Abstract
Naturally or surgically induced postmenopausal women are widely prescribed estrogen therapies to alleviate symptoms associated with estrogen loss and to lower the subsequent risk of developing metabolic diseases, including diabetes and nonalcoholic fatty liver disease. However, the molecular mechanisms by which estrogens modulate metabolism across tissues remain ill-defined. We have previously reported that 17β-estradiol (E2) exerts antidiabetogenic effects in ovariectomized (OVX) mice by protecting mitochondrial and cellular redox function in skeletal muscle. The liver is another key tissue for glucose homeostasis and a target of E2 therapy. Thus, in the present study we determined the effects of acute loss of ovarian E2 and E2 administration on liver mitochondria. In contrast to skeletal muscle mitochondria, E2 depletion via OVX did not alter liver mitochondrial respiratory function or complex I (CI) specific activities (NADH oxidation, quinone reduction, and H2O2 production). Surprisingly, in vivo E2 replacement therapy and in vitro E2 exposure induced tissue-specific effects on both CI activity and on the rate and topology of CI H2O2 production. Overall, E2 therapy protected and restored the OVX-induced reduction in CI activity in skeletal muscle, whereas in liver mitochondria E2 increased CI H2O2 production and decreased ADP-stimulated respiratory capacity. These results offer novel insights into the tissue-specific effects of E2 on mitochondrial function.
Keywords: estrogen, complex I, hydrogen peroxide, liver, skeletal muscle, mitochondrial respiratory chain complex, mitochondrial function, ovariectomy
Introduction
With menopause occurring at age ∼50 and a life expectancy in the United States of ∼80 years (1), women spend the latter third of their lives in a low 17β-estradiol (E2)3 state, which is associated with an elevated risk of developing obesity, type 2 diabetes, and cardiovascular disease (2). Estrogen-based hormone therapies (ET, estrogens ± progestins) have been recommended since the 1950s to help ameliorate menopausal symptoms and reduce the risk of developing chronic diseases (3). Although the use of ET curtailed in the early 2000s after the Women's Health Initiative Trial reported that ET increased the risks of oncogenicity and cardiovascular events (4), subsequent studies and meta-analyses later confirmed that ET does indeed reduce all-cause mortality (5). Today, the North American Menopause Society endorses ET based on an algorithm-derived benefit/risk ratio (6), especially for those <60 years of age and/or within <10 years postmenopause (7). However, the molecular mechanism(s) by which ET affects metabolism across tissues remains poorly understood. In addition, various formulations of custom-compounded “bioidentical” hormone therapies are used by 1–2.5 million women per year in the United States alone (8), despite a lack of evidence-based support for their safety and disease prevention claims (9) or U.S. Food and Drug Administration approval (10).
In a wide range of nonreproductive tissues, E2 has consistently been shown to modulate several parameters of mitochondrial function, including oxidative phosphorylation (11), ATP production, membrane potential (12, 13), Ca2+ homeostasis (14), and mitochondrial morphology dynamics (15, 16). In skeletal muscle, E2 localizes to the inner mitochondrial membrane, where it lowers membrane viscosity, which, in turn, promotes bioenergetic function, cellular redox balance, and insulin sensitivity (17). Many of the beneficial effects of E2 appear to be mediated through improved complex I (CI) activity, which is curious given that exposure of liver mitochondria to E2 reportedly inhibits CI activity (18). Combined, these findings raise the possibility that the effects of E2 on mitochondrial function may be tissue-specific (19). Similar to muscle, E2 given in vivo localizes to mitochondria in the liver (20), although the functional impact is unknown. Decreased circulating E2, as well as aging, is associated with declining liver function and increased risk of liver disease (21), particularly the development of nonalcoholic fatty liver disease (NAFLD) (22, 23). Although E2 administration in OVX rodents decreases adiposity (24) and reverses the development of fatty liver (25), postmenopausal women diagnosed with NAFLD do not show improvements with ET (26). In fact, ET is normally contraindicated in women with active liver disease or a history of liver disease (7, 27). Taken together, these data suggest that the effects of E2 may not only be tissue-specific but also dependent on the pathophysiology of the tissue.
In the present study, ex vivo and in vitro experiments were performed to assess and compare the targeted effects of E2 loss and E2 replacement on the kinetics and H2O2-emitting potential of CI in mitochondria from both liver and skeletal muscle. Overall, the findings suggest that E2 treatment is beneficial in restoring CI kinetics in skeletal muscle after ovarian E2 depletion. In contrast, liver mitochondrial function is minimally impacted by E2 depletion, whereas E2 treatment unexpectedly increases CI-mediated H2O2 production and decreases OXPHOS capacity.
Results
Hepatic cellular redox balance is not affected 4 weeks after OVX ±ET
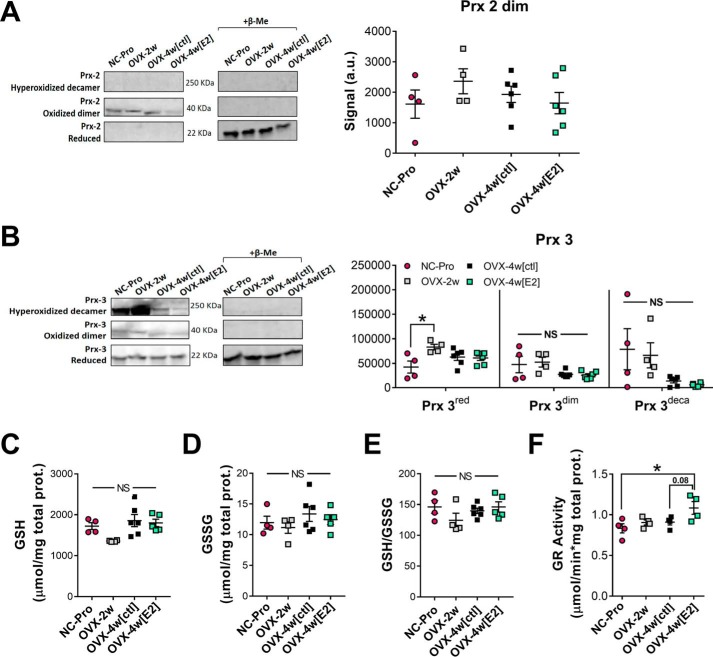
Young, sexually mature (10-week-old) female mice were studied 2 weeks after OVX (OVX-2w) and after an additional 2 weeks ± E2 treatment (OVX-4w(E2) and OVX-4w(ctl), respectively). Loss of circulating ovarian E2 was confirmed by reduced uterine weights relative to normally cycling females in the proestrus stage (NC-Pro) of the estrus cycle as previously described (17). In contrast with skeletal muscle (17), 2–4-week OVX did not affect cellular redox balance in the liver as evidenced by the lack of change in redox state of peroxiredoxins (Fig. 1, A and B) and the ratio of GSH/GSSG (Fig. 1, C–E). GSH reductase activity was increased in OVX mice treated with E2 (Fig. 1F), consistent with an E2-induced up-regulation of antioxidant enzymes in the liver (29).
Figure 1.
2–4-week OVX ± 2-week ET has no impact on hepatic cellular redox environment. A and B, relative levels of reduced (Prxred), oxidized dimer (Prxdim), and hyperoxidized decamer (Prxdeca) of Prx2 (A) and Prx 3 (B) measured in liver homogenates with NEM by nonreducing Western blotting analysis. As a positive control, samples were treated with β-mercaptoethanol (20%) to verify oxidized and reduced Prx bands (right panels). Representative blots are shown. See Fig. S1 for whole blot images. C and D, total GSH (C) and GSSG (D) concentration measured in liver homogenates by HPLC. E, resulting GSH/GSSG ratios. F, GSH reductase activity in liver homogenates. The values are means ± S.E. *, p < 0.05 (n = 4–6 mice/group).
ET, but not OVX, affects mitochondrial CI and CIII function in liver
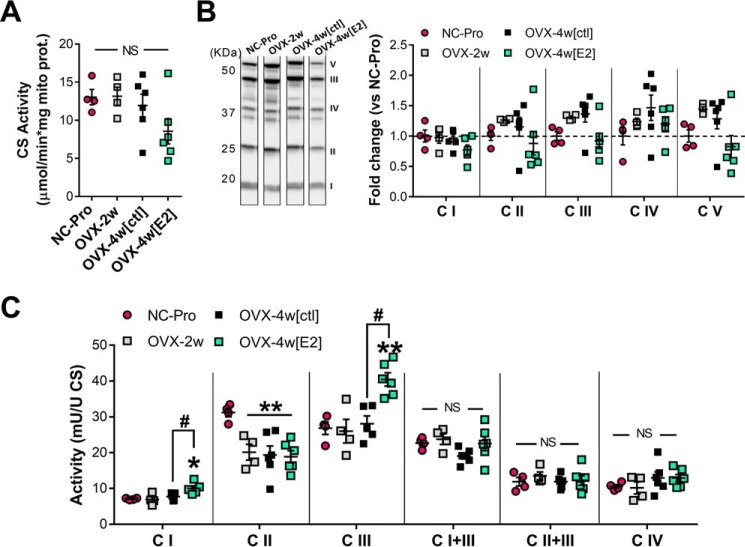
OVX ± E2 therapy did not alter mitochondrial content in liver, as demonstrated by unchanged citrate synthase activity and expression levels of the respiratory complexes (Fig. 2, A and B). We recently found that loss of E2 from OVX decreases mitochondrial CI, CIII, CI+III, and CII+III activity in skeletal muscle (17). In sharp contrast, the specific activities of CI, CIII, and CIV, as well as electron transfer between CI and CIII and between CII and CIII, were not affected in liver mitochondria after ovarian E2 withdrawal (Fig. 2C). Also in contrast to muscle (17), CII specific activity was decreased by ∼30% in liver mitochondria after OVX and was not rescued by E2. Although E2 treatment increased the individual specific activities of CI and CIII by ∼40% (p < 0.05) and ∼48% (p < 0.005), respectively, the accompanying CI to CIII activity remained the same.
Figure 2.
OVX + ET affects relative specific activity of mitochondrial electron transport complexes in liver. A, citrate synthase activity determined in isolated liver mitochondria. B, Western blotting analysis of mitochondrial OXPHOS complexes. Representative lanes loaded with 25 μg of mitochondrial protein from liver are shown. The data are expressed as fold change versus NC-Pro. No significant changes were detected. See Fig. S2 for full gel images. C, relative specific activities of electron transport complexes I–IV, I+III, and II+III determined spectrophotometrically and normalized to CS activity (1 unit = 1 μmol/min/mg protein). The values are means ± S.E. *, p < 0.05; **, p < 0.005 versus NC-Pro from one-way ANOVA analysis for each assay (n = 4–6 mice/group).
Tissue-specific effects of OVX and ET on CI activity
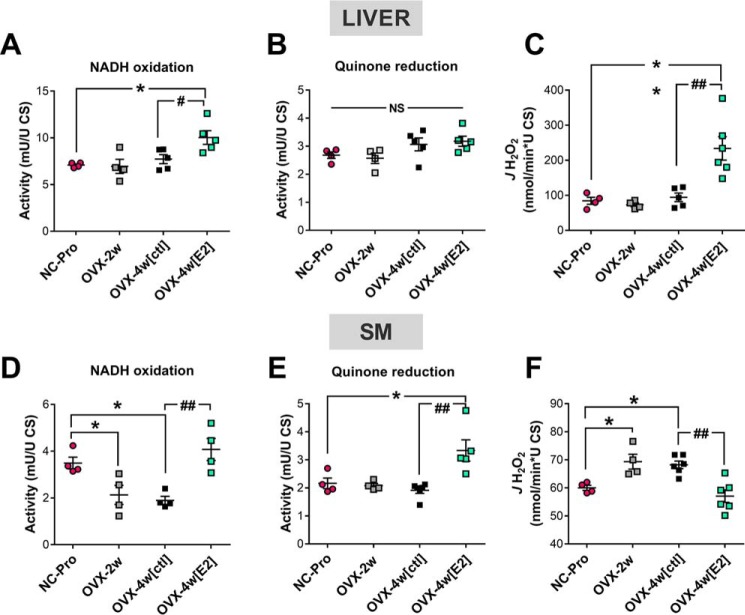
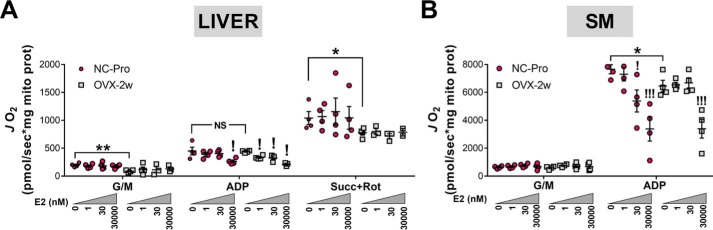
To further evaluate the potential tissue-specific effects of E2 on CI kinetics and JH2O2 production, the steady-state rates of NADH oxidation, decylubiquinone (DCU, a soluble substrate analog of coenzyme Q) reduction and H2O2 production were measured in fractured liver and skeletal muscle mitochondria from NC-Pro and OVX mice ± E2 treatment (Fig. 3). The NADH (IF) and quinone (IQ) binding sites are separated by a ∼90 Å tunnel containing eight redox-sensitive iron–sulfur clusters that connect the primary electron acceptor FMN to the quinone-binding site (30, 31). Overall, the maximal rate of CI-mediated NADH oxidation in NC-Pro control mice was ∼2-fold higher in liver compared with skeletal muscle mitochondria (Fig. 3, A versus D), whereas the maximal rate of quinone reduction was comparable between tissues (Fig. 3, B and E). Maximal rates of H2O2 production by CI were also higher in liver versus muscle mitochondria (Fig. 3, C versus F).
Figure 3.
Effects of ET in CI kinetics and JH2O2 emitting potential in liver versus skeletal muscle mitochondria. A and D, CI activity determined in liver (A) and skeletal muscle (SM) (D) following NADH oxidation, using DCUox as the electron acceptor. B and E, CI activity determined in liver (B) or SM (E) in the same conditions as in A and D but following the reduction of DCUox. C and F, JH2O2 emitting potential determined in parallel experiments to A and D and to B and E in the same conditions. The values are means ± S.E. *, p < 0.05; **, p < 0.005 versus NC-Pro from one-way ANOVA analysis (n = 4–6 mice/group).
CI activity responded differently to ovarian E2 depletion and E2 therapy in liver and skeletal muscle mitochondria. In liver, E2 depletion via OVX did not affect either the maximal rate of CI NADH oxidation or quinone reduction (Fig. 3, A–C). However, E2 treatment increased the maximal rate of NADH oxidation in OVX compared with NC-Pro control mice (Fig. 3A), whereas the maximal rate of quinone reduction remained unchanged (Fig. 3B). In skeletal muscle, the maximal rate of NADH oxidation was reduced in mitochondria isolated from OVX mice but was restored to NC-Pro levels with E2 treatment (Fig. 3D). In contrast, the maximal rate of quinone reduction remained unchanged after OVX but was significantly increased with E2 treatment (Fig. 3E). Finally, in liver mitochondria CI JH2O2 production was not affected after either 2- or 4-week OVX but was surprisingly increased by 2-fold after E2 treatment (Fig. 3C). Consistent with prior findings (17), JH2O2 production in mitochondria isolated from skeletal muscle was elevated in OVX mice relative to NC-Pro but restored to NC-Pro levels by E2 treatment (Fig. 3F).
Tissue-specific effects of OVX and in vitro E2 treatment on JH2O2 production at CI sites
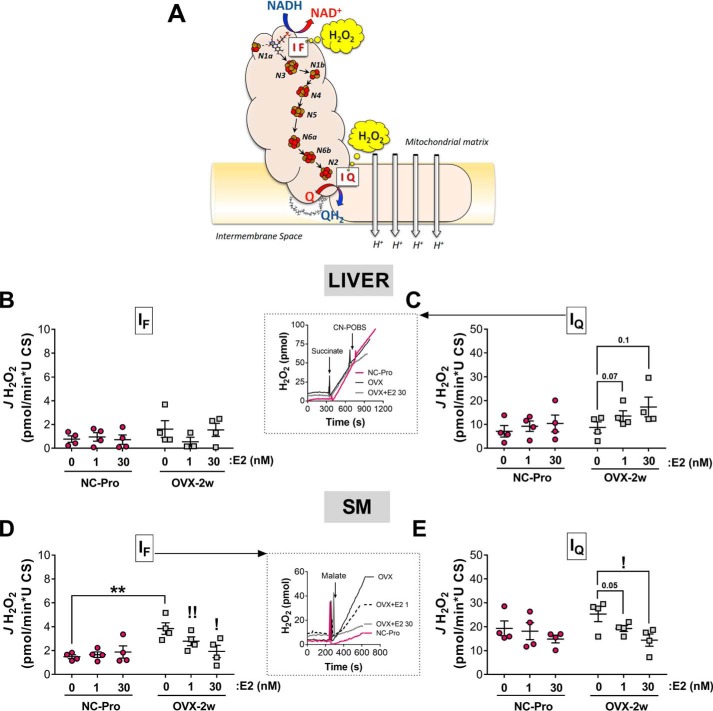
IF and IQ are the two major sites of superoxide/H2O2 production in CI (32) (Fig. 4A). In light of the tissue-specific response of CI activity, we next evaluated the influence of E2 on superoxide/H2O2 production at sites IF and IQ of CI in each tissue. Freshly isolated liver and skeletal muscle mitochondria from NC-Pro and OVX-2w mice were exposed to a low (1 nm) or high (30 nm) concentration of E2 in vitro, and JH2O2 production was determined at each of the respective CI sites. Inhibitors to the mitochondrial antioxidant enzymes thioredoxin reductase and GSH reductase were included to permit direct detection of JH2O2 production (33). The maximal JH2O2 production at site IF was measured using malate as substrate in the presence of rotenone (32). ATP and aspartate were included to prevent H2O2 generation from 2-oxoglutarate dehydrogenase, a potential site of electron leak when malate is the only substrate (32). For the IQ site, we determined the maximal succinate-supported JH2O2 production sensitive to N-cyclohexyl-4-(4-nitrophenoxy)-benzenesulfonamide, which inhibits electron leak at IQ without affecting oxidative phosphorylation (34).
Figure 4.
CI-derived JH2O2 emitting capacity and topology are differently affected by in vitro E2 exposure in liver and SM. A, schematic diagram of the electron transfer pathway in CI connecting the NADH oxidation with quinone reduction via seven iron-sulfur redox centers (Protein Data Bank code 2FUG) (30, 31, 52). H2O2 production occurs at two identified sites, the flavin- (IF) and quinone-binding sites (IQ). B and D, JH2O2 emitting capacity at site IF in liver (B) and SM (D) mitochondria preincubated for 15 min ± increasing concentrations of E2. JH2O2 rates were driven by 5 mm malate in the presence of 2.5 mm ATP, 1.5 mm aspartate, 4 μm rotenone, 1 μm bis-chloroethylnitrosourea, and 1 μm auranofin. C and E, JH2O2 emitting capacity at site IQ in liver (C) and SM (E) mitochondria preincubated for 15 min ± increasing concentrations of E2. N-Cyclohexyl-4-(4-nitrophenoxy)-benzenesulfonamide (CN-POBS)–sensitive JH2O2 rates were driven by 5 mm succinate in the presence of 1 μm bis-chloroethylnitrosourea and 1 μm auranofin. Insets in B and E show representative H2O2 traces of NC-Pro and OVX-2w plus E2. The data are means ± S.E. **, p < 0.01 versus NC-Pro from independent t test. Comparisons between increasing E2 concentrations within each group were performed using paired t tests versus 0 E2. !, p < 0.05 (n = 4 mice/group).
Consistent with a previous report (35), overall maximal JH2O2 production was an order of magnitude lower at site IF than IQ in both liver and skeletal muscle mitochondria from control NC-Pro mice (Fig. 4). Interestingly, despite differences in the catalytic rates of NADH oxidation and DCU reduction at sites IF and IQ, respectively (Fig. 3), maximal JH2O2 production was similar between the two tissues (Fig. 4, B versus D and C versus E). In liver mitochondria, OVX did not affect maximal JH2O2 production at either site IF or IQ (Fig. 4, B and C). Preincubation (15 min) of liver mitochondria from OVX mice with either low or high concentrations of E2 tended to increase maximal JH2O2 production at site IQ only (Fig. 4C). By contrast, in mitochondria from skeletal muscle, maximal JH2O2 production at site IF was ∼2-fold higher in OVX relative to NC-Pro mice (p < 0.05) but was restored by preincubation with E2 (Fig. 4D). Similarly, maximal JH2O2 production at site IQ was slightly elevated after OVX and restored by preincubation with E2 (Fig. 4E). Overall, these results suggest that loss of ovarian E2 in vivo and E2 replacement in vitro (at nanomolar concentrations as in Ref. 17) directly impacts CI-derived maximal JH2O2 production and topology in a tissue-specific manner.
Effects of in vitro E2 exposure on CI-supported respiration
To test whether in vitro treatment with E2 affects mitochondrial respiration supported by CI-linked substrates (glutamate/malate), oxygen consumption rates (JO2) were determined in liver and muscle mitochondria from NC-Pro and OVX-2w mice exposed (15 min) to increasing concentrations of E2. In control mice prior to addition of E2, maximal ADP-stimulated CI-supported JO2 was ∼14-fold lower in liver versus skeletal muscle mitochondria (Fig. 5, A and B). Addition of the CII-linked substrate succinate to liver mitochondria generated a higher maximal ADP-stimulated JO2 response, but this was still well below the respiratory capacity of skeletal muscle mitochondria. OVX-induced loss of E2 decreased maximal CI-supported JO2 in skeletal muscle, but not liver mitochondria.
Figure 5.
Effects of acute in vitro E2 exposure on CI-supported respiration in liver and SM mitochondria. JO2 measured in isolated mitochondria from liver (A) and SM (B) mitochondria from NC-Pro or OVW-2w mice preincubated for 15 min ± increasing concentrations of E2. The substrates added in sequence were 10 mm glutamate + 2 mm malate, 1 mm ADP and for liver 10 mm succinate, 10 μm rotenone. The data are means ± S.E. *, p < 0.05; **, p < 0.005 versus NC-Pro from independent t tests. Comparisons between increasing E2 concentrations within each group were performed using paired t tests versus 0 E2. !, p < 0.05; !!!, p < 0.005 (n = 4 mice/group).
We previously found that acute in vitro incubation of skeletal muscle mitochondria from OVX mice with nanomolar concentrations of E2 replenishes membrane E2 content but fails to restore maximal JO2 (17). In the present study, acute in vitro exposure to low nanomolar concentrations of E2 did not increase CI-supported JO2 in skeletal muscle mitochondria from OVX mice (Fig. 5B), consistent with the notion that restoration of mitochondrial respiratory capacity by in vivo E2 therapy may involve genomic mechanisms. Interestingly in liver, exposure to low nanomolar concentrations of E2 induced an inhibitory effect on maximal ADP-stimulated JO2 (Fig. 5A). Incubation with higher, supraphysiological E2 concentrations (30 μm) inhibited CI-linked JO2 by 35–50% in mitochondria from both liver and skeletal muscle of NC-Pro and OVX mice, possibly reflecting inhibition of the FMN cofactor in CI (18), F0F1-ATPase (12), and/or biophysical disruption of the membrane.
Discussion
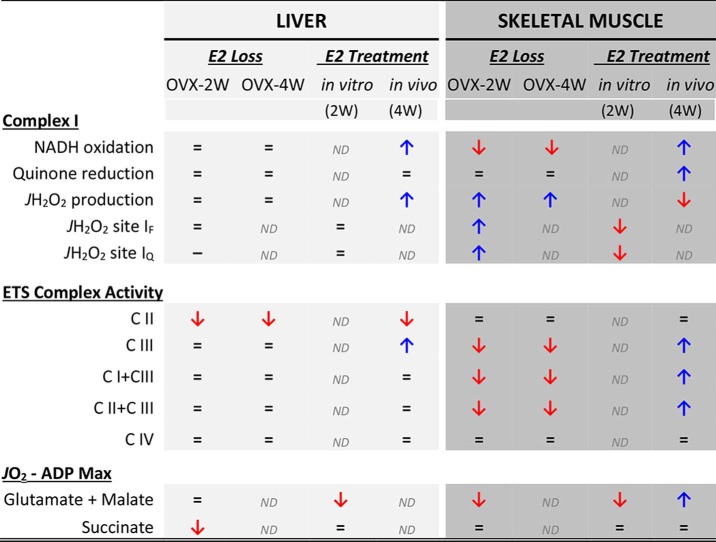
Loss of circulating ovarian E2 results in diverse physiological and biochemical responses across the body (36). In vitro, E2 elicits different effects on mitochondria depending on the tissue from which the mitochondria are isolated (19), suggesting that the influence of E2 on mitochondrial function may at least partially account for its diverse effects across tissues in vivo. To further explore this possibility, the present study investigated the effects of short-term ovarian E2 depletion and E2 therapy on mitochondrial redox function and respiratory complex activities in both liver and skeletal muscle. In contrast to the loss of function evident in mitochondria from skeletal muscle with E2 depletion (17), mitochondria from liver appear to be relatively resilient to the loss of E2, as evidenced by the lack of significant change in mitochondrial respiratory capacity or cellular redox state. A notable exception was a decrease (∼30%) in CII specific activity that persisted even after administration of E2. However, both liver and skeletal muscle mitochondria responded to E2 treatment with marked, albeit different, effects on CI activity and JH2O2 production. A summary of the effects of E2 loss and E2 replacement on indices of mitochondrial function from both tissues is shown in Table 1.
Table 1.
Summary of the effects of E2 loss and E2 treatment on indices of mitochondrial function in liver and skeletal muscle
The data were compiled from the present study and Ref. 28. ND, not determined.
The mechanism(s) behind tissue specificity of E2 action on mitochondrial function remains unknown. Our previous findings linked a direct effect of E2 on mitochondrial membrane viscosity to improved CI function in skeletal muscle (17). The extent to which a similar mechanism of action is present in other tissues is unclear, although E2-mediated increases in mitochondrial CI-supported JO2 have been previously reported in other tissues with high CI-linked mitochondrial respiratory capacity, including brain (11) and cardiac muscle (37, 38). The impact of E2 loss and replacement on liver mitochondrial function, however, appears to be comparatively modest and may simply reflect tissue-specific heterogeneity in inherent E2 content and/or varying dependence on membrane-bound estrogen receptor signaling (39, 40).
The potential effect of exogenously administered E2 on the liver also depends on the type of estrogen and the dose and route of administration (41, 42). In the present study, E2 was administered transdermally via a subcutaneous mini osmotic pump that, compared with oral administration, limits exposure of the liver and allows for a more physiological delivery of E2 to nonhepatic tissues (42). In fact, oral administration of conjugated estrogens has been associated with higher hepatic toxicity because of increased hepatic synthesis of triglycerides and coagulation and inflammatory factors (43). Inherent differences in mitochondrial lipid and/or protein composition (44–46), which influence the biophysical properties of membranes, may have also contributed to the different responses to E2 depletion and replacement between the two tissues.
The IF site in CI is a significant contributor to H2O2 production during forward electron flow (32). We previously found that OVX induces an increase in the mitochondrial H2O2 emitting potential and an oxidative shift in the cellular redox environment in skeletal muscle, accompanied by a decrease in CI function (17). In the present study, JH2O2 production was increased specifically at site IF after OVX, suggesting that loss of E2 increases the susceptibility of site IF to leak electrons in skeletal muscle mitochondria. Although the mechanism is unclear, either an increase in the sensitivity of site IF to the NADH/NAD+ ratio and/or a “backup” in downstream electron flow increasing the reducing pressure at site IF could explain this effect (47, 48). Regardless of the mechanism, E2 treatment in vitro reversed the increased susceptibility of site IF to leak electrons and in vivo enhanced both CI NADH oxidation and quinone reduction rates, lowering overall JH2O2 production. These effects of E2 loss and treatment were unique to skeletal muscle mitochondria and are consistent with prior findings, suggesting that the decrease in membrane microviscosity induced by the presence of E2 in mitochondrial membranes enhances coupling of the CI half-redox reactions, facilitating electron transfer and decreasing its net JH2O2 emitting potential (17).
Site IQ of CI does not normally produce H2O2 during forward electron flow (34) but can become a significant source under conditions that promote a high membrane potential and reduced ubiquinone pool (32), such as when electrons feed directly into the quinone pool from β-oxidation, succinate, etc., in excess of demand (49) and/or under various pathological states (50, 51). Surprisingly in liver mitochondria, E2 treatment of OVX mice in vivo increased both NADH oxidation and JH2O2 production from CI. E2 exposure of mitochondria from OVX mice in vitro also tended to increase JH2O2 production at site IQ but not site IF. Electron transfer through CI is thought to be limited by quinone binding and release at site IQ (52, 53). Thus, even a slight enhancement in NADH oxidation activity without a concomitant increase in quinone reduction activity could reduce the iron–sulfur centers along the peripheral arm of CI (52) and lead to higher JH2O2 production at the IQ site in hepatocytes.
Oxidative stress plays a key role in the pathophysiology of NAFLD (54–56) with elevated H2O2 production and decreased CI function thought to be primary underlying mechanisms (55, 57, 58). The surprisingly negative but relatively modest impact of E2 replacement on both CI kinetics and CI-derived JH2O2 in liver mitochondria in the present study raises the possibility that E2 therapy may promote H2O2 generation and potentially worsen the oxidative burden in hepatocytes, particularly with oral administration of E2. These findings, however, are early and should be interpreted cautiously given that in vivo protein markers of cellular redox state in liver were not affected by E2 loss and replacement. Thus, further research is needed to understand the full consequences of E2 loss and E2 therapy on liver mitochondrial function, especially in the context of other potential aggravating factors (i.e. high-fat diet, diabetes, etc.) to better define the risk-to-benefit ratio of ET in postmenopausal patients with NAFLD (7, 59).
Experimental procedures
Reagents
All chemicals were purchased from Sigma–Aldrich and of the highest purity available.
Animal use
All animal studies were approved by the East Carolina University Institutional Animal Care and Use Committee. C57BL-6N female mice were purchased from The Jackson Laboratory and housed in a 12-h light/dark cycle, temperature-controlled (22 °C) facility with free access to water and food (standard chow diet). The mice were ovariectomized at 10 weeks of age and sacrificed at 12 weeks old (OVX-2w) or implanted a subcutaneous micro-osmotic pump (Alzet model no. 1002) to receive an extra 2 weeks of E2 therapy (OVX-4w(E2)) or control saline solution (OVX-4w(ctl)). Water-soluble β-estradiol (Sigma catalog no. E4389) was dissolved in sterile saline, and the solution was sterilized by filtering through a 0.2-μm Millipore membrane. Each pump contained 17.5 μg of E2 (46.7 mg/g purity) in 100 μl of sterile saline, delivering ∼1 μg E2/day at 0.25 μl/h. To ensure high physiological E2 plasma levels, normally cycling females in the proestrus stage of the estrus cycle (NC-Pro) were selected as the control group. Estrus cycle was monitored daily via crystal violet staining and vaginal cytology evaluation (17). Liver was collected for mitochondria isolation, and quadriceps, plantaris, soleus, and whole gastrocnemius muscles were harvested for SM mitochondria isolation.
Mitochondrial isolation and relative specific activity of mitochondrial OXPHOS complexes
Liver and SM were harvested at sacrifice and immediately homogenized in mitochondrial isolation medium (0.3 m sucrose, 10 mm HEPES, 1 mm EGTA) containing 1 mg/ml BSA, on ice (adapted from Ref. 60). Aliquots of isolated mitochondria were diluted to 0.8 mg/ml in hypotonic medium (25 mm K2HPO4, 5.3 mm MgCl2, pH 7.2), and further subjected to three or four freeze–thaw cycles. CI activity was determined in 5 mm Tris, 0.5 mg/ml BSA, 34 μm KCN, 0.4 μm antimycin A, pH 8, by 1) following the oxidation of NADH (0.13 mm) at 340 nm (ϵ340 = 6220 m−1 cm−1) for 3 min using 50 μm oxidized decylubiquinone (DCUox) as the electron acceptor, or 2) following the reduction of DCUox at 280 nm (ϵ280 = 16,000 m−1 cm−1) in the presence of 0.13 mm NADH. 4 μm rotenone was added in the end to measure rotenone-sensitive NADH-DCU oxidoreductase activity. Specific activities of all other individual ETS complexes, I+III, and II+III were determined spectrophotometrically as described in Refs. 17 and 61).
Mitochondrial content
Citrate synthase activity was measured according to specifications by Sigma catalog no. CS0720 in isolated mitochondria. OXPHOS complexes were measured in isolated mitochondria by reducing Western blotting analysis using anti-Rt/Ms total OXPHOS complex mixture (Invitrogen catalog no. 458099). Equal protein load (25 μg/well) and consistent gel transfer was verified by Ponceau S stain. The blots were imaged using IRDye 680RD donkey anti-mouse IgG antibody (Licor Biosciences catalog no. 926-68072) in a Licor IR imager and quantified with Image Studio Lite within linear range of intensity (version 5.0, Licor Bioscience).
Mitochondrial respiration (JO2) and H2O2 emitting potential (JH2O2) measurements
JO2 was measured in fresh isolated mitochondria using high-resolution respirometers (O2K, OROBOROS, Innsbruck, Austria), in buffer Z supplemented with 20 mm creatine monohydrate and 25 μm blebbistatin. JH2O2 was measured using the Amplex UltraRed/horseradish peroxidase fluorescence system in FluoroMax/Fluorolog spectrofluorometers (HORIBA Jobin Yvon). All JH2O2 experiments were performed in buffer Z supplemented with 10 μm Amplex UltraRed (Invitrogen), and 20 units/ml copper–zinc SOD (Sigma). For CI-targeted experiments in freeze-fractioned mitochondria, assays were performed in the same way, in the presence of 5 mm Tris, 0.5 mg/ml BSA, 24 μm KCN, 0.4 μm antimycin A, pH 8, and 50 μm DCUox as the electron acceptor. The addition of NADH (0.13 mm) as the electron donor started the reaction.
Measurements of cellular and mitochondrial redox state
Adapting the protocol from Refs. 62 and 63, and as performed in Ref. 17, GSH and GSSG were measured by HPLC. GSH was measured as its conjugate with N-ethylmaleimide (NEM). The liver was homogenized immediately after collection in buffer containing NEM, and GS-NEM conjugate was measured by UV detection, in which two diastereomers are visible as equally sized peaks in the chromatogram. GSSG was measured in a parallel sample of the muscle homogenate using fluorescent detection of GSSG binding to o-phthalaldehyde at pH 12. Samples selected to measure GSH/GSSG by HPLC were also utilized for Western blotting analysis of Prx oxidation. Reduced, oxidized-dimer, and oxidized-decamer forms of peroxiredoxins (Prx2, cytosolic; and Prx3, mitochondrial) were measured in liver homogenates by standard nonreducing Western blotting analysis as described in Ref. 28. Ponceau S stain was used to verify equal protein load (45 μg/well) and consistent gel transfer. Anti-Prx2 (AbCam, ab109367) and anti-Prx3 (AbFrontier, LF-MA0044) were used for protein detection. The blots were imaged using a Licor IR imager and quantified using Image Studio Lite (version 5.0, Licor Bioscience). As a positive control, samples were treated with β-mercaptoethanol (20× dilution) to verify oxidized and reduced Prx bands. Oxidized decamer (250 kDa), oxidized dimer (45 kDa), and reduced (22–25 kDa) forms of Prx2 and Prx3 were quantified and presented as fold difference relative to NC-Pro.
GSH reductase activity
Determined in liver homogenates in buffer 100 mm Tris, 1 mm EGTA, 1 mm EDTA, 1% Tween, pH 8.35, as described in a Cayman chemical kit (catalog no. 703202).
Statistical analyses
The data are presented as means ± S.E. Comparisons between two groups were performed by independent (or paired, when applicable) Student's t tests. Comparisons between four groups were performed with one-way ANOVA, followed by Sidak's multiple comparisons test (versus NC-Pro). Graph Pad Prism 7 was used for statistical tests and data presentation. Statistical significance was set at p < 0.05. The p values are provided in the figure legends.
Author contributions
M. J. T., T. E. R., C.-T. L., T. N. Z., and P. D. N. conceptualization; M. J. T. data curation; M. J. T. and T. E. R. formal analysis; M. J. T., T. E. R., C.-T. L., T. N. Z., and P. D. N. investigation; M. J. T., T. E. R., C.-T. L., and T. N. Z. methodology; M. J. T. writing-original draft; M. J. T., T. E. R., C.-T. L., T. N. Z., and P. D. N. writing-review and editing; T. E. R., C.-T. L., T. N. Z., and P. D. N. supervision; T. N. Z. and P. D. N. project administration; P. D. N. funding acquisition.
Supplementary Material
Acknowledgment
We thank Dr. David A. Brown for providing materials.
The work was supported by National Institutes of Health Grants R01 DK096907 and DK110656 from the NIDDK (to P. D. N.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1 and S2.
- E2
- 17β-estradiol
- OVX
- ovariectomized
- CI
- complex I
- ET
- estrogen-based hormone therapies
- OXPHOS
- oxidative phosphorylation
- SM
- skeletal muscle
- NAFLD
- nonalcoholic fatty liver disease
- DCU
- decylubiquinone
- NEM
- N-ethylmaleimide
- ANOVA
- analysis of variance.
References
- 1. National Center for Health Statistics (2017) Health, United States, 2016: With Chartbook on Long-term Trends in Health, U.S. Government Printing Office, Washington, D. C. [PubMed] [Google Scholar]
- 2. Janssen I., Powell L. H., Crawford S., Lasley B., and Sutton-Tyrrell K. (2008) Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch. Intern. Med. 168, 1568–1575 10.1001/archinte.168.14.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lobo R. A., Pickar J. H., Stevenson J. C., Mack W. J., and Hodis H. N. (2016) Back to the future: hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 254, 282–290 10.1016/j.atherosclerosis.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 4. Rossouw J. E., Anderson G. L., Prentice R. L., LaCroix A. Z., Kooperberg C., Stefanick M. L., Jackson R. D., Beresford S. A., Howard B. V., Johnson K. C., Kotchen J. M., Ockene J., and Writing Group for the Women's Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- 5. Langer R. D., Simon J. A., Pines A., Lobo R. A., Hodis H. N., Pickar J. H., Archer D. F., Sarrel P. M., and Utian W. H. (2017) Menopausal hormone therapy for primary prevention: why the USPSTF is wrong. Menopause 24, 1101–1112 10.1097/GME.0000000000000983 [DOI] [PubMed] [Google Scholar]
- 6. Manson J. E., Ames J. M., Shapiro M., Gass M. L., Shifren J. L., Stuenkel C. A., Pinkerton J. V., Kaunitz A. M., Pace D. T., Kagan R., Schnatz P. F., Kingsberg S. A., Liu J. H., Joffe H., Richard-Davis G., et al. (2015) Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from the North American Menopause Society. Menopause 22, 247–253 10.1097/GME.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 7. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel (2017) The 2017 hormone therapy position statement of the North American Menopause Society. Menopause 24, 728–753 10.1097/GME.0000000000000921 [DOI] [PubMed] [Google Scholar]
- 8. Pinkerton J. V., and Santoro N. (2015) Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause 22, 926–936 10.1097/GME.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuksel N., Treseng L., Malik B., and Ogbogu U. (2017) Promotion and marketing of bioidentical hormone therapy on the internet: a content analysis of websites. Menopause 24, 1129–1135 10.1097/GME.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 10. Pinkerton J. V., and Pickar J. H. (2016) Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy. Menopause 23, 215–223 10.1097/GME.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brinton R. D. (2008) The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 31, 529–537 10.1016/j.tins.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreno A. J., Moreira P. I., Custódio J. B., and Santos M. S. (2013) Mechanism of inhibition of mitochondrial ATP synthase by 17β-estradiol. J. Bioenerg. Biomembr. 45, 261–270 10.1007/s10863-012-9497-1 [DOI] [PubMed] [Google Scholar]
- 13. Wang J., Green P. S., and Simpkins J. W. (2001) Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J. Neurochem. 77, 804–811 10.1046/j.1471-4159.2001.00271.x [DOI] [PubMed] [Google Scholar]
- 14. Ohya S., Kuwata Y., Sakamoto K., Muraki K., and Imaizumi Y. (2005) Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 289, H1635–H1642 10.1152/ajpheart.00016.2005 [DOI] [PubMed] [Google Scholar]
- 15. Arnold S., Victor M. B., and Beyer C. (2012) Estrogen and the regulation of mitochondrial structure and function in the brain. J. Steroid Biochem. Mol. Biol. 131, 2–9 10.1016/j.jsbmb.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 16. Ribas V., Drew B. G., Zhou Z., Phun J., Kalajian N. Y., Soleymani T., Daraei P., Widjaja K., Wanagat J., de Aguiar Vallim T. Q., Fluitt A. H., Bensinger S., Le T., Radu C., Whitelegge J. P., et al. (2016) Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 8, 334ra54 10.1126/scitranslmed.aad3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torres M. J., Kew K. A., Ryan T. E., Pennington E. R., Lin C. T., Buddo K. A., Fix A. M., Smith C. A., Gilliam L. A., Karvinen S., Lowe D. A., Spangenburg E. E., Zeczycki T. N., Shaikh S. R., and Neufer P. D. (2018) 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 27, 167–179.e7 10.1016/j.cmet.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreira P. I., Custódio J., Moreno A., Oliveira C. R., and Santos M. S. (2006) Tamoxifen and estradiol interact with the flavin mononucleotide site of complex I leading to mitochondrial failure. J. Biol. Chem. 281, 10143–10152 10.1074/jbc.M510249200 [DOI] [PubMed] [Google Scholar]
- 19. Moreira P. I., Custódio J. B., Nunes E., Oliveira P. J., Moreno A., Seiça R., Oliveira C. R., and Santos M. S. (2011) Mitochondria from distinct tissues are differently affected by 17β-estradiol and tamoxifen. J. Steroid Biochem. Mol. Biol. 123, 8–16 10.1016/j.jsbmb.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 20. Moats R. K. 2nd, and Ramirez V. D. (1998) Rapid uptake and binding of estradiol-17β-6-(O-carboxymethyl)oxime:125I-labeled BSA by female rat liver. Biol. Reprod. 58, 531–538 10.1095/biolreprod58.2.531 [DOI] [PubMed] [Google Scholar]
- 21. Brady C. W. (2015) Liver disease in menopause. World J. Gastroenterol. 21, 7613–7620 10.3748/wjg.v21.i25.7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark J. M. (2006) The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol 40, S5–S10 [DOI] [PubMed] [Google Scholar]
- 23. Wang Z., Xu M., Hu Z., and Shrestha U. K. (2015) Prevalence of nonalcoholic fatty liver disease and its metabolic risk factors in women of different ages and body mass index. Menopause 22, 667–673 10.1097/GME.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 24. D'Eon T. M., Souza S. C., Aronovitz M., Obin M. S., Fried S. K., and Greenberg A. S. (2005) Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 280, 35983–35991 10.1074/jbc.M507339200 [DOI] [PubMed] [Google Scholar]
- 25. Zhu L., Brown W. C., Cai Q., Krust A., Chambon P., McGuinness O. P., and Stafford J. M. (2013) Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62, 424–434 10.2337/db11-1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klair J. S., Yang J. D., Abdelmalek M. F., Guy C. D., Gill R. M., Yates K., Unalp-Arida A., Lavine J. E., Clark J. M., Diehl A. M., Suzuki A., and Nonalcoholic Steatohepatitis Clinical Research Network (2016) A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 64, 85–91 10.1002/hep.28514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stuenkel C. A., Davis S. R., Gompel A., Lumsden M. A., Murad M. H., Pinkerton J. V., and Santen R. J. (2015) Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 3975–4011 10.1210/jc.2015-2236 [DOI] [PubMed] [Google Scholar]
- 28. Cox A. G., Winterbourn C. C., and Hampton M. B. (2010) Measuring the redox state of cellular peroxiredoxins by immunoblotting. Methods Enzymol. 474, 51–66 10.1016/S0076-6879(10)74004-0 [DOI] [PubMed] [Google Scholar]
- 29. Abbas A. M., and Elsamanoudy A. Z. (2011) Effects of 17β-estradiol and antioxidant administration on oxidative stress and insulin resistance in ovariectomized rats. Can J. Physiol. Pharmacol. 89, 497–504 10.1139/y11-053 [DOI] [PubMed] [Google Scholar]
- 30. Wirth C., Brandt U., Hunte C., and Zickermann V. (2016) Structure and function of mitochondrial complex I. Biochim. Biophys. Acta 1857, 902–914 10.1016/j.bbabio.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 31. Sazanov L. A. (2015) A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 16, 375–388 10.1038/nrm3997, 10.1038/nrn3981, 10.1038/nrn3980 [DOI] [PubMed] [Google Scholar]
- 32. Brand M. D. (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 100, 14–31 10.1016/j.freeradbiomed.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 33. Fisher-Wellman K. H., Lin C. T., Ryan T. E., Reese L. R., Gilliam L. A., Cathey B. L., Lark D. S., Smith C. D., Muoio D. M., and Neufer P. D. (2015) Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem. J. 467, 271–280 10.1042/BJ20141447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orr A. L., Ashok D., Sarantos M. R., Shi T., Hughes R. E., and Brand M. D. (2013) Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free Radic. Biol. Med. 65, 1047–1059 10.1016/j.freeradbiomed.2013.08.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quinlan C. L., Goncalves R. L., Hey-Mogensen M., Yadava N., Bunik V. I., and Brand M. D. (2014) The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J. Biol. Chem. 289, 8312–8325 10.1074/jbc.M113.545301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wend K., Wend P., and Krum S. A. (2012) Tissue-specific effects of loss of estrogen during menopause and aging. Front. Endocrinol. (Lausanne) 3, 19 10.3389/fendo.2012.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rattanasopa C., Phungphong S., Wattanapermpool J., and Bupha-Intr T. (2015) Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol. 147, 1–9 10.1016/j.jsbmb.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 38. Ribeiro Junior R. F., Rodrigues P. L., Morra E. A., Ronconi K. S., Do Val Lima P. R., Porto M. L., Simões M. R., Vassallo D. V., Figueiredo S. G., and Stefanon I. (2017) Estrogen regulates spatially distinct cardiac mitochondrial subpopulations. Mitochondrion 35, 87–96 10.1016/j.mito.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 39. Levin E. R. (2015) Extranuclear steroid receptors are essential for steroid hormone actions. Annu. Rev. Med. 66, 271–280 10.1146/annurev-med-050913-021703 [DOI] [PubMed] [Google Scholar]
- 40. Gustafsson K. L., Farman H., Henning P., Lionikaite V., Movérare-Skrtic S., Wu J., Ryberg H., Koskela A., Gustafsson J. Å., Tuukkanen J., Levin E. R., Ohlsson C., and Lagerquist M. K. (2016) The role of membrane ERα signaling in bone and other major estrogen responsive tissues. Sci. Rep. 6, 29473 10.1038/srep29473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Schoultz B., Carlström K., Collste L., Eriksson A., Henriksson P., Pousette A., and Stege R. (1989) Estrogen therapy and liver function: metabolic effects of oral and parenteral administration. Prostate 14, 389–395 10.1002/pros.2990140410 [DOI] [PubMed] [Google Scholar]
- 42. Mauvais-Jarvis F., Manson J. E., Stevenson J. C., and Fonseca V. A. (2017) Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr. Rev. 38, 173–188 10.1210/er.2016-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elkik F., Gompel A., Mercier-Bodard C., Kuttenn F., Guyenne P. N., Corvol P., and Mauvais-Jarvis P. (1982) Effects of percutaneous estradiol and conjugated estrogens on the level of plasma proteins and triglycerides in postmenopausal women. Am. J. Obstet. Gynecol. 143, 888–892 10.1016/0002-9378(82)90468-9 [DOI] [PubMed] [Google Scholar]
- 44. Hovius R., Lambrechts H., Nicolay K., and de Kruijff B. (1990) Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta 1021, 217–226 10.1016/0005-2736(90)90036-N [DOI] [PubMed] [Google Scholar]
- 45. Mootha V. K., Bunkenborg J., Olsen J. V., Hjerrild M., Wisniewski J. R., Stahl E., Bolouri M. S., Ray H. N., Sihag S., Kamal M., Patterson N., Lander E. S., and Mann M. (2003) Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115, 629–640 10.1016/S0092-8674(03)00926-7 [DOI] [PubMed] [Google Scholar]
- 46. Phillips D., Covian R., Aponte A. M., Glancy B., Taylor J. F., Chess D., and Balaban R. S. (2012) Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1034–R1048 10.1152/ajpregu.00596.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kussmaul L., and Hirst J. (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. U.S.A. 103, 7607–7612 10.1073/pnas.0510977103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fisher-Wellman K. H., and Neufer P. D. (2012) Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 23, 142–153 10.1016/j.tem.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanderson T. H., Reynolds C. A., Kumar R., Przyklenk K., and Hüttemann M. (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 47, 9–23 10.1007/s12035-012-8344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C. T., Price J. W. 3rd, Kang L., Rabinovitch P. S., Szeto H. H., Houmard J. A., Cortright R. N., Wasserman D. H., and Neufer P. D. (2009) Mitochondrial H2O2 emission and cellular redox state link ex1s fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119, 573–581 10.1172/JCI37048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ransac S., Heiske M., and Mazat J. P. (2012) From in silico to in spectro kinetics of respiratory complex I. Biochim. Biophys. Acta 1817, 1958–1969 10.1016/j.bbabio.2012.03.037 [DOI] [PubMed] [Google Scholar]
- 53. Verkhovskaya M. L., Belevich N., Euro L., Wikström M., and Verkhovsky M. I. (2008) Real-time electron transfer in respiratory complex I. Proc. Natl. Acad. Sci. U.S.A. 105, 3763–3767 10.1073/pnas.0711249105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. García-Ruiz C., Baulies A., Mari M., García-Rovés P. M., and Fernandez-Checa J. C. (2013) Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radic. Res. 47, 854–868 10.3109/10715762.2013.830717 [DOI] [PubMed] [Google Scholar]
- 55. Hensley K., Kotake Y., Sang H., Pye Q. N., Wallis G. L., Kolker L. M., Tabatabaie T., Stewart C. A., Konishi Y., Nakae D., and Floyd R. A. (2000) Dietary choline restriction causes complex I dysfunction and increased H2O2 generation in liver mitochondria. Carcinogenesis 21, 983–989 10.1093/carcin/21.5.983 [DOI] [PubMed] [Google Scholar]
- 56. Pérez-Carreras M., Del Hoyo P., Martín M. A., Rubio J. C., Martín A., Castellano G., Colina F., Arenas J., and Solis-Herruzo J. A. (2003) Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38, 999–1007 10.1002/hep.1840380426 [DOI] [PubMed] [Google Scholar]
- 57. Paradies G., Paradies V., Ruggiero F. M., and Petrosillo G. (2014) Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 20, 14205–14218 10.3748/wjg.v20.i39.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Petrosillo G., Portincasa P., Grattagliano I., Casanova G., Matera M., Ruggiero F. M., Ferri D., and Paradies G. (2007) Mitochondrial dysfunction in rat with nonalcoholic fatty liver involvement of complex I, reactive oxygen species and cardiolipin. Biochim. Biophys. Acta 1767, 1260–1267 10.1016/j.bbabio.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki A., and Abdelmalek M. F. (2009) Nonalcoholic fatty liver disease in women. Womens Health (Lond.) 5, 191–203 10.2217/17455057.5.2.191 [DOI] [PubMed] [Google Scholar]
- 60. Frezza C., Cipolat S., and Scorrano L. (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295 10.1038/nprot.2006.478 [DOI] [PubMed] [Google Scholar]
- 61. Barrientos A. (2002) In vivo and in organello assessment of OXPHOS activities. Methods 26, 307–316 10.1016/S1046-2023(02)00036-1 [DOI] [PubMed] [Google Scholar]
- 62. Giustarini D., Dalle-Donne I., Milzani A., Fanti P., and Rossi R. (2013) Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 8, 1660–1669 10.1038/nprot.2013.095 [DOI] [PubMed] [Google Scholar]
- 63. Kand'ár R., Záková P., Lotková H., Kucera O., and Cervinková Z. (2007) Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J. Pharm. Biomed. Anal. 43, 1382–1387 10.1016/j.jpba.2006.11.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.