Abstract
Chemicals associated with unconventional oil and gas (UOG) operations have been shown to contaminate surface and ground water with a variety of endocrine disrupting compounds (EDCs) inducing multiple developmental alteration in mice. However, little is known about the impacts of UOG-associated contaminants on amphibian health and resistance to an emerging ranavirus infectious disease caused by viruses in the genus Ranavirus, especially at the vulnerable tadpole stage. Here we used tadpoles of the amphibian Xenopus laevis and the ranavirus Frog virus 3 (FV3) as a model relevant to aquatic environment conservation research for investigating the immunotoxic effects of exposure to a mixture of 23 UOG-associated chemicals with EDC activity. Xenopus tadpoles were exposed to an equimass mixture of 23 UOG-associated chemicals (range from 0.1 to 10 µg/l) for 3 weeks prior to infection with FV3. Our data show that exposure to the UOG chemical mixture is toxic for tadpoles at ecological doses of 5 to 10 µg/l. Lower doses significantly altered homeostatic expression of myeloid lineage genes and compromised tadpole responses to FV3 through expression of TNF-α, IL-1β, and Type I IFN genes, correlating with an increase in viral load. Exposure to a subset of 6 UOG chemicals was still sufficient to perturb the antiviral gene expression response. These findings suggest that UOG-associated water pollutants at low but environmentally relevant doses have the potential to induce acute alterations of immune function and antiviral immunity.
Keywords: water pollutants, ranavirus, antiviral immunity, immune toxicant
There is growing awareness that exposure to waterborne contaminant mixtures is an overlooked but important contributor to the burden of infectious diseases. This is particularly significant for emerging infectious diseases implicated in the dramatic worldwide amphibian declines that are of major concern for the maintenance of biodiversity (Daszak et al., 1999; Egea-Serrano et al., 2012; Stuart et al., 2004). Among pollutants, chemicals associated with and/or released during unconventional oil and gas (UOG) extraction are raising concern not only because of the physical and chemical damage they can cause to ecosystems, but also for their potential negative health impacts on wildlife.
UOG extraction involves high-pressure underground injection of millions of gallons of water mixed with over 1000 different undisclosed chemicals (including acids, friction reducers, and surfactants) to fracture the shale or coal bed layer, and release trapped natural oil and gas (Carpenter, 2016; Kassotis et al., 2016b; Mrdjen and Lee, 2015). Accumulating evidence indicates that UOG increases the potential for contamination of surface and ground water from chemicals used throughout the process, as more than 200 of these chemicals have been detected in wastewater, ground water, and surface water (Elsner and Hoelzer, 2016; Vengosh et al., 2014; Waxman et al., 2011; Webb et al., 2014). Among these chemicals identified, 23 chemicals were found consistently in water samples collected near sites with active UOG, with average concentrations in ground water ranging from 0.01 to 2.0 mg/l (Gross et al., 2013; Wilkin and Digiulio, 2010). Other studies showed that these chemicals exhibit significant endocrine disrupting activity (Kassotis et al., 2015, 2014). For example, that ability of constituents of this mixture to agonize and/or antagonize particular hormone receptors was determined using in vitro reporter assays (Table 1; Kassotis et al., 2016b, 2014). Also, a mixture of these 23 chemicals has been used as a realistic proxy for assessing in vivo effects of early life exposure in mouse models. This approach revealed that pups from dams exposed to representative environmental concentrations of this mixture of 23 chemicals via drinking water exhibited developmental defects such as decreased sperm counts and increased testes, body, heart, and thymus weights in the offspring, as well as elevated serum testosterone levels in male progeny (Kassotis et al., 2015) and pituitary hormones and mammary gland development in females (Kassotis et al., 2016a; Sapouckey et al., 2018).
Table 1.
List of the 23 UOG-Associated Chemicals With Endocrine Disruption Activitya
| Chemical Name | CAS # | Endocrine Activityb |
|---|---|---|
| 1,2,4-Trimethylbenzene | 95-63-6 | – |
| 2-(2-Methoxyethoxy)ethanol | 111-77-3 | AR, ER, GR |
| 2-Ethylhexanol | 104-76-7 | AR, ER, GR, PR |
| 2-Methyl-4-isothiazolin-3-one | 2682-20-4 | AR, ER, GR |
| Acrylamide | 79-06-1 | AR |
| Benzene | 71-43-2 | AR, ER |
| Bronopol | 52-51-7 | AR, ER, GR, PR |
| Cumenec | 98-82-8 | AR, ER, PR |
| Diethanolamine | 111-42-2 | AR, ER, PR |
| Ethoxylated nonylphenolc | 9016-45-9 | AR, ER, GR, PR, TR |
| Ethoxylated octylphenolc | 9036-19-5 | AR, ER, GR, PR, TR |
| Ethylbenzene | 100-41-4 | AR, ER |
| Ethylene glycolc | 107-21-1 | AR, ER, GR, PR, TR |
| Ethylene glycol monobutyl etherc | 111-76-2 | AR, ER, TR |
| Naphthalenec | 91-20-3 | AR, ER, GR, PR, TR |
| N,N-Dimethylformamide | 68-12-2 | AR, ER, PR |
| Phenol | 108-95-2 | AR, ER, PR |
| Propylene glycol | 57-55-6 | ER |
| Sodium tetraborate decahydrate (borax)c | 1303-96-4 | AR, ER |
| Styrene | 100-42-5 | AR, GR, TR |
| Toluene | 108-88-3 | AR, ER |
| Triethylene glycol | 112-27-6 | AR, ER |
| Xylenes | 1330-20-7 | AR, ER, PR |
Adapted from Kassotis et al. (2015).
Receptor abbreviations: AR, androgen receptor antagonist; ER, estrogen receptor antagonist; PR, progesterone receptor antagonist; TR, thyroid receptor antagonist; GR, glucocorticoid receptor antagonist.
Chemicals used in reduced mixture of 6 UOG chemicals.
To date, however, there is scant information regarding the potential immunotoxicity of water contaminated by UOG. In addition, health impacts of UOG-associated contaminants on aquatic vertebrates such as amphibians remain to be evaluated. Owing to their reliance on aquatic environments amphibians (especially fully aquatic anuran tadpoles) are highly susceptible to water contaminants, and are thus, at risk in water contaminated by UOG. Notably, endocrine disrupting compound (EDC) activity and developmental perturbations exerted by the 23 identified UOG-associated chemicals in mammalian models, raise the possibility of alterations of biological functions such as immunity. Indeed, we have recently shown that in the mouse, maternal exposure to this mixture of 23 UOG-associated chemicals durably affects the immune system of the offspring including the alteration of frequencies of certain T cell sub-populations and the exacerbated responses in an experimental model of autoimmune encephalitis (Boule et al., 2018). The possible negative impacts of immune dysregulation by UOG chemical mixtures on aquatic vertebrates merit full consideration because these animals are likely to be in direct contact with these pollutants at UOG sites. This is of particular relevance because even minute deregulation of the immune system can result, over time, in weakened defenses against pathogens such as ranavirus that plague amphibians worldwide (reviewed in Duffus et al. (2015)).
Ranaviruses are large DNA viruses (Iridoviridae) that are distributed globally and can cause severe disease, which sometimes has population-level impacts, in a wide range of vertebrates comprising amphibians, fish, and reptiles (Bandin and Dopazo, 2011; Chinchar, 2002; Chinchar et al., 2009; Greer et al., 2005; Jancovich et al., 2010; Price et al., 2017). One of the growing concerns is that environmental stressors, such as water pollutants, may increase host susceptibility to ranaviruses diseases. We have developed a model in Xenopus laevis to evaluate the effect of water contaminants on immune homeostasis and immune response against the ranavirus Frog Virus 3 (FV3; De Jesus Andino et al., 2017; Grayfer et al., 2012; Sifkarovski et al., 2014). Although we have previously used the Xenopus/FV3 model to examine the effects of individual contaminants, we reasoned that the effects of UOG-associated chemicals should be investigated in combination as a mixture because this more closely reflects the likely exposure of wild populations.
Here, we report the effects of exposure to mixtures of UOG-associated chemicals at environmentally relevant concentrations on immune homeostasis and antiviral immunity in X. laevis tadpoles.
MATERIALS AND METHODS
Chemical mixture preparation
Twenty-three chemicals (≥97% purity, Sigma Aldrich) listed in Table 1 were selected based on prior demonstration of endocrine activity, via the estrogen, androgen, progesterone, glucocorticoid, and/or thyroid receptors (Kassotis et al., 2015, 2014), and developmental effects on other physiological systems (Kassotis et al., 2016a, 2015). Stock solutions of chemicals were prepared in 100% ethanol (ThermoFisher Scientific, Waltham, MA), stored at –20°C, and used in experiments within 3 months of preparation.
Animal husbandry and exposure to water contaminants
All outbred X. laevis tadpoles were acquired from the X. laevis research resource for immunology at the University of Rochester (https://www.urmc.rochester.edu/microbiology-immunology/xenopus-laevis.aspx; last accessed July 18, 2018)). Three-week old (1.5 cm long) outbred tadpoles stage 51–52 (Nieuwkoop and Faber, 1967) were treated for 3 weeks by diluting an equimass amount of UOG-associated chemicals in the tadpole housing water (dechlorinated water at room temperature [22°C] and neutral pH 6.8–7.0) from a freshly prepared stock solution. Control tadpoles were kept in water spiked with the vehicle control (0.2% ethanol). The doses were chosen based on estimates of environmentally relevant exposures, such that the 2 concentrations are similar to levels detected in surface and groundwater in UOG production regions (Cozzarelli et al., 2017; DiGiulio and Jackson, 2016; Gross et al., 2013; Orem et al., 2017; United States Environmental Protection Agency, 2015). Animals were maintained at a density of 20 to 30 individuals in 4 l containers. Tadpoles were fed daily with food pellets (Purina Gel Tadpole Diet). The water and the chemicals were changed once a week because the chemicals are stable in water for 1 week (data not shown). Although the stability of all 23 chemicals in water is uncertain or unknown, the water and the chemicals were changed once a week. We then transferred these 6-week-old tadpoles still at premetamorphic stages (stage 55–56) to clean water and either monitored their survival for 3 weeks (Figure 1A) or used them for gene expression and FV3 infection. More advanced tadpoles that reached stage 56 were discarded to minimize effect of metamorphosis on gene expression. All animals were handled in accordance with stringent laboratory and University Committee on Animal Research regulations (Approval number 100577/2003-151). To assess potential effect of exposure to UOG chemicals on developmental rates the stage, body length (head to tail and head to belly), head width were measured for each individual before and after treatment.
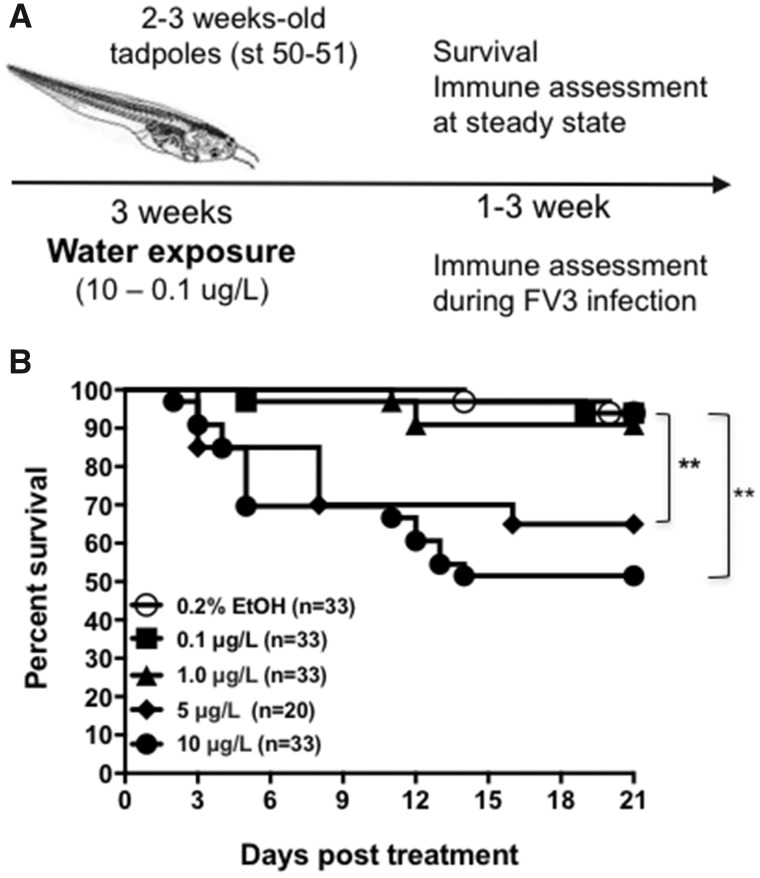
Figure 1.
A, Schematic of treatment strategy of tadpoles. B, Survival curve of tadpoles exposed for 3 weeks to 0.2% ethanol (vehicle control) or 0.1, 1, 5, or 10 μg/l of an equimass mixture of 23 UOG chemicals. Survival was monitored for 20 days following exposure during which tadpoles were checked daily. The data are pools of 2 independent experiments. **p < .005 (Log Rank Test).
Frog virus 3 stocks and infection
Baby hamster kidney cells (BHK-21, ATCC No. CCL-10) were maintained in DMEM (Invitrogen) containing 10% fetal bovine serum (Invitrogen), streptomycin (100 μg/ml), and penicillin (100 U/ml) with 5% CO2 at 37°C, then 30°C for infection. FV3 was grown using a single passage through BHK-21 cells and was subsequently purified by ultracentrifugation on a 30% sucrose cushion. Premetamorphic tadpoles (6-weeks old, stage 54–55) were infected by i.p. injection of 1 × 104 PFU in 10 µl of amphibian PBS (APBS). Uninfected control animals were mock-infected with an equivalent volume of amphibian APBS. Three days postinfection (dpi), animals were euthanized using 0.1 g/l tricaine methanesulfonate buffered with bicarbonate prior to dissection and extraction of nucleic acids from tissues (Figure 1A).
Tadpole survival studies
Following 3 weeks of exposure, stage 54–55 tadpoles were infected with FV3 by i.p. inoculation and moved to 4 l of clean water for monitoring. Tadpoles were checked daily; dead animals were immediately removed, frozen, and stored at –20°C.
Quantitative gene expression analyses
Total RNA was extracted from frog kidneys, livers, and spleens using Trizol reagent, following the manufacturer’s protocol (Invitrogen). cDNA was synthetized with 0.5 µg of RNA in 20 µl using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and 1 µl of cDNA template was used in all RT-PCRs and 150 ng DNA for PCR. Minus RT controls were included for every reaction. A water-only control was included in each reaction. The qPCR analysis was performed using the ABI 7300 real-time PCR system with PerfeCT SYBR Green FastMix, ROX (Quanta) and ABI sequence detection system software. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control was used for all samples in conjunction with the delta^delta CT method to analyze cDNA for gene expression. For tissue samples with enough material L13 was run as a second endogenous control. All primer sequences are listed in Table 2.
Table 2.
List of qPCR Primer Sequences
| Primer | Sequence (5′–3′) |
| CSF1 | F: ATCGAACTCTGTCCAAGCTGGATG |
| R: GGACGAAGCAAGCATCTGCCTTAT | |
| CSF-R1 | F: TGTATTCTTTGG ACT TGC CGT ATCTGG |
| R: TTGTTTAGCTTCAAATTCTGGGTAATA | |
| FV3 DNA POL II | F: ACGAGCCCGACGAAGACTACA |
| R: TGGTGGTCCTCAGCATCC T | |
| GAPDH | F: GACATCAAGGCCGCCATTAAGACT |
| R: AGATGGAGGAGTGAGTGTCACCAT | |
| GCSF-R1 | F: ACGTGCCAGCTAAACCTCACAGAT |
| R: TGACACAGCCTGGGCGAGAAATAA | |
| IL-1β | F: CATTCCCATGGAGGGCTACA |
| R: TGACTGCCACTGAGCAGCAT | |
| IL-10 | F: TGCTGGATCTTAAGCACACCCTGA |
| R: TGTACAGGCCTTGTTCACGCATCT | |
| IL-34 | F: AGCTCTTCTACGTGTATTCCTTGG |
| R: TGATAAGCGATTGACCTACCTGGG | |
| L13 | F: GGGAGGATTTCGGCAAGTTA |
| R: TTCTGGGAAGAGGTGCAATC | |
| TNF-α | F: TGTCAGGCAGGAAAGAAGCA |
| R: CAGCAGAGCAAAGAGGATGGT | |
| Type I IFN | F: GCTGCTCCTGCTCAGTCTCA |
| R: GAAAGCCTTCAGGATCTGTGTGT | |
| Type III IFN (λ) | F: TCCCTCCCAACAGCTCATG |
| R: CCGACACACTGAGCGGAAA |
Abbreviations: F, Forward; R, Reverse.
Viral load quantification by qPCR
FV3 viral loads were assessed by absolute qPCR by analysis of isolated DNA in comparison to a serially diluted standard curve. Briefly, an FV3 DNA Pol II PCR fragment was cloned into the pGEM-T vector (Promega). This construct was amplified in bacteria, quantified and serially diluted to yield 1010–101 plasmid copies of the vDNA POL II. These dilutions were employed as a standard curve in subsequent absolute qPCR experiments to derive the viral genome transcript copy numbers, relative to this standard curve.
Statistical analyses
The Mann–Whitney U and ANOVA test were used for statistical analysis of expression and viral load data. Analyses were performed using a Vassar Stat online resource (http://vassarstats.net/utest.html). Statistical analysis of survival data was performed using a Log-Rank Test (GraphPad Prism 6). A probability value of p < .05 was used in all analyses to indicate statistically significant differences in mean values. Error bars on all graphs represent the standard error of the mean (SEM).
RESULTS
Effects of Exposure to UOG Chemicals on X. laevis Tadpole Development and Survival
Given the range in amounts of UOG chemicals detected in water sources associated with UOG, we first determined how toxic these levels would be for fully aquatic animals such as X. laevis tadpoles. Based on the concentration range of chemicals in this mixture detected in various UOG sites from previous studies (Kassotis et al., 2015), we choose 3 concentrations of the mixture containing the 23 chemicals. Specifically, using equimass amounts, tadpoles were exposed to a high dose of 10 μg/l, a medium dose of 1 μg/l and a low dose of 0.1 μg/l. Given the thyroid-disrupting activity of some of the UOG chemicals in the mixture, effect on the developmental rate after 3 weeks of exposure to the UOG mixture was examined. There were no detectable differences in the increase body length (head to tail and head to belly) and width (head) as well as the developmental stage at the end of treatment for tadpoles exposed to 0.1 and 1.0 μg/l compared to controls (Table 3). However, tadpoles exposed at the high dose (10 μg/l) dose of the UOG mixture showed a significantly reduced body weight and more advanced developmental stage than controls. In addition, the high dose (10 μg/l) induced marked lethality cumulating to 40% death over 20 days, whereas exposure to 0.1 and 1.0 μg/l doses did not have detectable effect compared with animals treated with 0.2% ethanol (Figure 1B). To substantiate the toxicity of the UOG mixture, we exposed an independent group of tadpoles to 5 μg/l of the UOG mixture for 3 weeks, which also induced significant mortality (30%) compared to control group (no UOG exposure) but did not result in detectable effect on developmental rate (Figure 1B and Table 3). It is noteworthy that concentrations of these chemicals in ground water at sites contaminated by hydraulic fracturing operations reached 10 to 2000 μg/l (DiGiulio et al., 2011; Gross et al., 2013).
Table 3.
Changes in Body Length, Weight, and Stage in Xenopus Tadpoles Before and After UOG Treatment
| Before Treatment |
After Treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measurement | Vehicle Control | 0.1 µg/l | 1 µg/l | 5 µg/l | 10 µg/l | Vehicle Control | 0.1 µg/l | 1 µg/l | 5 µg/l | 10 µg/l |
| Weight (mg) | 279 ± 10 | 273 ± 10 | 254 ± 11 | 259 ± 16 | 293 ± 18 | 353 ± 17 | 346 ± 10 | 317 ± 11 | 275 ± 16 | 432 ± 180.05* |
| Stage | 52 ± 1 | 52 ± 1.0 | 52 ± 1 | 52 ± 1 | 52 ± 1.0 | 54 ± 1.0 | 54 ± 1 | 54 ± 1 | 54 ± 1 | 56 ± 10.05* |
| Size H-T (mm) | 38 ± 1 | 39 ± 1.0 | 38 ± 1 | N/A | 38 ± 1 | 40 ± 1.0 | 43 ± 1 | 39 ± 1 | N/A | 44 ± 1 |
| Size H-B (mm) | 12.9 ± 0.3 | 12.5 ± 0.3 | 12.2 ± 0.03 | N/A | 12.4 ± 0.3 | 12.9 ± 0.3 | 12.4 ± 0.3 | 12.1 ± 0.03 | N/A | 13.4 ± 0.3 |
| Size H (mm) | 7.9 ± 0.2 | 7.7 ± 0.2 | 7.5 ± 0.2 | N/A | 7.8 ± 0.2 | 7.9 ± 0.2 | 7.4 ± 0.2 | 7.3 ± 0.2 | N/A | 7.8 ± 0.2 |
Body length (mm), weight (mg), and stage from tadpoles before treatment or after treatment with 0.2% EtOH (n = 31–85 animals per group), 0.1 µg/l (n = 31–85 animals per group), 1 µg/l (n = 30–85 animals per group), 5 µg/l (n = 13–20 animals per group), or 10 µg/l (n = 17–48 animals per group) of UOG mixture for 3 weeks. p value determined—between vehicle control and treated animals. Abbreviations: H-T, length head to tail; H-B, length head to belly; H, head width; N/A, no data. *Significant.
Effects of Exposure to UOG Chemicals on Tadpole Immune Homeostasis
To evaluate the possible effects of low doses of UOG chemicals on the overall immune system at steady state, we determined the relative expression by qPCR of 11 immunologically relevant genes in the spleen, liver and the kidneys of tadpoles exposed for 3 weeks either to 0.2% ethanol or to the low (0.1 μg/l) or intermediate (1 μg/l) UOG mixtures, and then rested in normal water for 1 week. The high concentration of this mixture (10 µg/ml) was not assessed given the overt toxic effects. When analyzing the data, we noticed that the CT values for the GAPDH endogenous control were not uniform across the different treatment groups, although values were consistent across technical replicates and within treatment groups. In particular, GAPDH CT values were significantly different for tadpoles exposed to the low dose of UOG chemicals. For a subset of samples, we had enough material to perform qPCR using a second endogenous control gene, L13, which showed more uniformity between treatment groups, including the low dose group (see Tables 4 and 5).
Table 4.
Relative Gene Expression (RQ Value ± SD) in Kidneys for Experiments Using 23 UOG Chemicals Using L13 as Endogenous Control Gene
| Genes | Uninfecteda | p values | FV3 infectedb | p values (3 dpi) | p values 3 dpi/Uninfc |
|---|---|---|---|---|---|
| Arg I | C: 5 ± 1 | C: 115 ± 66 | C: NS | ||
| L: 598 ± 330 | NS | L: 2343 ± 962 | 0.02* | L: NS | |
| H: 147 ± 84 | NS | H: 157 ± 70 | NS | H: NS | |
| CSF-1 | 97 ± 32 | C: 3922 ± 2326 | C: NS | ||
| 17 ± 8 | NS | L: 58 ± 26 | NS | L: NS | |
| 2670 ± 1717 | NS | H: 2610 ± 658 | NS | H: NS | |
| GCSFR | C: 72 ± 29 | C: 366 ± 127 | C: 0.04* | ||
| L: 107 ± 57 | NS | L: 160 ± 118 | NS | L: NS | |
| H: 332 ± 103 | 0.04* | H: 215 ± 100 | NS | H: NS | |
| IFN type I | C: 25 ± 12 | C: 1617 ± 501 | C: 0.005* | ||
| L: 85 ± 30 | NS | L: 553 ± 404 | NS | L: NS | |
| H: 14 ± 4 | NS | H: 88 ± 37 | 0.02* | H: NS | |
| IFN type II (γ) | C: 116 ± 47 | C: 1185 ± 645 | C: NS | ||
| L: 20 ± 10 | NS | L: 64 ± 27 | NS | L: NS | |
| H: 64 ± 25 | NS | H: 53 ± 15 | NS | H: NS | |
| IFN type III (λ) | C: 79 ± 42 | C: 702 ± 330 | C: NS | ||
| L: 2357 ± 1961 | NS | L: 829 ± 516 | NS | L: NS | |
| H: 146 ± 83 | NS | H: 296 ± 144 | NS | H: NS | |
| IL-1β | C: 170 ± 73 | C: 7271 ± 3160 | C: 0.04* | ||
| L: 167 ± 127 | NS | L: 363 ± 283 | 0.03* | L: NS | |
| H: 60 ± 21 | NS | H: 190 ± 85 | 0.03* | H: NS | |
| IL-10 | C: 25 ± 17 | C: 135 ± 58 | C: NS | ||
| L: 67 ± 43 | NS | L: 81 ± 49 | NS | L: NS | |
| H: 30 ± 10 | NS | H: 37 ± 16 | NS | H: NS | |
| IL-34 | C: 13 ± 4 | C: 37 ± 22 | C: NS | ||
| L: 26 ± 11 | NS | L: 91 ± 64 | NS | L: NS | |
| H: 83 ± 19 | 0.002* | H: 78 ± 29 | NS | H: NS | |
| CSF-R1 | C: 21 ± 7 | C: 165 ± 80 | C: NS | ||
| L: 79 ± 40 | NS | L: 64 ± 37 | NS | L: NS | |
| H: 44 ± 16 | NS | H: 15 ± 6 | NS | H: NS | |
| Mx1 | C: 35 ± 17 | C: 3931 ± 1289 | C: 0.007* | ||
| L: 23 ± 11 | NS | L: 4480 ± 3479 | NS | L: NS | |
| H: 647 ± 341 | NS | H: 31 719 ± 13801 | NS | H: 0.04* | |
| TNFα | C: 22 ± 8 | C: 909 ± 578 | C: 0.05* | ||
| L: 21 ± 12 | NS | L: 62 ± 42 | NS | L: NS | |
| H: 235 ± 57 | 0.001* | H: 965 ± 413 | NS | H: NS |
NS, nonsignificant; *,significant.
Gene expression RQ values from kidneys of tadpoles (10 per group) treated with 0.2% EtOH, 0.1 μg/l (L), or 1 μg/l (H) of UOG mixture for 3 weeks using L13 as reference gene. p value determined between vehicle control and treated animals.
Gene expression RQ values from kidneys of tadpoles (10 per group) treated with 0.2% EtOH, 0.1 μg/l (L), or 1 μg/l (H) of UOG mixture then FV3 infected for 3 days using L13 as reference gene. p value determined by between vehicle control and treated animals.
p value determined between each treated and corresponding infected group.
Table 5.
Relative Gene Expression (RQ Value ± SD) in Liver for Experiments Using 23 UOG Chemicals Using L13 as Endogenous Control Gene
| Genes | Uninfecteda | p values | FV3 infectedb | p values (3 dpi) | p values Uninf/3dpic |
|---|---|---|---|---|---|
| Arg I | C: 71 ± 34 | C: 98 ± 36 | C: NS | ||
| L: 41 ± 12 | NS | L: 214 ± 118 | NS | L: NS | |
| H: 1565 ± 630 | 0.03* | H: 3348 ± 1726 | NS | H: NS | |
| CSF-1 | C: 806 ± 304 | C: 11231 ± 5928 | C: NS | ||
| L: 3 ± 1 | 0.05* | L: 67 ± 42 | 0.05* | L: NS | |
| H: 3434 ± 1388 | NS | H: 23472 ± 15642 | NS | H: NS | |
| GCSFR | C: 2377 ± 806 | C: 7157 ± 2826 | C: NS | ||
| L: 34 ± 12 | 0.01* | L: 486 ± 269 | 0.03* | L: NS | |
| H: 7337 ± 3664 | NS | H: 40037 ± 20416 | NS | H: NS | |
| IFN type I | C: 88 ± 57 | C: 865 ± 527 | C: NS | ||
| L: 40 ± 24 | NS | L: 6141 ± 5615 | NS | L: NS | |
| H: 170 ± 68 | NS | H: 371 ± 270 | NS | H: NS | |
| IFN type II (γ) | C: 239 ± 143 | C: 1941 ± 727 | C: 0.05* | ||
| L: 3 ± 1 | NS | L: 123 ± 82 | 0.03* | L: NS | |
| H: 28 ± 14 | NS | H: 28 ± 11 | 0.02* | H: NS | |
| IL-1β | C: 315 ± 128 | C: 4607 ± 2732 | C: NS | ||
| L: 323 ± 87 | NS | L: 6543 ± 3153 | NS | L: NS | |
| H: 2521 ± 1182 | 0.05* | H: 2553 ± 1378 | NS | H: NS | |
| IL-10 | C: 469 ± 162 | C: 556 ± 268 | C: NS | ||
| L: 44 ± 24 | NS | L: 52 ± 42 | 0.05* | L: NS | |
| H: 532 ± 344 | NS | H: 51 ± 28 | 0.05* | H: NS | |
| IL-34 | C: 967 ± 402 | C: 4970 ± 2466 | C: NS | ||
| L: 5 ± 1 | 0.03* | L: 81 ± 36 | 0.05* | L: NS | |
| H: 1804 ± 746 | NS | H: 15451 ± 7235 | NS | H: NS | |
| CSF-R1 | C: 156 ± 85 | C: 621 ± 268 | C: NS | ||
| L: 21 ± 14 | NS | L: 96 ± 61 | NS | L: NS | |
| H: 10316 ± 4728 | 0.05* | H: 18982 ± 7568 | 0.03* | H: NS | |
| Mx1 | C: 86 ± 64 | C: 639 ± 445 | C: NS | ||
| L: 56 ± 36 | NS | L: 247 ± 180 | NS | L: NS | |
| H: 19 ± 10 | NS | H: 129 ± 99 | NS | H: NS | |
| TNF-α | C: 138 ± 41 | C: 1245 ± 428 | C: 0.02* | ||
| L: 7 ± 2 | 0.02* | L: 76 ± 37 | 0.02* | L: NS | |
| H: 2925 ± 1190 | 0.02* | H: 649 ± 211 | NS | H: NS |
NS, nonsignificant; *,significant.
Gene expression RQ values from livers of tadpoles (10 per group) treated with 0.2% EtOH, 0.1 μg/l (L), or 1 μg/l (H) of UOG mixture for 3 weeks using L13 as reference gene. p value determined between vehicle control and treated animals.
Gene expression RQ values from livers of tadpoles (10 per group) treated with 0.2% EtOH, 0.1 μg/l (L), or 1 μg/l (H) of UOG mixture then FV3 infected for 3 days using L13 as reference gene. p value determined between vehicle control and treated animals.
p value determined by between each treated and corresponding infected group.
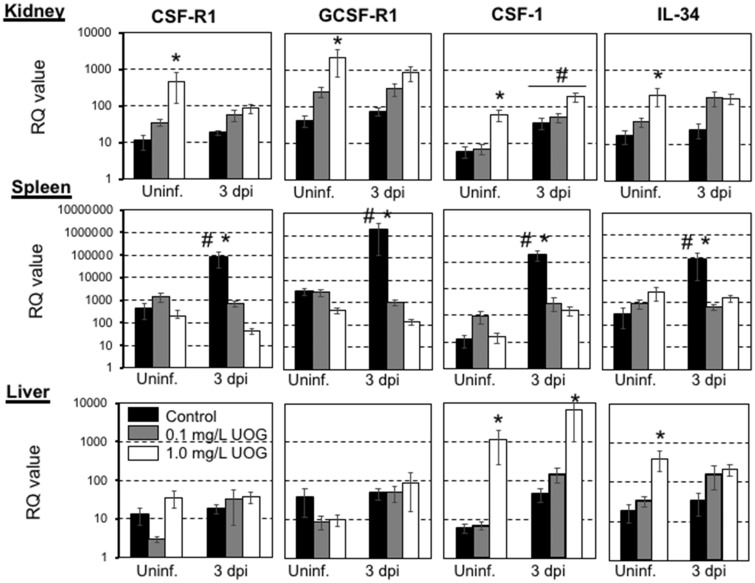
Using GAPDH asendogenous control, most genes tested including TNFα, IL-1β, IL-10, Mx1, IFN-I, II and III did not show statistically significant differences in expression between control and UOG-exposed groups. However, several genes relevant to macrophage/monocytic function did show altered expression (Figure 2). The transcript levels of the master macrophage colony stimulator factor (CSF-1) as well as IL-34, which is a critical ligand for the CSF-1 receptor (CSF-1R), were significantly higher in livers and kidneys of tadpoles exposed to the intermediate dose of UOG chemicals, but intriguingly this difference was not observed in the spleen. No changes were observed in the 0.1 μg/l treated group. In addition, CSF-1R gene expression itself was markedly increased in the kidneys, but not in the liver or the spleen. The transcript levels of another leukocyte receptor, the granulocyte colony stimulator receptor (GCSF-R1), were also increased at steady state in the kidneys. Using L13 as endogenous control, GCSF-R and IL-34 (but not CSF-R1) were significantly differentially expressed in kidneys, and differential expression of CSF-1 and IL-34 in the liver was not statistically significant for the medium dose (Tables 4 and 5).
Figure 2.
Relative expression of CSF-R1, GCSFR, CSF-1, and IL-34 genes from kidney, spleen, and liver tissue harvested from premetamorphic tadpoles (stage 54–55) exposed to either 0.2% ethanol (vehicle control), 0.1, or 1.0 μg/l of an equimass mixture of 23 UOG chemicals for 3 weeks. After chemical exposure, tadpoles were either ip injected with 10 000 pfu of FV3 or with amphibian PBS (Uninf.), and then euthanized after 3 days. Results are means ± SEM of 12 individuals per group from 2 different experiments (6 per experiment). Gene expression is represented as fold increase (RQ: relative quantification) relative to GAPDH endogenous control. Statistical significance (ANOVA and Mann-Whitney U Test): *p < .05 between control and treated groups, #p < .05 between uninfected and infected groups.
Effects of Exposure to UOG Chemicals on Tadpole Susceptibility to FV3 Infection
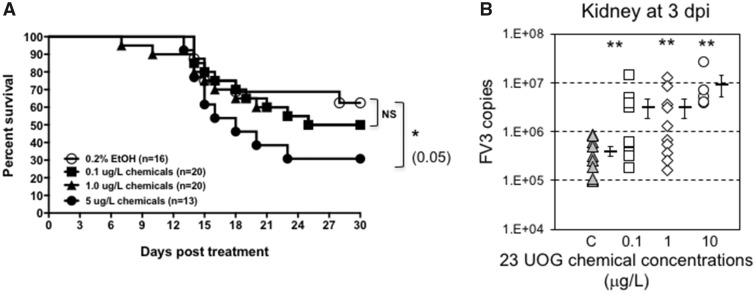
Immune responses trigger a complex set of activation, regulation and effector molecule production processes. Thus, we next determined whether exposure to UOG chemicals could alter antiviral host immune defenses. To examine potential effects on global host resistance, we first conducted a survival experiment. Tadpoles were exposed for 3 weeks with different doses of the UOG mixture and rested for 1 week in clean water to minimize possible stress from the exposure. Developmentally matched stage 54–55 tadpoles were then infected with 10 000 PFU of FV3 by ip injection. Because of the high mortality rate induced at early stage, the 10 μg/l of mixture was not used. However, survivors from the group exposed to 5 μg/l of UOG chemicals were infected with FV3 and showed a statistically significant increased susceptibility compared to controls treated tadpoles (Figure 3A). The mortality rate over 30 days post-FV3 infection did not significantly differ between the groups exposed to 1 and 0.1 μg/l of UOG chemical and control.
Figure 3.
A, Survival curve of FV3-infected tadpoles (stage 50–52; n = 13 to 20 individuals) that were exposed for 3 weeks to 0.2% ethanol (vehicle control) or 0.1, 1.0, 5, or 10 μg/l of an equimass mixture of 23 UOG chemicals. Survival was monitored for 30 days following infection, during which tadpoles were checked daily. *p < .005 (Log Rank Test). B, FV3 copy number 3 days after FV3 infection as determined by absolute qPCR. For each group, the viral genome copy number of each individual is depicted by different symbols as well as a horizontal barre indicating the average ± SD. Statistical significance: **p < .005 (ANOVA and Mann-Whitney U Test).
To further investigate whether exposure to UOG chemicals altered tadpole host anti-viral immune defenses, we determined the viral load at the peak of the immune response, 6 dpi. Viral genomes were detected in all the samples indicating successful infection all tadpoles. Notably, exposure at both intermediate (1 μg/l) and low (0.1 μg/l) concentrations of this mixture resulted in significantly increased FV3 genome copy numbers (by roughly 10-fold on average) compared to controls, as determined by qPCR (Figure 3B).
This increase in viral load is indicative of a less efficient control of viral replication by tadpoles. To investigate further the potential effects of exposure to UOG chemicals on antiviral immune response, we determined the changes in relative gene expression following FV3 infection by qPCR. As a representative time point we choose 3 dpi, an intermediate time point where expression of many antiviral and pro-inflammatory genes is significantly increased by viral infection, especially in kidneys that is the main site of FV3 replication (De Jesus Andino et al., 2012). We also monitored changes in gene expression in the spleen and liver that are 2 important immune organs but not the major site of infection.
In contrast to gene expression patterns at steady state, expression of several key immune genes induced upon FV3 infection was affected in the spleen (the only secondary immune tissue in absence of lymph nodes) and the kidney (main site of FV3 infection and replication) of tadpoles that were exposed to the UOG mixture. Notably, transcript levels of the prominent pro-inflammatory cytokine TNFα were markedly decreased in the spleen at 1 μg/l UOG exposed dose, although slightly but significantly increased in the kidneys for the group exposed to 1 μg/l (Figure 4). Similarly, lower transcript levels were found for Type I IFN and IL-1β in tadpole exposed to 1 μg/l of UOG chemical at 3 dpi (Figure 4). In addition, exposure to 1 μg/l UOG chemical abrogated the FV3-induced gene expression response of CSF-1 and CSF-1R in the spleen compared to control vehicle (Figure 2). A similar ablated gene expression response for GCSFR, the receptor typifying granulocytes, was noted (Figure 2). Normalizing transcript levels in kidney samples with reference to L13 confirmed the defect in expression response of TNF-α, Type I IFN and IL-1β but not CSF-1 or CSF-R1 (Tables 4 and 5).
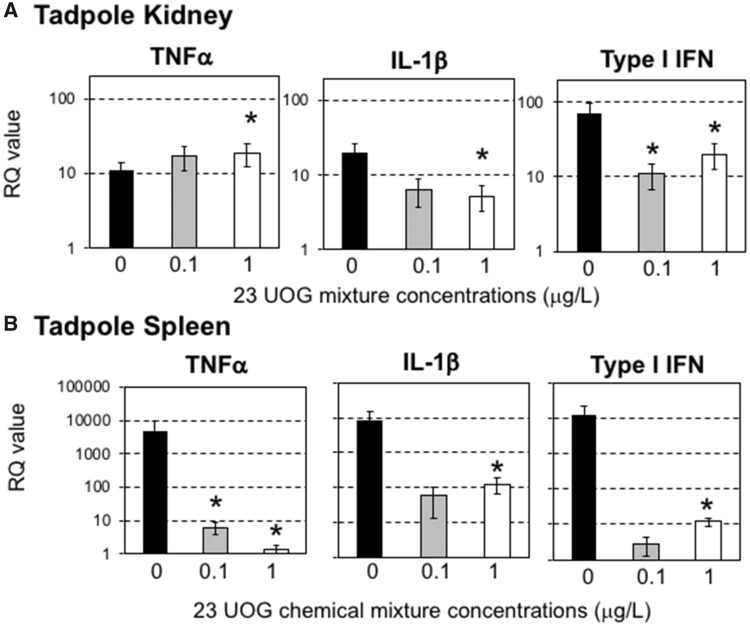
Figure 4.
Relative expression of TNF-α, IL-1β, and Type I IFN genes from kidney and spleen tissue harvested from premetamorphic tadpoles (stage 54–55; n = 6 individuals) exposed to either 0.2% ethanol (vehicle control), 0.1, or 1.0 μg/l of an equimass mixture of 23 UOG chemicals for 3 weeks. After chemical exposure, tadpoles were either ip injected with 10 000 pfu of FV3 or with amphibian PBS (vehicle control), and then euthanized after 3 days. Results are means ± SEM of 12 individuals per group from 2 different experiments (6 per experiment) using GAPDH as endogenous control. *p < .05 significant differences between controls and UOG exposed groups using one-way ANOVA test and Tukey’s post hoc test.
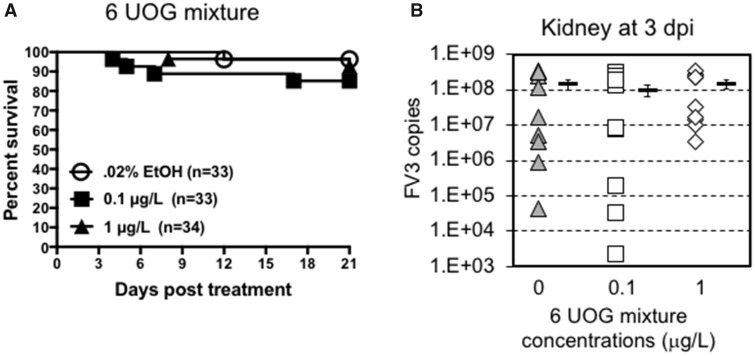
Effects of Exposure to a Mixture of 6 UOG Chemicals
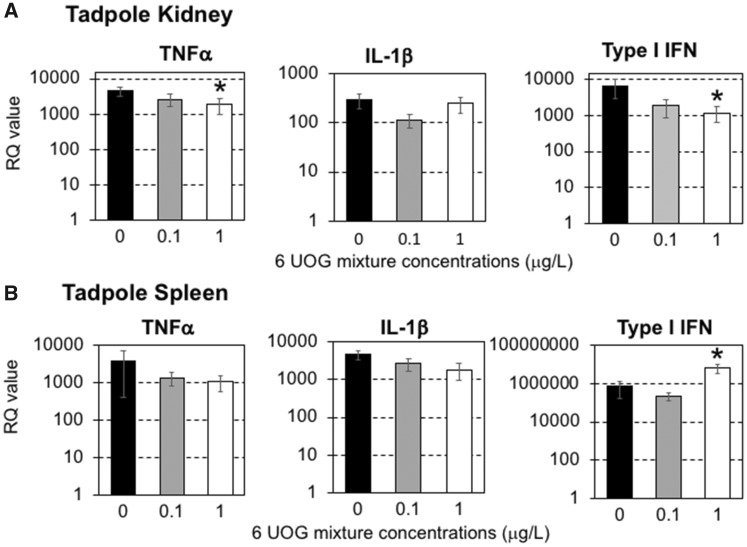
To reduce the complexity of the UOG mixture, we selected 6 of the 23 chemicals based on their putative thyroid related activity on tadpole developmental stage. Thyroid hormone signaling is critical for metamorphosis and development, and thyroid disrupting compounds have been reported to affect frog development (Miyata and Ose, 2012). Four chemicals (ethoxylated nonylphenol, ethoxylated octylphenol, ethylene glycol, and napthalene) exhibit wide EDC activity including thyroid receptor antagonism (Kassotis et al., 2015, 2016c), cumene can induce thyroid tumor (2009), and sodium tetraborate decahydrate is implicated in birth defects (Fail et al., 1998). Accordingly, we tested an equimass mixture of these 6 compounds using the same experimental procedure followed in experiments with the 23-chemical mixture. Tadpoles were exposed to 2 different equimass concentrations of these 6 UOG-associated chemicals (1 and 0.1 μg/l) for 3 weeks. Then, survival was recorded for 20 days and immune response at steady state and at 3 dpi following FV3 infection was assessed. Exposure to these 6 UOG chemicals at both doses did not induce significant death (Figure 5A). Similarly, there were no significant differences in viral loads at 3 dpi in tadpoles infected with FV3 between controls and those exposed to the 6 UOG chemicals (Figure 5B). However, when assessing changes in gene expression 3 days following FV3 infection, the gene expression response for the pro-inflammatory cytokine TNF-α and the antiviral cytokine type I IFN were significantly reduced in the 1 µg/ml treated group compared to vehicle controls (Figure 6A). In the spleen, although TNF-α gene expression was not affected, type I IFN expression was significantly elevated in the higher exposure group (Figure 6B). No significant differences in the expression of IL-1β (Figure 6), or CSF-1R, IL-34 and GSFR1 (data not shown) genes were observed in either organ. Similar results were obtained using L13 as endogenous control (Table 6).
Figure 5.
A, Survival curve of tadpoles exposed for 3 weeks to 0.2% ethanol (vehicle control) or 0.1, or 1.0 μg/l of an equimass mixture of 6 UOG chemicals. Survival was monitored for 20 days following exposure during which tadpoles were checked daily. The data are pools of 2 independent experiments. No statistical significance (Log Rank Test). B, FV3 copy number 3 days after FV3 infection as determined by absolute qRT-PCR. For each group, the viral genome copy number of each individual is depicted by different symbol as well as a horizontal barre indicating the average ± SD. No statistical significance (ANOVA and Mann-Whitney U Test). n = 10 animals per group from 2 different experiments (5 per experiment).
Figure 6.
Relative expression of TNF-α, IL-1β, and Type I IFN genes from kidney and spleen tissue harvested from premetamorphic tadpoles (stage 54–55; n = 6 individuals) exposed to either 0.2% ethanol (vehicle control), 0.1, or 1.0 μg/l of an equimass mixture of 6 UOG chemicals for 3 weeks. After chemical exposure, tadpoles were either ip injected with 10 000 pfu of FV3 or with amphibian PBS (vehicle control), and then euthanized after 3 days. Results are means ± SEM of 12 individuals per group from 2 different experiments (6 per experiment) using GAPDH as endogenous control. *p < 0.05 significant differences between controls and UOG exposed groups using one-way ANOVA test and Tukey’s post hoc test.
Table 6.
Relative Gene Expression (RQ Value ± SD) in Kidneys for Experiments Using 6 UOG Chemicals Using L13 as Endogenous Control Gene
| Genes | Uninfecteda | p values | 3 dpi FV3b | p values | p values Uninf/3 dpic |
|---|---|---|---|---|---|
| GCSFR | C: 276 ± 290 | C: 1107 ± 1502 | C: NS | ||
| L: 102 ± 89 | NS | L: 186 ± 250 | NS | L: NS | |
| H: 2240 ± 3921 | NS | H: 1114 ± 1048 | NS | H: NS | |
| IFN type I | C: 178 ± 162 | C: 2715 ± 3419 | C: 0.01 | ||
| L: 274 ± 231 | NS | L: 107 ± 116 | 0.001 | L: NS | |
| H: 204 ± 363 | NS | H: 199 ± 389 | 0.01 | H: NS | |
| IFN type III (λ) | C: 415 ± 444 | C: 470 ± 536 | C: NS | ||
| L: 170 ± 227 | NS | L: 252 ± 354 | NS | L: NS | |
| H: 521 ± 224 | NS | H: 33 ± 37 | NS | H: NS | |
| IL-1β | C: 60 ± 76 | C: 157 ± 166 | C: NS | ||
| L: 33 ± 23 | NS | L: 192 ± 218 | NS | L: NS | |
| H: 131 ± 156 | NS | H: 14 ± 8 | 0.003 | H: NS | |
| IL-10 | C: 33 ± 50 | C: 268 ± 58 | C: NS | ||
| L: 4 ± 6 | NS | L: 620 ± 543 | NS | L: 0.02 | |
| H: 103 ± 139 | NS | H: 34 ± 46 | NS | H: NS | |
| IL-34 | C: 3 ± 2 | C: 53 ± 53 | C: 0.04 | ||
| L: 1 ± .1 | NS | L: 20 ± 16 | NS | L: NS | |
| H: 11 ± 5 | NS | H: 63 ± 47 | NS | H: NS | |
| CSF-1R | C: 17 ± 10 | C: 211 ± 273 | C: 0.06 | ||
| L: 6 ± 6 | NS | L: 105 ± 125 | NS | L: 0.02 | |
| H: 416 ± 697 | 0.03 | H: 366 ± 556 | NS | H: NS | |
| TNF-α | C: 1859 ± 3409 | C: 127659 ± 174670 | C: 0.02 | ||
| L: 4269 ± 4709 | NS | L: 64516 ± 79618 | NS | L: NS | |
| H: 4704 ± 5643 | NS | H: 2881 ± 2635 | 0.06 | H: NS |
NS, nonsignificant; *,significant.
Gene expression RQ values from kidneys of tadpoles (10 per group) treated with 0.2% EtOH, 0.1 μg/l (L), or 1 μg/l (H) of UOG mixture for 3 weeks using L13 as reference gene. p value determined between vehicle control and treated animals.
Gene expression RQ values from kidneys of tadpoles (10 per group) treated with 0.2% EtOH, 0.1 μg/l (L), or 1 μg/l (H) of UOG mixture then FV3 infected for 3 days using L13 as reference gene. p value determined between vehicle control and treated animals.
p value determined by between each treated and corresponding infected group.
DISCUSSION
This study provides evidence that in addition to overt toxicity, mixtures of UOG-associated water pollutants likely have subtler deleterious potential to disrupt tadpole immune homeostasis and antiviral immune responses. Our data indicate that a relatively short 3-week exposure of X. laevis tadpoles to a mixture of 23 EDC chemicals associated with UOG results in significant acute alterations of several immunologically relevant genes at steady state and negatively affects antiviral immune response. The similarly altered expression response of several antiviral genes in tadpoles exposed to a more limited mixture of only 6 UOG chemicals further supports the immunomodulatory activity of UOG-associated water contaminants. Collectively, our findings suggest that human introduced UOG-associated chemicals can negatively impact the health and resistance to infection of amphibians, which may be representative of other aquatic species groups.
The objective of this study was 2-fold, first to examine the immunotoxicity of a combination EDCs released at UOG sites rather than single chemical in order to better reflect real risk of exposure; and second to use a relevant amphibian model to conservation research of aquatic environment. Although representing what happens in reality, consequences of exposure to multiple EDCs simultaneously are not well understood. Exposure to EDCs in contaminated waters of UOG sits occur in the form of mixtures where combinations of EDCs may produce a significant effect, although the doses of each individual chemical would not induce observable effects. To circumvent the multiple sources of variability (geography, half-life, concentrations of various pollutants, time of release, etc.), we used a means of exposure sufficiently defined, titratable and controllable, consisting of a well-defined mixture containing an equimass amount of 23 different UOG chemicals. These UOG contaminants were selected based on their demonstrable endocrine disruptor activity in vitro and their consistent detection in ground and surface water collected near or downstream across multiple UOG sites. Because the average concentration of each of these chemicals in ground water near hydraulic fracturing operations ranges from 0.01 to 2.0 mg/l (Wilkin et al., 2010; Gross et al., 2013), a mixture containing equimass amounts of all 23 compounds serves as a useful proxy to capture environmentally relevant exposure levels for humans and wild-life living in dense-drilling regions. This mixture of 23 EDCs has been rigorously characterized in the mouse model and showed to induce multiple developmental defects in the offspring of exposed pregnant females (Kassotis et al., 2016a; Sapouckey et al., 2018). Notably, developmental exposure to the mixture during pregnancy in mice affects immune functions of the offspring (Boule et al., 2018). Parenthetically, the same mixture concentration range shown to induce immune alterations in mouse (1 and 0.1 μg/l) was effective in X. laevis, which give a point of comparison. As such, the combination of this defined EDC mixture with our X. laevis-FV3 immunological model provides a reliable way for evaluating the impacts of EDCs released by UOG on the immune status of aquatic vertebrates.
A first finding of this study is the startling toxicity of the UOG mixture to tadpoles. Exposure to an equimass of both 10 and even 5 μg/l induced marked lethality over a 2-week period. This is notable when one considers that these different chemicals have been reported to reach up to 2 mg/l in ground water at fracturing spill sites (DiGiulio et al., 2011; Gross et al., 2013) and surface water concentrations are likely higher. Although chemicals such as acrylamide or phenol are notoriously toxic, LD50 values of most of the 23 UOG chemicals are 150 mg/kg of body weight or higher in rats. Thus, even if little is known about Xenopus sensitivity for these chemicals, it seems likely that the lethal effect results from the combined activity of some or all these chemicals. Based on these results, it stands to reason that one should consider UOG polluted water especially harmful for amphibian tadpoles and that more detailed toxicity assays utilizing different combinations of UOG-associated chemicals should be performed to identify those compounds to eliminate in order to minimize harmful effects.
Concerning the observed immunomodulation of the UOG mixture in tadpoles, several points merit further discussion. It should first be noted that little is known about the immunotoxicity of most of the 23 compounds used in the mixture. Benzene and styrene are considered strongly or moderately toxic to the mammalian immune system (Veraldi et al., 2006). The combination of benzene, toluene, ethylbenzene, and xylenes has carcinogenic effects on many tissues and organs as well as the vertebrate immune system (BTEX; Bahadar et al., 2014; Bolden et al., 2015).
Our data indicate that a relatively short exposure (3 weeks) to UOG chemicals at a rather low dose of 1 μg/l is sufficient to induce perturbation of genes involved in differentiation and function of myeloid lineage cells. Similar to mammals, Xenopus myeloid lineage cell includes neutrophils, basophils, eosinophils, polymorphonuclear cells, monocytes, and macrophages. Peritoneal macrophages play crucial role during infection by ranaviruses exhibiting active phagocytic activity and producing pro-inflammatory cytokines (TNFα, IL1-β) and type I IFN (Edholm et al., 2017). We have previously shown that CSF-R1 is a master gene that drives the differentiation and function of macrophages (Grayfer and Robert, 2013), which interact in Xenopus as in mammals, with 2 distinct, evolutionarily unrelated ligands, CSF-1 and IL-34 (Grayfer and Robert, 2014, 2015). In the absence of bone marrow, it is currently unclear whether monocytic lineage differentiation is limited to the liver or may also involve other sites such as the spleen and/or kidneys. Although exposure to UOG chemicals did not consistently alter CSF-R1 or CSF-1 gene expression, transcript levels of IL-34 were significantly increased in the kidney of tadpoles exposed to 1 μg/l of UOG mixture. In addition, GCSF-R transcripts, a gene typically expressed by cells of the granulocyte lineage such as neutrophils, had raised transcript levels in kidneys compared to controls. This suggests that UOG-associate EDC chemicals have the potential to acutely perturb myeloid cells in this organ. The ablated expression response triggered by FV3 infection in the spleen for CSF-1R, GCSF-R, CSF1, and IL-34 would be consistent with the perturbation of myeloid cell effectors from exposure to UOG chemicals, although we were not able to substantiate these results when normalizing with a second endogenous control (L13). Thus, more extensive and mechanistic studies will be needed to elucidate to what extent the UOG mixtures affect the myeloid lineage and whether these effects are long lasting.
However, whether related or not to the perturbations of myeloid lineage cells, it is clear that exposure to the UOG mixture negatively affected the immune response against FV3. This was revealed by a significant increase in the viral load as well as by a concomitant defect in type I IFN and IL-1β gene expression response in both the kidneys (the main site of viral replication) and the spleen (the main immune organ). It is noteworthy that type I IFN induction was also reduced following treatment with a lower dose (0.1 μg/l) of the UOG mixture, which corresponded to an increased viral load. An efficient type I IFN response is critical for host defense to control initial viral burden (Grayfer et al., 2014). Thus, even at scant amount, EDCs released at UOG sites can compromise antiviral immune defenses. Although the decreased survival to FV3 infection of UOG-exposed tadpoles did not reach statistical significance, it is reasonable to assume that the alterations of the antiviral immune response would negatively impact hosts fitness in the wild. Indeed, correlation between increased viral load and increased probability of mortality of tadpoles is well documented in Xenopus (Grayfer et al., 2014, 2015; Koubourli et al., 2017) and other anuran species (Brand et al., 2016; Yu et al., 2017).
As mentioned before, our rationale for using a mixture of 23 chemicals consistently present in water contaminated by UOG is that it more realistically represents the type of exposure that wild animals are encountering. However, for future mechanistic studies it would be useful to reduce the number of chemical in the mixture tested. With this idea in mind, we have focused on 6 of the 23 chemicals based on their putative action on development of tadpoles, which is controlled by thyroid hormone. We also included 2 compounds, cumene and sodium tetraborate decahydrate, because of the possible susceptibility of tadpoles for these compounds. Although cumene did not show much antagonistic activity, it can induce hemangiosarcomas of the spleen and follicular cell adenomas of the thyroid gland (1999) and sodium tetraborate decahydrate as a Borax has been implicated in birth defects (Pongsavee, 2009). We realize of course the limitations of this approach, but we posit that attempting to reduce the complexity of the UOG mixture is an important step toward a better understanding the impacts. In any case, exposure to an equimass mixture of these 6 UOG chemicals, although not as potent as the 23 UOG chemicals still induced significant alteration of antiviral immunity. It will be interesting in future experiments to further reduce the number of these chemicals, so that possible interactions and synergetic effects can be investigated.
ACKNOWLEDGMENTS
We thank Tina Martin for animal husbandry.
FUNDING
National Institute of Allergy and Infectious Diseases at the National Institutes of Health (R24-AI-059830) and a Pilot Project Grant from the Rochester Environmental Health Sciences Center (P30-ES01247). C.M. is supported by the Toxicology Program (T32-ES07026) and S.P by the Natural Environment Research Council (NE/M000338/1; NE/M000591/1; NE/M00080X/1).
REFERENCES
- 1999. Complete sequence and gene map of a human major histocompatibility complex. The MHC sequencing consortium. Nature 401, 921–923. [DOI] [PubMed] [Google Scholar]
- 2009. Toxicology and carcinogenesis studies of cumene (CAS No. 98-82-8) in F344/N rats and B6C3F1 mice (inhalation studies). National Toxicology Program technical report series, 2009/04/03 ed, pp. 1–200. [PubMed]
- Bahadar H., Mostafalou S., Abdollahi M. (2014). Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharmacol. 276, 83–94. [DOI] [PubMed] [Google Scholar]
- Bandin I., Dopazo C. P. (2011). Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet. Res. 42, 67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A. L., Kwiatkowski C. F., Colborn T. (2015). New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol. 49, 5261–5276. [DOI] [PubMed] [Google Scholar]
- Boule L. A., Chapman T., Hillman S., Balise V., O'Dell C., Robert J., Georas S., Nagel S., Lawrence P. (2018). Developmental exposure to a mixture of 23 chemicals associated with unconventional oil and gas operations alters the immune system of mice. Toxicol. Sci. 163, 639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., Hill R. D., Brenes R., Chaney J. C., Wilkes R. P., Grayfer L., Miller D. L., Gray M. J. (2016). Water temperature affects susceptibility to ranavirus. Ecohealth 13, 350–359. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O. (2016). Hydraulic fracturing for natural gas: Impact on health and environment. Rev. Environ. Health 31, 47–51. [DOI] [PubMed] [Google Scholar]
- Chinchar V. G. (2002). Ranaviruses (family Iridoviridae): Emerging cold-blooded killers. Arch. Virol. 147, 447–470. [DOI] [PubMed] [Google Scholar]
- Chinchar V. G., Hyatt A., Miyazaki T., Williams T. (2009). Family Iridoviridae: Poor viral relations no longer. Curr. Top. Microbiol. 328, 123–170. [DOI] [PubMed] [Google Scholar]
- Cozzarelli I. M., Skalak K. J., Kent D. B., Engle M. A., Benthem A., Mumford A. C., Haase K., Farag A., Harper D., Nagel S. C., et al. (2017). Environmental signatures and effects of an oil and gas wastewater spill in the Williston Basin, North Dakota. Sci. Total Environ. 579, 1781–1793. [DOI] [PubMed] [Google Scholar]
- Daszak P., Berger L., Cunningham A. A., Hyatt A. D., Green D. E., Speare R. (1999). Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 5, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus Andino F., Chen G., Li Z., Grayfer L., Robert J. (2012). Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology 432, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus Andino F., Lawrence B. P., Robert J. (2017). Long term effects of carbaryl exposure on antiviral immune responses in Xenopus laevis. Chemosphere 170, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiulio D. C., Jackson R. B. (2016). Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the Pavillion, Wyoming, Field. Environ. Sci. Technol. 50, 4524–4536. [DOI] [PubMed] [Google Scholar]
- DiGiulio D. C., Wilkin R. T., Miller C., Oberley G.. 2011. Investigation of ground water contamination near Pavillion. Environmental Protection Agency, Wyoming, Draft Report, p. 121.
- Duffus A., Waltzek T., Stöhr A., Allender M., Gotesman M., Whittington R., Hick P., Hines M., Marschang R.. 2015. Distribution and host range of Ranaviruses In Gray M.J., Chinchar V.G. (Eds.), Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates. Springer Open, pp. 9–59. [Google Scholar]
- Edholm E. S., Rhoo K. H., Robert J. (2017). Evolutionary aspects of macrophages polarization. Results Probl Cell Differ 62, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Serrano A., Relyea R. A., Tejedo M., Torralva M. (2012). Understanding of the impact of chemicals on amphibians: A meta-analytic review. Ecol. Evol. 2, 1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner M., Hoelzer K. (2016). Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ. Sci. Technol. 50, 3290–3314. [DOI] [PubMed] [Google Scholar]
- Fail P. A., Chapin R. E., Price C. J., Heindel J. J. (1998). General, reproductive, developmental, and endocrine toxicity of boronated compounds. Reprod. Toxicol. 12, 1–18. [DOI] [PubMed] [Google Scholar]
- Grayfer L., Andino F. D. J., Chen G., Chinchar G. V., Robert J. (2012). Immune evasion strategies of ranaviruses and innate immune responses to these emerging pathogens. Viruses 4, 1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayfer L., De Jesus Andino F., Robert J. (2014). The amphibian (Xenopus laevis) type I interferon response to frog virus 3: New insight into ranavirus pathogenicity. J. Virol. 88, 5766–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayfer L., De Jesus Andino F., Robert J. (2015). Prominent amphibian (Xenopus laevis) tadpole type III interferon response to the frog virus 3 ranavirus. J. Virol. 89, 5072–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayfer L., Robert J. (2013). Colony-stimulating factor-1-responsive macrophage precursors reside in the Amphibian (Xenopus laevis) bone marrow rather than the hematopoietic sub-capsular liver. J. Innate Immun. 5, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayfer L., Robert J. (2014). Divergent antiviral roles of amphibian (Xenopus laevis) macrophages elicited by colony-stimulating factor-1 and interleukin-34. J. Leukoc. Biol. 96, 1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayfer L., Robert J. (2015). Distinct functional roles of amphibian (Xenopus laevis) colony-stimulating factor-1- and interleukin-34-derived macrophages. J. Leukoc. Biol. 98, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer A. L., Berrill M., Wilson P. J. (2005). Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Dis. Aquat. Organ. 67, 9–14. [DOI] [PubMed] [Google Scholar]
- Gross S. A., Avens H. J., Banducci A. M., Sahmel J., Panko J. M., Tvermoes B. E. (2013). Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J. Air Waste Manage. Assoc. (1995) 63, 424–432. [DOI] [PubMed] [Google Scholar]
- Jancovich J. K., Bremont M., Touchman J. W., Jacobs B. L. (2010). Evidence for multiple recent host species shifts among the Ranaviruses (Family Iridoviridae). J. Virol. 84, 2636–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis C. D., Bromfield J. J., Klemp K. C., Meng C. X., Wolfe A., Zoeller R. T., Balise V. D., Isiguzo C. J., Tillitt D. E., Nagel S. C. (2016). Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology 157, 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis C. D., Iwanowicz L. R., Akob D. M., Cozzarelli I. M., Mumford A. C., Orem W. H., Nagel S. C. (2016). Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Sci. Total Environ. 557-558, 901–910. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Klemp K. C., Vu D. C., Lin C. H., Meng C. X., Besch-Williford C. L., Pinatti L., Zoeller R. T., Drobnis E. Z., Balise V. D., et al. (2015). Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 156, 4458–4473. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Tillitt D. E., Davis J. W., Hormann A. M., Nagel S. C. (2014). Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 155, 897–907. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Tillitt D. E., Lin C. H., McElroy J. A., Nagel S. C. (2016c). Endocrine-Disrupting chemicals and oil and natural gas operations: Potential environmental contamination and recommendations to assess complex environmental mixtures. Environ. Health Perspect. 124, 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubourli D. V., Wendel E. S., Yaparla A., Ghaul J. R., Grayfer L. (2017). Immune roles of amphibian (Xenopus laevis) tadpole granulocytes during Frog Virus 3 ranavirus infections. Dev. Comp. Immunol. 72, 112–118. [DOI] [PubMed] [Google Scholar]
- Miyata K., Ose K. (2012). Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J. Toxicol. Pathol. 25, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen I., Lee J. (2015). High volume hydraulic fracturing operations: Potential impacts on surface water and human health. Int. J. Environ. Health Res. 1, 23. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P., Faber J.. 1967. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis, 2nd ed.Amsterdam: North Holland. [Google Scholar]
- Orem W., Varonka M., Crosby L., Haase K., Loftin K., Hladik M., Akob D. M., Tatu C., Mumford A., Jaeschke J., et al. (2017). Organic geochemistry and toxicology of a stream impacted by unconventional oil and gas wastewater disposal operations. Appl. Geochem. 80, 155–167. [Google Scholar]
- Pongsavee M. (2009). Genotoxic effects of borax on cultured lymphocytes. Southeast Asian J. Trop. Med. Public Health 40, 411–418. [PubMed] [Google Scholar]
- Price S. J., Ariel E., Maclaine A., Rosa G. M., Gray M. J., Brunner J. L., Garner T. W. J. (2017). From fish to frogs and beyond: Impact and host range of emergent ranaviruses. Virology 511, 272–279. [DOI] [PubMed] [Google Scholar]
- Sapouckey S. A., Kassotis C. D., Nagel S. C., Vandenberg L. N. (2018). Prenatal exposure to unconventional oil and gas operation chemical mixtures altered mammary gland development in adult female mice. Endocrinology 159, 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifkarovski J., Grayfer L., De Jesus Andino F., Lawrence B. P., Robert J. (2014). Negative effects of low dose atrazine exposure on the development of effective immunity to FV3 in Xenopus laevis. Dev. Comp. Immunol. 47, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S., Fischman D. L., Waller R. W. (2004). Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786. Epub 2004 Oct 1714. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. (2015). Assessment of the potential impacts of hydraulic fracturing for oil and gas on drinking water resources. [DOI] [PubMed]
- Vengosh A., Jackson R. B., Warner N., Darrah T. H., Kondash A. (2014). A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 48, 8334–8348. [DOI] [PubMed] [Google Scholar]
- Veraldi A., Costantini A. S., Bolejack V., Miligi L., Vineis P., van Loveren H. (2006). Immunotoxic effects of chemicals: A matrix for occupational and environmental epidemiological studies. Am. J. Ind. Med. 49, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Waxman H. A., Markey E. J., DeGette D.. 2011. Chemicals used in hydraulic fracturing. US House of Representatives Council Committee on Energy and Commerce Minority Staff Report.
- Webb E., Bushkin-Bedient S., Cheng A., Kassotis C. D., Balise V., Nagel S. C. (2014). Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev. Environ. Health 29, 307–318. [DOI] [PubMed] [Google Scholar]
- Wilkin R. T., Digiulio D. C. (2010). Geochemical impacts to groundwater from geologic carbon sequestration: Controls on pH and inorganic carbon concentrations from reaction path and kinetic modeling. Environ. Sci. Technol. 44, 4821–4827. [DOI] [PubMed] [Google Scholar]
- Yu Y., Huang Y., Ni S., Zhou L., Liu J., Zhang J., Zhang X., Hu Y., Huang X., Qin Q. (2017). Singapore grouper iridovirus (SGIV) TNFR homolog VP51 functions as a virulence factor via modulating host inflammation response. Virology 511, 280–289. [DOI] [PubMed] [Google Scholar]