Abstract
Seed germination and early seedling development have been studied in the recalcitrant species Quercus ilex using targeted transcriptional, hormonal, and sugar analysis. Embryos and seedlings were collected at eight morphologically defined developmental stages, S0–S7. A typical triphasic water uptake curve was observed throughout development, accompanied by a decrease in sucrose and an increase in glucose and fructose. Low levels of abscisic acid (ABA) and high levels of gibberellins (GAs) were observed in mature seeds. Post-germination, indole-3-acetic acid (IAA), increased, whereas GA remained high, a pattern commonly observed during growth and development. The abundance of transcripts from ABA-related genes was positively correlated with the changes in the content of the phytohormone. Transcripts of the drought-related genes Dhn3 and GolS were more abundant at S0, then decreased in parallel with increasing water content. Transcripts for Gapdh, and Nadh6 were abundant at S0, supporting the occurrence of an active metabolism in recalcitrant seeds at the time of shedding. The importance of ROS during germination is manifest in the high transcript levels for Sod and Gst, found in mature seeds. The results presented herein help distinguish recalcitrant (e.g., Q. ilex) seeds from their orthodox counterparts. Our results indicate that recalcitrance is established during seed development but not manifest until germination (S1–S3). Post-germination the patterns are quite similar for both orthodox and recalcitrant seeds.
Keywords: Quercusilex, recalcitrant, germination, early seedling growth, phytohormone
Introduction
Quercus ilex subsp. ballota [Desf.] Samp. is the dominant tree in forest ecosystems over large areas of the Western Mediterranean Basin (Pulido et al., 2001; de Rigo and Caudullo, 2016). As a Quercus species, it produces recalcitrant seeds, which are shed, and germinate, at a relatively high-water content (Connor and Sowa, 2003; Pasquini et al., 2012; Joët et al., 2013; Sghaier-Hammami et al., 2016). They are susceptible to desiccation injury, and become inviable when stored for long periods (Pasquini et al., 2012). Problems associated with loss of viability during collection and, processing, and the short storage life of these seeds, can lead to serious difficulties with seed conservation and propagation.
A number of studies focused on germination, storage, desiccation sensitivity, and viability after storage of Quercus spp. seeds have been previously published (Bonner and Vozzo, 1987; Finch-Savage and Blake, 1994; Finch-Savage et al., 1996; Connor and Sowa, 2003; Goodman et al., 2005; Berjak and Pammenter, 2008; Ntuli et al., 2011; Liu et al., 2012; Pasquini et al., 2012; Xia et al., 2014; Pariona et al., 2017; Shi et al., 2017). However, specific differences between recalcitrant and orthodox species (Weitbrecht et al., 2011; Rajjou et al., 2012; Swigonska and Weidner, 2013), and the molecular bases of Q. ilex recalcitrance remain enigmatic. In a previous study of Quercus robur it was found that recalcitrance does not on accumulation of dehydrins, abscisic acid (ABA), or soluble sugars during seed maturation (Finch-Savage and Blake, 1994).
Development of orthodox seeds ends at the desiccation stage, when the dry seeds enter a quiescent phase prior to germination. Major changes that occur during seed maturation involve multiple cellular processes including gene expression reprogramming which is under strict hormonal control. These include: synthesis of storage, heat-shock, and osmoprotective proteins and carbohydrates, activation of antioxidant defenses, and reduction of metabolism (Angelovici et al., 2010). In contrast, recalcitrant seeds are able to germinate immediately after shedding, without a quiescent phase, and continuously maintain high metabolic activity (Berjak and Pammenter, 2013; Caccere et al., 2013; Parkhey et al., 2014).
A complex interacting set of phytohormones, including ABA, gibberellins (GAs), ethylene, brassinosteroids (BR), auxin, and cytokinins (CK), control seed germination. ABA and GA are the prominent inhibitor and promoter, respectively, of germination (Rajjou et al., 2012). The ABA/GA ratio is thought to regulate the metabolic transition required for germination, but this has been largely studied in orthodox seeds and poorly investigated in recalcitrant seeds. It has been proposed that precocious germination of recalcitrant seeds is because of low levels of ABA in the mature embryos (Farrant et al., 1993; Prewein et al., 2006).
High metabolic activity during germination is generally accompanied by an increase in reactive oxygen species (ROS) (especially H2O2) (Kranner et al., 2010; Roach et al., 2010). ROS can act as secondary messengers in signal transduction pathways, that control several processes, including germination (Oracz and Karpiñski, 2016). Also, the overproduction of ROS has been recognized as a main cause of the deterioration associated with loss of seed vigor (Jeevan Kumar et al., 2015). Therefore, antioxidant defense is required to reduce the level of ROS produced during imbibition and germination (Bailly et al., 2008). An increase in anti-oxidant metabolites (ascorbate and glutathione) and enzymes (APX, SOD, and CAT) during seed imbibition and germination has been described in different species of orthodox seeds (Gara et al., 1997; Tommasi et al., 2001; Bogdanović, 2008; Lee et al., 2010; Wojtyla et al., 2016) but data from studies of recalcitrant species are scarce.
In this study, we have targeted transcriptional, hormonal, and metabolic analyses of mature, and germinating seeds, and young seedlings of Q. ilex. The transcript profiles of eleven genes coding proteins involved in: desiccation tolerance (DHN3 and GOLS) (Nishizawa et al., 2008; Hanin et al., 2011), ABA-signaling regulation (OCP3, SKP1, and SDIR1) (Cutler et al., 2010), metabolism (FDH, GAPDH, RBLC, and NADH6) (Muñoz-Bertomeu et al., 2009; David et al., 2010) and oxidative stress (SOD1 and GST) (Giannopolitis and Ries, 1977; Schopfer et al., 1985) were determined. Our results contribute to understanding the physiological changes that take place during seed germination and seedling growth of recalcitrant species such as Q. ilex.
Materials and Methods
Plant Material
Acorns, in accordance with the maturity indexes described by Bonner and Vozzo (1987) and used by Pasquini et al. (2012), were harvested from 10 Q. ilex trees randomly selected (Cerro Muriano, province of Córdoba, Spain; 37°59′57.74″N, 04°46′57.93″W) in December 2010. Climate data can be found at https://es.climate-data.org/location/657770/. Undamaged acorns were sterilized by immersion in 2.5% sodium hypochlorite for 10 min, abundantly rinsed with water, and dried by air circulation at room temperature on filter paper.
Seed Germination
Acorns were hand dehulled in order to perform a synchronized germination, as suggested by Liu et al. (2012) (Supplementary Figure S1). Germination was performed in darkness at 22°C for a total period of 216 h. Seed embryos or germinated seedlings, were collected at 0, 6, 12, 24, 48, 72, 144, and 216 h after imbibition (Supplementary Figure S2), and immediately frozen in liquid nitrogen and stored at −70°C. Three biological replicates were performed for each sampling time, each one containing ten-to fifty, depending on the analysis and sampling time, individual embryonic axes or germinated seedlings.
Relative Water Content
The relative water content (RWC) was determined for all developmental stages (S0–S7) according to the following equation: RWC = [(FW–DW)/FW] × 100, where FW and DW are fresh and dry weight, respectively. FW and DW were measured using an analytical balance. Dry weight was obtained after sample drying in an oven at 70°C for 48 h.
Sugar Analysis
Sugar content was determined by gas chromatography coupled to mass spectrometry (GC-MS). Sugars were extracted as described (Gómez-González et al., 2010) using 100 mg of lyophilized plant tissue and analyzed by GC-MS as described by Cerdán-Calero et al. (2012). Calibration curves from standards D-(+)-glucose, D-(−) fructose and D-(+)-sucrose (5, 10, 20, 30, 40, and 50 μg) were prepared for identification and quantification. In every case, 10 μg of xylitol was added as reference to the samples. GC-MS was performed on an Agilent Technologies GC system 7890A coupled to a mass spectrometer 5975C fitted with a column HP-5MS (30 m × 0.25 mm, Agilent). The temperature program was isothermal at 150°C for 3 min, followed by a 5°C/min gradient up to 210°C, a 15°C/min gradient up to 310°C, and isothermal for 2 min. The ionization potential was 70 eV, and spectra were recorded in low-resolution mode.
Phytohormone Analysis
The following phytohormones were analyzed by using liquid chromatography coupled to mass spectrometry (LC/MS): abscisic acid (ABA), gibberellins (GA3 and GA4), indole-3-acetic acid (IAA), brassinolide (CS: castasterone), cytokinins [CKs: dihydrozeatin riboside, (DHZR), dihydrozeatin (DHZ), isopentenyladenine (Ip), isopentenyladenine riboside (iPR), trans-zeatin (tZ), trans-zeatin riboside (tZR)]. Phytohormones were extracted from 120 mg of lyophilized tissue (20–50 individuals, depending on the sampling time, 0, 24, and 216 h post-imbibition) as described by Pan et al. (2008). Phytohormones were separated and quantified by ultra-high performance liquid chromatography (UHPLC) in a 6460 Triple Quad LC/MS (Agilent Technologies) using the protocol described by Novák et al. (2008).
RNA Extraction and cDNA Synthesis Analysis
Total RNA was extracted from 20 mg of embryos and germinated seeds by using the InviTrap® Spin Plant RNA Mini Kit (Invitek), following the manufacturer’s directions with minor modifications (Echevarría-Zomeño et al., 2012). RNA quantitation was performed with the Qubit® RNA Assay Kit in a Qubit® 2.0 Fluorometer (Invitrogen) and RNA quality was checked electrophoretically (Agilent 2100 Bioanalyzer). Only high-quality RNAs with RIN values >8 and A260:A280 ratios of approximately 2.0 were used for subsequent experiments (Fleige and Pfaffl, 2006). cDNAs were generated from 1 μg of total RNA using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instructions. Reverse transcriptions were set up from RNA samples of identical concentrations in order to add the same volume to the RT reaction (Taylor and Mrkusich, 2014). An RNA “calibrator” with a known number of transcripts of the A170 gene was introduced in each experiment to guarantee the quality of the reverse-transcription, and to set the threshold in the different qRT-PCR plates (inter-plate calibrator).
Primer Design and Sequencing
Sequences from Q. ilex Dhn3, Gapdh, Sod1 and Rblc genes, or corresponding to Fdh, Gst, GolS, Nadh6, Ocp3, Sdir1 and Skp1 orthologs from different phylogenetically related species (preferentially Quercus > Fagaceas > Fagales > other plants) were obtained from the GenBank database1 (Supplementary Table S1). Alignments were performed by using the ClustalW software (MegAlign, DNASTAR Lasergene, v.6). Primer pairs were designed over conserved sequences and obtained using the Primer-BLAST tool of NCBI2. The proposed primer pairs were analyzed with the OLIGO Primer Analysis Software v 7.58 (Molecular Biology Insights, Inc.) and one pair for each gene, with high Tm and free from hairpin and duplex structures, was chosen. These primers were used to amplify synthesized cDNA from pooled Q. ilex RNAs and to obtain sequences from the species. PCR amplification was achieved by mixing 50 ng of Q. ilex cDNA with 0.75U of iTaqTM DNA Polymerase (Bio-Rad) and following the manufacturer’s recommendations. Amplifications were carried out using an iCycler iQ Real-Time PCR System (Bio-Rad). PCR products were electrophoretically separated and visualized on agarose gels (2%) containing GelRedTM (Biotium). The DNA bands of the predicted size were excised from the gels, purified (Wizard® SV Gel and PCR Clean-Up System kit, Promega) and sequenced on an ABI PRISMTM 3130 XL sequencer (Applied Biosystems). The identity of the trimmed sequences was confirmed using tBLASTx algorithm on the BLAST server at the NCBI databank, and the Q. ilex sequences were deposited in the GenBank Database (accession numbers in Supplementary Table S2). These sequences were used to design primers that exactly complemented the Q. ilex genes for the absolute quantification of transcript levels by real-time RT-PCR (qRT-PCR). To obtain a high specificity and a better performance, primers, free from hairpin and duplex structures, were required to have high Tm (≥70°C), and an optimal 3′-ΔG (≤−3 kcal/mol) value to be used in two-step PCR reactions. All primer pairs produced amplicons of the predicted size (Supplementary Table S2). All PCR products were further verified by nucleotide sequencing.
qRT-PCR
Real-time PCR reactions were performed in quadruplicate with 50 ng cDNA per reaction, using an iCycler iQ thermocycler (Bio-Rad) and the iQ SYBR Green SuperMix (Bio-Rad), following the manufacturer’s directions. The amplification program consisted of one cycle at 95°C for 3 min, and 40 two-step amplification cycles at 95°C for 15 s and 68°C for 30 s, respectively. After 1 min at 95°C, a melting curve was obtained by following the fluorescence intensity during gradual cooling from 95 to 65°C.
To carry out absolute qRT-PCR, a calibration curve was constructed with an in vitro synthesized RNA, as previously detailed by Prieto-Alamo et al. (2003).
Protein Immunoblot Analysis
Two hundred milligrams of plant material (corresponding to 20–50 embryos or seedlings) was employed for western blot analysis. Tissue was trichloroacetic–acetone–phenol extracted as previously described (Wang et al., 2006). The final pellet was suspended in 9 M urea containing 4% CHAPS, 0.5% Triton X-100 and 100 mM DTT, and insoluble material was eliminated by centrifugation. Protein concentrations were determined by the Bradford method (Bradford, 1976), using bovine albumin as standard. Proteins (25 μg) were resolved by SDS-PAGE, using 12% polyacrylamide gels (Mini-PROTEAN® TGX Stain-FreeTM Precast Gels, Bio-Rad) with a Mini Protean Tetra-Cell (Bio-Rad). After electrophoresis, the gel image was captured and analyzed using a ChemiDocTM MP Imaging System (Bio-Rad). Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes by using a Trans-Blot® TurboTM Transfer System (Bio-Rad). After transfer, both gels and PVDF membranes were briefly rinsed with distilled water and the captured images were analyzed (ChemiDocTM MP Imaging System, Bio-Rad) to assess the quality of the transfers. Images were used to normalize band intensity after incubating membranes with the corresponding primary and secondary antibodies (Lee et al., 2005). PVDF membranes were blocked with 2% non-fat milk powder. Later, the membranes were incubated with rabbit antibodies raised against GAPDH (Sigma-Aldrich; dilution: 1:500), Dehydrin (Agrisera; dilution: 1:100) or RBCL (Agrisera; dilution: 1:100), for 1–3 h, washed and incubated with the secondary goat anti-rabbit IgG antibody (Sigma: A-3687, 1:2000), and conjugated to alkaline phosphatase for an additional 1 h. Image acquisitions and densitometric analyses were performed with the ChemiDoc MP Imaging system and ImageLab 4.1 software (Bio-Rad), respectively.
Superoxide Dismutase Assays
Protein extracts for superoxide dismutase (SOD) assays were obtained from cryo homogenized tissue, by mixing 500 mg of each sample with 1 mL of 10 mM Tris–HCl buffer (pH 7.4), 5 mM DTT, 2 mM EDTA; 0.5% (v/v) Triton X-100, 5 mM ascorbic acid, 100 mM PMSF and 10% (w/v) PVPP. The mixtures were vortexed and sonicated four times (10 s at 6 W). Supernatants were collected by centrifugation (10000g, 10 min at 4°C) and used for enzyme assays. Protein concentrations were quantified by the Bradford method (Bradford, 1976), using bovine albumin as the standard. To preserve the enzymatic activity, the extracts were supplemented with 30% (w/v) sucrose and stored at −80°C until used, for no more than 1 week. SOD isoforms were separated on 10% non-denaturing polyacrylamide gels at 4°C, by using a mini protean electrophoresis unit (Bio-Rad, United States) and loading 20 μg of protein per well. After electrophoresis, the gels were stained for SOD activity as described by Weydert and Cullen (2010). The SOD isoforms were differentiated as described in Rowe et al. (1997).
Statistical Analysis
In all the experiments, data from moisture, sugar, phytohormone, qRT-PCR, protein immunoblot, and SOD analyses were subjected to a univariate analysis of variance (ANOVA) and mean values were compared by Tukey’s test for P ≤ 0.05 with the software InStat v2.05/00 (GraphPad).
Results and Discussion
Germination Stages and Relative Water Content
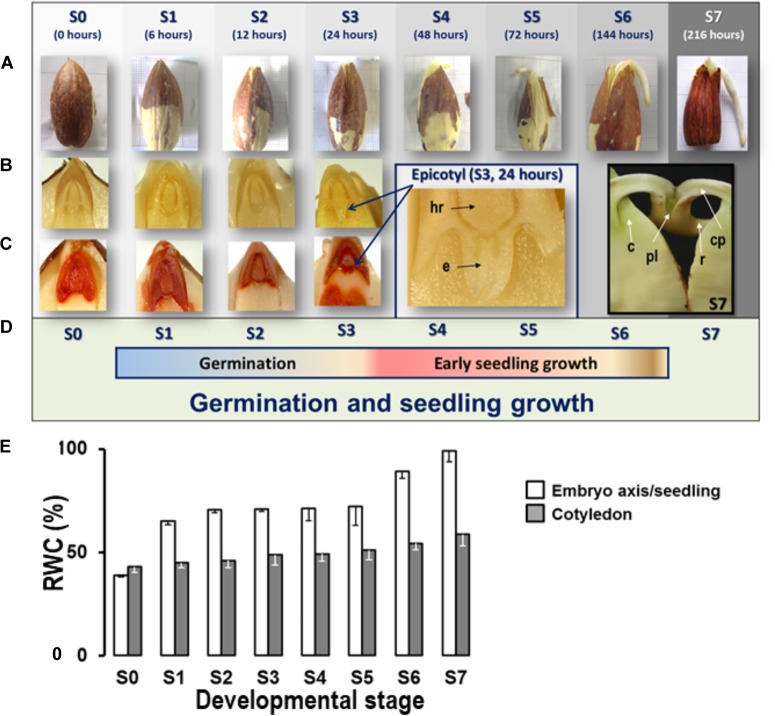
Seed germination is a complex process, comprising events from seed imbibition to radicle emergence. Morphologically, initiation of growth corresponds to radicle emergence; and subsequent development is generally defined as seedling growth (Bewley, 1997). In all the replicates, synchronized germination of 100% of the seeds was reached if they were previously dehulled. Eight developmental stages referred to as S0 to S7, were differentiated (Figure 1). Changes in morphology were observed during germination; at stage S2, the rupture of the testa was appreciable, with radicle emergence starting to be visible at stage S3, and plumule emergence from cotyledonary petioles at 216 h (S7) (Figures 1A–C). These changes indicate that the Q. ilex seed germination covers the first 24 h after imbibition, under our experimental conditions; followed by early seedling growth (from S4 to S7) (Figure 1D).
FIGURE 1.
Morphology and physiology of Quercus ilex seeds germinating at different developmental stages. (A) Morphology of germinating acorns and young seedlings at each developmental stage; the relative time taken for low hydrated seeds to reach each stage is also shown; germinating dehulled seeds shows important morphological changes after imbibition at S2: testa rupture at 12 h of germination. S3: radicle emergence at 24 h; S5: epicotyl emergence from the embryonic axis; and S6: plumule emergence from differentiated cotyledonary petioles. (B) Seed sections showing the embryonic axes and the progressively differentiated parts of the embryo: hr, hypocotyle radicle; e, epicotyl; pl, plumule; c, cotyledon; cp, cotyledonary petiole; r, radicle; (C) TZ staining was used to facilitate the visualization of the morphological changes produced in the embryonic axes during germination. (D) Correspondence among the eight developmental stages and the phases of Q. ilex seed germination and seedling growth: germination (S0–S3) and early seedling growth (S4–S7). (E) Percentage of relative water content (RWC %) of complete germinating Q. ilex seeds or their cotyledons at the different developmental stages. The fresh (FW) and dried (DW) weights (70°C for 48 h) of 10–15 germinating acorns or seedlings were plotted per stage, with vertical bars representing ± SE of the mean. All mass measurements were made using an analytical scale, with precision of 0.0001 g. The relative water content (RWC) in germinating seeds was expressed as percentage of lost weight [(FW–DW) × 100] relative to fresh weight (FW). All values were rounded to the nearest milligram.
Relative water content was determined at the different developmental stages (Figure 1E), values for mature Q. ilex seeds were of 38%, which is in accordance with the data previously reported for Quercus spp. (i.e., Echevarría-Zomeño et al., 2009; Tilki, 2010). Then, the typical triphasic water uptake curve reported in germinating seeds (Finch-Savage and Leubner-Metzger, 2006; Rajjou et al., 2012; Bewley et al., 2013), was observed, including rapid initial water uptake (phase I, i.e., imbibition; S0–S2), followed by a plateau phase (phase II, S2–S5), and a further increase (phase III S5 and forward) (Figure 1E).
Sucrose, Glucose, and Fructose Content
Soluble carbohydrates have been proposed to play an important role in desiccation tolerance (Finch-Savage et al., 1996) and are associated with metabolic activity during germination and early seedling growth (Białecka and Kȩpczyński, 2007). Sucrose, fructose, and glucose were analyzed in embryos or seedlings at three stages, S0, S3, and S7, by means of GC-MS/MS. Soluble carbohydrate analysis in Q. ilex and other recalcitrant seeds during germination is scant in the literature. The level of sucrose (83 ± 0.4 μg g−1, <1% of DW; Figure 2) accumulated in the embryonic axis of Q. ilex seeds (S0 stage) was in the lowest range, even compared with other recalcitrant seeds (Steadman et al., 1996). Data reported for sucrose in orthodox seeds range from 33% in African oil bean (Pentaclethra macrophylla) to 0.3–3% in the seeds of some crops such as chick pea (Cicer arietinum) or black gram (Vigna mungo) (Black and Bewley, 2000), respectively. The amount of glucose or fructose in Q. ilex seed (3.0 ± 0.7 and 6.7 ± 0.7 μg g−1 DW, respectively) at S0 stage is very small, similar to that described for orthodox seeds by Black and Bewley (2000). Several factor are involved in the recalcitrant character of seeds (Pammenter and Berjak, 2014). In Q. ilex seeds the low content of these three sugars might contribute to this trait.
FIGURE 2.
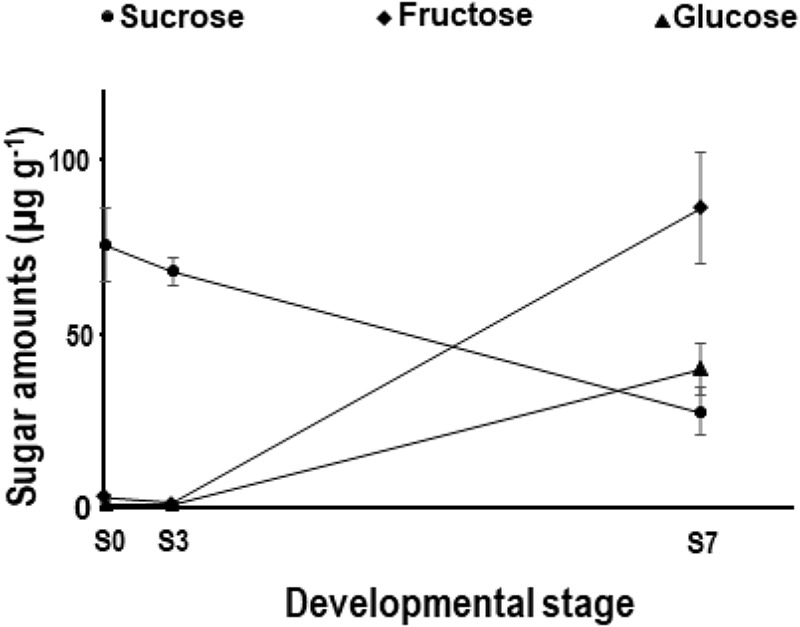
Sugar content in germinating Q. ilex seeds. Sucrose, glucose, and fructose content in the embryo axis at the S0, S3, and S7 stages were determined by GC-MS. Data are means ± SE of measurements made on 20–50 seeds per sampling time, grouped in three pools. Changes between the S3 and S7 stages were statistically significantly different at the P < 0.001 value (Student’s t-test).
The levels of both monosaccharides increased dramatically (10–20-folds) during the late S7 stage and were accompanied by a statistically significant decrease in the amount of sucrose, from 83 ± 0.4 to 28 ± 6.6 μg g−1 DW. Similar changes have been reported in germinating Glycine max (Kuo et al., 1990) and Moringa oleifera (Tesfay et al., 2016) seeds. The germination and early seedling growth processes are characterized by a high metabolic activity in order to satisfy the energy required by them for development and growth (Nkang, 2002; Rajjou et al., 2012). In support of this hypothesis, a decrease in sucrose and an increase in monosaccharides was observed at stages S3 to S7.
Phytohormone Profiling
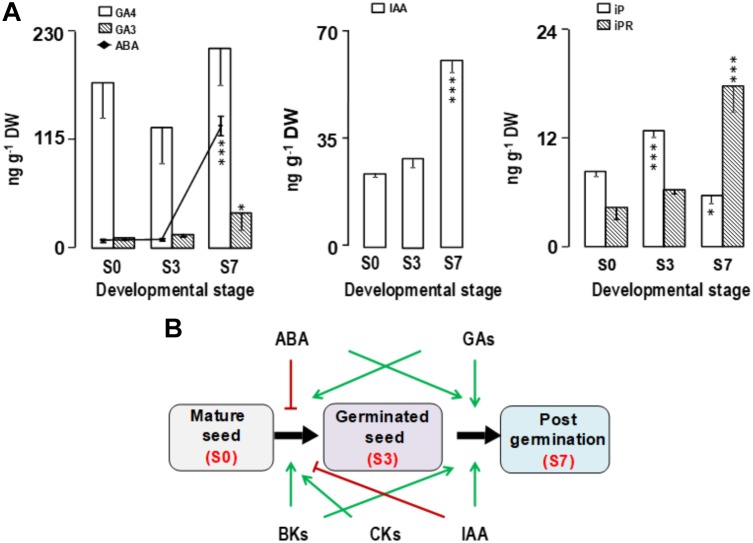
The ABA content in mature Q. ilex embryo axes (6.4 ± 1.0 ng g−1 DW) reported here was similar to that described for the embryo axis of Q. robur (Prewein et al., 2006) and other recalcitrant seeds (Pieruzzi et al., 2011). However, this value is considerably lower than those reported for orthodox seeds of Arabidopsis thaliana (140 ng g−1 DW) (Liu et al., 2009) or Solanum lycopersicum (50 ng g−1 DW) (Yang et al., 2014). The desiccation sensitivity and absence of dormancy in Q. ilex seeds are probably related to their low ABA levels, emphasizing the role of this phytohormone in preventing premature germination and desiccation tolerance acquisition (Ali-Rachedi et al., 2004). In fact, in orthodox species A. thaliana, aba mutants unable to synthesize ABA and ABA signaling mutants are less desiccation tolerant and less dormant (Parcy et al., 1994; Lefebvre et al., 2006; Nakashima et al., 2009).
The amount of ABA was constant during the germination process (S0 to S3), although the ABA amount increased considerably (>20-fold) during early seedling growth (from S3 to S7; Figure 3A). This fact has also been described for the recalcitrant species Araucaria angustifolia (Pieruzzi et al., 2011). The high levels of ABA at the S7 stage might be related to the development of Q. ilex seedlings. Recent studies indicated a positive role for ABA in promoting root meristem maintenance and root growth in Arabidopsis, Zea mays, Vicia faba and Helianthus annuus, but the underlying molecular mechanisms remain unknown (Finkelstein et al., 2002; Zhang et al., 2010; McAdam et al., 2016).
FIGURE 3.
(A) Changes in the endogenous concentrations of phytohormones in Q. ilex embryo axis and seedling during germination and post-germination stages. The levels of abscisic acid (ABA), gibberellins (GA3, GA4), auxin (IAA), and cytokinins (iP, iPR,) were determined by using GC-MS/MS. Data are means ± SE of measurements made on 20–50 seeds per sampling time, grouped in three pools. Statistical significance was determined using the one-way ANOVA Tukey’s test. The P-values are given as ∗P < 0.05 level and ∗∗∗P < 0.001 level. (B) Phytohormone interactions during germination and seedling development.
The level of GAs is usually undetectable in orthodox non-germinating seeds but its accumulation is a prerequisite for dormancy breaking (Toh et al., 2008; Chen et al., 2010; Weitbrecht et al., 2011), with GA4 being the major endogenous active GA in germinating seeds and shoots (Ogawa and Hanada, 2003). Related to the absence of dormancy, Q. ilex seeds showed high levels of gibberellins throughout all the developmental stages surveyed, from mature seeds (S0) to early growth seedlings (S7) (Figure 3A). GA4 was the major gibberellin in Q. ilex seeds (174.8 ± 37.1 ng/g DW), with much higher values than those found in germinated orthodox seeds (Ogawa and Hanada, 2003; Chen et al., 2010), and remained unchanged throughout the course of the experiment. A 15 times lower content was found in mature seeds for GA3 (10.6 ± 2.9 ng/g DW), that increased up to 3-times (37.0 ± 18.1 ng/g DW) in S7, early growth seedlings. An increase in GA3 has also been reported in orthodox seeds of Phellodendron amurense (Chen et al., 2010) and recalcitrant Avicennia marina (Farrant et al., 1993), although different amounts of the hormone at the mature seed stage were provided. The significant increase of GA3 in early seedling growth of Q. ilex could be related to growth promotion, a biological function described for active GAs (Zhang, 2007; Vishal and Kumar, 2018).
Small amounts of ABA and large ones of active GAs are required for seed germination (Shu et al., 2016). GA does not appear to control dormancy per se but acts by stimulating radicle emergence (Bewley, 1997). Our findings in the embryonic axes of mature Q. ilex seeds showed this pattern, therefore indicating that Q. ilex seeds have the hormone balance necessary to proceed to germination at the time of shedding.
This ABA and GA pattern in mature seeds are one explanation of the non-dormant character of Q. ilex seeds. During seedling growth (from S3 to S7; Figure 3A), increases in ABA and GAs were observed; the high amount of ABA in seedling (S7) of Q. ilex could be necessary for the root development. This result is in accordance with that reported by McAdam et al. (2016), where ABA-biosynthetic mutants of Pisum sativum and S. lycopersicum showed reduced root biomass highlighting the importance of ABA in root development.
Indole-3-acetic acid is involved in seed developmental processes, interacting with other hormones and controlling dormancy and germination (Figure 3B; Finkelstein et al., 2008; Miransari and Smith, 2014; Shu et al., 2016). We found an increase in the amount of IAA during the experiment (Figure 3A), which was expected, since IAA has often been implicated in plant growth (Santner et al., 2009) and root development (Tromas and Perrot-Rechenmann, 2010). The IAA levels in Q. ilex (23.9 ± 1.4 ng g−1 DW) mature seeds were lower than those reported in A. marina (500 ng g−1 DW) (Farrant et al., 1993). Similar IAA increases during germination and early seedling development to those reported here have been described for several other recalcitrant seeds (Farrant et al., 1993; Pieruzzi et al., 2011). The increase in IAA and ABA content during Q. ilex seedling growth supports the idea that there is crosstalk between IAA and ABA (Liu et al., 2007; Nguyen et al., 2016), and that both hormones are involved in the processes occurring during Q. ilex seed germination and seedling growth (Figure 3B).
We also analyzed the levels of some cytokinins and the brassinolide castasterone (CS), in Q. ilex embryo axes and seedlings during germination and early seedling growth. Cytokinins play an important role in promoting cell division and elongation in the embryo and providing positional information for the developing embryo (Leubner-Metzger, 2006). It is also known that cytokinins antagonize ABA during seed germination (Wang et al., 2011). Among the CKs and brassinolide measured here (tZ, iP, DHZ, iPR, tZR and DHZR, and CS), only iP and iPR showed significant differences during the stages studied (P < 0.001; Figure 3A). The tZ, tZR, DZ and DZR levels (data not shown) do not shown significant variation during germination as observed in recalcitrant seeds of A. marina (Farrant et al., 1993), and the most abundant cytokinin was tZR. In orthodox seeds the predominant free cytokinin was DHZ-in Medicago sativa L. while cZ was predominant in Z. mays and Avena sativa. Their levels shown some variation during germination (Stirk et al., 2012).
The iP content in Q. ilex seeds/seedlings increased from 8.5 ± 0.5 to 12.6 ± 0.6 ng g−1 DW during germination (S0–S3), and then decreased at S7 stage (Figure 3A), reaching lower levels than in the S0 stage. The Q. ilex seed content of iP was lower than that described in other recalcitrant species, but showed a similar time-course profile (Farrant et al., 1993; Farrant and Moore, 2011). In contrast to iP, iPR exhibited a continuous increase during germination and post-germination stages (from 4.2 ± 1.3 ng g−1 DW in S0 to 17.7 ± 2.9 ng g−1 DW in S7). To our knowledge, this is the first study reporting the time-course increase in iPR in this type of seed and demonstrating that, in recalcitrant Q. ilex seeds, the increase in-dormancy-releasing hormones accompanies germination, and that the mature Q. ilex seed has the phytohormone balance necessary for germination.
Our data support a model for non-dormant recalcitrant seeds of Q. ilex, low ABA content is insufficient to prevent premature germination, while high levels of GAs, auxin, and CKs promote germination.
Targeted Transcriptional Profiling
A targeted transcriptional analysis was performed on 11 genes of relevance in seed viability and germination, whose expression is expected to be different between orthodox and recalcitrant species (Supplementary Table S2). The list included genes related to desiccation tolerance (Nishizawa et al., 2008; Hanin et al., 2011), ABA-signaling regulation (Cutler et al., 2010), metabolism (Muñoz-Bertomeu et al., 2009; David et al., 2010), and oxidative stress (Giannopolitis and Ries, 1977; Roxas et al., 1997).
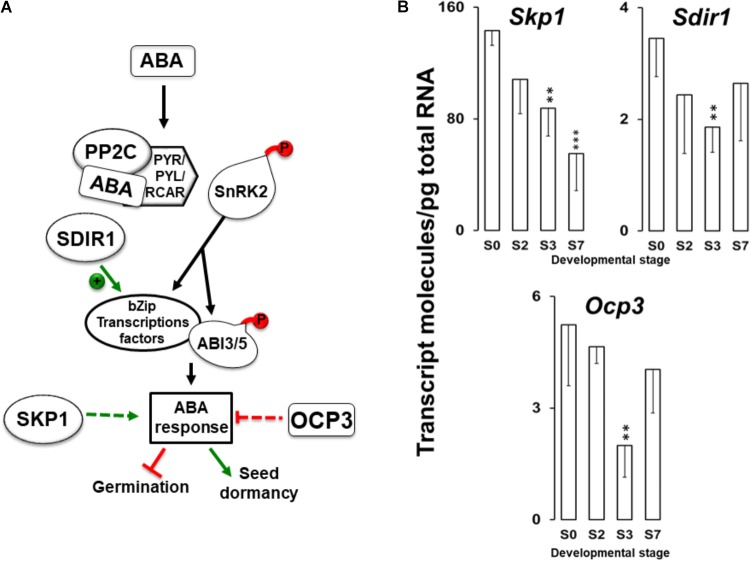
We analyzed Skp1, Sdir1 and Ocp3 genes coding for regulatory elements involved in the ABA signaling pathways, among which Ocp3 is a negative regulator (Ramírez et al., 2009) while, Skp1 and Sdir promote ABA effects (Zhang et al., 2007; Hu et al., 2013; Figure 4A). It is well established that ABA-dependent signaling is a main response to drought, salt stresses, and the maintenance of seed dormancy (Cutler et al., 2010 and ref. therein).
FIGURE 4.
(A) A simplified working model of the ABA signaling pathway controlling the germination process indicating the participation of the Skp1, Sdir1, and Ocp3 gene products. (B) Absolute quantitation of Skp1, Sdir1, and Ocp3 transcript molecules in embryo axis tissue isolated from non-imbibed seed (S0) or after 10 h (S2), 24 h (S3), and 216 h (S7) of germination. Transcript data are the means ± SD of transcript molecules/pg of total RNA from three biological replicates made in quadruplicate. Each biological replicate was a pool generated by mixing equal amounts of homogenized tissue from 10 to 30 embryo axis of the same sampling time. Statistical significances were determined by a one-way ANOVA. Differences between S0 samples and between each other were statistically significant at the level ∗∗P < 0.01 and ∗∗∗P < 0.001. Dashed lines indicate that multiple stages are included.
In a previous study, overexpression of Skp1 led to a delay in germination, improved drought tolerance, and root growth inhibition in the presence of ABA (Li et al., 2012). Values for the Skp1 transcript decreased as the germination and seedling growth progressed (from S0 to S7 stages) (Figure 4B), in parallel with the rise in ABA levels, (Figure 3A). Therefore, the decrease in Skp1 expression from S0 to S3 could facilitate germination, and the continued decrease in Skp1 expression continues to S7 stage might promote root growth.
In addition to SKP1, many other E3 ligases have been found to be involved in ABA responses. The RING type E3 Salt and Drought Induced RING Finger 1 (SDIR1) acts as an active RING-type E3 ubiquitin ligase, upstream of ABA-responsive transcription factors, in a feedback mechanism that enhances the ABA-signal (Lyzenga and Stone, 2012). Sdir1 expression has been reported to be upregulated by drought and salt stress, but not by ABA. Overexpression of Sdir1 leads to ABA hypersensitivity and drought tolerance (Zhang et al., 2007). In Q. ilex samples, Sdir1 transcript values decreased ∼2-fold from S0 (3.5 molecules/pg total RNA) to S3 (1.8 molecules/pg total RNA), resulting in a promotion of germination. A slight increase in Sdir1 expression was observed at the S7 stage (Figure 4B), but this expression level was slightly lower than the S0 stage. This result suggests that Sdir1 could be involved in some extent in seedling development.
The Ocp3 mRNAs diminished twofold during the germination (S0 to S3, from 5.2 to 2.3 molecules/pg total RNA) and increased thereafter during the post-germination phase by up to 3 molecules/pg total RNA at S7 stage. OCP3 is a member of the homeobox transcription factor family, and is considered as a negative regulator of the early response of the plant to drought stress since the Ocp3 loss of function yields a hyper-susceptibility to the ABA hormone in Arabidopsis, but does not affect germination rate (Ramírez et al., 2009). Considering that Ocp3 is a negative regulator of ABA signaling, its expression was unexpectedly decreased at S3 stage where ABA response should be lower to promote germination and there is no dehydration stress due to the imbibition of seeds. This result suggests that Ocp3 has little or no effect on modulation of ABA response during the germination and rehydration process.
Partial desiccation of up to 38% RWC takes place during maturation of Q. ilex seeds so some dehydration stress is expected. This can be confirmed by the presence of dehydration-responsive genes such as Dhn3 and GolS. Figure 5 shows changes in Dhn3 and GolS transcript abundance throughout the different developmental stages. Both are ABA-dependent dehydration-responsive genes, whose protein products protect plant proteins and membranes from water loss and help to maintain cell integrity during seed desiccation. Dehydrin DHN3 is a Group II Late Embryogenesis Abundant (LEA) family member, acting as a potent chaperone under dehydration stress conditions (Eriksson and Harryson, 2011). The accumulation of dehydrins was also reported previously in the recalcitrant Q. robur seeds (Šunderlíková et al., 2009), so their presence, at least at certain levels, cannot be correlated with desiccation tolerance and the orthodox character. Dhn3 transcript abundance dropped dramatically and constantly (>120-fold) from the S0 stage up to the end of the experiment (S7), and showed an inverse pattern with the RWC (Figure 5A). That decrease was also observed for the DHN3 protein, as was revealed by western blot assay (Figure 5B).
FIGURE 5.
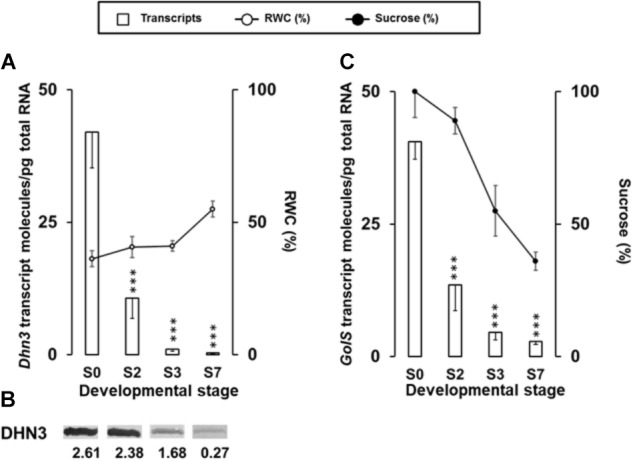
(A) Absolute quantitation of Dhn3 transcript molecules in embryo axis tissue isolated from non-imbibed seed (S0) or after 10 h (S2), 24 h (S3), and 216 h (S7) of germination, compared with the relative water content (RWC) in germinating seeds. Transcript data are the means ± SD of transcript molecules/pg of total RNA from three biological replicates made in quadruplicate. Each biological replicate was a pool generated by mixing equal amounts of homogenized tissue from 10 to 30 embryo axis of the same sampling time. Statistical significances were determined by a one-way ANOVA. Differences between S0 samples and each other were all statistically significant (∗∗∗P < 0.001). RWC data, expressed as percentage of lost weight relative to fresh weight, correspond to those in Figure 1E and are included for correlation with Dhn3 transcript amount. (B) Western blotting of DHN3 protein in Q. ilex seeds samples. Proteins were extracted from the same pools used in the transcriptional analysis. Numbers indicate the arbitrary Western blotting signal intensities normalized to the total protein contents, using Stain-Free Technology for total protein quantification. (C) Absolute quantitation of GolS transcript amounts in the same samples S0, S2, S3, and S7 and conditions described in panel (A), compared with the sucrose content in germinating seeds. Sucrose data correspond to those in Figure 2 and included for correlation with GolS transcript levels.
Gols is an enzyme involved in the synthesis of the raffinose series oligosaccharides, which are osmoregulators associated with seed desiccation tolerance (Li et al., 2011). We analyzed the GolS mRNA pattern during germination and seedling growth (Figure 5C). Transcripts accumulated at high levels (>40 transcript/pg total RNA) in mature Q. ilex embryo axes (S0), dramatically dropped (>14-fold decrease) after imbibition (S2–S7), changing in parallel with the relative sucrose content in germinating seeds (Figure 5C). A decrease in the expression of GolS mRNA during germination process has been reported in chickpea (C. arietinum). The raffinose series oligosaccharides have also been reported as ROS scavengers protecting cellular components from oxidative stress (Nishizawa et al., 2008). Therefore, they might improve seed vigor and longevity through limiting the age-induced excess ROS and subsequent lipid peroxidation (Salvi et al., 2016).
During seed maturation in many species (mostly orthodox seeds), there are two peaks of ABA accumulation: the first is maternally derived and promotes reserve accumulation and inhibits vivipary, and the second has embryonic origin and is important for LEA synthesis, desiccation tolerance, and dormancy (Finkelstein et al., 2002). In the recalcitrant Q. robur seeds, a peak of ABA accumulation was observed at the end of the maturation process (Prewein et al., 2006). This accumulation of ABA could occur during Q. ilex seed maturation, leading to the expression of Sdir1, Skp1, Dhn3, and GolS which are present in the mature seeds (S0). Although these genes can contribute to the tolerance of partial desiccation of Q. ilex seed, their accumulation levels together with the low ABA, sucrose, glucose, and fructose content are not sufficient to confer orthodox seed character. Because the ABA signaling pathway is involved in acquisition of dormancy and desiccation tolerance and both occur during seed development, it is difficult to evaluate the contribution of Sdir1, Skp1, and Ocp3 in dormancy on the one hand and in desiccation tolerance for the other hand, at least in our experimental conditions.
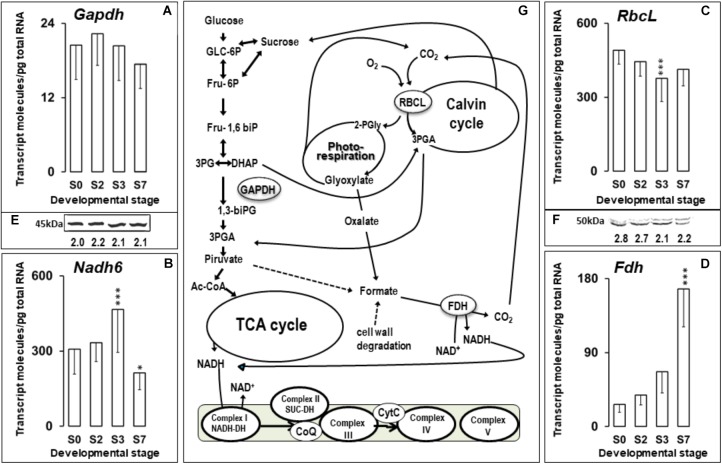
A very active metabolism is a characteristic of germination and growth processes in order to satisfy the high energy and biomolecule demand of the growing tissues. In orthodox seeds, germination represents a switch from quiescence in mature seeds to a metabolism reactivation under a low oxygen concentration in germinating ones (Logan et al., 2001). In contrast, in recalcitrant seeds, maturation and germination is continuous, with an active metabolism also after shedding (Dias et al., 2010; Sghaier-Hammami et al., 2016). Figure 6 shows the variation in the transcript abundance of four genes representing metabolic activity, Gapdh, Nadh6, Rbcl, and Fdh, throughout the germination and early post-germination stages. Gapdh transcripts and proteins remained almost constant during germination and early post-germination stages of Q. ilex seeds (Figures 6A,E). Recent studies have shown that GAPDH has multiple functions independently of its role in energy metabolism. An increased GAPDH gene expression and enzymatic function is associated with cell proliferation, transcriptional, and posttranscriptional gene regulation, vesicular transport, receptor-mediated cell signaling, chromatin structure and the maintenance of DNA integrity (O’Halloran and Culotta, 2000; Aoki et al., 2006). Nadh6 transcript numbers peaked early during acorn germination (Figure 6B), coinciding with radicle emergence (S3), and dropped later to the levels found in mature seeds. A similar increase in the abundance of Nadh6 and other transcripts encoding mitochondrial proteins has been reported for Arabidopsis during the maturation of the mitochondria that occurs after imbibition (Howell et al., 2009). These changes were correlated with the decrease in sucrose (Figure 2) at germination (S0 to S3 steps) and the increases in glucose and fructose levels at post-germination (S3 to S7) stages. This indicated a modulation of the sugar catabolism and the adaptation to the energy and carbon demands by the developing tissues, similarly to those described for orthodox seeds (Downie and Bewley, 2000; Nkang, 2002).
FIGURE 6.
(A–D) Absolute quantitation of Gapdh, Nadh6, RbcL, and Fdh transcript molecules in embryo axis tissue isolated from non-imbibed seed (S0) or after 10 h (S2), 24 h (S3), and 216 h (S7) of germination. Transcript data are the means ± SD of transcript molecules/pg of total RNA from three biological replicates made in quadruplicate. Each biological replicate was a pool generated by mixing equal amounts of homogenized tissue from 10 to 30 embryo axis of the same sampling time. Statistical significance was determined by a one-way ANOVA. Differences between S0 samples and between each other were statistically significant at the level ∗P < 0.05; ∗∗∗P < 0.001. (E,F) Western blotting of GAPDH and RBCL proteins in Q. ilex seed samples. Proteins were extracted from the same pools used in the transcriptional analysis. Numbers indicate the arbitrary Western blotting signal intensities normalized to the total protein contents, using Stain-Free Technology for total protein quantification. (G) A simplified working model of some metabolic pathways indicating the participation of the Gapdh, Nadh6, RbcL and Fdh gene products.
The controversial presence of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) in non-photosynthetic tissue, such as seeds (Sghaier-Hammami et al., 2016) and roots (Simova-Stoilova et al., 2015), has been proven by proteomics in Q. ilex seeds and confirmed by data presented here on transcript abundance of the RbcL gene that codes for the RuBisCO large subunit. Speculating on the physiological role of the RuBisCO enzyme, it can be proposed as being implicated in the re-assimilating CO2 from decarboxylation reactions. In Q. ilex seeds, RbcL transcript abundance provided one of the highest values for the genes analyzed (∼400 molecules/ng total RNA) (Figure 6C). Transcription of RbcL has been shown to be repressed by soluble sugars (Kersten et al., 2006), which might explain the decrease in the RbcL transcript and protein levels during Q. ilex germination, when the soluble sugars increase (Figures 2, 6C,F).
The Fdh mRNA levels (Figure 6D) showed a constant increase throughout the different developmental stages studied here, becoming an abundant transcript, with more than 150 molecules/pg total RNA, at the end of the experiment. FDH is one of the enzymes whose gene is dependent on ABA (Alekseeva et al., 2011). The increase in endogenous concentrations of this phytohormone in Q. ilex seedlings during post-germination stages (Figure 3A) might explain this dramatic increase in Fdh mRNA.
Reactive oxygen species, exert a dual effect on germination, acting as messengers and causing oxidative damage to cell macromolecules and structures (Bailly et al., 2008). In orthodox seeds, elevated rates of ROS production upon seed imbibition has been suggested to be involved in cell wall loosening, and in defense of emerging seedlings against pathogens (Roach et al., 2010), and are necessary for proceeding to germination (Bailly et al., 2008). To keep ROS in balance, different tightly regulated mechanisms do exist, with redox enzymes acting as scavengers playing an important role. The transcript abundance of two of them, superoxide dismutase (SOD1) and glutathione-S-transferase (GST), was analyzed in the present work. Sod1 transcriptional profile (Figure 7A) shows a significant decrease in the amount of Sod1 mRNA molecules throughout the germination process. The decrease in the number of Sod1 transcripts was initiated at S2 stage and might contribute to the drop in SOD1 activity detected later (S7 stage). These results suggest that ROS scavenging mechanisms have been activated during recalcitrant seed maturation, leading to the accumulation of SOD, one of the enzymes implicated in the balance of ROS, and allowing progression to germination (Barba-Espin et al., 2010). Three types of SODs have been classified on the basis of the metal present at the catalytic site: Cu/Zn-SOD (SOD1, located in the cytoplasm and chloroplasts), Mn-SOD (in the mitochondrial matrix and peroxisomes), and Fe-SOD (in chloroplasts and cytoplasm) (Redgwell and Fry, 1993; Myouga et al., 2008). By means of an in gel enzyme activity assay (Weydert and Cullen, 2010) and the SOD-inhibitors H2O2 and KCN, we identified three MnSOD isoforms (resistant to H2O2 and KCN), a single Cu/ZnSOD (sensitive to H2O2 and KCN) and a single FeSOD (resistant to KCN and sensitive to H2O2) in Q. ilex seeds (Figure 7B and Supplementary Figure S3). Bands of the five isoforms were present in all the stages studied.
FIGURE 7.
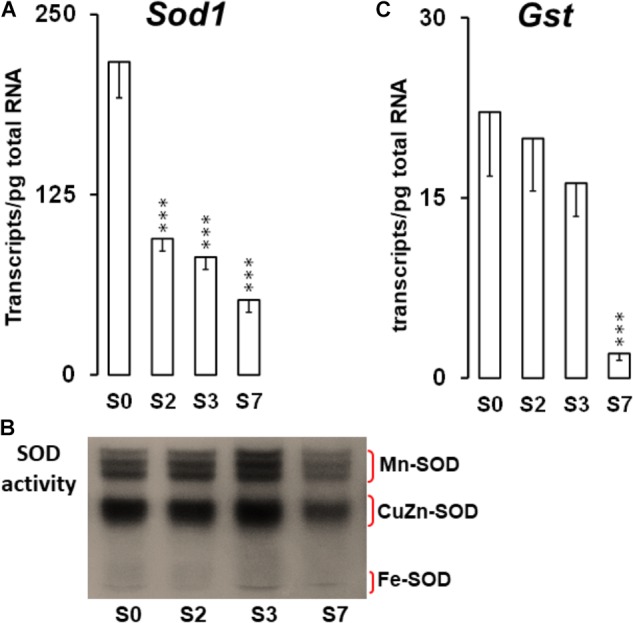
(A,C) Absolute quantitation of Sod and Gst transcript molecules in embryo axis tissue isolated from non-imbibed seed (S0) or after 10 h (S2), 24 h (S3), and 216 h (S7) of germination. Transcript data are the means ± SD of transcript molecules/pg of total RNA from three biological replicates made in quadruplicate. Each biological replicate was a pool generated by mixing equal amounts of homogenized tissue from 10 to 30 embryo axis of the same sampling time. Statistically significant differences between S0 samples were determined by a one-way ANOVA (∗∗∗P < 0.001). (B) SOD activity gel assay showing the SOD protein profiles on native PAGE of different Q. ilex seeds germination stages. Proteins were extracted from the same pools used in the transcript analysis. A 20 μg sample of total soluble proteins was loaded onto a 10% acrylamide gel. The image of the gel was inverted to obtain a better visualization of the bands.
Plant GSTs are a super-family of proteins of a divergent sequence but conserved structure, which catalyze the conjugation of electrophilic xenobiotic substrates with the tripeptide glutathione (GSH), and which are selectively stress-inducible. Some GSTs can also act as glutathione peroxidases, protecting cells from oxygen toxicity (Binder et al., 2004). Our results revealed that the abundance of Gst transcripts decreases during germination in Q. ilex acorns (Figure 7C). Our findings also suggest, that the accumulation of SOD and GST transcripts, implicated in ROS balance in mature seeds, could be necessary to protect the tissue from ROS damage with other antioxidant enzyme such as catalase, peroxidase, etc. Although these enzymes were not analyzed in this work, their activity was previously described during recalcitrant seed germination (Gumilevskaya and Azarkovich, 2007). ROS also act as messengers in signal transduction pathways during germination (Bailly et al., 2008; Wojtyla et al., 2016).
Conclusion
The germination and early seedling growth process, and the non-orthodox, non-dormant character of Q. ilex seeds, have been partially characterized at the molecular level.
The main differences with orthodox seeds are already established in mature ones, and are manifested at the germination (S1–S3) stage. Once germinated, the patterns are quite similar between both types of seeds.
The hormonal balance (low ABA and high GA, IAA and cytokinin content) and the presence of enzyme machinery ensuring an active metabolism (i.e., GAPDH and NADH) seem to contribute to the non-dormant character.
Although desiccation tolerance genes were expressed, their accumulation level together with the low ABA, sucrose, glucose, and fructose content are not sufficient to confer orthodox seed character.
The high transcript levels of two ROS scavenging enzymes found in mature seeds, suggest that these enzymes are important in maintaining ROS balance in Q. ilex seeds.
Author Contributions
JJ-N conceived the work, examined, and evaluated the data. MR-R and AA-Y carried out the gene expression and protein immunoblot assays, and measurements of enzyme activities. NA examined and evaluated the gene expression data. AG-S performed the sugar analysis. MM conducted the hormone analysis. The manuscript was written by JJ-N and MR-R, and finally approved by the rest of the authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank J. Valero-Galván, J. Ruiz-Laguna, and I. Redondo-López for their technical assistance. This manuscript is part of the MR-R Ph.D. Thesis (Romero-Rodríguez, 2015), which can be accessed online, in line with the University of Córdoba policy.
Funding. This work was supported by the Spanish “Ministry of Science and Innovation”, project AGL2009-12243-C02-02 (FEDER Cofinanced), “Junta de Andalucía” and University of Córdoba. AA-Y and MR-R were recipients of a grant from Spanish “Ministry of Education and Science” and a predoctoral fellowships “Itaipu Binacional” from Paraguay, respectively.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01508/full#supplementary-material
Process to acorn germination. (A) Acorn with pericarp, (B) acorn peeled, (C) acorn cut at the distal end and (D) boxes contain filter paper, perlite, and a germinated acorn.
Morphological aspects of acorns and seeds at different stages. In brackets the approximate time in hours post-imbibition to obtain the indicated stages.
SOD in-gel activity assay of embryo axis and shoot seedling of Q. ilex. (A) Inhibitor test for SOD isoforms was conducted by application of 2 mM KCN or 5 mM H2O2 prior to activity staining. (B) SOD isoforms are labeled S1–S5 in order of increasing mobility.
Primers used in this work for the determination of Q. ilex sequences. Primers based on orthologous sequences were designed to obtain partial coding sequences of Q. ilex genes.
Primers used for absolute quantification by real-time PCR of Q. ilex transcripts.
References
- Alekseeva A. A., Savin S. S., Tishkov V. I. (2011). NAD (+) -dependent formate dehydrogenase from plants. Acta Nat. 3 38–54. [PMC free article] [PubMed] [Google Scholar]
- Ali-Rachedi S., Bouinot D., Wagner M. H., Bonnet M., Sotta B., Grappin P., et al. (2004). Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219 479–488. 10.1007/s00425-004-1251-4 [DOI] [PubMed] [Google Scholar]
- Angelovici R., Galili G., Fernie A. R., Fait A. (2010). Seed desiccation: a bridge between maturation and germination. Trends Plant Sci. 15 211–218. 10.1016/j.tplants.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Aoki N., Scofield G. N., Wang X.-D., Offler C. E., Patrick J. W., Furbank R. T. (2006). Pathway of sugar transport in germinating wheat seeds. Plant Physiol. 141 1255–1263. 10.1104/pp.106.082719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C., El-Maarouf-Bouteau H., Corbineau F. (2008). From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 331 806–814. 10.1016/j.crvi.2008.07.022 [DOI] [PubMed] [Google Scholar]
- Barba-Espin G., Diaz-Vivancos P., Clemente-Moreno M. J., Albacete A., Faize L., Faize M., et al. (2010). Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 33 981–994. 10.1111/j.1365-3040.2010.02120.x [DOI] [PubMed] [Google Scholar]
- Berjak P., Pammenter N. W. (2008). From Avicennia to Zizania: seed recalcitrance in perspective. Ann. Bot. 101 213–228. 10.1093/aob/mcm168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjak P., Pammenter N. W. (2013). Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 4:478. 10.3389/fpls.2013.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J. D. (1997). Seed germination and dormancy. Plant Cell 9 1055–1066. 10.1105/tpc.9.7.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J. D., Bradford K. J., Kent J., Hilhorst H. W. M., Nonogaki H. (2013). “Germination”, in Seeds: Physiology of Development, Germination and Dormancy. New York, NY: Springer, 133–181. 10.1007/978-1-4614-4693-4_4 [DOI] [Google Scholar]
- Białecka B., Kȩpczyński J. (2007). Changes in concentrations of soluble carbohydrates during germination of Amaranthus caudatus L. seeds in relation to ethylene, gibberellin A3and methyl jasmonate. Plant Growth Regul. 51 21–31. 10.1007/s10725-006-9145-z [DOI] [Google Scholar]
- Binder B. M., Malley R. C. O., Wang W., Moore J. M., Parks B. M., Spalding E. P., et al. (2004). Arabidopsis seedling growth response and recovery to ethylene a kinetic analysis. Plant Physiol. 136 2913–2920. 10.1104/pp.104.050369.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M., Bewley J. D. (eds) (2000). Seed Technology and its Biological Basis. Sheffield: Sheffield Academic Press. [Google Scholar]
- Bogdanović J., Radotić K., Mitrović A. (2008). Changes in activities of antioxidant enzymes during Chenopodium murale seed germination. Biol. Plant. 52 396–400. 10.1007/s10535-008-0083-7 [DOI] [Google Scholar]
- Bonner F. T., Vozzo J. A. (1987). Seed Biology and Technology of Quercus. Gen. Technical Report. SO-66. New Orleans, LA: U.S. Department of Agriculture, Forest Service, 1–21. 10.2737/SO-GTR-66 [DOI] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Caccere R., Teixeira S. P., Centeno D. C., Figueiredo-Ribeiro Rde C., Braga M. R. (2013). Metabolic and structural changes during early maturation of Inga vera seeds are consistent with the lack of a desiccation phase. J. Plant Physiol. 170 791–800. 10.1016/j.jplph.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Cerdán-Calero M., Sendra J. M., Sentandreu E. (2012). Gas chromatography coupled to mass spectrometry analysis of volatiles, sugars, organic acids and amino acids in valencia late orange juice and reliability of the automated mass spectral deconvolution and identification system for their automatic identification. J. Chromatogr. A 1241 84–95. 10.1016/j.chroma.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Chen S.-Y., Chien C.-T., Baskin J. M., Baskin C. C. (2010). Storage behavior and changes in concentrations of abscisic acid and gibberellins during dormancy break and germination in seeds of Phellodendron amurense var. wilsonii (Rutaceae). Tree Physiol. 30 275–284. 10.1093/treephys/tpp111 [DOI] [PubMed] [Google Scholar]
- Connor K. F., Sowa S. (2003). Effects of desiccation on the physiology and biochemistry of Quercus alba acorns. Tree Physiol. 23 1147–1152. 10.1093/treephys/23.16.1147 [DOI] [PubMed] [Google Scholar]
- Cutler S. R., Rodriguez P. L., Finkelstein R. R., Abrams S. R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61 651–679. 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- David P., des Francs-Small C. C., Sévignac M., Thareau V., Macadré C., Langin T., et al. (2010). Three highly similar formate dehydrogenase genes located in the vicinity of the B4 resistance gene cluster are differentially expressed under biotic and abiotic stresses in Phaseolus vulgaris. Theor. Appl. Genet. 121 87–103. 10.1007/s00122-010-1293-x [DOI] [PubMed] [Google Scholar]
- de Rigo D., Caudullo G. (2016). “Quercus ilex in Europe: distribution, habitat, usage and threats,” in European Atlas of Forest Tree Species, eds San-Miguel-Ayanz J., de Rigo D., Caudullo G., Houston Durrant T., Mauri A. (Luxemburgo: Publ. Off. EU; ), 152–153. [Google Scholar]
- Dias L. L. C., Balbuena T. S., Silveira V., Santa-Catarina C., Schevchenko A., Floh E. I. S. (2010). Two-dimensional gel electrophoretic protein profile analysis during seed development of Ocotea catharinensis: a recalcitrant seed species. Braz. J. Plant Physiol. 22 23–33. 10.1590/S1677-04202010000100003 [DOI] [Google Scholar]
- Downie B., Bewley J. D. (2000). Soluble sugar content of white spruce (Picea glauca) seeds during and after germination. Physiol. Plant. 110 1–12. 10.1034/j.1399-3054.2000.110101.x [DOI] [Google Scholar]
- Echevarría-Zomeño S., Abril N., Ruiz-Laguna J., Jorrín-Novo J., Maldonado-Alconada A. M. (2012). Simple, rapid and reliable methods to obtain high quality RNA and genomic DNA from Quercus ilex L. leaves suitable for molecular biology studies. Acta Physiol. Plant. 34 793–805. 10.1007/s11738-011-0880-z [DOI] [Google Scholar]
- Echevarría-Zomeño S., Ariza D., Jorge I., Lenz C., Del Campo A., Jorrín J. V., et al. (2009). Changes in the protein profile of Quercus ilex leaves in response to drought stress and recovery. J. Plant Physiol. 166 233–245. 10.1016/j.jplph.2008.05.008 [DOI] [PubMed] [Google Scholar]
- Eriksson S. K., Harryson P. (2011). “Dehydrins: molecular biology, structure and function,” in Plant Desiccation Tolerance. Ecological Studies Analysis and Synthesis Vol. 215 eds Lüttge U., Beck E., Bartels D. (Berlin: Springer; ), 289–305. 10.1007/978-3-642-19106-0_14 [DOI] [Google Scholar]
- Farrant J. M., Berjak P., Cutting J. G. M., Pammenter N. W. (1993). The role of plant growth regulators in the development and germination of the desiccation sensitive recalcitrant seeds of Avicennia Marina. Seed Sci. Res. 3 55–63. 10.1017/S0960258500001562 [DOI] [Google Scholar]
- Farrant J. M., Moore J. P. (2011). Programming desiccation-tolerance: from plants to seeds to resurrection plants. Curr. Opin. Plant Biol. 14 340–345. 10.1016/j.pbi.2011.03.018 [DOI] [PubMed] [Google Scholar]
- Finch-Savage W., Blake P. S., Clay H. A. (1996). Desiccation stress in recalcitrant Quercus robur L. seeds results in lipid peroxidation and increased synthesis of jasmonates and abscisic acid. J. Exp. Bot. 47 661–667. 10.1093/jxb/47.5.661 [DOI] [Google Scholar]
- Finch-Savage W. E., Blake P. S. (1994). Indeterminate development in desiccation-sensitive seeds of Quercus robur L. Seed Sci. Res. 4 127–133. 10.1017/S0960258500002129 [DOI] [Google Scholar]
- Finch-Savage W. E., Leubner-Metzger G. (2006). Seed dormancy and the control of germination. New Phytol. 171 501–523. 10.1111/j.1469-8137.2006.01787.x [DOI] [PubMed] [Google Scholar]
- Finkelstein R., Reeves W., Ariizumi T., Steber C. (2008). Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 59 387–415. 10.1146/annurev.arplant.59.032607.092740 [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Gampala S. S. L., Rock C. D. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl.), S15–S45. 10.1105/tpc.010441.would [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleige S., Pfaffl M. W. (2006). RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 27 126–139. 10.1016/j.mam.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Gara L., Pinto M. C., Arrigoni O. (1997). Ascorbate synthesis and ascorbate peroxidase activity during the early stage of wheat germination. Physiol. Plant. 100 894–900. 10.1111/j.1399-3054.1997.tb00015.x [DOI] [Google Scholar]
- Giannopolitis C. N., Ries S. K. (1977). Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 59 309–314. 10.2307/4264724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González S., Ruiz-Jiménez J., Priego-Capote F., Luque De Castro M. D. (2010). Qualitative and quantitative sugar profiling in olive fruits, leaves, and stems by gas chromatography-tandem mass spectrometry (GC-MS/MS) after ultrasound-assisted leaching. J. Agric. Food Chem. 58 12292–12299. 10.1021/jf102350s [DOI] [PubMed] [Google Scholar]
- Goodman R. C., Jacobs D. F., Karrfalt R. P. (2005). Evaluating desiccation sensitivity of Quercus rubra acorns using X-ray image analysis. Can. J. For. Res. 35 2823–2831. 10.1139/x05-209 [DOI] [Google Scholar]
- Gumilevskaya N. A., Azarkovich M. I. (2007). Physiological and biochemical characteristics of the recalcitrant seeds having dormancy: a review. Appl. Biochem. Microbiol. 43 332–340. 10.1134/S0003683807030167 [DOI] [PubMed] [Google Scholar]
- Hanin M., Brini F., Ebel C., Toda Y., Takeda S., Masmoudi K. (2011). Plant dehydrins and stress tolerance. Plant Signal. Behav. 6 1503–1509. 10.4161/psb.6.10.17088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K. A., Narsai R., Carroll A., Ivanova A., Lohse M., Usadel B., et al. (2009). Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol. 149 961–980. 10.1104/pp.108.129874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D.-L., Chen Q.-Z., Zhang C.-J., Wang Y., Zhang B.-J., Tang C.-M. (2013). Identification of cotton SKP1-like gene GhSKP1 and its function in seed germination and taproot growth in tobacco. Can. J. Plant Sci. 93 817–825. 10.4141/CJPS2012-312 [DOI] [Google Scholar]
- Jeevan Kumar S. P., Rajendra Prasad S., Banerjee R., Thammineni C. (2015). Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann. Bot. 116 663–668. 10.1093/aob/mcv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët T., Ourcival J. M., Dussert S. (2013). Ecological significance of seed desiccation sensitivity in Quercus ilex. Ann. Bot. 111 693–701. 10.1093/aob/mct025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten B., Agrawal G. K., Iwahashi H., Rakwal R. (2006). Plant phosphoproteomics: a long road ahead. Proteomics 6 5517–5528. 10.1002/pmic.200600232 [DOI] [PubMed] [Google Scholar]
- Kranner I., Roach T., Beckett R. P., Whitaker C., Minibayeva F. V. (2010). Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant Physiol. 167 805–811. 10.1016/j.jplph.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Kuo T. M., Doehlert D. C., Crawford C. G. (1990). Sugar metabolism in germinating soybean seeds: evidence for the sorbitol pathway in soybean axes. Plant Physiol. 93 1514–1520. 10.1104/pp.93.4.1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Polisensky D. H., Braam J. (2005). Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol. 165 429–444. 10.1111/j.1469-8137.2004.01238.x [DOI] [PubMed] [Google Scholar]
- Lee Y. P., Baek K.-H., Lee H.-S., Kwak S.-S., Bang J.-W., Kwon S.-Y. (2010). Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 61 2499–2506. 10.1093/jxb/erq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., North H., Frey A., Sotta B., Seo M., Okamoto M., et al. (2006). Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 45 309–319. 10.1111/j.1365-313X.2005.02622.x [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G. (2006). “Hormonal interactions during seed dormancy release and germination,” in Handbook of Seed Science and Technology, ed. Basra A. S. (New York, NY: Food Products Press; ), 303–341. [Google Scholar]
- Li C., Liu Z., Zhang Q., Wang R., Xiao L., Ma H., et al. (2012). SKP1 is involved in abscisic acid signalling to regulate seed germination, stomatal opening and root growth in Arabidopsis thaliana. Plant Cell Environ. 35 952–965. 10.1111/j.1365-3040.2011.02464.x [DOI] [PubMed] [Google Scholar]
- Li X., Zhuo J. J., Jing Y., Liu X., Wang X. F. (2011). Expression of a Galactinol Synthase gene is positively associated with desiccation tolerance of Brassica napus seeds during development. J. Plant Physiol. 168 1761–1770. 10.1016/j.jplph.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Liu P. P., Montgomery T. A., Fahlgren N., Kasschau K. D., Nonogaki H., Carrington J. C. (2007). Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 52 133–146. 10.1111/j.1365-313X.2007.03218.x [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu G., Li Q., Liu Y., Hou L., Li G. L. (2012). Influence of pericarp, cotyledon and inhibitory substances on sharp tooth oak (Quercus aliena var. acuteserrata) germination. PLoS One 7:e47682. 10.1371/journal.pone.0047682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shi L., Ye N., Liu R., Jia W., Zhang J. (2009). Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol. 183 1030–1042. 10.1111/j.1469-8137.2009.02899.x [DOI] [PubMed] [Google Scholar]
- Logan D. C., Millar A. H., Sweetlove L. J., Hill S. A., Leaver C. J. (2001). Mitochondrial biogenesis during germination in maize embryos. Plant Physiol. 125 662–672. 10.1104/pp.125.2.662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzenga W. J., Stone S. L. (2012). Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 63 599–616. 10.1093/jxb/err310 [DOI] [PubMed] [Google Scholar]
- McAdam S. A. M., Brodribb T. J., Ross J. J. (2016). Shoot-derived abscisic acid promotes root growth. Plant. Cell Environ. 39 652–659. 10.1111/pce.12669 [DOI] [PubMed] [Google Scholar]
- Miransari M., Smith D. L. (2014). Plant hormones and seed germination. Environ. Exp. Bot. 99 110–121. 10.1016/j.envexpbot.2013.11.005 [DOI] [Google Scholar]
- Muñoz-Bertomeu J., Cascales-Minana B., Mulet J. M., Baroja-Fernandez E., Pozueta-Romero J., Kuhn J. M., et al. (2009). Plastidial Glyceraldehyde-3-Phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol. 151 541–558. 10.1104/pp.109.143701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myouga F., Hosoda C., Umezawa T., Iizumi H., Kuromori T., Motohashi R., et al. (2008). A heterocomplex of oron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20 3148–3162. 10.1105/tpc.108.061341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Fujita Y., Kanamori N., Katagiri T., Umezawa T., Kidokoro S., et al. (2009). Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 50 1345–1363. 10.1093/pcp/pcp083 [DOI] [PubMed] [Google Scholar]
- Nguyen T. C. T., Obermeier C., Friedt W., Abrams S. R., Snowdon R. J. (2016). Disruption of germination and seedling development in Brassica napus by mutations causing severe seed hormonal imbalance. Front. Plant Sci. 7:322. 10.3389/fpls.2016.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa A., Yabuta Y., Shigeoka S. (2008). Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 147 1251–1263. 10.1104/pp.108.122465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkang A. (2002). Carbohydrate composition during seed development and germination in two sub-tropical rainforest tree species (Erythrina caffra and Guilfoylia monostylis). J. Plant Physiol. 159 473–483. 10.1078/0176-1617-00516 [DOI] [Google Scholar]
- Novák O., Hauserová E., Amakorová P., Doležal K., Strnad M. (2008). Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69 2214–2224. 10.1016/j.phytochem.2008.04.022 [DOI] [PubMed] [Google Scholar]
- Ntuli T. M., Finch-Savage W. E., Berjak P., Pammenter N. W. (2011). Increased drying rate lowers the critical water content for survival in embryonic axes of english oak (Quercus robur L.) seeds. J. Integr. Plant Biol. 53 270–280. 10.1111/j.1744-7909.2010.01016.x [DOI] [PubMed] [Google Scholar]
- Ogawa M., Hanada A. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15 1591–1604. 10.1105/tpc.011650.ble [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran T. V., Culotta V. C. (2000). Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275 25057–25060. 10.1074/jbc.R000006200 [DOI] [PubMed] [Google Scholar]
- Oracz K., Karpiñski S. (2016). Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 7:864. 10.3389/fpls.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter N. W., Berjak P. (2014). Physiology of desiccation-sensitive (recalcitrant) seeds and the implications for cryopreservation. Int. J. Plant Sci. 175 21–28. 10.1086/673302 [DOI] [Google Scholar]
- Pan X., Welti R., Wang X. (2008). Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69 1773–1781. 10.1016/j.phytochem.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Parcy F., Valon C., Raynal M., Gaubier-Comella P., Delseny M., Giraudat J. (1994). Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6 1567–1582. 10.1105/tpc.6.11.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariona N., Martínez A. I., Hernandez-Flores H., Clark-Tapia R. (2017). Effect of magnetite nanoparticles on the germination and early growth of Quercus macdougallii. Sci. Total Environ. 575 869–875. 10.1016/j.scitotenv.2016.09.128 [DOI] [PubMed] [Google Scholar]
- Parkhey S., Naithani S. C., Keshavkant S. (2014). Protein metabolism during natural ageing in desiccating recalcitrant seeds of Shorea robusta. Acta Physiol. Plant. 36 1649–1659. 10.1007/s11738-014-1540-x [DOI] [Google Scholar]
- Pasquini S., Mizzau M., Petrussa E., Braidot E., Patui S., Gorian F., et al. (2012). Seed storage in polyethylene bags of a recalcitrant species (Quercus ilex): analysis of some bio-energetic and oxidative parameters. Acta Physiol. Plant. 34 1963–1974. 10.1007/s11738-012-0996-9 [DOI] [Google Scholar]
- Pieruzzi F. P., Dias L. L., Balbuena T. S., Santa-Catarina C., Dos Santos A. L., Floh E. I. (2011). Polyamines, IAA and ABA during germination in two recalcitrant seeds: Araucaria angustifolia (Gymnosperm) and Ocotea odorifera (Angiosperm). Ann. Bot. 108 337–345. 10.1093/aob/mcr133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewein C., Endemann M., Reinohl V., Salaj J., Sunderlikova V., Wilhelm E. (2006). Physiological and morphological characteristics during development of pedunculate oak (Quercus robur L.) zygotic embryos. Trees Struct. Funct. 20 53–60. 10.1007/s00468-005-0012-8 [DOI] [Google Scholar]
- Prieto-Alamo M. J., Cabrera-Luque J.-M., Pueyo C. (2003). Absolute quantitation of normal and ROS-induced patterns of gene expression: an in vivo real-time PCR study in mice. Gene Expr. 11 23–34. 10.3727/000000003783992315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido F., Díaz M., Hidalgo S. (2001). Size-structure and regeneration of Spanish holm oak Quercus ilex forest and dehesas: effects of agroforestry use on their long-term sustainability. For. Ecol. Manage. 146 1–13. 10.1016/S0378-1127(00)00443-6 [DOI] [Google Scholar]
- Rajjou L., Duval M., Gallardo K., Catusse J., Bally J., Job C., et al. (2012). Seed germination and vigor. Annu. Rev. Plant Biol. 63 507–533. 10.1146/annurev-arplant-042811-105550 [DOI] [PubMed] [Google Scholar]
- Ramírez V., Coego A., López A., Agorio A., Flors V., Vera P. (2009). Drought tolerance in Arabidopsis is controlled by the OCP3 disease resistance regulator. Plant J. 58 578–591. 10.1111/j.1365-313X.2009.03804.x [DOI] [PubMed] [Google Scholar]
- Redgwell R. J., Fry S. C. (1993). Xyloglucan endotransglycosylase activity increases during kiwifruit (Actinidia deliciosa) ripening (implications for fruit softening). Plant Physiol. 103 1399–1406. 10.1104/pp.103.4.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T., Beckett R. P., Minibayeva F. V., Colville L., Whitaker C., Chen H., et al. (2010). Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant Cell Environ. 33 59–75. 10.1111/j.1365-3040.2009.02053.x [DOI] [PubMed] [Google Scholar]
- Romero-Rodríguez M. C. (2015). Integrated “omics” Approaches to Study Non-Orthodox Seed Germination: The Case of Holm Oak (Quercus Ilex Subsp. Ballota [Desf.] Samp). Ph.D. thesis, Universidad de Córdoba, Córdoba IL. [Google Scholar]
- Rowe H. A., Knox D. P., Poxton I. R., Donachie W. (1997). Divergent activity and function of superoxide dismutases in Pasteurella haemolytica serotypes A1 and A2 and Pasteurella trehalosi serotype T10. FEMS Microbiol. Lett. 150 197–202. 10.1016/S0378-1097(97)00113-4 [DOI] [PubMed] [Google Scholar]
- Roxas V. P., Smith R. K., Smith R. K., Allen R. D. (1997). Overexpression of glutathione s-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat. Biotechnol. 15 988–991. 10.1038/nbt1097-988 [DOI] [PubMed] [Google Scholar]
- Salvi P., Saxena S. C., Petla B. P., Kamble N. U., Kaur H., Verma P., et al. (2016). Differentially expressed galactinol synthase(s) in chickpea are implicated in seed vigor and longevity by limiting the age induced ROS accumulation. Sci. Rep. 6 1–15. 10.1038/srep35088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner A., Calderon-Villalobos L. I. A., Estelle M. (2009). Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 5 301–307. 10.1038/nchembio.165 [DOI] [PubMed] [Google Scholar]
- Schopfer P., Plachy C., Fraga C. G., Puntarulo S., Sevilla F., Corpas F., et al. (1985). Control of seed germination by abscisic acid: III. effect on embryo growth potential (minimum turgor pressure) and growth coefficient (cell wall extensibility) in Brassica napus L. Plant Physiol. 77 676–686. 10.1104/pp.77.3.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sghaier-Hammami B., Redondo-López I., Valero-Galvàn J., Jorrín-Novo J. V. (2016). Protein profile of cotyledon, tegument, and embryonic axis of mature acorns from a non-orthodox plant species: Quercus ilex. Planta 243 369–396. 10.1007/s00425-015-2404-3 [DOI] [PubMed] [Google Scholar]
- Shi W., Bloomberg M., Li G., Su S., Jia L. (2017). Combined effects of cotyledon excision and nursery fertilization on root growth, nutrient status and outplanting performance of Quercus variabilis container seedlings. PLoS One 12:e0177002. 10.1371/journal.pone.0177002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K., Liu X., Xie Q., He Z. (2016). Two faces of one seed: hormonal regulation of dormancy and germination. Mol. Plant 9 34–45. 10.1016/J.MOLP.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Simova-Stoilova L. P., Romero-Rodríguez M. C., Sánchez-Lucas R., Navarro-Cerrillo R. M., Medina-Aunon J. A., Jorrín-Novo J. V. (2015). 2-DE proteomics analysis of drought treated seedlings of Quercus ilex supports a root active strategy for metabolic adaptation in response to water shortage. Front. Plant Sci. 6:627. 10.3389/fpls.2015.00627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman K. J., Pritchard H. W., Dey P. M. (1996). Tissue-specific soluble sugars in seeds as indicators of storage category. Ann. Bot. 77 667–674. 10.1006/anbo.1996.0083 [DOI] [Google Scholar]
- Stirk W. A., Václavíková K., Novák O., Gajdošová S., Kotland O., Motyka V., et al. (2012). Involvement of cis-zeatin, dihydrozeatin, and aromatic cytokinins in germination and seedling establishment of maize, oats, and lucerne. J. Plant Growth Regul. 31 392–405. 10.1007/s00344-011-9249-1 [DOI] [Google Scholar]
- Šunderlíková V., Salaj J., Kopecky D., Salaj T., Wilhem E., Matušíková I. (2009). Dehydrin genes and their expression in recalcitrant oak (Quercus robur) embryos. Plant Cell Rep. 28 1011–1021. 10.1007/s00299-009-0710-6 [DOI] [PubMed] [Google Scholar]
- Swigonska S., Weidner S. (2013). Proteomic analysis of response to long-term continuous stress in roots of germinating soybean seeds. J. Plant Physiol. 170 470–479. 10.1016/j.jplph.2012.11.020 [DOI] [PubMed] [Google Scholar]
- Taylor S. C., Mrkusich E. M. (2014). The state of RT-quantitative PCR: firsthand observations of implementation of minimum information for the publication of quantitative real-time PCR experiments (MIQE). J. Mol. Microbiol. Biotechnol. 24 46–52. 10.1159/000356189 [DOI] [PubMed] [Google Scholar]
- Tesfay S. Z., Modi A. T., Mohammed F. (2016). The effect of temperature in moringa seed phytochemical compounds and carbohydrate mobilization. S. Afr. J. Bot. 102 190–196. 10.1016/j.sajb.2015.07.003 [DOI] [Google Scholar]
- Tilki F. (2010). Influence of acorn size and storage duration on moisture content, germination and survival of Quercus petraea (Mattuschka). J. Environ. Biol. 31 325–328. [PubMed] [Google Scholar]
- Toh S., Imamura A., Watanabe A., Nakabayashi K., Okamoto M., Jikumaru Y., et al. (2008). High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 146 1368–1385. 10.1104/pp.107.113738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi F., Paciolla C., de Pinto M. C., De Gara L. (2001). A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J. Exp. Bot. 52 1647–1654. 10.1093/jexbot/52.361.1647 [DOI] [PubMed] [Google Scholar]
- Tromas A., Perrot-Rechenmann C. (2010). Recent progress in auxin biology. C. R. Biol. 333 297–306. 10.1016/J.CRVI.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Vishal B., Kumar P. P. (2018). Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 9:838. 10.3389/fpls.2018.00838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Vignani R., Scali M., Cresti M. (2006). A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27 2782–2786. 10.1002/ELPS.200500722 [DOI] [PubMed] [Google Scholar]
- Wang Y., Li L., Ye T., Zhao S., Liu Z., Feng Y. Q., et al. (2011). Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 68 249–261. 10.1111/j.1365-313X.2011.04683.x [DOI] [PubMed] [Google Scholar]
- Weitbrecht K., Müller K., Leubner-Metzger G. (2011). First off the mark: early seed germination. J. Exp. Bot. 62 3289–3309. 10.1093/jxb/err030 [DOI] [PubMed] [Google Scholar]
- Weydert C. J., Cullen J. J. (2010). Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 5 51–66. 10.1038/nprot.2009.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtyla Ł, Lechowska K., Kubala S., Garnczarska M. (2016). Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 7:66. 10.3389/fpls.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K., Hill L. M., Li D. Z., Walters C. (2014). Factors affecting stress tolerance in recalcitrant embryonic axes from seeds of four Quercus (Fagaceae) species native to the USA or China. Ann. Bot. 114 1747–1759. 10.1093/aob/mcu193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Yang T., Zhang H., Qi Y., Xing Y., Zhang N., et al. (2014). Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. 77 23–34. 10.1016/j.plaphy.2014.01.015 [DOI] [PubMed] [Google Scholar]
- Zhang C. (2007). Biologically active gibberellins and abscisic acid in fruit of two late-maturing japanese pear cultivars with contrasting fruit size. J. Am. Soc. Hortic. Sci. 132 452–458. [Google Scholar]
- Zhang H., Han W., De Smet I., Talboys P., Loya R., Hassan A., et al. (2010). ABA promotes quiescence of the quiescent center and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 64 764–774. 10.1111/j.1365-313X.2010.04367.x [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang C., Li Y., Zheng N., Chen H., Zhao Q., et al. (2007). SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 19 1912–1929. 10.1105/tpc.106.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Process to acorn germination. (A) Acorn with pericarp, (B) acorn peeled, (C) acorn cut at the distal end and (D) boxes contain filter paper, perlite, and a germinated acorn.
Morphological aspects of acorns and seeds at different stages. In brackets the approximate time in hours post-imbibition to obtain the indicated stages.
SOD in-gel activity assay of embryo axis and shoot seedling of Q. ilex. (A) Inhibitor test for SOD isoforms was conducted by application of 2 mM KCN or 5 mM H2O2 prior to activity staining. (B) SOD isoforms are labeled S1–S5 in order of increasing mobility.
Primers used in this work for the determination of Q. ilex sequences. Primers based on orthologous sequences were designed to obtain partial coding sequences of Q. ilex genes.
Primers used for absolute quantification by real-time PCR of Q. ilex transcripts.