 | 5.6 | 0 | 10 | 5.6 | 0.2 |
| Aluminum hydroxide | 0 | 125 | 125 | 125 | 125 | 0 |
| Other antigens/adjuvants | 0 | 20 | 0 | 0 | 0 | 62 |
All masses of antigens or adjuvant are indicated in μg.
This estimate is based on the number of peptides identified by mass spectrometry for each antigen in the wP (data not shown). The percentage is then used to estimate the potential mass based on the fact that the wP dose used in this study contained 66 μg of total protein.
For total pertussis toxin, the sum of PtxA, -B, -C, and -D peptides was combined.
FIG 1.
RTX-only immunization does not protect against B. pertussis challenge in CD-1 mice, and inclusion of RTX with 1/5 dose of aP does not improve clearance kinetics, but ACT-neutralizing antibodies are produced. (A) CD-1 mice were vaccinated with PBS vehicle, 1/5 human dose of aP, RTX (5.6 μg) plus MPLA (20 μg), RTX (5.6 μg) plus alum (125 μg), or PBS as a control vehicle (naive) (n = 4, with 4 technical replicates). Mice were intranasally challenged with 2 × 107 CFU of B. pertussis (UT25Sm1). At days 1 and 3 postchallenge the bacterial burdens were determined by culturing of homogenates of the lungs on BG agar. The dashed line represents the lower limit of detection due to plating. Bacterial loads in mice vaccinated with aP were significantly lower than those of the PBS control group at day 1 (P < 0.0001) and day 3 (P < 0.0001) in the lung. (B) CD-1 mice were vaccinated with PBS control vehicle, wP, aP, aP plus ACT, or aP plus RTX and challenged intranasally with UT25Sm1 (n = 4, with 4 technical replicates). Mice vaccinated with aP, aP plus ACT, and aP plus RTX had a significantly lower bacterial burden than the PBS control group at day 1 (P < 0.0003, P < 0.0006, and P < 0.0013) in the lung. Bacterial loads in all immunized mice were significantly lower than those of the PBS group at days 3 and 8 (P < 0.0001 for all groups) in the lung. Data in each group were compared to those of every other group at days 1, 3, or 8 postchallenge using one-way ANOVA with Tukey's post hoc test. (C) Serum from vaccinated/challenged mice was used to determine if ACT-neutralizing antibodies were produced following RTX or ACT inclusion in aP by adenylate cyclase toxin neutralization assay. J774A.1 macrophages were cultured with active ACT and serum from mice. Neutralization of ACT results in an increase of macrophage survival. Serum from vaccinated mice with aP plus ACT and aP plus RTX resulted in neutralization of ACT, unlike the case for unvaccinated, aP, and wP groups. Results are shown as means ± standard errors of the means (SEM). ****, P < 0.0001 by one-way ANOVA; ***, P < 0.001 by one-way ANOVA.
At 1 and 3 days after B. pertussis challenge, we enumerated the viable bacteria in the lungs, nares, and trachea of naive and aP-, RTX-plus-alum-, and RTX-plus-MPLA-immunized CD-1 mice (Fig. 1A; also see Fig. S1 in the supplemental material). The aP-immunized mice had 99.75% less B. pertussis at day 1 postchallenge than naive mice (Fig. 1A), and by day 3, the bacterial load was at or below our detection limit of 100 CFU. However, mice immunized with RTX plus alum or RTX plus MPLA were not significantly protected, as they contained the same amount of viable B. pertussis as naive mice (Fig. 1A and Fig. S1A and B). Interestingly, enzyme-linked immunosorbent assays (ELISAs) were performed to measure anti-ACT antibodies and showed that mice vaccinated with RTX in combination with alum or MPLA had detectable levels of antibodies in sera and lung homogenates (data not shown).
Vaccination with wP, aP, aP plus RTX, or aP plus ACT protects against B. pertussis challenge.
We hypothesized that inclusion of RTX into a standard acellular formulation would enhance protection against B. pertussis challenge in mice. To test this hypothesis, we compared aP and wP to aP supplemented with ACT or RTX in a murine vaccination challenge study. CD-1 mice were immunized as indicated in Table 1 and boosted 3 weeks after initial vaccination. At day 38 after initial vaccination, the mice were challenged with the B. pertussis strain UT25Sm1. At 1, 3, and 8 days postchallenge, mice were euthanized for determination of bacterial burden and collection of tissues and samples for analysis. Naive mice harbored similar amounts of B. pertussis in the lungs (Fig. 1B), trachea, and nares (Fig. S1C and D) as those observed previously (Fig. 1A). wP- and aP-vaccinated mice had significantly lower bacterial loads in the airways than nonvaccinated mice, and aP mice had the lowest bacterial burden at days 1 and 3. By day 8, all wP- or aP-immunized mice had cleared the infection to our detection limit (Fig. 1B). Overall these data show that, in CD-1 mice with UT25Sm1 as the challenge strain, the 1/5 human dose of aP is protective and that at that dose, adding RTX or ACT does not enhance bacterial clearance.
Serological analysis of naive and wP-, aP-, aP-plus-ACT-, and aP-plus-RTX-immunized mice.
ELISAs were performed, and we observed that alum- and MPLA-formulated RTX or ACT vaccines induced antibodies that recognize ACT (data not shown). Additionally, 1/5 aP supplemented with either RTX or ACT induced detectable antibody production. Surprisingly, we did not observe any antibodies that would recognize ACT in the wP-immunized mice (data not shown). The lack of anti-ACT in wP-immunized mice is likely due to the fact that when we analyze the wP with shotgun proteomics, we only observe 4 peptides of ACT per 1,000 total peptides in the whole-cell vaccine, which shows that ACT makes up a minor amount of the total protein content (data not shown).
AC toxin-neutralizing capacity of serum antibodies.
Due to the lack of noticeable effects on the rate of clearance of B. pertussis, it was unclear that the anti-ACT and anti-RTX antibodies influenced clearance of B. pertussis in the mouse. ACT has potent toxic effects against CD-11b/CD-18-expressing phagocytes, such as macrophages or neutrophils (32–34). To determine the functional capacity of the serum antibodies induced via immunization, we utilized the AC toxin neutralization assay with J774A.1 cells (35). Only sera obtained from mice vaccinated with aP plus ACT or aP plus RTX were able to neutralize the toxicity of ACT in J774A.1 macrophages (Fig. 1C). We did not observe detectable anti-ACT antibodies in the serum from wP mice, and this serum did not neutralize ACT in vitro (Fig. 1C). The AC toxin neutralization data suggest that immunization with RTX can induce production of antibodies that can neutralize the AC toxin in vitro.
Identification of the optimal aP murine dose to determine synergy between aP and RTX.
Our data and previously published studies (9) show that RTX is immunogenic and could contribute to protection against B. pertussis when used as an antigen. However, we hypothesized that the dose of aP used (1/5 human dose) in these studies is too high to observe synergy between aP and RTX. Therefore, we sought to define a physiologically appropriate aP dose for the mouse that could be used to evaluate the effects of inclusion of new antigens into the aP. Guiso et al. demonstrated that 1 full human dose of DTaP was most protective (SmithKline Beecham Biologicals) (36) and that 1/4 and 1/10 of the human dose were protective yet required more time than a full human dose to result in sterilizing immunity. Alvarez Hayes et al. (37) used 1/5 human dose and supplemented it with an iron-regulated protein, AfuA (B. pertussis 1605), and observed modest improvement in bacterial clearance compared to 1/5 human DTaP (Infanrix; GSK). In other recent studies, 1/50 human DTaP (Infanrix; GSK) was partially protective in BALB/c mice challenged with B3 lineage strain B1917 (26, 38). These two studies encouraged us to determine if lowering the aP dose could allow us to detect measurable improvements in bacterial clearance when additional antigens improve the vaccine.
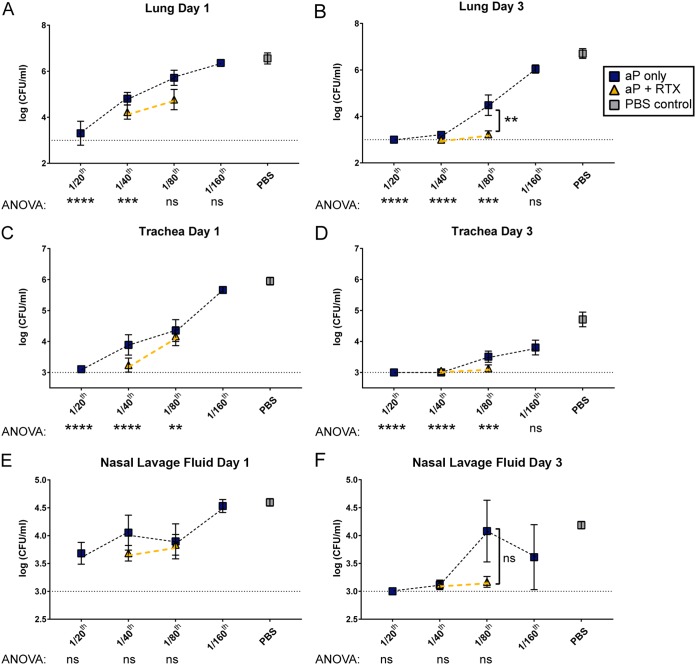
CD-1 mice were immunized with aP doses of 1/20, 1/40, 1/80, and 1/160 human dose (see Table 1) to titrate the vaccine and identify both the effective and noneffective doses. These mice were challenged with B. pertussis, and viable bacteria in the lungs, trachea, and nasal lavage fluid were determined at 1 and 3 days postchallenge (Fig. 2). At day 1 postchallenge, the 1/20 aP group had the lowest bacterial burden, and at day 3, both the 1/20 and 1/40 doses were near our detection limit (Fig. 2A and B) in the lungs. A similar trend was observed in the trachea and nasal lavage fluid (Fig. 2C to F). However, the 1/80 aP group had 3.5 logs more viable B. pertussis in the lungs than the 1/40 aP group, suggesting that the 1/80 dose was below the aP dose for maximum protection in CD-1 mice (Fig. 2B). The noneffective dose for trachea seemed to be higher than 1/160 (Fig. 2C and D), whereas the noneffective dose for the nasal lavage fluid was 1/160 (Fig. 2E and F). Based on these data, we selected the 1/40 (minimal effective dose) and 1/80 (maximal noneffective dose) doses to supplement with AC toxoid antigen (RTX).
FIG 2.
Respiratory tract bacterial burden of CD-1 mice immunized with aPs or aPs supplemented with RTX antigen. Immunization with 1/20, 1/40, 1/80, and 1/160 human dose of aP was used to titrate the minimal effective dose and the maximum noneffective dose. RTX (5.6 μg) was included in 1/40 and 1/80 aP doses. Vaccinated CD-1 mice were challenged with 2 × 107 CFU of B. pertussis, and bacterial burden was determined at days 1 and 3. CFU counts were determined from lung homogenate (A and B), trachea homogenate (C and D), and nasal lavage fluid (E and F). Results are means ± SEM (n = 4 to 8, with 4 technical replicates) from independent experiments. ns, not significant; ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05. P values were determined by one-way ANOVA with Tukey's post hoc test and compared to naive mice (PBS control). The dashed line represents the lower limit of detection due to plating. The blue boxes indicate aP groups, whereas aP-plus-RTX groups are indicated by gold triangles. Gray squares indicate mock-vaccinated and -challenged mice. In panels E and F there were no statistically significant comparisons between the aP titration doses, as determined by ANOVA.
We hypothesized that neutralization of ACT in vivo would enhance bacterial clearance and that inclusion of RTX would improve the protection provided by the 1/80 dose, as demonstrated by decreasing viable bacteria in the lung. Testing the inclusion of RTX in the 1/40 dose would allow for evaluation of other subtler variables, such as cytokine production, phagocyte recruitment, and serology. CD-1 mice were therefore immunized with either a 1/40 or a 1/80 dose of aP supplemented with RTX antigen (see Table 2). Inclusion of RTX into the 1/40 dose of aP did not affect the bacterial burden at day 3 in the lungs (Fig. 2B) compared to 1/40 aP alone, as we predicted. When RTX was added to 1/80 aP, we saw a significant decrease in viable bacteria in the lungs at day 3 (Fig. 2B). In light of this observation, it was necessary to measure other metrics in addition to viable bacteria to further support this finding.
TABLE 2.
Compositions of vaccines used in the titration of aP and RTX supplementation studies for Fig. 2 to 6
| Vaccine component | Value for vaccine groupa: |
|||||
|---|---|---|---|---|---|---|
| 1/20 aP | 1/40 aP | 1/40 aP + RTX | 1/80 aP | 1/80 aP + RTX | 1/160 aP | |
| Pertussis toxoid | 1.25 | 0.63 | 0.63 | 0.33 | 0.33 | 0.16 |
| Filamentous hemagglutinin | 1.25 | 0.63 | 0.63 | 0.33 | 0.33 | 0.16 |
| Pertactin | 0.4 | 0.2 | 0.2 | 0.1 | 0.1 | 0.05 |
| Adenylate cyclase toxin or RTX | 0 | 0 | 5.6 | 0 | 5.6 | 0 |
| Aluminum hydroxide | 31.25 | 15.63 | 15.63 | 7.81 | 7.81 | 3.91 |
All masses of antigens or adjuvant are indicated in μg.
In the trachea and nasal lavage fluid, we observed an inverse relationship between bacterial burden and decreasing vaccine concentration similar to that in the lungs. In the trachea, there was minimal improvement of bacterial clearance with inclusion of RTX to the 1/40 or 1/80 base aP. In the nares, adding RTX to 1/40 aP decreased the bacterial burden at day 1, but this difference was not significant (P = 0.29), and no differences were observed at day 3. Overall, these data highlight the need to consider the location of B. pertussis in the host with respect to vaccine efficacy in a challenge trial and also the utility of identifying subeffective murine vaccine doses to determine if there are effects of adding new antigens to a formulation.
Serological analysis of antibodies recognizing PT and RTX antigens.
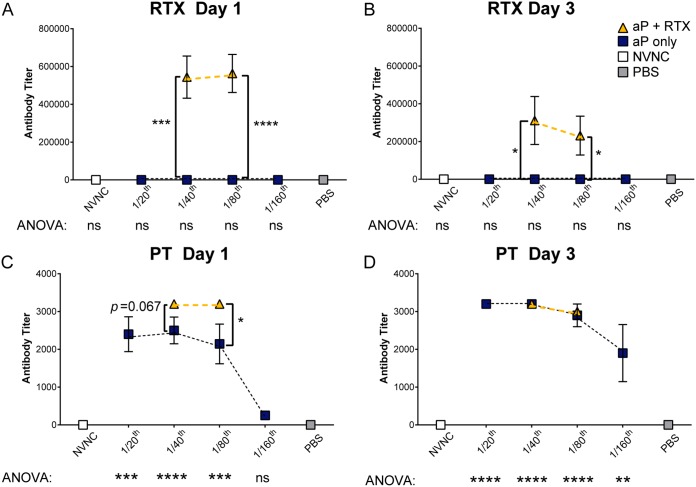
In our aP immunization titration strategy, we expected that as the aP dose decreased, the concentration of anti-PT in the serum of immunized and challenged mice would also decrease. Given that mice immunized with 1/40 aP had minimal bacterial burden in all respiratory tract locations (Fig. 2), we hypothesized that 1/40 aP would induce production of high concentrations of anti-PT antibodies in serum. We also hypothesized that inclusion of the RTX antigen in the 1/40 dose would result in production of a significant amount of anti-RTX antibodies. As expected, immunization with RTX in both 1/40 and 1/80 aP doses induced production of antibodies that recognize the RTX antigen (Fig. 3A and B). Surprisingly, there was very little difference between the amounts of detectable anti-RTX in 1/40 and 1/80 doses despite the fact that there was 50% less alum adjuvant in the 1/80 than the 1/40 dose. We also observed that as we decreased PT antigen and alum adjuvant by diluting the vaccine, less anti-PT was produced in response to B. pertussis challenge (Fig. 3C and D). At day 1 we saw a dose-response curve for anti-PT respective of the aP dose (Fig. 3C). Anti-PT decreased, as would be expected, since PT antigen and alum adjuvant are decreasing respective to the vaccine dose.
FIG 3.
Serological responses from mice immunized with aP titrations or aPs supplemented with RTX. (A and B) ELISA was used to determine the anti-RTX IgG titers in serum of immunized and challenged mice at 1 and 3 days postchallenge. (C and D) Anti-PT IgG titers in serum of immunized and challenged mice at the indicated time points. Titrations of aP were compared by one-way ANOVA with Tukey's post hoc test, shown below graphs, and compared to the PBS group. Unpaired two-way t test was used to compare addition of RTX to aP titrations, shown by brackets. Results plotted on the graph are means ± SEM. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
The addition of RTX in the 1/40 and 1/80 doses significantly increased antibody production to PT compared to the same dilution of aP alone (P = 0.067 and P = 0.049, respectively) at day 1 postchallenge. However, when antibody concentrations were calculated, we observed anti-PT antibody concentrations decreasing at day 3 (Fig. S5). This may be due to clearance of antigen-antibody by day 3 postchallenge. More kinetics studies should be performed on these dual-antigen considerations, especially with antigens like PT and RTX, which both are classified as immunogens due to their adjuvant properties.
Analysis of cytokine secretion as a function of aP immunization and inclusion of RTX antigen.
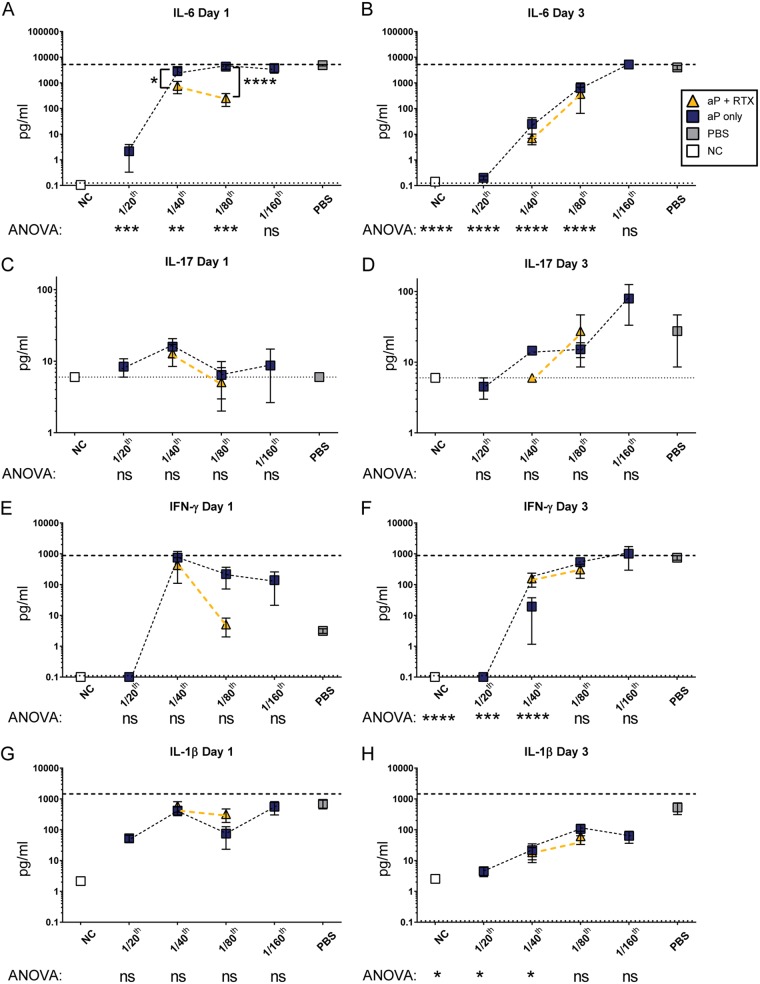
Because including RTX in the 1/80 aP dose helps lower the bacterial burden in the lungs, we then characterized lung cytokine profiles to better understand the type of immune signaling factors released during infection and their association with bacterial clearance. To do so, we measured cytokines in lung homogenate of vaccinated and naive mice at 1 and 3 days postchallenge. Interleukin-6 (IL-6) is one of the most important cytokines involved in the immune response to B. pertussis. Respiratory epithelial cells and macrophages have increased IL-6 mRNA and protein in response to B. pertussis in vitro (39, 40), and C57BL/6 mice have high IL-6 secretion in response to B. pertussis infection (40). Specifically, ACT has been shown to induce IL-6 expression (41). Furthermore, IL-6 knockout mice have increased B. pertussis burden in the respiratory tract compared to that of wild-type mice due to decreased recruitment of leukocytes (40). Based on these studies and our preliminary cytokine data (Fig. S2), we anticipated that as we titrated down the aP dose, we would observe increased IL-6 correlating with the increased bacterial burden (Fig. 4A and B). If antibodies against RTX can neutralize ACT in vivo, we expected to observe decreased IL-6 levels in RTX-vaccinated mice. Our data confirmed this idea at day 1, but at day 3 postchallenge, RTX did not affect IL-6 secretion in the 1/80 vaccine dilution group (Fig. 4B).
FIG 4.
Analysis of proinflammatory cytokines from lungs of mice immunized with aP titrations or aPs supplemented with RTX. (A to H) Th1-associated cytokines from supernatant of lung homogenates analyzed at days 1 and 3 postchallenge. Cytokines IL-6 (A and B), IL-17 (C and D), IFN-γ (E and F), and IL-1β (G and H) are shown. The blue boxes indicate aP groups, whereas aP-plus-RTX groups are indicated by gold triangles. Gray squares indicate mock-vaccinated and -challenged mice, while white squares represent nonvaccinated and nonchallenged mice (NC). Results are means ± SEM. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05. P values were determined by one-way ANOVA with Tukey's post hoc test compared to the PBS (naive) group of challenged mice. Brackets between two points represent unpaired two-way t test. A dashed line indicates the upper limit of detection, and a dotted line indicates the lower limit of detection.
IL-6 is related to induction of Th17 responses, and in the case of B. pertussis infection, PT facilitates induction of IL-17 production typically around 7 days after B. pertussis challenge (42). At day 1, no statistically significant changes were observed in IL-17 across the groups. However, at day 3, as the aP dose decreased, the IL-17 level was slightly increased until it was significantly different at 1/160 human dose (Fig. 4D). It was surprising that naive infected mice did not have significantly more IL-17 than mice vaccinated with the 1/160 dose. We interpret these data to mean that (i) 1 and 3 days are early for IL-17 responses to B. pertussis, as we saw biological variability within the groups, and (ii) these diluted vaccines likely are capable of eliciting PT-neutralizing antibodies. We did not observe any effect on IL-17 concentrations due to including RTX in either the 1/40 or the 1/80 dose (Fig. 4C and D).
B. pertussis challenge of mice induces interferon gamma (IFN-γ) expression, which is necessary for clearance of B. pertussis from mice (43). Furthermore, IFN-γ receptor-deficient mice challenged with B. pertussis display atypical disease, leading to dissemination of B. pertussis and death of the mice within several weeks (44). Since aP immunization results in Th2 polarization, we expected that as the aP was titrated to less effective doses, we would see an increase in IFN-γ induced by the natural Th1 response to B. pertussis infection (45). At day 1, the 1/40 aP and the 1/80 aP doses displayed similar IFN-γ levels in the lung. However, when RTX was added to the 1/80 dose, we observed a striking reduction of IFN-γ at day 1 only. It is also interesting that naive infected mice had lower IFN-γ levels than 1/40, 1/80, or 1/160 aP groups at day 1 (Fig. 4E), yet a high IFN-γ level was observed in the naive infected mice at day 3 (Fig. 4F).
IL-1β mediates clearance of B. pertussis and resolution of inflammation in mice (46). IL-1β-deficient mice have large amounts of IL-17 that are observed at 7 days postchallenge and high IFN-γ levels at 14 days postchallenge (46). We wondered if neutralization of ACT in vivo would alter IL-1β production in the lungs of the mice. All challenged mice had very similar IL-1β levels at day 1 (Fig. 4G), and the levels of IL-1β were lowest in the 1/20 aP group at day 3 (Fig. 4H). Addition of RTX did not significantly decrease IL-1β, which suggests that IL-1β is induced independently of ACT activity or bacterial burden.
We measured other cytokines, such as IL-5, tumor necrosis factor alpha (TNF-α), KC Gro, IL-12p70, IL-10, IL-2, and IL-4, in the lung homogenates of the vaccine groups (Fig. S3 and S4). Vaccination with aP plus RTX resulted in lower IL-5 levels at day 1 in both the 1/40 and 1/80 groups, yet these groups returned to base aP IL-5 levels at day 5 (Fig. S4). IL-2 followed a trend similar to that of IL-5 (Fig. S4). We observed that Th1-related responses, such as those of IFN-γ, were decreased by the addition of RTX to the aP.
Neutrophil infiltration kinetics are not affected by inclusion of RTX in the aP.
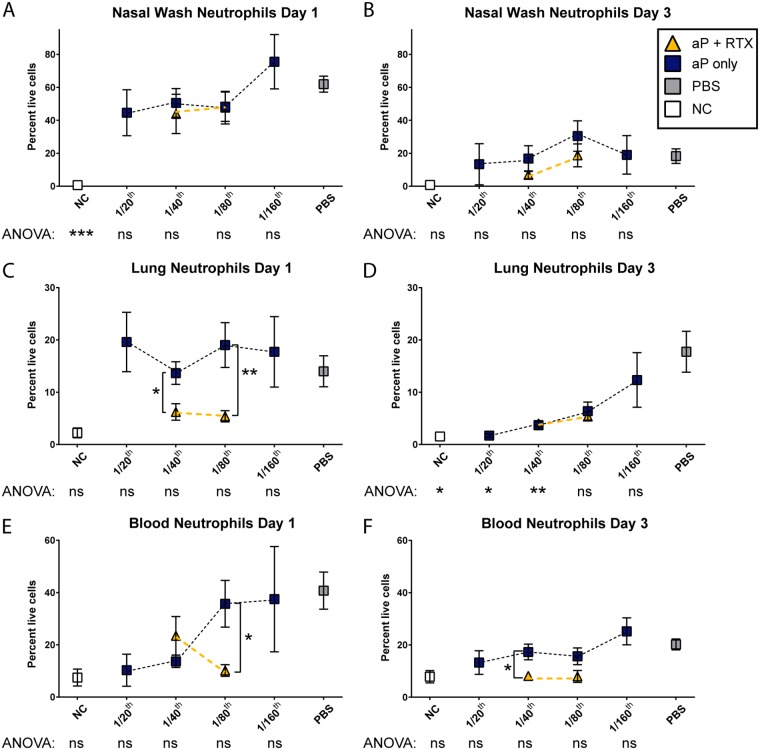
Pertussis is characterized by an increase in the number of circulating white blood cells. This phenomenon, known as leukocytosis, can lead to lung hypertension and death in the most severe cases. ACT targets the CR3 receptor expressed on many cell types, including neutrophils (47). Based on this, we hypothesize that neutralization of ACT in vivo would alter neutrophil kinetics and recruitment profiles in the blood and airway. Andreasen et al. reported that in naive mice, neutrophils did not play a role in clearance of B. pertussis from the airway (42, 48). On the other hand, neutrophils played an essential role in immune mice in the same study. Another study showed that PT inhibited recruitment of neutrophils to the lung and that neutrophils played a role in antibody-directed clearance of B. pertussis by the Fcγ receptor (49). As the dose of the aP decreased, we observed an increase in lung and blood neutrophils with the 1/160 dose, and neutrophil percentages were similar to those of naive B. pertussis-challenged mice (Fig. 5); however, this increase was not observed in the nasal lavage fluid (Fig. 5A and B). It is likely that as the aP dose decreased, there was less PT neutralization due to a decrease in anti-PT titers. Lung neutrophil populations of mice immunized with inclusion of RTX into the 1/40 and 1/80 doses were significantly reduced (P = 0.0142 and P = 0.008, respectively) compared to those of mice immunized with aP only at the same doses (Fig. 5C); however, by day 3, this reduction was no longer significant, because neutrophil levels had returned to levels observed in nonchallenged mice (Fig. 5D). We observed lower circulating neutrophil populations in the blood at both days when RTX was included than with the aP-only doses (Fig. 5F) (1/40, P = 0.018; 1/80, P = 0.071). Collectively, these data suggest that inclusion of RTX into the aP affects the neutrophil populations in the respiratory tissues and circulating neutrophils in the blood.
FIG 5.
Flow cytometric analysis of blood, pulmonary, and nasal lavage fluid neutrophils from mice immunized with aP titrations or aPs supplemented with RTX. Quantification of the percentage of live, single cells that were classified as neutrophils (GR-1hi CD-11b+) from nasal lavage fluid (A and B), lung homogenate (C and D), or blood (E and F). Results are means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05. P values were determined by one-way ANOVA with Tukey's post hoc test compared to the PBS (naive) group of challenged mice. Brackets between two points represent unpaired two-way t test.
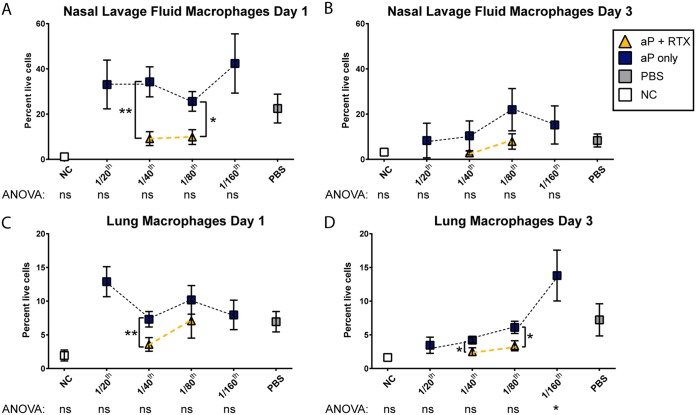
Decreased macrophages in the respiratory tract due to inclusion of RTX into the aP.
Macrophages are a primary target of ACT and are readily killed by the toxin (32, 34). We hypothesized that as ACT is neutralized in vivo, there is an increase in macrophages due to their increased survival. However, our data did not support this hypothesis, and instead we saw that both RTX-supplemented aP groups had lower levels of macrophages in the nasal lavage fluid at day 1 than did the aP-only groups (Fig. 6A). Inclusion of RTX decreased the overall numbers of macrophages in both the 1/40 and 1/80 base aP groups (Fig. 6C and D). At day 3, we observed that mice vaccinated with 1/40, 1/80, and 1/160 aP showed increasing amounts of macrophages compared to naive and naive challenged mice (Fig. 6B and D). As observed for other parameters measured, this effect might be due to an induction of a naive Th1 response due to insufficient aP-driven Th2 as a result of our decrease in aP dose.
FIG 6.
Flow cytometric analysis of macrophages from lung and nasal lavage fluid of mice immunized with aP titrations or aPs supplemented with RTX. (A to D) Percentages of macrophages (defined as CD-11b+ F4/80+) from live, single cells from nasal lavage fluid (A and B) and single-cell suspensions prepared from lung homogenate (C and D). Results shown are means ± SEM. **, P < 0.01; *, P < 0.05. P values were determined by one-way ANOVA with Tukey's post hoc test compared to the PBS (naive) group of challenged mice. Brackets between two points represent unpaired two-way t test.
Modeling the relationships among antibodies against B. pertussis toxins, proinflammatory signals, phagocytes, and clearance of B. pertussis from the murine respiratory tract.
The Kendrick test was used to establish units of efficacy for whole-cell vaccines in mice to estimate the dose to be used in humans (2). The murine intranasal infection model was then developed to validate aPs (36, 50). Since the introduction of Tdap, numerous preclinical studies have been performed to evaluate new antigens or adjuvants to improve aPs. However, we argue that there is a need for standardization of the murine evaluation platform to facilitate the development of these formulations and transition to other models, such as the baboon. In this study, we titrated DTaP and found a dose (1/80 human) that was partially protective with the B. pertussis strain (UT25Sm1) and the challenge dose of 2 × 107 CFU that were used. It is clear that decreases in bacterial burden are indicative of protection; however, we aimed to visually represent all of the data collected to support our conclusions. For this comparison, we represented the increases and decreases for each variable relative to the no vaccine/no challenge group using a 64-color scheme (Fig. S7). If the relative units increase, the color changes to red; if they decrease, then the value is represented in blue. We then plotted the average values per immunization group (1/20 to 1/160) in relation to the RTX-supplemented groups. As one visually compares 1/80 to 1/80 plus RTX at day 1 or 3, it is apparent that adding RTX decreases all values toward the green or no relative change, which we interpret as overall protection. It is also important that values at day 1 are relatively higher than those at day 3, and this relates to the fact that the total amount of bacteria is decreasing. The amount of anti-PT or anti-RTX is shown in a color scale of black to gray to white (Fig. S7). There is a clear correlation between amount of anti-PT and overall protection. In addition, it is interesting that inclusion of RTX enhanced the anti-PT levels in the aP-plus-RTX groups. Overall, variables associated with inflammation, such as cytokines or phagocytes, tend to return to green or naive noninfected levels in mice vaccinated with aP plus RTX. We only observed five relatively large decreases (indicated in blue). We also saw a relative decrease in the number of macrophages present in the nasal lavage fluid at day 3 in the naive group. This decrease could be attributed to large amounts of PT and ACT secreted during infection, which could impair normal macrophage recruitment in the airway. We also saw large relative decreases in macrophages in the RTX-supplemented 1/40 and 1/80 groups at day 3, and reasons for these changes are described above. Combining these data allows us to consider the overall inflammatory response in relation to the vaccination status to further demonstrate the effects of including RTX in aP formulations and to begin to establish a multivariable approach to determining the efficacy of experimental vaccines in the mouse.
DISCUSSION
There is a resurgence of whooping cough that coincides with the switch to the use of aPs. In this study, we aimed to accomplish two goals. First, we sought to define an aP dose or doses that allow for the evaluation of synergy between the current aP and potential new antigens. The second goal was to evaluate RTX as an antigen for inclusion in acellular pertussis vaccines in the murine challenge model. As a result of these studies, we now better appreciate the need to continue to refine the methodologies of improving aPs in the murine model.
We initiated this study by evaluating the efficacy of ACT and RTX as single vaccine antigens. Immunization with ACT or RTX alone did not induce significant protection in CD-1 mice with the UT25Sm1 B. pertussis challenge strain (Fig. 1). The AC domain of ACT was first described as a protective antigen by Guiso et al. (17). In that study, the antigen preparation that was used consisted of two polypeptides of 43 and 45 kDa. We now know that these polypeptides corresponded to the AC domain of ACT. In those studies, BALB/c mice were vaccinated twice with AC polypeptide absorbed on aluminum hydroxide adjuvant, and it was observed that 80% of the AC-vaccinated mice survived B. pertussis challenge with strain 18323 (17). The aforementioned study was then further supported by a subsequent study by the same group that showed the protective capacity of the AC domain and full-length recombinant ACT protein (27). Several more studies showed that (i) CyaC acylation of ACT was required for the protective activity (18) and (ii) the C-terminal domain was also required for protection with recombinant ACT. Full-length recombinant ACT was protective when 15 μg of antigen was mixed and absorbed with 250 μg of alum (19). We used less antigen and adjuvant than Betsou et al. (either 10 μg of ACT or 5.6 μg of RTX with 125 μg of alum), and we did not observe protection in CD-1 mice (Fig. 1A). The use of the MPLA adjuvant did not improve bacterial clearance compared to that of alum. An apparent difference between our study and that of Betsou et al. is that strain 18323 was used, which has been suggested to be an outlier compared to most other global B. pertussis strains. Guiso et al. noted that strain 18323 produces less PT than the Tohama I type strain (27). From our own studies, we know that UT25Sm1 produces more PT (data not shown) and ACT (51) than Tohama I. This information highlights the importance of the challenge strain. Another caveat of our study is that Betsou et al. absorbed the ACT antigen to alum, which we did not do with RTX. It is possible that absorbing RTX to the aP could further enhance the synergistic protection observed in this study when RTX was combined with the alum-containing aP. An additional key aspect to consider is the potential endotoxin contamination in recombinant ACT preparations. Villarino Romero et al. used recombinant ACT but did not observe the same bacterial clearance as Betsou et al. (19, 31). ACT used by Cheung et al. was highly purified of endotoxin (21), as was the RTX that we used in this study (9). It is possible that endotoxin levels in ACT preparations influence the overall protection afforded by such antigens, as they may induce a more Th1-biased response.
Several of the aforementioned studies indicated that single-antigen vaccines containing ACT were protective, but the study by Cheung et al. and our RTX-only study (Fig. 1) were not in concordance. Other recent studies testing single-antigen vaccines composed of proteins expressed during biofilm formation or in response to iron starvation showed that very few of these single-antigen formulations were protective on their own (26, 38). Given that the aP antigens of either DTaP or Tdap (PT, FHA, PRN, and/or FIM) do provide some protection against disease, we hypothesize that new antigens to be included in the aP should be tested not only alone but also in combination with aP antigens. Only one study has investigated ACT as an antigen in combination with the aP in mice (21). Cheung et al. reported that adding 12.5 or 25 μg to 1/8 aP significantly decreased bacterial burden in mice at 7 days postchallenge with strain 18323 (21). PT and FHA are formulated in the DTaP (Infanrix) at 25 μg each in 1 human dose. Therefore, the use of 25 μg ACT is roughly equal to 1 human dose of antigen (assuming no adjustment for molar mass of each antigen). We chose to test the utility of RTX antigen with lower antigen doses in an effort to best model what would be scaled toward a commercial vaccine dose. To do so, we identified a partially protective dose of aP so that synergistic effects of addition of the antigen could be tested. We titrated the aP from 1/20 to 1/160 human dose. As decreasing aP doses were tested, we saw an increase in innate immune responses to levels observed in naive challenged mice (see Fig. S6 in the supplemental material). From the bacterial burden in the lung, we determined that 1/40 dose of aP is protective, while 1/80 is only partially protective. However, it is important to note that 1/40 aP immunization was nonprotective in the nares at day 1, but at that dose, B. pertussis was cleared by day 3 (Fig. 2E and F). These data show that the protective capacity of an aP dose in the mouse is dependent on the location in the airway and the time elapsed since B. pertussis challenge. Using total clearance in all locations, 1/40 aP immunization results in sterilizing immunity against our challenge dose, as observed at 3 days postchallenge. Based on these data, we selected the 1/40 (minimal effective dose) and 1/80 (maximal noneffective dose) doses to supplement with AC toxoid antigen (RTX).
We identified the 1/80 aP dose as one that would allow us to tease out RTX antigen effects that would otherwise be masked by the use of a high mouse-to-human aP dose (e.g., 1/5). With the 1/80 aP dose, it is clear that innate responses are occurring due to the lack of protection provided by the immunization. We hypothesized that decreases in innate responses would correlate with induction of antibody production that either neutralizes the effects of the pathogen (e.g., toxin PT or ACT) or results in killing of the pathogen (e.g., opsonization, phagocytosis, or complement killing). In our study, we detected antibodies that recognized RTX (Fig. 3A and B), and we saw that adding RTX enhanced production of anti-PT (Fig. 3A). Additionally, when we supplemented the 1/80 aP dose with RTX, we were able to observe positive effects on the response to B. pertussis challenge, such as decreased bacterial burden (Fig. 2B) and proinflammatory cytokine secretion (Fig. 4 and Fig. S4). We speculate that the neutralization of ACT in vivo leads to a decrease in the innate immune responses at day 1 postchallenge, but at day 3 the levels of these proinflammatory cytokines were not affected by the inclusion of RTX (Fig. 4B and F and Fig. S4). The decrease in innate immune response was supported by the reduction in neutrophils in the lungs and nares by day 3 postchallenge in the RTX-supplemented groups compared to those for nonvaccinated mice (Fig. 5). Overall, by first optimizing murine immunization with aP, we were able to induce a protective response with RTX at an immunization dose that, without RTX, was not protective.
B. pertussis strains have evolved in response to aP immunization (52). Pertactin is no longer expressed by circulating B. pertussis strains (53), and recently a B. pertussis strain that lacked PT was isolated and sequenced (54). B. parapertussis is not capable of expressing PT (55) but causes as many as ∼15% of whooping cough cases. Bordetella holmesii is an underappreciated pathogen that causes whooping cough-like illness and does not express PT (56). In addition, there are unpublished reports of strains sequenced by the CDC that lack expression of filamentous hemagglutinin. Given the potential rise of triple-mutant strains that do not make pertactin, FHA, or PT, it is possible that the efficacy of aP protection will further decline if strains arise that lack the main antigens that most aPs contain. Based on these possibilities, we propose that adding antigens that are both immunogenic and important for bacterial virulence is necessary to slow the progression of B. pertussis evolution (57) and the resistance to aP-mediated immunity.
We aimed to investigate ACT as a vaccine antigen, and we developed a new evaluation approach involving analysis of multiple correlates of protection (Fig. S7). Our data, based on what we observed in CD-1 mice, suggest that RTX antigen enhances protection of aPs. The different protection levels observed in each of the regions of the airway (nares, trachea, and lungs) suggest that it also is advantageous to consider the development of mucosal B. pertussis vaccines. This would allow for the pathogen to be neutralized rapidly in the upper airway before infection is fully established, when highly potent PT and ACT are impairing the innate and adaptive immune responses. Higgs et al. (58) proposed that aPs containing antigens that direct a more diverse response are more effective. While we have focused on adding RTX to a Th2-inducing aP, it would be very interesting to formulate and test RTX-containing vaccines with Th1 adjuvants. Neutralization of ACT along with a cellular response would likely be an ideal protection profile and possibly address the waning protection issue of the aP. Our study was focused on RTX, but there are other candidates that should be evaluated as well. Furthermore, this study highlights the importance of titrating the vaccine dose for the model system, and we expect this will provide new methodologies for the development of pertussis vaccines. Significant research is still necessary to formulate and evaluate safe and more effective next-generation pertussis vaccines, which could include ACT as an antigen.
MATERIALS AND METHODS
ACT and RTX antigen expression and purification.
ACT and RTX antigens were expressed and purified as previously described (9). Following purification, antigens were stripped of endotoxin with Dextoxi-Gel endotoxin-removing gel (20399; Thermo Scientific) and Zeba Spin columns (89896; Thermo Scientific). Endotoxin purity was quantified with a Pierce LAL chromogenic endotoxin quantitation kit (88282; Thermo Scientific).
Composition of vaccines used in study and administration.
The Infanrix (GSK) human vaccine (diphtheria, tetanus, and pertussis) was the aP used in this study. For the purpose of vaccinating mice, 1/5 of the human dose was used (100 μl). To test ACT as an additional antigen, full-length ACT or RTX-ACT was purified as previously described (9). Full-length ACT (10 μg) or RTX (5.6 μg) was used for vaccination. Vaccine compositions are shown in Tables 1 and 2. Alum and MPLA were obtained from InvivoGen. The National Institute for Biological Standards and Control WHO standard whole-cell pertussis vaccine (NIBSC code 94/532) was used as the wP. One ampoule of NIBSC wP contains 40 IU (10 human doses). For murine vaccination, the whole ampoule was diluted in 1 ml of sterile PBS, and 20 μl (1/5 of the human dose, 66 μg of total protein) was used. The NIBSC wP standard used in this study has been used in other studies as a wP (59). The NIBSC wP does not contain alum adjuvant, whereas commercial wP contains alum to induce antibody production to the diphtheria and tetanus toxoids. The adjuvant alum was only added to single-antigen vaccines (Table 1). Infanrix is formulated with alum, so we did not include additional alum for aP titration and aP supplementation with RTX. Unvaccinated mice received 200 μl of sterile PBS as a vehicle control. Prior to administration, aP, aP-ACT, aP-RTX, and wP doses were all diluted into a final volume of 200 μl with sterile PBS and administered by intraperitoneal injection.
B. pertussis strains and growth conditions.
B. pertussis strain UT25Sm1 (60) was used in all experiments of this study. UT25Sm1 was cultured on Bordet Gengou (BG) agar (Remel) (61) supplemented with 15% defibrinated sheep blood (Hemostat Laboratories) for 48 h at 36°C. Bacteria were transferred from BG plates to a new, previously unused glass flask with 20 ml of modified Stainer-Scholte liquid medium (SSM) (62). SSM cultures were not supplemented with cyclodextrin (heptakis). B. pertussis cultures in SSM were grown for ∼22 h at 36°C with shaking at 180 rpm, at which time the optical density at 600 nm (OD600) was ∼0.6.
Vaccination and challenge with B. pertussis.
Four-week-old CD-1 (outbred; strain code 022) mice were obtained from Charles River Laboratories. Mice were vaccinated at 5 weeks of age with the vaccines described (Tables 1 and 2) and then were boosted with the same vaccines 21 days later. Thirty-eight days after initial vaccination, B. pertussis UT25Sm1 was grown as described above and cultures were diluted to provide a challenge dose of 2 × 107 CFU in 20 μl. Mice were anesthetized by intraperitoneal injection of 200 μl of ketamine (6.7 mg/ml) and xylazine (1.3 mg/ml) in 0.9% saline. Mice received a 20-μl total challenge dose by pipetting 10 μl directly into each nostril of the mouse. Four to eight mice per group were challenged, and at 1, 3, and 8 days postinfection mice were euthanized. Blood was collected by cardiac puncture, and serum was separated by centrifugation through a BD Microtainer blood collector and stored at −80°C until analysis. Trachea and lungs were removed and separately homogenized with Dounce homogenizers for determination of bacterial burden in each tissue. To determine the number of B. pertussis organisms in the nares, 1 ml of PBS was flushed through the nares and collected. Serial dilutions in PBS were plated on BG containing streptomycin (100 μg/ml) to ensure that only UT25Sm1 B. pertussis was cultured. All murine infection experiments were performed according to protocols approved by the West Virginia University Animal Care and Use Committee (protocol number 14-1211.10).
Cytokine analysis.
Lung homogenates were pelleted by centrifugation, and the supernatants were collected and stored at −80°C until analysis. The concentration of cytokines in the lungs and serum of vaccinated and challenged mice was determined by a Raybiotech mouse inflammation array C1 (AAM-INF-1-8) by following the manufacturer's instructions. A total of 100 μl of lung homogenate supernatants or serum was pooled from 4 mice for each analysis. Quantitative analysis of the concentration of cytokines in the lungs of mice was determined with the Meso Scale Discovery V-PLEX proinflammatory panel (K15048G-1) or mouse IL-17 ultrasensitive kits (K152ATC-1) per the manufacturer's instructions.
ACT neutralization assay.
ACT is, through a combination of mechanisms, cytotoxic for J774A.1 (ATCC TIB-67) macrophage-like cells, and protection against this effect is the basis for the toxin neutralization assay (35). J774A.1 cells were cultured in Dulbecco's modified Eagle's medium with 25 mM glucose (Gibco). J774A.1 cells (30,000 in 90 μl) were seeded in each well of a 96-well plate and allowed to attach overnight. ACT was incubated with 1/5 dilution of serum samples for 10 min with mixing at 4°C and added to the cells at a final ACT concentration of 80 ng/ml. Cells were then incubated at 37°C for 2 h. The number of viable cells was determined using the CCK8 assay (Dojindo Laboratories), which measures reduction of WST-8, a water-soluble tetrazolium salt, by dehydrogenases in viable cells. Percent viable cells was determined for ACT alone and for serum plus ACT compared to control cells by the following equation: [(treatment − medium without cells)/(control − medium without cells)] × 100.
Preparation of flow cytometry samples and analysis.
Blood, lung, and cells isolated from nasal lavage fluid or lung were analyzed by flow cytometry. Lung tissue was homogenized with Dounce homogenizers and filtered through a 70-μm strainer and then centrifuged at 1,000 × g for 5 min to pellet cells. Supernatant was removed, Pharmlyse red blood cell (RBC) lysis buffer (BD Biosciences) was added, and samples were incubated at 37°C for 2 min before being pelleted by centrifugation at 1,000 × g for 5 min. Cells were resuspended in 500 μl PBS plus 1% fetal bovine serum (FBS). To prepare blood samples for flow cytometry, blood collected by cardiac puncture was placed in EDTA-containing tubes (BD Biosciences), and RBC were lysed using Pharmlyse with a 15-min room temperature incubation. Cells were then pelleted by centrifugation at 1,000 × g for 5 min and resuspended in PBS plus 1% FBS. Cells isolated by nasal lavage are in a single-cell suspension by nature, and therefore only centrifugation at 1,000 × g for 5 min was required to concentrate samples. Aliquots of single-cell suspensions were incubated on ice in PBS and 1% FBS for blocking for 15 min, and a 1/5 dilution of lung, a 1/10 dilution of blood, and total nasal lavage fluid were reconstituted in 100 μl. Antibodies against specific cell surface markers were added to cell suspensions and incubated in the dark for 1 h at 4°C: phycoerythrin-conjugated GR-1 (553128; BD), Alexa Fluor 700-conjugated CD-11b (101222; BioLegend), and peridinin chlorophyll protein/Cy5.5-conjugated F4/80 (123128; BioLegend). Lung, blood, and nasal lavage fluid suspensions were pelleted and resuspended in PBS prior to analysis. Samples were read using an LSR Fortessa (BD) flow cytometer and analyzed using the software FlowJo v10 (FlowJo, LLC). Neutrophils and macrophages were classified as CD-11b+ Gr-1+ single, live cells or CD-11b+ F4/80+ single, live cells, respectively.
Serological analysis of B. pertussis-specific antibodies.
Vaccinated and challenged mouse serological responses to RTX (9) and PT (LIST Biologicals) were determined by enzyme-linked immunosorbent assays (ELISA). High-binding 96-well ELISA plates were coated overnight at 4°C with 50 μl of purified RTX or PT in PBS at a concentration of 1 μg/ml. IgG2a monoclonal m1B7 (63) (anti-PT) and IgG2a monoclonal M1H5 (9) (anti-RTX) were obtained from Jennifer Maynard. Plates were washed with PBS plus 1% Tween 20 (PBS-T) and then blocked with 5% milk in PBS-T for 2 h at 37°C. Sera were diluted to a concentration in the linear detection range for each antigen. Plates were incubated for 2 h at 37°C with serially diluted serum samples from vaccinated groups. Following 3 PBS-T washes, goat anti-mouse IgG secondary antibody conjugated to alkaline phosphatase (AP) (Southern Biotech) was diluted at 1:2,000 in blocking buffer, added to each well, and incubated for 1 h at 37°C. Antibody standards were prepared from anti-PT or anti-RTX by 2-fold dilutions from 100 μg/ml. Plates were washed and then developed for 30 min with 100 μl p-nitrophenyl phosphate. Colorimetric signal was determined by measuring sample absorbance at 450 nm using a SpectraMax i3 (Molecular Devices). Antibody titers were determined by establishing a minimum detection limit greater than 2-fold over the standard deviation of the average of negative controls containing no antigen. Serum antibody concentrations were calculated by plotting absorbance values of samples to absorbance of known concentrations of control antibodies. Values were plotted using a logarithmic scale and fit to a four-parameter equation. Antibody concentrations were determined by plotting the calculated antibody sample concentrations to sample dilution factors and fit to a four-parameter logistic curve (64). Dilutions were accounted for by multiplying antibody concentration by dilution factor.
Statistical analysis.
Experiments in this study were performed with a minimum of four biological replicates. Data were statistically analyzed with GraphPad Prism 7. Comparisons between single groups were performed using unpaired two-tailed t tests, and comparisons between multiple groups were performed using one-way analysis of variance (ANOVA) with a Tukey's test for multiple comparisons.
Heat map modeling and visualization of the effects of immunization on correlates of protection.
A master file containing all of the data collected during this project was compiled, and linear regressions were performed for each variable measured in response to vaccination and challenge (Matlab; MathWorks) to identify variables that correlate with overall protection against B. pertussis challenge. A subset of those variables was then extracted and used to plot visual representations of the overall correlates of protection. For each variable, modulations were calculated as the difference between the averaged responses of the nonchallenged/nonvaccinated mice and the averaged responses of the mice in each experimental group. To establish a colorimetric scale, green was set as equal to the nonchallenged/nonvaccinated mice, red was used for the maximal relative increase for that variable, and blue was used for the minimal relative value for that variable.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kathleen Brundage for assistance with flow cytometry, Josh Eby of University of Virginia for critical discussions that contributed to this project, John Barnett for providing institutional support and mentorship, and the anonymous reviewers for providing input that improved the manuscript.
D.T.B. was funded by a West Virginia Space Grant Consortium Graduate Student Fellowship and the Jennifer Gossling Fellowship. F.H.D. and the Vaccine Development center at WVU-HSC are funded by Research Challenge Grant no. HEPC.dsr.18.6 from the Division of Science and Research, WV Higher Education Policy Commission. This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272201200005C. Additional support was provided by NIH/NIAID grant 5 RO1 AI1018000 (E.L.H.), NIH/NIAID grant RO1 A1122753 (J.A.M.), and laboratory startup funds from West Virginia University to F.H.D. Flow cytometry experiments were performed in the West Virginia University Flow Cytometry Core Facility, which is supported by the National Institutes of Health equipment grant number S10OD016165 and the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P30GM103488 (CoBRE) and P20GM103434 (INBRE).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00857-17.
REFERENCES
- 1.Madsen T. 1933. Vaccination against whooping cough. JAMA 101:187–188. doi: 10.1001/jama.1933.02740280007003. [DOI] [Google Scholar]
- 2.Kendrick PL, Eldering G, Dixon MK, Misner J. 1947. Mouse protection tests in the study of pertussis vaccine: a comparative series using the intracerebral route for challenge. Am J Public Health Nations Health 37:803–810. doi: 10.2105/AJPH.37.7.803-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato Y, Sato H. 1999. Development of acellular pertussis vaccines. Biologicals 27:61–69. doi: 10.1006/biol.1999.0181. [DOI] [PubMed] [Google Scholar]
- 4.Cherry JD. 2012. Why do pertussis vaccines fail? Pediatrics 129:968–970. doi: 10.1542/peds.2011-2594. [DOI] [PubMed] [Google Scholar]
- 5.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. 2013. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131:e1716-. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 6.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 367:1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 7.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebo P, Osicka R, Masin J. 2014. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev Vaccines 13:1215–1227. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Maynard JA, Hewlett EL, Maynard JA. 2015. The Bordetella adenylate cyclase repeat-in-toxin (RTX) domain is immunodominant and elicits neutralizing antibodies. J Biol Chem 290:3576–3591. doi: 10.1074/jbc.M114.585281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrmann IE, Gray MC, Gordon VM, Gray LS, Hewlett EL. 1991. Hemolytic activity of adenylate cyclase toxin from Bordetella pertussis. FEBS Lett 278:79–83. doi: 10.1016/0014-5793(91)80088-K. [DOI] [PubMed] [Google Scholar]
- 11.Khelef N, Sakamoto H, Guiso N. 1992. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb Pathog 12:227–235. doi: 10.1016/0882-4010(92)90057-U. [DOI] [PubMed] [Google Scholar]
- 12.Skopova K, Tomalova B, Kanchev I, Rossmann P, Svedova M, Adkins I, Bibova I, Tomala J, Masin J, Guiso N, Osicka R, Sedlacek R, Kovar M, Sebo P. 2017. Cyclic AMP-elevating capacity of adenylate cyclase toxin-hemolysin is sufficient for lung infection but not for full virulence of Bordetella pertussis. Infect Immun 85:e00937-. doi: 10.1128/IAI.00937-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eby JC, Gray MC, Warfel JM, Paddock CD, Jones TF, Day SR, Bowden J, Poulter MD, Donato GM, Merkel TJ, Hewlett EL. 2013. Quantification of the adenylate cyclase toxin of Bordetella pertussis in vitro and during respiratory infection. Infect Immun 81:1390–1398. doi: 10.1128/IAI.00110-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherry JD, Xing DX, Newland P, Patel K, Heininger U, Corbel MJ. 2004. Determination of serum antibody to Bordetella pertussis adenylate cyclase toxin in vaccinated and unvaccinated children and in children and adults with pertussis. Clin Infect Dis 38:502–507. doi: 10.1086/381204. [DOI] [PubMed] [Google Scholar]
- 15.Farfel Z, Konen S, Wiertz E, Klapmuts R, Addy PA, Hanski E. 1990. Antibodies to Bordetella pertussis adenylate cyclase are produced in man during pertussis infection and after vaccination. J Med Microbiol 32:173–177. doi: 10.1099/00222615-32-3-173. [DOI] [PubMed] [Google Scholar]
- 16.Hewlett EL, Manclark CR, Wolff J. 1977. Adenyl cyclase in Bordetella pertussis vaccines. J Infect Dis 136(Suppl):S216–S219. doi: 10.1093/infdis/136.Supplement.S216. [DOI] [PubMed] [Google Scholar]
- 17.Guiso N, Rocancourt M, Szatanik M, Alonso JM. 1989. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb Pathog 7:373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 18.Betsou F, Sebo P, Guiso N. 1993. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun 61:3583–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betsou F, Sebo P, Guiso N. 1995. The C-terminal domain is essential for protective activity of the Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun 63:3309–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hormozi K, Parton R, Coote J. 1999. Adjuvant and protective properties of native and recombinant Bordetella pertussis adenylate cyclase toxin preparations in mice. FEMS Immunol Med Microbiol 23:273–282. [DOI] [PubMed] [Google Scholar]
- 21.Cheung GY, Xing D, Prior S, Corbel MJ, Parton R, Coote JG. 2006. Effect of different forms of adenylate cyclase toxin of Bordetella pertussis on protection afforded by an acellular pertussis vaccine in a murine model. Infect Immun 74:6797–6805. doi: 10.1128/IAI.01104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray MC, Donato GM, Jones FR, Kim T, Hewlett EL. 2004. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol Microbiol 53:1709–1719. doi: 10.1111/j.1365-2958.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- 23.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. 1988. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol 2:19–30. doi: 10.1111/j.1365-2958.1988.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 24.Hewlett EL, Gordon VM, McCaffery JD, Sutherland WM, Gray MC. 1989. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem 264:19379–19384. [PubMed] [Google Scholar]
- 25.Hewlett EL, Gray L, Allietta M, Ehrmann I, Gordon VM, Gray MC. 1991. Adenylate cyclase toxin from Bordetella pertussis. Conformational change associated with toxin activity. J Biol Chem 266:17503–17508. [PubMed] [Google Scholar]
- 26.de Gouw D, de Jonge MI, Hermans PW, Wessels HJ, Zomer A, Berends A, Pratt C, Berbers GA, Mooi FR, Diavatopoulos DA. 2014. Proteomics-identified Bvg-activated autotransporters protect against Bordetella pertussis in a mouse model. PLoS One 9:e105011. doi: 10.1371/journal.pone.0105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guiso N, Szatanik M, Rocancourt M. 1991. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb Pathog 11:423–431. doi: 10.1016/0882-4010(91)90038-C. [DOI] [PubMed] [Google Scholar]
- 28.Hormozi K, Parton R, Coote J. 1999. Toxicity tests on native and recombinant Bordetella pertussis adenylate cyclase toxin preparations. Dev Biol Stand 101:147–154. [PubMed] [Google Scholar]
- 29.Macdonald-Fyall J, Xing D, Corbel M, Baillie S, Parton R, Coote J. 2004. Adjuvanticity of native and detoxified adenylate cyclase toxin of Bordetella pertussis towards co-administered antigens. Vaccine 22:4270–4281. doi: 10.1016/j.vaccine.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Orr B, Douce G, Baillie S, Parton R, Coote J. 2007. Adjuvant effects of adenylate cyclase toxin of Bordetella pertussis after intranasal immunisation of mice. Vaccine 25:64–71. doi: 10.1016/j.vaccine.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Villarino Romero R, Bibova I, Cerny O, Vecerek B, Wald T, Benada O, Zavadilova J, Osicka R, Sebo P. 2013. The Bordetella pertussis type III secretion system tip complex protein Bsp22 is not a protective antigen and fails to elicit serum antibody responses during infection of humans and mice. Infect Immun 81:2761–2767. doi: 10.1128/IAI.00353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basler M, Masin J, Osicka R, Sebo P. 2006. Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes. Infect Immun 74:2207–2214. doi: 10.1128/IAI.74.4.2207-2214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Confer DL, Eaton JW. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 34.Hewlett EL, Donato GM, Gray MC. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol Microbiol 59:447–459. [DOI] [PubMed] [Google Scholar]
- 35.Eby JC, Gray MC, Warfel JM, Merkel TJ, Hewlett EL. 2017. Use of a toxin neutralization assay to characterize the serologic response to adenylate cyclase toxin after infection with Bordetella pertussis. Clin Vaccine Immunol 24:e00370-. doi: 10.1128/CVI.00370-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guiso N, Capiau C, Carletti G, Poolman J, Hauser P. 1999. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine 17:2366–2376. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez Hayes J, Erben E, Lamberti Y, Principi G, Maschi F, Ayala M, Rodriguez ME. 2013. Bordetella pertussis iron regulated proteins as potential vaccine components. Vaccine 31:3543–3548. doi: 10.1016/j.vaccine.2013.05.072. [DOI] [PubMed] [Google Scholar]
- 38.de Gouw D, Serra DO, de Jonge MI, Hermans PW, Wessels HJ, Zomer A, Yantorno OM, Diavatopoulos DA, Mooi FR. 2014. The vaccine potential of Bordetella pertussis biofilm-derived membrane proteins. Emerg Microbes Infect 3:e58. doi: 10.1038/emi.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belcher CE, Drenkow J, Kehoe B, Gingeras TR, McNamara N, Lemjabbar H, Basbaum C, Relman DA. 2000. The transcriptional responses of respiratory epithelial cells to Bordetella pertussis reveal host defensive and pathogen counter-defensive strategies. Proc Natl Acad Sci U S A 97:13847–13852. doi: 10.1073/pnas.230262797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Goel T, Goodfield LL, Muse SJ, Harvill ET. 2011. Decreased leukocyte accumulation and delayed Bordetella pertussis clearance in IL-6−/− mice. J Immunol 186:4895–4904. doi: 10.4049/jimmunol.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins DJ, Gray MC, Hewlett EL, Vogel SN. 2007. Bordetella pertussis adenylate cyclase toxin (ACT) induces cyclooxygenase-2 (COX-2) in murine macrophages and is facilitated by ACT interaction with CD11b/CD18 (Mac-1). Mol Microbiol 66:1003–1015. doi: 10.1111/j.1365-2958.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- 42.Andreasen C, Powell DA, Carbonetti NH. 2009. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One 4:e7079. doi: 10.1371/journal.pone.0007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbic J, Leef MF, Burns DL, Shahin RD. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect Immun 65:4904–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J Exp Med 186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnard A, Mahon BP, Watkins J, Redhead K, Mills KHG. 1996. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology 87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Place DE, Muse SJ, Kirimanjeswara GS, Harvill ET. 2014. Caspase-1-independent interleukin-1beta is required for clearance of Bordetella pertussis infections and whole-cell vaccine-mediated immunity. PLoS One 9:e107188. doi: 10.1371/journal.pone.0107188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J Exp Med 193:1035–1044. doi: 10.1084/jem.193.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreasen C, Carbonetti NH. 2009. Role of neutrophils in response to Bordetella pertussis infection in mice. Infect Immun 77:1182–1188. doi: 10.1128/IAI.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirimanjeswara GS, Agosto LM, Kennett MJ, Bjornstad ON, Harvill ET. 2005. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J Clin Investig 115:3594–3601. doi: 10.1172/JCI24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boursaux-Eude C, Thiberge S, Carletti G, Guiso N. 1999. Intranasal murine model of Bordetella pertussis infection. II. Sequence variation and protection induced by a tricomponent acellular vaccine. Vaccine 17:2651–2660. [DOI] [PubMed] [Google Scholar]
- 51.Barbier M, Boehm DT, Sen-Kilic E, Bonnin C, Pinheiro T, Hoffman C, Gray M, Hewlett E, Damron FH. 2017. Modulation of Pertussis and adenylate cyclase toxins by sigma factor RpoE in Bordetella pertussis. Infect Immun 85:e00565-. doi: 10.1128/IAI.00565-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weigand MR, Peng Y, Loparev V, Batra D, Bowden KE, Burroughs M, Cassiday PK, Davis JK, Johnson T, Juieng P, Knipe K, Mathis MH, Pruitt AM, Rowe L, Sheth M, Tondella ML, Williams MM. 2017. The history of Bordetella pertussis genome evolution includes structural rearrangement. J Bacteriol 199:e00806-16. doi: 10.1128/JB.00806-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, Miller L, Selvage D, Lee A, Skoff TH, Kamiya H, Cassiday PK, Tondella ML, Clark TA. 2015. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis 60:223–227. doi: 10.1093/cid/ciu788. [DOI] [PubMed] [Google Scholar]
- 54.Williams MM, Sen K, Weigand MR, Skoff TH, Cunningham VA, Halse TA, Tondella ML, CDC Pertussis Working Group. 2016. Bordetella pertussis strain lacking pertactin and pertussis toxin. Emerg Infect Dis 22:319–322. doi: 10.3201/eid2202.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arico B, Rappuoli R. 1987. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol 169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pittet LF, Emonet S, Schrenzel J, Siegrist CA, Posfay-Barbe KM. 2014. Bordetella holmesii: an under-recognised Bordetella species. Lancet Infect Dis 14:510–519. doi: 10.1016/S1473-3099(14)70021-0. [DOI] [PubMed] [Google Scholar]
- 57.Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, Cassiday PK, Chiang CS, Dalby T, Fry NK, Gaillard ME, van Gent M, Guiso N, Hallander HO, Harvill ET, He Q, van der Heide HG, Heuvelman K, Hozbor DF, Kamachi K, Karataev GI, Lan R, Lutynska A, Maharjan RP, Mertsola J, Miyamura T, Octavia S, Preston A, Quail MA, Sintchenko V, Stefanelli P, Tondella ML, Tsang RS, Xu Y, Yao SM, Zhang S, Parkhill J, Mooi FR. 2014. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 5:e01074-14. doi: 10.1128/mBio.01074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgs R, Higgins SC, Ross PJ, Mills KH. 2012. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol 5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong ME, Loscher CE, Lynch MA, Mills KH. 2003. IL-1beta-dependent neurological effects of the whole cell pertussis vaccine: a role for IL-1-associated signalling components in vaccine reactogenicity. J Neuroimmunol 136:25–33. doi: 10.1016/S0165-5728(02)00468-X. [DOI] [PubMed] [Google Scholar]
- 60.Brickman TJ, Armstrong SK. 1996. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J Bacteriol 178:54–60. doi: 10.1128/jb.178.1.54-60.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bordet J. 1906. Le microbe de la coqueluche. Ann Inst Pasteur (Paris) 20:731–741. [Google Scholar]
- 62.Stainer DW, Scholte MJ. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen AW, Wagner EK, Laber JR, Goodfield LL, Smallridge WE, Harvill ET, Papin JF, Wolf RF, Padlan EA, Bristol A, Kaleko M, Maynard JA. 2015. A cocktail of humanized anti-pertussis toxin antibodies limits disease in murine and baboon models of whooping cough. Sci Transl Med 7:316ra195. doi: 10.1126/scitranslmed.aad0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner EK, Wang X, Bui A, Maynard JA. 2016. Synergistic neutralization of pertussis toxin by a bispecific antibody in vitro and in vivo. Clin Vaccine Immunol 23:851–862. doi: 10.1128/CVI.00371-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.