ABSTRACT
NADPH oxidase AtrbohD plays very important roles in modulating many cellular processes through production of signal molecules reactive oxygen species in Arabidopsis. However, whether it regulates the response to waterlogging stress is unclear. In this report, we showed that expression of AtrbohD was markedly induced by waterlogging stress, and mutation in AtrbohD led to clear sensitivity of Arabidopsis plants to waterlogging stress. Moreover, waterlogging-promoted increases in alcohol dehydrogenase (ADH) activity, ADH1 expression and H2O2 accumulation were significantly attenuated in two mutant lines of AtrbohD. These results indicate that AtrbohD is required for Arabidopsis tolerance to waterlogging stress. Besides, GUS staining experiments revealed that disruption of small G protein ROP2 encoding gene evidently suppressed the increase of AtrbohD expression while defect of AtrbohD did not prominently affect the abundance enhancements of ROP2 transcripts under waterlogged conditions. Together, these data suggest that AtrbohD functions downstream of ROP2 to positively regulate the response to waterlogging stress in Arabidopsis.
KEYWORDS: AtrbohD, watterlogging stress, ROP2, reactive oxygen species, Arabidopsis
Introduction
Waterlogging is one of the most important environmental stresses for plants that typically results from excessive rainfall and flooding. It causes oxygen deficiency, remarkable reduction in stomata conductance and root hydraulic conductivity, and strong inhibition of photosynthesis, thereby significant suppression of plant growth.1 Under oxygen deprivation conditions, plants suffer from energy crisis, which leads to overproduction of reactive oxygen species (ROS) including hydrogen peroxide (H2O2) and superoxide.2,3 During evolution, plants have developed effective adaptive strategies to cope with hypoxic stresses. One of the key adaptation responses is the activation of fermentative metabolism to generate ATP, which involves in the enhancements of alcohol dehydrogenase (ADH) activity and of ADH1 expression levels in Arabidopsis.2
To date, many components including RHO-like small G protein ROP2 and ROS have been demonstrated to mediate the responses to oxygen deficiency in Arabidopsis.4,5 Under hypoxia conditions, ROP2 is activated, and further stimulates the production of H2O2. H2O2 then promotes the increases in the expression level of ADH1 and the activity of ADH.4 H2O2 also play roles in responding to oxygen starvation stress through affecting ethylene signaling.6
Evidence indicated that NADPH oxidase is an important source of H2O2. In Arabidopsis, 10 NADPH oxidase genes (AtrbohA-J) have been identified. Among these, AtrbohD and AtrbohF have been addressed to function in adaptation to hypoxic stress through regulating the expression of multiple hypoxia-inducible genes and Ca2+ signaling, and the action of AtrbohD is particularly important.7–9 AtrbohD is also activated by the hypoxia responsive universal stress protein 1 (HRU1), which interacts with both ROP2 and AtrbohD in Arabidopsis under low oxygen conditions.5 Although AtrbohD plays an essential role in acclimation to oxygen deficiency, whether it exerts effects under waterlogged conditions is unclear. The underlying mechanism also remains to be elucidated. Here, we provided evidence that AtrbohD functions downstream of ROP2, and positively regulates the generation of H2O2 and the increases in expression of ADH1 and the activity of ADH in response to waterlogging stress in Arabidopsis.
Results
The expression of atrbohD was increased under waterlogged conditions
To determine whether AtrbohD plays a role in response to waterlogging stress, the expression pattern of AtrbohD under the stress was examined in Arabidopsis wild type (WT) seedlings using quantitative PCR (QPCR) methods. Treatment with waterlogging significantly elevated the transcriptional levels of AtrbohD in a time-dependent manner, and the transcripts of AtrbohD were most abundant at 24 h, more than 4 folds of the untreated control (Figure 1). The results indicate that AtrbohD was upregulated under waterlogged conditions.
Figure 1.
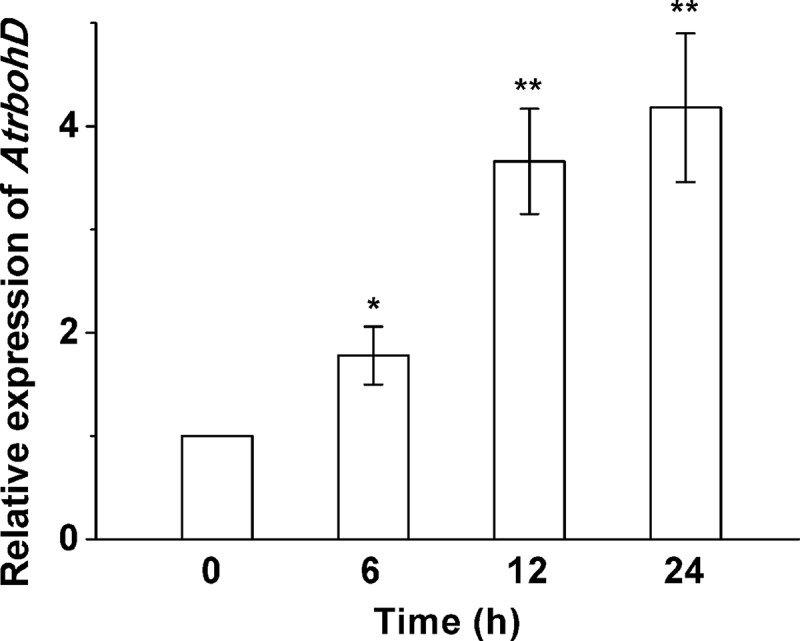
Expression patterns of AtrbohD in response to waterlogging stress. Ten-day-old Arabidopsis WT seedlings grown on MS medium were waterlogged for 6, 12 and 24 h, respectively. QPCR experiments were performed to detect the expression of AtrbohD. Actin2 was used as an internal control.
Null mutant atrbohd1 and atrbohd2 were sensitive to waterlogging stress
Now that waterlogging remarkably promoted the expression of AtrbohD, we next investigated whether the mutants of AtrbohD and the transgenic plants overexpressing AtrbohD differ from WT in responding to waterlogging stress. Two null T-DNA mutants atrbohD1 (SALK_120299) and atrbohD2 (SALK_083046) were identified, and two transgenic lines overexpressing AtrbohD driven by 35S promoter in WT, named OE1 and OE2, were obtained. Expression analysis showed that the transcripts of AtrbohD was eliminated in these mutants (Figure 2A), and was enriched in the overexpression lines (Figure 2B). Then, phenotypes of atrbohD1, atrbohD2, OE1 and OE2 plants under waterlogged conditions were analyzed. All the seedlings grew well in nutrient soil under normal conditions. However, growth of the plants was clearly inhibited after 4 d of waterlogging, followed by recovery for another 10 d. Noteworthily, the two mutants displayed more serious growth suppression than WT, OE1 and OE2. Most leaves from atrbohD1 and atrbohD2 were withered and died while majority of leaves from WT, OE1 and OE2 were still green and alived; and the two overexpression plants grew observably better than WT after waterlogging treatment followed by recovery (Figure 2F). Survival rate results indicated that about 33%, 40%, 87%, 100% and 100% seedlings survived in atrbohD1, atrbohD2, WT, OE1 and OE2 respectively under waterlogging conditions (Figure 2G). These data suggest that atrbohD1 and atrbohD2 are sensitive to waterlogging stress, and AtrbohD positively modulates flooding tolerance in Arabidopsis.
Figure 2.
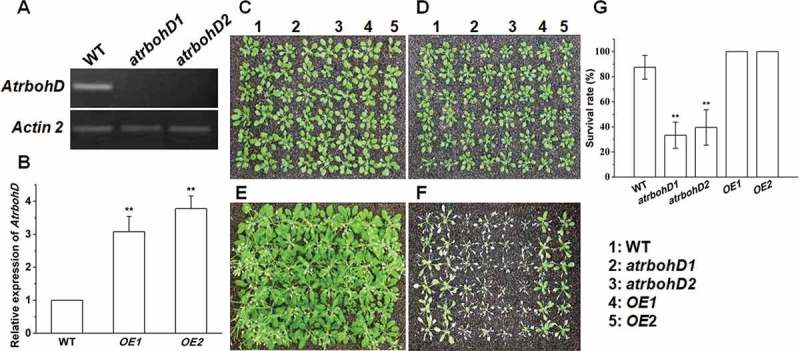
Effects of waterlogging on growth of WT, the mutants and overexpressors of AtrbohD. (A) Expression of AtrbohD in atrbohD1 and atrbohD2 plants. (B) Relative transcriptional levels of AtrbohD in two transgenic lines overexpressing AtrbohD in WT (OE1 and OE2). Actin2 was applied as an internal control. (C) and (D) Growth performances of various plants before waterlogging treatment. (E) The plants in C grew under normal condition for 14 d. (F) The plants in D were waterlogged for 4 d and grown for another 10 d for recovery. (G) Survival rate of various plants as described in F (n ≥ 30).
Waterlogging-triggered increases in ADH activity and ADH1 expression were attenuated in atrbohd1 and atrbohd2
Changes in ADH activity and expression of ADH genes are key indicators for measuring plant tolerance to oxygen deprivation. We therefore compared the differences in these parameters among WT, atrbohD1 and atrbohD2 under waterlogged conditions. Treatments with waterlogging markedly enhanced the activities of ADH in WT in a time-dependent manner. However, the enhanced effects of waterlogging on ADH activity in atrbohD1 and atrbohD2 were prominently arrested at 12 h and 24 h post treatment (Figure 3A). Likewise, waterlogging significantly elevated the expression levels of ADH1 in WT in a time-dependent pattern. In contrast, the increments in transcriptional abundances of ADH1 in atrbohD1 and atrbohD2 were remarkably attenuated (Figure 3B). These findings imply that AtrbohD regulates ADH activity and ADH1 expression in responding to waterlogging stress.
Figure 3.
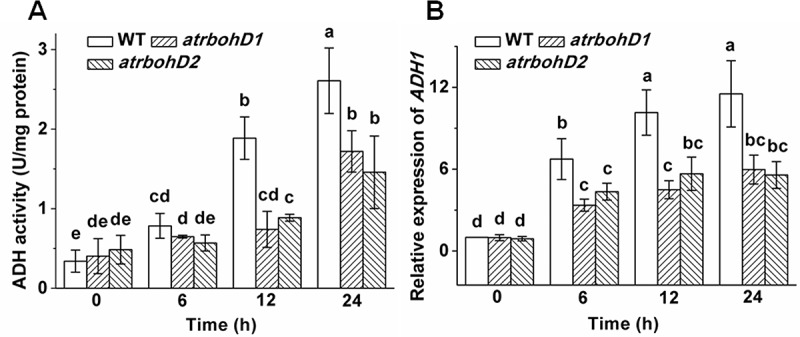
Effects of waterlogging on the ADH activities and expression of ADH1 in WT and the AtrbohD mutants. Changes in ADH activities (A) and ADH1 expression (B) in WT, atrbohD1 and atrbohD2.
Waterlogging-induced H2O2 accumulation was arrested in atrbohD1 and atrbohD2
AtrbohD has been addressed to be an important generator of ROS. Hence, we monitored the contents of H2O2 in WT and the two mutants of AtrbohD after waterlogging treatment. The results revealed that the levels of H2O2 in atrbohD1 and atrbohD2 were similar to those in WT under untreated conditions. Waterlogging resulted in clear increases in H2O2 contents at 12 h and 24 h. However, H2O2 levels were not significantly elevated in the two mutants under waterlogged conditions for the same time periods. These results indicate that H2O2 derived from AtrbohD is involved in the acclimation to flooding stress in Arabidopsis (Figure 4).
Figure 4.
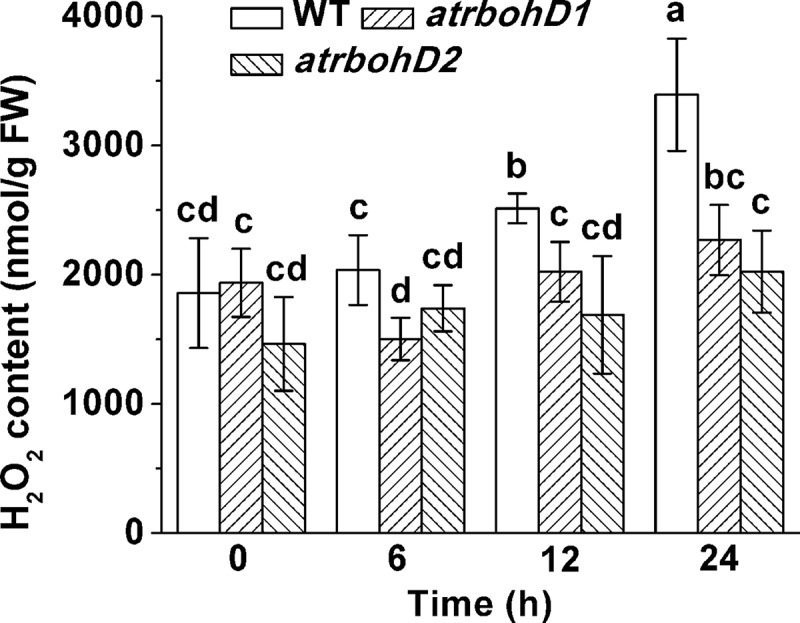
Changes in H2O2 contents of the WT and the mutant plants in response to waterlogging stress.
Waterlogging stress-stimulated increases in ADH activity and ADH1 expression were abolished in ROP2 mutant
ROP2 has been demonstrated to participate in adaptation to oxygen deficiency in plants. Accordingly, we studied whether ROP2 regulates ADH activity and ADH1 abundances under waterlogged conditions. Waterlogging treatment caused markedly elevation in the ADH activity and ADH1 expression levels in WT in a time dependent manner. However, the enhancements in these parameters were evidently inhibited in the line expressing a dominant negative form of ROP2 [35S::DN-rop2]4 after exposure to waterlogging (Figure 5). These data hint that ROP2 is required for Arabidopsis response to waterlogging stress.
Figure 5.
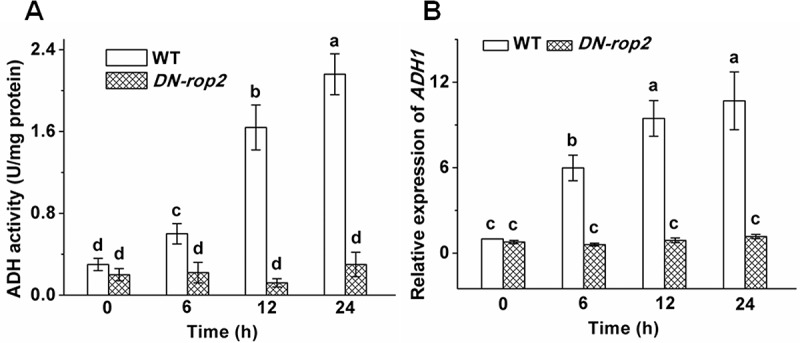
Effects of waterlogging on the ADH activities and expression of ADH1 in WT and DN-rop2. Changes in ADH activities (A) and ADH1 expression (B) of WT, atrboD1 and atrboD2 under waterlogged conditions.
AtrbohD may act downstream of ROP2 under waterlogging stress
To gain insight into the regulatory relationship of ROP2 and AtrbohD in acclimation to waterlogging stress, we generated multiple transgenic lines expressing AtrbohDpro::GUS in backgrounds of WT and DN-ROP2, and expressing ROP2pro::GUS in contexts of WT and atrbohD1. GUS staining results showed that waterlogging treatment clearly enhanced the acitivity of AtrbohD promoter in WT, but not in DN-ROP2 plants (Figure 6A, B). Waterlogging stress prominently increased the GUS activity of ROP2 promoter in WT and atrbohD1. However, no marked differences in the increment of the GUS activity were observed among WT and atrbohD1 (Figure 6C, D). Together, these results suggest that AtrbohD acts downstream of ROP2 in response to waterlogging stress in Arabidopsis.
Figure 6.
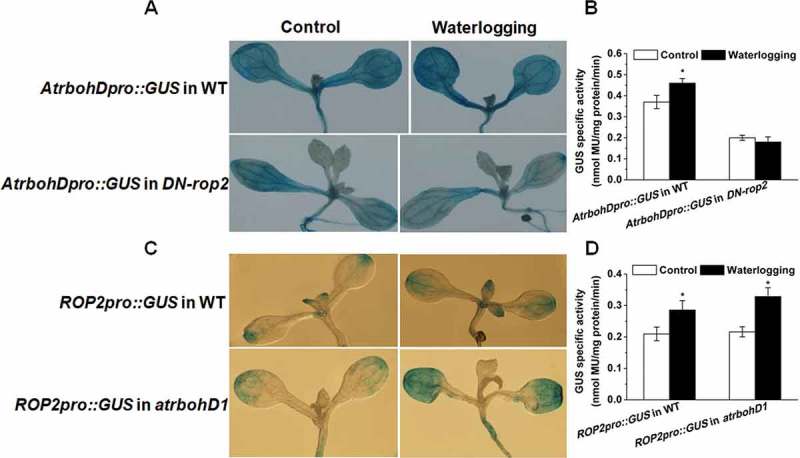
Effects of waterlogging on the GUS activities in various seedlings. (A, B) Changes in AtrbohD promoter GUS activities in WT (AtrbohDpro::GUS in WT) and DN-rop2 seedlings (AtrbohDpro::GUS in DN-rop2). (C, D) Changes in ROP2 promoter GUS activities in WT (ROP2pro::GUS in WT) and atrbohD1 (ROP2pro::GUS in atrbohD1).
Discussion
NADPH oxidase AtrbohD has been documented to modulate many cellular processes such as pathogen resistance, ABA-promoted stomatal closure, salt tolerance, root development, and so on.10–14 It also plays important roles in adaptation to oxygen deprivation in Arabidopsis.5,8,9 Although plants will suffer from hypoxia stress under waterlogging condition, effects of waterlogging on plant growth are different from those of oxygen starvation stress.1 To date, whether AtrbohD regulates Arabidopsis response to waterlogging stress is unknown. In this report, we found that the expression level of AtrbohD was clearly elevated under waterlogged conditions (Figure 1), and two mutant atrbohD1 and atrbohD2 were sensitive to waterlogging stress (Figure 2). Furthermore, waterlogging-induced increases in ADH activity, ADH1 expression and H2O2 generation were evidently arrested in the mutants of AtrbohD relative to WT (Fig. 3, 4). These findings indicate that AtrbohD is essential for Arabidopsis tolerance to waterlogging stress.
Evidence shows that ROP2 regulates ROS production, and HRU1 can interact with both ROP2 and AtrbohD under oxygen deficiency in Arabidopsis.4,5 However, whether ROP2 modulates AtrbohD in responding to hypoxic stress or waterlogging stress is unclear. In this study, we demonstrated that ROP2 was involved in the modulation of waterlogging-stimulated increases of ADH activity and ADH1 expression (Figure 5), implying that ROP2 is of great importance in response to waterlogging stress in Arabidopsis. Further, we observed that loss of function of ROP2 prominently hindered the rise of AtrbohD expression while mutation of AtrbohD failed to inhibit the acceleration of ROP2 promoter activity under waterlogged conditions (Figure 6). Collectively, these data suggest that AtrbohD acts downstream of ROP2 to positively regulate the tolerance to waterlogging stress in Arabidopsis. The mechanism of ROP2 modulating AtrbohD under waterlogged conditions will be thoroughly investigated in the future.
Plant materials and growth
Arabidopsis thaliana wild type (WT) (Columbia 0, Col-0), single mutants atrbohD1 (SALK_120299)12 and atrbohD2 (SALK_083046) of AtrbohD gene, as well as the line expressing a dominant negative form of ROP2 gene [35S::DN-rop2]4 (kindly provided by Ying Fu from China Agriculural University) were applied. Seeds of these plants were sterilized using 0.1% HgCl2, washed clearly with sterile ddH2O, and planted on solid Murashige-Skoog (MS) medium. After germination for about two weeks, the seedlings were grown in a growth chamber.12
Analysis of gene expression by quantitative real-time PCR
Ten-day-old seedlings were waterlogged with ddH2O for indicated hours in figure legends. Total RNA was extracted and the cDNA was generated according to the method described by Ma et al.12 The QPCR experiments were conducted, and Actin2 was used as the internal control.
Phenotype analysis
Twelve-day-old seedlings grown in MS medium were transferred to nutrient soil for another two weeks. Then, the plants were waterlogged with ddH2O for 4 days and grown for another 10 days for recovery. Photographs were taken and the survival rate was calculated.
Measurement of ADH activity
Ten-day-old seedlings were waterlogged with ddH2O for indicated hours. ADH activity was assayed as described previously.9
Assay of H2O2 content
Ten-day-old seedlings were waterlogged with ddH2O for indicated hours. H2O2 content was measured using peroxidase (POD)-coupled method following Ma et al.12
Aanalysis of β-glucuronidase (GUS) expression and GUS activity
The promoter regions of AtrbohD14 and ROP2 (1600 bp upstream of the initiation codon) were respectively amplified, sequenced, and cloned into the GUS gene-contained pCAMBIA1381 vector. Transgenic Arabidopsis plants expressing AtrbohDpro::GUS or ROP2pro::GUS were obtained. GUS expression of seedlings was determined as described previously.14 GUS activities of seedlings were monitored following Blázquez.15
Statistical analysis
Each experiment was repeated at least three times. Data are mean ± SE. Statistical analyses were carried out by student’s t test or one-way ANOVA and Tukey’s honestly significant difference test (Tukey’s HSD test) (P < 0.05). For student’s t test, the single and double asterisks above the error bars in figures indicate P ≤ 0.05 and P ≤ 0.01, respectively. For Tukey’s HSD test, different lowercase letters above the error bars in figures show significant differences among different plant lines, and among the control and various treatments.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31070239 and 30970235) and by the Program for young backbone teachers in universities of Henan Province (2016GGJS-024).
Disclosure of potential conflicts of interest
The authors declare that there are no potential conflicts of interest to disclose.
References
- 1.Ashraf MA. Waterlogging stress in plants: A review. Afr J Agric Res. 2012;7(13):1976–1981. [Google Scholar]
- 2.Gibbs J, Greenway H.. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- 3.Bailey-Serres J, Chang R. Sensing and signaling in response to oxygen deprivation in plants and other organisms. Ann Bot. 2005;96:507–518. doi: 10.1093/aob/mci206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science. 2002;296:2026–2028. doi: 10.1126/science.1071505. [DOI] [PubMed] [Google Scholar]
- 5.Gonzali S, Loreti E, Cardarelli F, Novi G, Parlanti S, Pucciariello C, Bassolino L, Banti V, Licausi F, Perata P. Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat Plants. 2015;1(11):15151. doi: 10.1038/nplants.2015.176. [DOI] [PubMed] [Google Scholar]
- 6.Yang CY. Hydrogen peroxide controls transcriptional responses of ERF73/HRE1 and ADH1 via modulation of ethylene signaling during hypoxic stress. Planta. 2014;239:877–885. doi: 10.1007/s00425-013-2020-z. [DOI] [PubMed] [Google Scholar]
- 7.Pucciariello C, Parlanti S, Banti V, Novi G, Perata P. Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol. 2012;159:184–196. doi: 10.1104/pp.111.191122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CY, Hong CP. The NADPH oxidase RbohD is involved in primary hypoxia signalling and modulates expression of hypoxia-inducible genes under hypoxic stress. Environ Exp Bot. 2015;115:63–72. doi: 10.1016/j.envexpbot.2015.02.008. [DOI] [Google Scholar]
- 9.Liu B, Sun L, Ma L, Hao FS, Both Atrboh D. AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep. 2017;36(6):947–957. doi: 10.1007/s00299-017-2128-x. [DOI] [PubMed] [Google Scholar]
- 10.Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. PNAS. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma LY, Zhang H, Sun LR, Jiao YH, Zhang GZ, Miao C, Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+homeostasis in Arabidopsis under salt stress. J Exp Bot. 2012;63:305–317. doi: 10.1093/jxb/err280. [DOI] [PubMed] [Google Scholar]
- 13.Jiao YH, Sun LR, Song YL, Wang LM, Liu LP, Zhang LY, Liu B, Li N, Miao C, Hao F. AtrbohD and AtrbohF positively regulate abscisic acid inhibited primary root growth by affecting Ca2+ signaling and auxin response of roots in Arabidopsis. J Exp Bot. 2013;64:4183–4192. doi: 10.1093/jxb/ert228. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Sun LR, Zhang LY, Song YL, Hu PP, Li C, Hao FS. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta. 2015;241:591–602. doi: 10.1007/s00425-014-2204-1. [DOI] [PubMed] [Google Scholar]
- 15.Blázquez M. Quantitative GUS activity assay of plant extracts. Cold Spring Harb Protoc. 2007;2007(2):pdb.prot4690. [DOI] [PubMed] [Google Scholar]


