ABSTRACT
Cytokinin (CK) is one of key phytohormones for de-differentiation and de novo organogenesis in plants. During the CK-mediated organogenesis not only genes in CK homeostasis, perception and signal transduction, but also factors regulating basic transcription, splicing and chromatin remodeling contribute to coordinate a sequence of events leading to formation of new organs. We have found that silencing of RNA polymerase II CTD-phosohatase-like 4 (CPL4RNAi) in Arabidopsis induces CK-oversensitive de novo shoot organogenesis (DNSO) from roots, partly by early activation of transcription factors such as WUSCHEL and SHOOT MERISTEMLESS during pre-incubation on callus induction media. Here we show that a cluster of thalianol-biogenesis genes is highly expressed in the CPL4RNAi during DNSO, implying involvement of CPL4 in transcriptional regulation of the thalianol pathway in DNSO.
KEYWORDS: Arabidopsis, de novo shoot organogenesis, regeneration, Pol II-CTD phosphatase-like 4
Plant cells have remarkable plasticity in that they can de-differentiate (callus formation) and re-differentiate into a whole plant through de novo organogenesis of shoot and root. This regenerative capacity has been widely explored in the field of biotechnology and agriculture. De novo shoot/root organogenesis is orchestrated by an interplay between two major phytohormones auxin and cytokinin (CK).1–3 Incubating excised plant tissue (explant) on media with auxin induces de novo root formation. Presence of CK together with high concentration of auxin (i.e. callus induction media, CIM) results in formation of callus through the same pathway for lateral root primordia development.4 Incubating explants on media with high CK/auxin ratio (i.e. shoot induction media, SIM) after pre-incubation on CIM triggers de novo shoot organogenesis (DNSO). Consequently, many genes involved in CK-sensing,5,6 signaling,6,7 and CK-responsive transcription factors,8-12 have been implicated in the regulation of DNSO. In addition, genes involved in basic transcriptional and post-transcriptional regulation have been identified in callus formation and DNSO processes;13,14 for example, TATA-binding protein complex associated factor 12B (TAF12B) as CYTOKININ HYPERSENSITIVE 1 (CKH1);15 CHD3-type SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling factor, chromatin remodeler PICKEL (PKL) as CYTOKININ HYPERSENSITIVE 2 (CKH2);16 a DEAH-box RNA helicase involved in pre-mRNA splicing as ROOT INITIATION DEFECTIVE 1 (RID1);17,18 and a small nuclear RNA (snRNA) activator protein complex subunit SNAP50 homolog as SHOOT REDIFFERENTIATION DEFECTIVE 2 (SRD2).19,20
RNA polymerase II CTD-phosphatase-like 4 (CPL4), a homolog of yeast TFIIF-interacting CTD-phosphatase FCP1, is an essential regulator of Pol II-CTD phosphoregulation in Arabidopsis. We have shown that CPL4 suppresses xenobiotic stress responsive genes21 and mediates snRNA transcription termination/3ʹ-end formation.22 In our recent report,23 we showed that silencing of CPL4 induces CK-oversensitive DNSO from Arabidopsis roots. DNSO from CPL4 knock-down (CPL4RNAi) root explants are more vigorous than wild type (WT) in standard SIM condition. Moreover, CPL4RNAi root explants can show DNSO even with 1/10 concentration of CK (0.05 mg/L 2iP, low-CK) in SIM, while WT cannot. RNA-seq analysis on WT (W) and CPL4RNAi (C4) root explants on SIM with (+) or without (-) low-CK at 3-day-post-transfer identified 2,838 genes significantly up-regulated (> 1.5-fold) in CPL4RNAi on low-CK SIM [C4(+)] compared to WT on SIM without CK [W(-)] (referred to as C4(+)UT, C4(+)-Upregulated Transcripts). We further classified them into four classes based on expression pattern in each sample; class I genes require both CPL4RNAi and low-CK to be up-regulated; class II genes can be up-regulated solely by CPL4RNAi; class III genes are low-CK-responsive; class IV genes can be up-regulated either by CPL4RNAi or low-CK. Transcription factors essential for DNSO, such as WUSCHEL (WUS), SHOOT MERISTEMLESS (STM) and CUP-SHAPED COTYLEDON 2 (CUC2), were all found in class II, indicating that these gene expression in CPL4RNAi did not rely on CK in SIM. Class II genes were most abundant (1,594 out of 2,383) and enriched with gene ontologies related to DNA replication and cell cycle regulation, suggesting that CPL4RNAi root explants after pre-incubation on CIM are highly proliferative even without CK in the SIM. RT-qPCR on root explants during pre-incubation on CIM confirmed that indeed WUS, STM and CUC2 are up-regulated by CIM-preincubation, presumably by oversensitivity to low-CK (0.05 mg/L kinetin) in the CIM.
During the course of the analysis, we found that number of genes were induced in C4(-) and in W(+), but induced to higher levels in the C4(+) condition. Because qualitative classification adapted in the above analysis does not highlight this behavior, we also evaluated quantitative behavior of each gene and identified 248 genes (61, 71, 28, 88 genes from class I, II, III and IV, respectively) expressed higher in C4(+) than any other conditions thus possibly contributing to low-CK DNSO. Among them, 26 are transcription factors (GO:0003700), and 14 of them, including ENHANCER OF SHOOT REGENERATION 1 (ESR1),8 are associated with a developmental process GO (Table 1; GO0037502).
Table 1.
Twenty-six transcription factors in the 248 genes highly expressed in low-CK CPL4RNAi.
| AGI | Symbol | Class | Gene description | FPKM |
|||
|---|---|---|---|---|---|---|---|
| W(-) | C4(-) | W(+) | C4(+) | ||||
| DNA binding transcription factor activity (GO:003700); Developmental Process (GO:0032502) | |||||||
| AT5G65640 | bHLH093 | I-a | beta HLH protein 93 | 27.19 | 40.65 | 33.08 | 61.60 |
| AT1G75390 | bZIP44 | IV | basic leucine-zipper 44 | 11.19 | 23.93 | 21.48 | 45.74 |
| AT1G10480 | ZFP5 | IV | zinc finger protein 5 | 9.27 | 17.16 | 17.42 | 38.20 |
| AT5G64060 | NAC103 | IV | NAC domain containing protein 103 | 1.24 | 14.53 | 3.32 | 23.98 |
| AT2G21650 | MEE3 | IV | Homeodomain-like superfamily protein | 1.16 | 3.93 | 3.95 | 23.09 |
| AT4G37750 | ANT | IV | Integrase-type DNA-binding superfamily protein | 3.83 | 9.68 | 14.78 | 22.70 |
| AT5G03790 | HB51 | II | homeobox 51 | 0.56 | 3.70 | 1.18 | 8.21 |
| AT3G50060 | MYB77 | II | myb domain protein 77 | 1.42 | 3.24 | 2.22 | 5.45 |
| AT3G12720 | MYB67 | IV | myb domain protein 67 | 1.88 | 2.99 | 3.14 | 4.88 |
| AT1G12980 | ESR1 | III | Integrase-type DNA-binding superfamily protein | 0.40 | 0.77 | 1.39 | 4.54 |
| AT1G02250 | NAC005 | III | NAC domain containing protein 5 | 0.88 | 0.37 | 2.65 | 3.98 |
| AT5G03680 | PTL | IV | Duplicated homeodomain-like superfamily protein | 0.50 | 2.01 | 1.09 | 3.60 |
| AT1G01060 | LHY | II | Homeodomain-like superfamily protein | 1.01 | 2.24 | 0.74 | 3.44 |
| AT4G17785 | MYB39 | I | myb domain protein 39 | 1.31 | 0.69 | 1.66 | 2.83 |
| DNA binding transcription factor activity (GO:0003700) | |||||||
| AT5G25190 | ESE3 | IV | Integrase-type DNA-binding superfamily protein | 12.76 | 41.18 | 28.78 | 77.40 |
| AT1G14600 | - | II | Homeodomain-like superfamily protein | 3.02 | 15.34 | 4.37 | 23.96 |
| AT3G07340 | - | IV | basic helix-loop-helix (bHLH) DNA-binding superfamily protein | 3.87 | 11.56 | 8.46 | 19.64 |
| AT2G17770 | BZIP27 | II | basic region/leucine zipper motif 27 | 1.62 | 3.10 | 2.52 | 7.42 |
| AT4G39410 | WRKY13 | II | WRKY DNA-binding protein 13 | 0.52 | 4.82 | 0.69 | 7.41 |
| AT2G46130 | WRKY43 | II | WRKY DNA-binding protein 43 | 1.53 | 3.45 | 2.82 | 6.72 |
| AT3G60390 | HAT3 | I | homeobox-leucine zipper protein 3 | 1.81 | 1.62 | 2.39 | 4.94 |
| AT5G11060 | KNAT4 | II | homeobox protein knotted-1-like 4 | 0.85 | 1.91 | 1.19 | 3.50 |
| AT1G80590 | WRKY66 | I | WRKY DNA-binding protein 66 | 1.54 | 1.38 | 2.10 | 3.46 |
| AT2G47520 | ERF71 | II | Integrase-type DNA-binding superfamily protein | 0.48 | 1.71 | 0.57 | 3.19 |
| AT1G75240 | HB33 | III | homeobox protein 33 | 0.23 | 0.11 | 1.10 | 2.10 |
| AT5G03720 | HSFA3 | II | heat shock transcription factor A3 | 0.45 | 0.94 | 0.50 | 1.67 |
W(-), wild type on SIM without 2iP; W(+), wild type on SIM with 0.05 mg/L 2iP; C4(-); CPL4RNAi on SIM without 2iP; C4(+), CPL4RNAi on SIM with 0.05 mg/L 2IP.
Co-expression analysis by ATTED-II identified only one co-expression network consisting of more than 10 genes. The network includes three genes from a gene cluster for synthesizing and metabolizing thalianol, a tricyclic triterpene compound synthesized from 2,3-oxidosqualene (Figure 1a). The genes are oxidosqualene cyclase thalianol synthase 1 (THAS1), cytochrome P450 thalianol Hydroxylase (THAH) and a BADH acyltransferase (ACT), which are the three of four thalianol synthesis/metabolism genes tandemly arranged in an operon-like organization on chromosome 524 (Figure 1b). The missing gene, thalian-diol desaturase (THAD), does not pass the new criteria but shows significantly higher expression in low-CK CPL4RNAi over low-CK WT by 1.38-fold (Figure 1b). Because of the unique gene structure, we also checked expression behavior of 34 potential operon-like gene clusters involving 308 genes in total.25 Only five genes passed the criteria described above; three are from the thalianol cluster, and two are each from independent clusters (AT1G63480 and AT3G16430). Therefore, the thalianol cluster is the sole operon-like cluster over-induced in CPL4RNAi during low-CK DNSO. THAS1 catalyzes the conversion of oxidosqualene to thalianol; THAH is responsible for converting thalianol into thalian-diol, which can be desaturated by THAD.24 Overaccumulation of thalianol or thalian-diol results in shoot dwarf phenotype.24 The thalianol cluster genes have been identified as CK-responsive.26-32 Additionally, ACT is directly bound and regulated by a chromatin remodeler PICKLE/CYTOKININ-HYPERSENSITIVE 2 (PKL/CKH2)33 Mutations in PKL/CKH2 result in CK-hypersensitive greening of callus, indicating a potential interplay between chromatin regulation and CK-sensitivity.14,16
Figure 1.
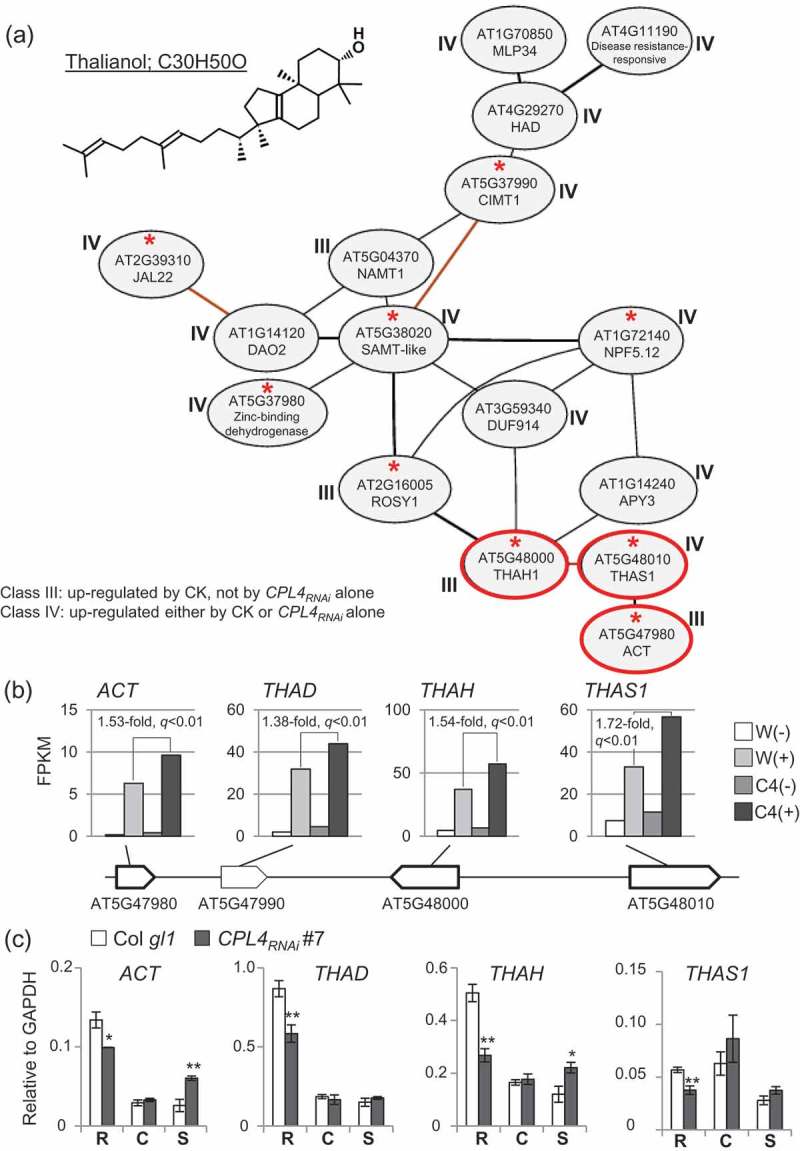
Thalianol biosynthesis-related co-expression network observed among the 248 genes. (a) A microarray-based (Ath-m) co-expression cluster comprised of more than 10 genes among the 248 genes highly expressed in CPL4RNAi during DNSO, detected by NetworkDrawing tool in ATTED-II ver.9.2 (http://atted.jp/). Genes with a red asterisk show up in the same network based on RNA-seq (Ath-r). An associated class is indicated for each gene. The three genes from a thalianol-biosynthesis operon-like regulon on chromosome 5 are circled in red. The chemical structure of thalianol (C30H50O) is shown, drawn by PubChem Sketcher V2.4, based on the CID25229600. Full name of each gene is described in Abbreviations. (b) FPKM values of the four thalianol biosynthesis genes arranged in an operon-like manner on chromosome 5. C4(+)/W(+) fold change values and associated q-values from Cuffdiff are shown. (c) Expression level of each gene relative to GAPDH [2(Cp of GAPDH – Cp of target)] was measured by RT-qPCR. Roots from 10-day-old seedlings (labeled as “R”) were subjected to CIM pre-incubation for 5 days, then moved to SIM containing 0.05 mg/L 2iP (“S”) for 3 days. Root samples right after the CIM pre-incubation were harvested and denoted as “C”. Bars represent standard error of means from biological triplicates. *p < 0.05, **p < 0.01 by one-tailed Student’s t-test between wild type (Col gl1) and each CPL4RNAi line in each condition.
We also examined expression levels of the thalianol cluster genes before and during pre-incubation on CIM. In roots before treatments, expression levels of all four thalianol cluster genes were lower in CPL4RNAi than in wild type (Figure 1c). During CIM pre-incubation, there was substantial decrease in ACT, THAD and THAH expression both in wild type and CPL4RNAi, compared to intact roots. Expression levels of ACT and THAH during low-CK SIM incubation were higher in CPL4RNAi than wild type (Figure 1c), confirming RNA-seq analysis data (Figure 1b). Between CIM pre-incubation and low-CK SIM, increase of ACT and THAH expression was observed in CPL4RNAi but not in wild type (Figure 1c). Such CPL4RNAi -specific re-activation of thalianol pathway genes might play a role in low-CK DNSO, which is also specific to CPL4RNAi. Thalianol pathway intermediates differentially affect roots and shoot development.24 High levels of thalianol in thah1 and thad1 mutants as well as in THAS overexpressing plants resulted in production of longer roots, whereas THAS overexpressing plants showed shoot dwarfism.24 The shoot phenotype was further enhanced by co-expressing THAS and THAD, suggesting thalian-diol and/or desaturated thalian-diol are more active than thalianol.24 Although no functional link between thalianol and DNSO has been made, the inhibitory effect of thalianol to shoot growth could be related to reduction of cell proliferation during DNSO, for example, decline in cycB expression in CPL4RNAi after explants were transferred to low-CK-containing SIM medium.23 Alternatively, thalianol may function as a negative feedback mechanism for DNSO. At molecular level, how CPL4 is involved in thalianol cluster regulation is still unknown, as CPL4RNAi did not show notable transcription initiation or termination defects within the locus. PKL directly binds ACT33 and with PICKLE RELATED 2 (PKR2) it positively regulates expression of ACT .34 Also, PKL has been shown to associate with elongating Pol II co-purified with a transcription elongation factor SPT4.35 It is tempting to speculate that CPL4 as a global Pol II-CTD phosphatase might regulate PKL recruitment to the thalianol cluster via modulating Pol II-CTD phosphorylation of elongating Pol II during DNSO. Taken together, these analyses identified the thalianol biosynthesis cluster as a potential regulator of low-CK DNSO in CPL4RNAi. It will be an attractive target for further dissection of DNSO, as it might mediate interplay of CK, chromatin remodeling and Pol II-CTD phosphoregulation mediated by CPL4.
Funding Statement
This work was supported by the National Science Foundation [MCB0950459]; Texas A and M University [Genomics of Plant Water Use Seed Grant]; U.S. Department of Agriculture [2010-34402-20875]
Acknowledgments
The authors thank Dr. Charles D. Johnson and Dr. Richard Metz (Texas A&M AgriLife Genomics and Bioinformatics Service) for their support in the RNA-seq experiment. This work was supported by the National Science Foundation (MCB0950459, to H.K.), USDA-CSREES (2010-34402-20875 to H.K.) “Designing Food for Health”, and Genomics of Plant Water Use Seed Grant (Texas AgriLife Research to H.K.). A.F. was supported by the Student Exchange Support Program (Scholarship for long-term study abroad from Japan Student Services Organization) and Dissertation Fellowship from Office of Graduate and Professional Studies, Texas A&M University. The authors declare no conflict of interest.
Abbreviations
- CPL4
RNA polymerase II CTD-phosphatase-like 4
- CTD
carboxyl-terminal domain
- CK
cytokinin
- DNSO
de novo shoot organogenesis
- SIM
shoot induction medium
- CIM
callus induction medium
- 2iP
2-isopentenyladenine
- GO
gene ontology
- MLP34
major latex protein-like protein 34
- HAD
haloacid dehalogenase
- CIMT1
Cd-inducible methyltransferase 1
- NAMT1
nicotinic acid methyltransferase 1
- JAL22
jacalin-related lectin 22
- DAO2
dioxygenase for auxin oxidation 2
- SAMT-like
S-adenosyl-L-methionine-dependent methyltransferase-like
- NPF5.12
nitrate transporter 1/peptide transporter family 5.12
- DUF914
solute carrier family 35 protein
- ROSY1
interactor of synaptotagmin-1
- APY3
apyrase 3
- cycB
cyclin B
References
- 1.Skoog F. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–131. [PubMed] [Google Scholar]
- 2.Skoog F. Chemical control of growth and organ formation in plant tissues. L’Annee Biologique. 1950;54:545–562. [PubMed] [Google Scholar]
- 3.Christianson ML, Warnick DA. Competence and determination in the process of in vitro shoot organogenesis. Dev Biol. 1983;95:288–293. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto K, Jiao YL, Meyerowitz EM. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell. 2010;18:463–471. doi: 10.1016/j.devcel.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang I, Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. 2001;413:383–389. doi: 10.1038/35096500. [DOI] [PubMed] [Google Scholar]
- 7.Che P, Lall S, Howell SH. Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta. 2007;226:1183–1194. doi: 10.1007/s00425-007-0565-4. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo N, Banno H. The Arabidopsis transcription factor ESR1 induces in vitro shoot regeneration through transcriptional activation. Plant Physiol Biochem. 2008;46:1045–1050. doi: 10.1016/j.plaphy.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda Y, Banno H, Niu QW, Howell SH, Chua NH. The ENHANCER OFSHOOT REGENERATION 2 gene in Arabidopsis regulates CUP-SHAPED COTYLEDON 1 at the transcriptional level and controls cotyledon development. Plant Physiol. 2006;47:1443–1456. doi: 10.1093/pcp/pcl023. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo N, Mase H, Makino M, Takahashi H, Banno H. Identification of ENHANCER OF SHOOT REGENERATION 1-upregulated genes during in vitro shoot regeneration. Plant Biotechnol. 2009;26:385–393. doi: 10.5511/plantbiotechnology.26.385. [DOI] [Google Scholar]
- 11.Motte H, Verstraeten I, Werbrouck S, Geelen D. CUC2 as an early marker for regeneration competence in Arabidopsis root explants. J Plant Physiol. 2011;168:1598–1601. doi: 10.1016/j.jplph.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Cary AJ, Che P, Howell SH. Developmental events and shoot apical meristem gene expression patterns during shoot development in Arabidopsis thaliana. Plant J. 2002;32:867–877. [DOI] [PubMed] [Google Scholar]
- 13.Yasutani I, Ozawa S, Nishida T, Sugiyama M, Komamine A. Isolation of temperature-sensitive mutants of arabidopsis-thaliana that are defective in the redifferentiation of shoots. Plant Physiol. 1994;105:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo M, Kakimoto T. The CYTOKININ-HYPERSENSITIVE genes of Arabidopsis negatively regulate the cytokinin-signaling pathway for cell division and chloroplast development. Plant J. 2000;23:385–394. [DOI] [PubMed] [Google Scholar]
- 15.Kubo M, Furuta K, Demura T, Fukuda H, Liu YG, Shibata D, Kakimoto T. The CKH1/EER4 gene encoding a TAF12-like protein negatively regulates cytokinin sensitivity in Arabidopsis thaliana. Plant Physiol. 2011;52:629–637. doi: 10.1093/pcp/pcr021. [DOI] [PubMed] [Google Scholar]
- 16.Furuta K, Kubo M, Sano K, Demura T, Fukuda H, Liu YG, Shibata D, Kakimoto T. The CKH2/PKL chromatin remodeling factor negatively regulates cytokinin responses in Arabidopsis Calli. Plant Physiol. 2011;52:618–628. doi: 10.1093/pcp/pcr022. [DOI] [PubMed] [Google Scholar]
- 17.Ohtani M, Demura T, Sugiyama M. Arabidopsis ROOT INITIATION DEFECTIVE1, a DEAH-box RNA helicase involved in pre-mRNA splicing, is essential for plant development. Plant Cell. 2013;25:2056–2069. doi: 10.1105/tpc.113.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konishi M, Sugiyama M. Genetic analysis of adventitious root formation with a novel series of temperature-sensitive mutants of Arabidopsis thaliana. Development. 2003;130:5637–5647. doi: 10.1242/dev.00794. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani M, Sugiyama M. Involvement of SRD2-mediated activation of snRNA transcription in the control of cell proliferation competence in Arabidopsis. Plant J. 2005;43:479–490. doi: 10.1111/j.1365-313X.2005.02469.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani M, Takebayashi A, Hiroyama R, Xu B, Kudo T, Sakakibara H, Sugiyama M, Demura T. Cell dedifferentiation and organogenesis in vitro require more snRNA than does seedling development in Arabidopsis thaliana. J Plant Res. 2015;128:371–380. doi: 10.1007/s10265-015-0704-0. [DOI] [PubMed] [Google Scholar]
- 21.Fukudome A, Aksoy E, XQ Wu, Kumar K, Jeong IS, May K, Russell WK, Koiwa H. Arabidopsis CPL4 is an essential C-terminal domain phosphatase that suppresses xenobiotic stress responses. Plant J. 2014;80:27–39. [DOI] [PubMed] [Google Scholar]
- 22.Fukudome A, Sun D, Zhang XR, Koiwa H. Salt stress and CTD PHOSPHATASE-LIKE4 mediate the switch between production of small nuclear RNAs and mRNAs. Plant Cell. 2017;29:3214–3233. doi: 10.1105/tpc.17.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukudome A, Goldman JS, Finlayson SA, Koiwa H. Silencing Arabidopsis CARBOXYL-TERMINAL DOMAIN PHOSPHATASE-LIKE 4 induces cytokinin-oversensitive de novo shoot organogenesis. Plant J Cell Mol Biol. 2018;94:799–812. doi: 10.1111/tpj.13895. [DOI] [PubMed] [Google Scholar]
- 24.Field B, Osbourn AE. Metabolic diversification - Independent assembly of operon-like gene clusters in different plants. Science. 2008;320:543–547. doi: 10.1126/science.1154990. [DOI] [PubMed] [Google Scholar]
- 25.Wada M, Takahashi H, Altaf-Ul-Amin M, Nakamura K, Hirai MY, Ohta D, Kanaya S. Prediction of operon-like gene clusters in the Arabidopsis thaliana genome based on co-expression analysis of neighboring genes. Gene. 2012;503:56–64. doi: 10.1016/j.gene.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 26.Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ. A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proc Natl Acad Sci U S A. 2006;103:11081–11085. doi: 10.1073/pnas.0602038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiba T, Naitou T, Koizumi N, Yamashino T, Sakakibara H, Mizuno T. Combinatorial microarray analysis revealing Arabidopsis genes implicated in cytokinin responses through the His -> Asp phosphorelay circuitry. Plant Physiol. 2005;46:339–355. doi: 10.1093/pcp/pci033. [DOI] [PubMed] [Google Scholar]
- 28.Lee DJ, Park JY, Ku SJ, Ha YM, Kim S, Kim MD, Oh MH, Kim J. Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7(ARR7) overexpression in cytokinin response. Mol Genet Genomics. 2007;277:115–137. doi: 10.1007/s00438-006-0177-x. [DOI] [PubMed] [Google Scholar]
- 29.Heyl A, Ramireddy E, Brenner WG, Riefler M, Allemeersch J, Schmulling T. The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiol. 2008;147:1380–1395. doi: 10.1104/pp.107.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner WG, Ramireddy E, Heyl A, Schmulling T. Gene regulation by cytokinin in Arabidopsis. Front Plant Sci. 2012;3:22. doi: 10.3389/fpls.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner WG, Schmulling T. Summarizing and exploring data of a decade of cytokinin-related transcriptomics. Front Plant Sci. 2015;6:13. doi: 10.3389/fpls.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhargava A, Clabaugh I, To JP, Maxwell BB, Chiang YH, Schaller GE, Loraine A, Kieber JJ. Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-seq in Arabidopsis. Plant Physiol. 2013;162:272–294. doi: 10.1104/pp.113.217026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aichinger E, Villar CBR, Farrona S, Reyes JC, Hennig L, Kohler C. CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet. 2009;5:12. doi: 10.1371/journal.pgen.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu N, Nutzmann HW, MacDonald JT, Moore B, Field B, Berriri S, Trick M, Rosser SJ, Kumar SV, Freemont PS, et al. Delineation of metabolic gene clusters in plant genomes by chromatin signatures. Nucleic Acids Res. 2016;44:2255–2265. doi: 10.1093/nar/gkw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antosz W, Pfab A, Ehrnsberger HF, Holzinger P, Kollen K, Mortensen SA, Bruckmann A, Schubert T, Langst G, Griesenbeck J, et al. The composition of the Arabidopsis RNA polymerase II transcript elongation complex reveals the interplay between elongation and mRNA processing factors. Plant Cell. 2017;29:854–870. doi: 10.1105/tpc.16.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]


