Abstract
The direct C–H functionalization methodology has first been applied to perform transition metal-free C–H/C–Li cross-couplings of 2H-imidazole 1-oxides with carboranyllithium. This atom- and step-economical approach, based on one-pot reactions of nucleophilic substitution of hydrogen (SNH) in non-aromatic azaheterocycles, affords novel imidazolyl-modified carboranes of two types (N-oxides and their deoxygenative analogues), which are particularly of interest in the design of advanced materials.
Keywords: carboranes, C–H functionalization, C–H/C–Li cross-coupling, 2H-imidazole 1-oxide, nucleophilic substitution of hydrogen (SNH)
Introduction
Dicarbadodecaboranes (carboranes) are known to be 3D polyhedral clusters with a special type of structural organization, relative thermal and chemical stabilities, as well as unique physicochemical properties [1–3]. The functional derivatives of carboranes, in particular heterocyclic ones, are undeniably of increased interest in the chemistry of organoboron compounds due to wide opportunities to use these boron-enriched substances as diagnostic tools for tumor radio imaging [4–7], promising agents for boron neutron capture therapy (BNCT) of cancer [8–12], as well as agonists and antagonists of biological receptors [13–17], etc. In addition, azaheterocyclic carboranes are actively used as ligands in the synthesis of metal complexes of various architectures, possessing catalytic activity [18–21], as well as unique photophysical properties [22–26]. Thus, the development of effective approaches to azaheterocyclic carboranes is currently considered to be one of the most important synthetic challenges.
At present, there are three main synthetic strategies in the design of promising heterocyclic carboranes: (i) condensation of decaborane (B10H14) with substituted acetylenes [27–30], (ii) carboryne-based cycloaddition reactions [31–33], and (iii) C–X/C–M cross coupling of halogenated azaheterocycles (X = Br, Cl, F) with carborane organometallic derivatives (M = Li, Cu) [11,22,34–35]. Despite the fact that the latter synthetic strategy is in common use, it has significant limitations. In particular, the preliminary functionalization of heterocyclic substrates is required to apply this cross-coupling methodology.
Thus, the development of pot-, atom- and stage-economical (PASE) [36–38] methods leading to novel C-modified ortho-carboranes, as well as elucidation of scope and mechanistic features of these transformations, is one of the crucial steps to obtain promising boron-enriched heterocyclic ensembles and functional materials based on them. An alternative approach to exploit the C–X/C–M cross-coupling reactions, leading to heterocyclic boron clusters, is based on the C–H/C–M coupling strategy. One of the ways to realize these cross couplings is the transition metal-free methodology for direct C–H functionalization of azaheterocyclic substrates [39–42], which can be carried out by using SNH reactions (nucleophilic substitution of hydrogen) [43–48]. The SNH methodology corresponds to the basic principles of green chemistry [49–53], and it is now considered to be one of the most valuable approaches to functionally substituted azaheterocycles, since these transformations can be performed without any catalysis by transition metals, neither they need preliminary introduction of good leaving groups into heterocyclic substrates.
It is known that SNH reactions have successfully been applied in the chemistry of π-deficient (hetero)aromatic systems as an effective and useful synthetic approach [43–48]. These transformations have been used for the synthesis of various mono-, di-, and triazinyl-modified carboranes [54–56]. At the same time, a limited number of protocols describe application of SNH reactions to functionalize non-aromatic heterocycles. In particular, only few examples of SNH reactions are available in the chemistry of 2H-imidazole 1-oxides [57–60] that are of interest as promising pharmacoactive azaheterocyclic compounds [61–65].
In this paper, we wish to report the first examples of the SNH methodology for the synthesis of new heterocyclic carboranes by means of direct C–H/C–Li coupling of non-aromatic 2H-imidazole 1-oxides with carboranyllithium. A growing interest in bifunctional compounds, bearing both the pharmacoactive imidazole motif and a polyhedral boron-enriched scaffold, is likely to be due to unique properties of these organoboron compounds and materials based on them.
Results and Discussion
2H-Imidazole 1-oxides are known to be non-aromatic heterocyclic compounds, bearing the electrophilic center C(5)–H, which is active for interaction with nucleophilic reagents. This feature enables one to carry out the direct nucleophilic functionalization of the C(sp2)–H bond, thus leading to novel C(5)-modified 2H-imidazoles. We have established that carboranyllithium, generated in situ from 1,2-dicarba-closo-dodecaborane [66], can be involved in the above mentioned C–H/C–Li coupling reactions successfully as a nucleophilic partner.
The direct transition metal-free C–H/C–Li cross-coupling reactions of 2H-imidazole 1-oxides 1a–d with 2 have been found to result in the novel carboranes 4a–d and 5a–d of various architectures. These transformations are able to be considered as nucleophilic substitution of hydrogen (SNH) in non-aromatic N-oxides of 2H-imidazoles 1a–d. It has also been shown that the SNH reactions can be realized via either an "addition–elimination" SNH(AE) mechanism, or through an "addition–oxidation" SNH(AO) scheme to form imidazolyl carbonanes 4a–d and 5a–d, respectively.
In accordance with the current SNH concept, the first step of both protocols, SNH(AE) and SNH(AO), involves a reversible formation of unstable anionic σH-adducts 3(a–d)-OLi as a result of nucleophilic attack of carboranyllithium 2 at the CH=N+–O− bond of 2H-imidazole 1-oxides 1a–d. The second step can be carried out in both eliminative and oxidative versions, the structure of the final carboranylated 2H-imidazoles 4a–d and 5a–d being controlled by the reaction conditions used.
In order to accomplish the nucleophilic substitution of hydrogen according to the "addition–elimination" scheme SNH(AE), the key factor has been shown to be the presence of a deoxygenating reagent in the reaction mixtures, containing the σH-adducts 3(a–d)-OLi. Indeed, it has been found that addition of acylating agents to intermediates 3(a–d)-OLi facilitates elimination of hydrogen from the σH-adducts along with the oxygen-containing fragment from the N+–O− moiety to form imidazolyl-substituted carboranes 4a–d with the loss of the N-oxide function (Scheme 1).
Scheme 1.
C–H/C–Li cross-coupling reactions of 2H-imidazole 1-oxides 1a–d and carboranyl lithium 2. The reactions were carried out in accordance with the optimized coupling conditions according to the "addition–elimination" SNH(AE) or "addition–oxidation" SNH(AO) pathways (Table 1 and Table 2).
In order to find out the optimal reaction conditions for the SNH(AE) pathway, affording the maximum yields of the target products 4, the effects of various factors (type of acylating agents, exposure time for which the reaction mass is kept after mixing 2H-imidazole N-oxide 1 with carboranyllithium 2 and quenching with an acylating agent, as well as the temperature regime, at which the deoxygenative agent is added) have been studied. The cross-coupling reaction of 2,2-dimethyl-4-phenyl-2H-imidazole 1-oxide (1a) with carboranyllithium 2 was chosen as a model reaction. It has finally been found that the best yield of 4a is achieved using AcCl as a deoxygenative agent at room temperature with stirring of the resulted reaction mixture for 15 min (Table 1, entry 11). Thus, a number of novel heterocyclic carboranes 4a–d have been synthesized in 40–55% yields following the optimized reaction conditions (Scheme 1).
Table 1.
Optimization of the reaction conditions for the C–H/C–Li cross-coupling of 2H-imidazole 1-oxides 1a–d with carboranyllithium 2 according to the "addition–elimination" protocola.
| entry | temperature, °Cb | exposure time (min)c | acylating agent | yield of 4a (%) |
| 1 | −78 | 15 | Ac2O | 9 |
| 2 | −78 | 30 | Ac2O | 9 |
| 3 | 0 | 15 | Ac2O | 17 |
| 4 | 0 | 30 | Ac2O | 17 |
| 5 | rt | 15 | Ac2O | 28 |
| 6 | rt | 30 | Ac2O | 27 |
| 7 | −78 | 15 | AcCl | 12 |
| 8 | −78 | 30 | AcCl | 12 |
| 9 | 0 | 15 | AcCl | 23 |
| 10 | 0 | 30 | AcCl | 23 |
| 11 | rt | 15 | AcCl | 43 |
| 12 | rt | 30 | AcCl | 40 |
| 13 | −78 | 15 | TFAA | 7 |
| 14 | −78 | 30 | TFAA | 7 |
| 15 | 0 | 15 | TFAA | 14 |
| 16 | 0 | 30 | TFAA | 14 |
| 17 | rt | 15 | TFAA | 18 |
| 18 | rt | 30 | TFAA | 18 |
aThe reaction was carried out in dry THF using 2H-imidazole 1-oxide 1a (1.1 equiv), carboranyllithium 2 prepared from o-carborane (1.0 equiv) and n-BuLi (1.1 equiv) at −78 °C. bThe temperature at which the acylating agent was added. cBetween addition of 2H-imidazole 1-oxide 1a to carboranyllithium 2 and quenching with the acylating agent.
In case of the "addition–oxidation" protocol realization for the SNH (AO) reactions, an oxidative agent to convert the σH-adducts 3(a–d)-OLi into the corresponding imidazolyl carboranes 5a–d with retention of the N-oxide function in the imidazole moiety, has been found to play a key role. It should be noted that optimization of the reaction conditions for oxidative C–C couplings has been carried out by using the model reaction of 2,2-dimethyl-4-phenyl-2H-imidazole 1-oxide (1a) with carboranyllithium 2. The experiments performed have shown effects of the used oxidants, temperature regime, and exposure time after addition of an oxidant into the reaction mixture. As a result, the optimal conditions have been found to involve the use of DDQ as oxidant and refluxing of the reaction mixture in argon atmosphere for 1 h (Table 2, entry 12). It has also been observed that further increase in the exposure time does not improve yields (39–52%) of the target carboranyl-substituted imidazole 1-oxides 5a–d (Scheme 1). Besides compounds 5a–d, the formation of their deoxygenated analogues 4a–d has been shown to take place in trace amounts under the optimized reaction conditions. The latter are supposed to be derived from elimination of hydrogen and the oxygen-containing moiety from the corresponding σH-adducts.
Table 2.
Optimization of the reaction conditions for the C–H/C–Li cross-coupling of 2H-imidazole 1-oxides 1a–d with carboranyllithium 2 according to the "addition–oxidation" protocola.
| entry | oxidant | temperature, °Cb | exposure time (min)c | yield of 5a (%) |
| 1 | – | Rt | 30 | trace |
| 2 | – | Rt | 60 | trace |
| 3 | – | 40 | 30 | 5 |
| 4 | – | 40 | 60 | 7 |
| 5 | – | reflux | 30 | 9 |
| 6 | – | reflux | 60 | 12 |
| 7 | DDQ | Rt | 30 | 7 |
| 8 | DDQ | Rt | 60 | 7 |
| 9 | DDQ | 40 | 30 | 9 |
| 10 | DDQ | 40 | 60 | 11 |
| 11 | DDQ | reflux | 30 | 35d |
| 12 | DDQ | reflux | 60 | 42d |
| 13 | DDQ | reflux | 120 | 42d |
| 14 | p-chloranyl | rt | 60 | 5 |
| 15 | p-chloranyl | 40 | 60 | 7 |
| 16 | p-chloranyl | reflux | 30 | 17 |
| 17 | p-chloranyl | reflux | 60 | 31 |
| 18 | p-chloranyl | reflux | 120 | 30 |
aThe reaction was carried out in dry THF using 2H-imidazole 1-oxide 1a (1.1 equiv), carboranyllithium 2 prepared from o-carborane (1.0 equiv) and n-BuLi (1.1 equiv) at −78 °C. bThe temperature at which the reaction mixture was stirred after addition of oxidant. cExposure time after addition of oxidant to the reaction mixture. dImidazolyl carborane 4a was isolated in trace amounts.
It is worth mentioning that the C–H/C–Li coupling reactions of 2H-imidazole 1-oxides 1c,d with carboranyllithium 2 have been found to result in the formation of p-bromophenyl derivatives 4c,d and 5c,d, which are of particular interest as valuable synthons for further modifications, for instance by means of transition metal-catalyzed C–Br/C–M (M = Li, Mg, Zn, etc.) and C–Br/C–H cross-couplings reactions leading to more complex organic compounds.
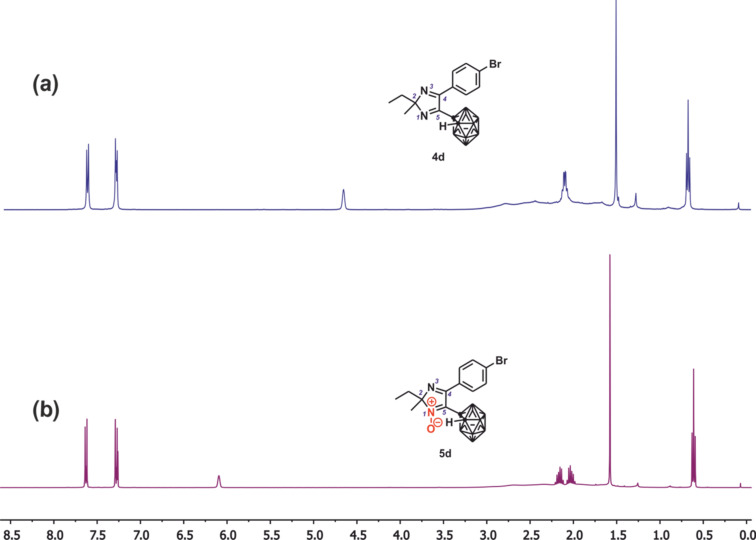
Mono-substituted imidazolyl carboranes 4a–d and their N-oxide analogues 5a–d were characterized by the data of elemental analysis, IR, NMR spectroscopy (1H, 13C{1H} (APT), 11B, 11B{1H} experiments), and mass spectrometry. In the IR spectra of 4a–d and 5a–d, there are absorption bands corresponding to the stretching vibrations of carborane B–B atoms (ν 713–721 cm−1), B–H (ν 2561–2605 cm−1) and C–H (ν 3026–3075 cm−1). In the 1H NMR spectra of 4a–d and 5a–d the resonance signals of both carborane and imidazole fragments were observed. Typical 1H NMR spectra of carboranyl-substituted imidazoles and their N-oxides (4d versus 5d) are shown in Figure 1. In these spectra, the characteristic signals of C-substituted carborane are registered as two sets, corresponding to CCarb–H and B–H protons. The chemical shift of unsubstituted carborane Ccarb–H proton resonance has been shown to depend on the presence of the N+–O– moiety in the imidazole ring. In particular, a broadened singlet of the Ccarb–H proton is exhibited at δ 4.63–4.58 ppm in the 1H NMR spectra of 4a–d, while in the spectra of their N-oxide analogues 5a–d the corresponding singlet is observed at δ 6.09–6.03 ppm. Such difference in chemical shifts is likely to be due to the effect of the imidazole N+–O– group oxygen. Carborane B–H protons are observed as 10 H broadened multiplets at δ 3.08–1.61 ppm. The proton resonances of C(2)-alkyl and C(4)-aryl substituents are observed in the appropriate fields: at δ 2.17–0.61 ppm and δ 7.67–7.22 ppm, respectively.
Figure 1.
The 1H NMR spectra of 1-(5-(4-bromophenyl)-2-ethyl-2-methyl-2H-imidazol-4-yl)-1,2-dicarba-closo-dodecaborane (4d) and 1-(4-(4-bromophenyl)-2-ethyl-2-methyl-1-oxido-2H-imidazol-5-yl)-1,2-dicarba-closo-dodecaborane (5d) in CDCl3 at 295 K.
In the 13C NMR spectra, one can also see the difference in chemical shifts for carbon-13 resonance signals of imidazoles 4a–d and their N-oxide analogues 5a–d. Indeed, in the 13C NMR spectra of 4a–d the carbon-13 resonances of organoboronic Ccarb–H fragments are observed at δ 61.75–61.47 ppm, while in the spectra of their N-oxides 5a–d the corresponding signals are exhibited at δ 57.64–57.63 ppm. The carbon resonances associated with the imidazole ring have been recorded at δ 69.36–69.12 ppm and at δ 65.66–65.41 ppm in the 13C NMR spectra of compounds 4a–d and 5a–d, respectively. In the mass spectra of carboranylimidazoles the corresponding molecular ion [M]+ peaks have also been registered. It should be mentioned that [M]+ values, observed for the N-oxide series of compounds 5a–d, proved to differ from those registered for the deoxygenated analogues 4a–d by atomic oxygen mass (16 amu).
The novel imidazolyl carboranes 4a–d and 5a–d obtained are considered to be complex closo-cluster structures formed by polyboron and heterocyclic scaffolds linked to each other through the C–C bond. In order to correlate the resonance signals with the assumed structure unambiguously, two-dimensional NMR correlation 1H–13C spectra, showing direct (HSQC) and distant (HMBC) spin–spin constants, have been recorded for compound 5d (Figure 2). The presence of the cross-peak {6.09, 57.64} in the HSQC spectrum of 5d and absence of any signals in these regions in the HMBC spectrum enables one to state that these signals belong to proton (δ 6.09 ppm) and carbon (δ 57.64 ppm) resonances of the CCarb–H fragment. Also, the 2D spectra allow one to distinguish the carbon resonances of 2H-imidazole methyl (δ 24.06 ppm) and ethyl (δ 6.74 and 31.12 ppm) groups. In addition, the interaction observed in the HMBC spectra of quaternary carbons with protons of methyl and ethyl groups through the long-range constants allows the signal at δ 103.85 ppm in the 13C NMR spectra of 5d to be attributed to C-2 of the imidazole ring.
Figure 2.
Fragment of the 2D 1H–13C{1H} HSQC (a) and HMBC (b) spectra of imidazolyl carborane 5d in CDCl3 at 295 K (the whole spectra are shown in Supporting Information File 1).
The structure of imidazolyl carborane 5d has also been proved by the X-ray analysis (Figure 3). The single crystals were obtained by slow evaporation of imidazolyl carborane 5d from a mixture of CH2Cl2/heptane, 8:2. According to the XRD data, two independent molecules have been found to be crystallized in the P1211 chiral space group of the monoclinic system. General views for molecules 1 and 2 are shown in Figure 3, atoms of the molecule 2 being labeled with the additional index “A”. Both independent molecules proved to be characterized by the (R)-configuration of the C(2)-chiral center and the appropriate bond and angle distances. Both heterocyclic rings have been found to be planar with 4-BrC6H4 substituent being turned towards the heterocyclic fragments, thus forming the angles of 74° and 80°, respectively. In the crystals of molecule 1, the 2H-imidazole 1-oxide fragment is disordered into two positions with occupancy coefficient of 0.8/0.2. The minor disordered moiety has been confirmed to be in the (S)-configuration. Also it is worth mentioning that no shortened contacts have been observed in the crystals.
Figure 3.
Molecular structure of 5d. Selected bond distances (Å) and angles (deg) for molecule 1: C(3)–C(14), 1.665; C(3)–C(5), 1.491; C(5)–N(1), 1.338; C(5)–C(4), 1.472; Br(1)–C(9), 1.910; O(1)–N(1), 1.281; N(3)–C(4), 1.30; N(3)–C(2), 1.45; C(2)–C(1), 1.54; C(2)–C(12), 1.53; C(6)–C(4), 1.499; C(4)–N(3)–C(2), 108.2; O(1)–N(1)–C(2), 120.3; O(1)–N(1)–C(5), 130.0; C(5)–C(3)–C(14), 116.6; C(12)–C(2)–N(1), 108.4; C(1)–C(2)–N(3), 111.4; C(1)–C(2)–C(12), 113.8; for molecule 2 “A”: C(3A)–C(15), 1.65; C(3A)–C(5A), 1.500; C(5A)–N(1A), 1.326; C(5A)–C(4A), 1.468; Br(2)–C(9A), 1.904; O(1A)–N(1A), 1.267; N(3A)–C(4A), 1.300; N(3A)–C(2A), 1.441; C(2A)–C(1A), 1.523; C(2A)–C(12A), 1.532; C(6A)–C(4A), 1.491; C(4A)–N(3A)–C(2A), 108.6; O(1A)–N(1A)–C(2A), 120.4; O(1A)–N(1A)–C(5A), 130.4; C(5A)–C(3A)–C(15), 116.5; C(12A)–C(2A)–N(1A), 107.1; C(1A)–C(2A)–N(3A), 113.0; C(1A)–C(2A)–C(12A), 112.0.
Conclusion
The direct C(sp2)–H functionalization methodology has first been applied to design the synthesis of polyhedral boron closo-clusters. Novel heterocyclic carboranes have been obtained through a one pot-, atom- and stage-economical approach, based on nucleophilic substitution of hydrogen (SNH) in 2H-imidazole 1-oxides by action of carboranyllithium, generated in situ from commercially available o-carborane. It has also been found that the SNH(AE, «addition–elimination») scheme leads to carboranyl-substituted 2H-imidazoles, while alternative SNH(AO, «addition–oxidation») results in the corresponding N-oxide analogues. In summary, novel organoelement bifunctional ensembles, bearing heterocyclic and carborane moieties, which are of particular interest in the design of advanced materials, have been obtained in good yields.
Supporting Information
Experimental procedures, characterization data, copies of the 1H, 13C, 11B NMR spectra and X-ray diffraction studies.
Acknowledgments
This study was supported by the Russian Ministry of Education and Science (State contract 4.6351.2017/8.9) and the Russian Foundation for Basic Research (grant 16-03-00958).
References
- 1.Grimes R N. Carboranes. 3rd ed. Elsevier Inc.; 2016. [Google Scholar]
- 2.Calabrese G, Daou A, Rova A, Tseligka E, Vizirianakis S I, Fatouros G D, Tsibouklis J. Med Chem Commun. 2017;8:67–72. doi: 10.1039/c6md00383d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Núñez R, Tarrés M, Ferrer-Ugalde A, de Biani F F, Teixidor F. Chem Rev. 2016;116:14307–14378. doi: 10.1021/acs.chemrev.6b00198. [DOI] [PubMed] [Google Scholar]
- 4.Paxton R J, Beatty B G, Hawthorne M F, Varadarajan A, Williams L E, Curtis F L, Knobler C B, Beatty J D, Shively J E. Proc Natl Acad Sci U S A. 1991;88:3387–3391. doi: 10.1073/pnas.88.8.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawthorne M F, Maderna A. Chem Rev. 1999;99:3421–3434. doi: 10.1021/cr980442h. [DOI] [PubMed] [Google Scholar]
- 6.Genady A R, Tan J, El-Zaria M E, Zlitni A, Janzen N, Valliant J F. J Organomet Chem. 2015;798:278–288. doi: 10.1016/j.jorganchem.2015.10.030. [DOI] [Google Scholar]
- 7.Gómez-Vallejo V, Vázquez N, Gona K B, Puigivila M, González M, Sebastián E S, Martin A, Llop J. J Labelled Compd Radiopharm. 2014;57:209–214. doi: 10.1002/jlcr.3159. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese G, Daou A, Barbu E, Tsibouklis J. Drug Discovery Today. 2018;23:63–75. doi: 10.1016/j.drudis.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Khalil A, Ishita K, Ali T, Tjarks W. Future Med Chem. 2013;5:677–692. doi: 10.4155/FMC.13.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva A F F, Seixas R S G R, Silva A M S, Coimbra J, Fernandes A C, Santos J P, Matos A, Rino J, Santos I, Marques F. Org Biomol Chem. 2014;12:5201–5211. doi: 10.1039/c4ob00644e. [DOI] [PubMed] [Google Scholar]
- 11.Lee C-H, Jin G F, Yoon J H, Jung Y J, Lee J-D, Cho S, Nakamura H, Kang S O. Tetrahedron Lett. 2008;49:159–164. doi: 10.1016/j.tetlet.2007.10.145. [DOI] [Google Scholar]
- 12.Genady A R. Eur J Med Chem. 2009;44:409–416. doi: 10.1016/j.ejmech.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Issa F, Kassiou M, Rendina L M. Chem Rev. 2011;111:5701–5722. doi: 10.1021/cr2000866. [DOI] [PubMed] [Google Scholar]
- 14.Couto M, García M F, Alamón C, Cabrera M, Cabral P, Merlino A, Teixidor F, Cerecetto H, Viñas C. Chem – Eur J. 2018;24:3122–3126. doi: 10.1002/chem.201705181. [DOI] [PubMed] [Google Scholar]
- 15.Neumann W, Xu S, Sárosi M B, Scholz M S, Crews B C, Ghebreselasie K, Banerjee S, Marnett L J, Hey-Hawkins E. ChemMedChem. 2016;11:175–178. doi: 10.1002/cmdc.201500199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii S, Ohta K, Goto T, Kagechika H, Endo Y. Bioorg Med Chem. 2009;17:344–350. doi: 10.1016/j.bmc.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Ohta K, Goto T, Fujii S, Suzuki T, Ohta S, Endo Y. Bioorg Med Chem. 2008;16:8022–8028. doi: 10.1016/j.bmc.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Selg C, Neumann W, Lönnecke P, Hey-Hawkins E, Zeitler K. Chem – Eur J. 2017;23:7932–7937. doi: 10.1002/chem.201700209. [DOI] [PubMed] [Google Scholar]
- 19.Tsang M Y, Viñas C, Teixidor F, Planas J G, Conde N, SanMartin R, Herrero M T, Domínguez E, Lledós A, Vidossich P, et al. Inorg Chem. 2014;53:9284–9295. doi: 10.1021/ic5013999. [DOI] [PubMed] [Google Scholar]
- 20.Estrada J, Lavallo V. Angew Chem, Int Ed. 2017;56:9906–9909. doi: 10.1002/anie.201705857. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Jin G-X. Chem – Eur J. 2005;11:5758–5764. doi: 10.1002/chem.200500280. [DOI] [PubMed] [Google Scholar]
- 22.Axtell J C, Kirlikovali K O, Djurovich P I, Jung D, Nguyen V T, Munekiyo B, Royappa A T, Rheingold A L, Spokoyny A M. J Am Chem Soc. 2016;138:15758–15765. doi: 10.1021/jacs.6b10232. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Tong X, Yan H, Lu C, Zhao Q, Huang W. Chem – Eur J. 2016;22:17282–17290. doi: 10.1002/chem.201603340. [DOI] [PubMed] [Google Scholar]
- 24.Nghia N V, Oh J, Jung J, Lee M H. Organometallics. 2017;36:2573–2580. doi: 10.1021/acs.organomet.7b00139. [DOI] [Google Scholar]
- 25.Bae H J, Chung J, Kim H, Park J, Lee K M, Koh T-W, Lee Y S, Yoo S, Do Y, Lee M H. Inorg Chem. 2014;53:128–138. doi: 10.1021/ic401755m. [DOI] [PubMed] [Google Scholar]
- 26.Berksun E, Nar I, Atsay A, Özçeşmeci I, Gelir A, Hamuryudan E. Inorg Chem Front. 2018;5:200–207. doi: 10.1039/C7QI00608J. [DOI] [Google Scholar]
- 27.Neirynck P, Schimer J, Jonkheijm P, Milroy L-G, Cigler P, Brunsveld L. J Mater Chem B. 2015;3:539–545. doi: 10.1039/C4TB01489H. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J, Jiang S, Chen Y, Chen X, Yang Q, Yin C. Polyhedron. 2018;142:105–109. doi: 10.1016/j.poly.2017.12.026. [DOI] [Google Scholar]
- 29.Mourier N S, Eleuteri A, Hurwitz S J, Tharnish P M, Schinazi R F. Bioorg Med Chem. 1999;7:2759–2766. doi: 10.1016/S0968-0896(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 30.Tanui H K, Hao E, Ihachi M I, Fronczek F R, Smith K M, Vicente G H. J Porphyrins Phthalocyanines. 2011;15:412–420. doi: 10.1142/S1088424611003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao D, Zhang J, Xie Z. J Am Chem Soc. 2015;137:9423–9428. doi: 10.1021/jacs.5b05426. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Zhang J, Xie Z. J Am Chem Soc. 2015;137:13938–13942. doi: 10.1021/jacs.5b09074. [DOI] [PubMed] [Google Scholar]
- 33.Zhao D, Xie Z. Coord Chem Rev. 2016;314:14–33. doi: 10.1016/j.ccr.2015.07.011. [DOI] [Google Scholar]
- 34.Alekseyeva E S, Batsanov A S, Boyd L A, Fox M A, Hibbert T G, Howard J A K, MacBride J A H, Mackinnon A, Wade K. Dalton Trans. 2003:475–482. doi: 10.1039/B209931D. [DOI] [Google Scholar]
- 35.Weber L, Kahlert J, Brockhinke R, Böhling L, Brockhinke A, Stammler H-G, Neumann B, Harder R A, Fox M A. Chem – Eur J. 2012;18:8347–8357. doi: 10.1002/chem.201200390. [DOI] [PubMed] [Google Scholar]
- 36.Clarke P A, Zaytzev A V, Whitwood A C. Tetrahedron Lett. 2007;48:5209–5212. doi: 10.1016/j.tetlet.2007.05.141. [DOI] [Google Scholar]
- 37.Clarke P A, Ermanis K. Curr Org Chem. 2013;17:2025–2037. doi: 10.2174/13852728113179990095. [DOI] [Google Scholar]
- 38.Elinson M N, Ryzhkov F V, Korolev V A, Egorov M P. Heterocycl Commun. 2016;22:11–15. doi: 10.1515/hc-2015-0232. [DOI] [Google Scholar]
- 39.Li J J, editor. C–H Bond Activation in Organic Synthesis. New York: CRC Press; 2015. [Google Scholar]
- 40.Dixneuf P H, Doucet H, editors. C–H Bond Activation and Catalytic Functionalization I and II. Berlin: Springer; 2016. [Google Scholar]
- 41.Murakami K, Yamada S, Kaneda T, Itami K. Chem Rev. 2017;117:9302–9332. doi: 10.1021/acs.chemrev.7b00021. [DOI] [PubMed] [Google Scholar]
- 42.Shang R, Ilies L, Nakamura E. Chem Rev. 2017;117:9086–9139. doi: 10.1021/acs.chemrev.6b00772. [DOI] [PubMed] [Google Scholar]
- 43.Terrier F. Modern Nucleophilic Aromatic Substitution. Weinheim, Germany: Wiley-VCH; 2013. [Google Scholar]
- 44.Charushin V, Chupakhin O. Metal Free C-H Functionalization of Aromatics Nucleophilic Displacement of Hydrogen. In: Maes B U W, Cossy J, Polanc S, editors. Topics in Heterocyclic Chemistry. Switzerland: Springer; 2014. [Google Scholar]
- 45.Chupakhin O N, Charushin V N. Tetrahedron Lett. 2016;57:2665–2672. doi: 10.1016/j.tetlet.2016.04.084. [DOI] [Google Scholar]
- 46.Błaziak K, Danikiewicz W, Makosza M. J Am Chem Soc. 2016;138:7276–7281. doi: 10.1021/jacs.5b13365. [DOI] [PubMed] [Google Scholar]
- 47.Kiriazis A, Aumüller I B, Arnaudova R, Brito V, Rüffer T, Lang H, Silvestre S M, Koskinen P J, Yli-Kauhaluoma J. Org Lett. 2017;19:2030–2033. doi: 10.1021/acs.orglett.7b00588. [DOI] [PubMed] [Google Scholar]
- 48.Chupakhin O N, Charushin V N. Pure Appl Chem. 2017;89:1195–1208. doi: 10.1515/pac-2017-0108. [DOI] [Google Scholar]
- 49.Sheldon R A, Arends I, Hanefeld U. Green Chemistry and Catalysis. Weinheim, Germany: John Wiley & Sons; 2007. [Google Scholar]
- 50.Mulvihill M J, Beach E S, Zimmerman J B, Anastas P T. Annu Rev Environ Resour. 2011;36:271–293. doi: 10.1146/annurev-environ-032009-095500. [DOI] [Google Scholar]
- 51.Lancaster M. Green Chemistry. An Introductory Text. 2nd ed. Cambridge, UK: RSC Publishing; 2010. [Google Scholar]
- 52.Anastas P, Eghbali N. Chem Soc Rev. 2010;39:301–312. doi: 10.1039/b918763b. [DOI] [PubMed] [Google Scholar]
- 53.Dicks A P, Hent A. SpringerBriefs in Molecular Science. 2015: Springer; Green Chemistry Metrics; pp. 17–44. [Google Scholar]
- 54.Prokhorov A M, Hofbeck T, Czerwieniec R, Suleymanova A F, Kozhevnikov D N, Yersin H. J Am Chem Soc. 2014;136:9637–9642. doi: 10.1021/ja503220w. [DOI] [PubMed] [Google Scholar]
- 55.Prokhorov A M, Slepukhin P A, Rusinov V L, Kalinin V N, Kozhevnikov D N. Chem Commun. 2011;47:7713–7715. doi: 10.1039/C1CC12230D. [DOI] [PubMed] [Google Scholar]
- 56.Galliamova L A, Varaksin M V, Chupakhin O N, Slepukhin P A, Charushin V N. Organometallics. 2015;34:5285–5290. doi: 10.1021/acs.organomet.5b00736. [DOI] [Google Scholar]
- 57.Varaksin M, Moseev T, Chupakhin O, Charushin V, Trofimov B. Org Biomol Chem. 2017;15:8280–8284. doi: 10.1039/c7ob01999h. [DOI] [PubMed] [Google Scholar]
- 58.Zubenko D, Kirilyuk I, Roschchupkina G, Zhurko I, Reznikov V, Marque S R A, Bagryanskaya E. Helv Chim Acta. 2006;89:2341–2353. doi: 10.1002/hlca.200690218. [DOI] [Google Scholar]
- 59.Edeleva M V, Parkhomenko D A, Morozov D A, Dobrynin S A, Trofimov D G, Kanagatov B, Kirilyuk I A, Bagryanskaya E G. J Polym Sci, Part A: Polym Chem. 2014;52:929–943. doi: 10.1002/pola.27071. [DOI] [Google Scholar]
- 60.Varaksin M V, Utepova I A, Chupakhin O N, Charushin V N. J Org Chem. 2012;77:9087–9093. doi: 10.1021/jo301618b. [DOI] [PubMed] [Google Scholar]
- 61.You Y, Kim H S, Bae I H, Lee S G, Jee M H, Keum G, Jang S K, Kim B M. Eur J Med Chem. 2017;125:87–100. doi: 10.1016/j.ejmech.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 62.Tsay S C, Lin S Y, Huang W C, Hsu M H, Hwang K C, Lin C C, Horng J C, Chen I C, Hwu J R, Shieh F K. Molecules. 2016;21:No. 228. doi: 10.3390/molecules21020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelazeem A H, El-Saadi M T, Safi El-Din A G, Omar H A, El-Moghazy S M. Bioorg Med Chem. 2017;25:665–676. doi: 10.1016/j.bmc.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Shen Y, Wu X, Tu X, Wang G-X. Eur J Med Chem. 2018;143:958–969. doi: 10.1016/j.ejmech.2017.11.100. [DOI] [PubMed] [Google Scholar]
- 65.Ali I, Lone M N, Aboul-Enein H Y. Med Chem Commun. 2017;8:1742–1773. doi: 10.1039/C7MD00067G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popescu A-R, Musteti A D, Ferrer-Ugalde A, Viñas C, Núñez R, Teixidor F. Chem – Eur J. 2012;18:3174–3184. doi: 10.1002/chem.201102626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, copies of the 1H, 13C, 11B NMR spectra and X-ray diffraction studies.