Abstract
Nutrient sensing is a critical cell function that regulates survival and growth by adjusting metabolism. During nutrient shortage, autophagy enables the recycling of major cellular components to prevent cell death. Understanding the mechanisms that trigger and control autophagy is of fundamental importance, as this degradative pathway plays a pivotal role in many diseases. Gubbiotti et al. report the identification of a new player, the proteoglycan decorin, which functions as a nutrient sensor in the extracellular matrix and controls autophagy in the heart.
To stay healthy, cells and tissues constantly need energy. A regular nutrient supply ensures adequate cellular ATP levels and provides the building blocks to synthesize proteins, lipids, and carbohydrates. Accordingly, to maintain energy homeostasis, cells reprogram their metabolism in response to internal and external nutrient levels. During starvation, autophagy is the main strategy cells adopt for survival (1) and results in cytoplasmic materials undergoing degradation in lysosomes for production of new building blocks. This permits fine balancing between catabolism and anabolism and increases cell survival in stressful conditions. Interestingly, autophagy plays critical roles not only in nutrient deprivation, but also in other physiological processes such as development, differentiation, and aging. Moreover, aberrant autophagy seems to have a central role in several pathologies, including cancer and vascular, neurodegenerative, infectious, and metabolic diseases (1). Therefore, the understanding of the mechanisms that trigger and regulate autophagy could lead to the design of new drugs to target this process.
Autophagy is a multistep process that culminates in the transfer of cytosolic materials and organelles to the lumen of the lysosomes for degradation (2). Briefly, the initiation step requires the engagement of nutrient sensors such as AMP-activated protein kinase (AMPK),2 whose activity depends on the ATP:AMP ratio, or mTOR complex 1 (mTORC1) (3), whose activity as an autophagy repressor is blocked in response to growth factor deficiency or amino acid shortage. Autophagy initiation leads to the formation of a double-membrane vesicle (i.e. autophagosome) that surrounds cytoplasmic material and eventually fuses with lysosomes.
Recently, a lot of attention has been dedicated to protein O-GlcNAcylation, a post-translational modification of GlcNAc O-linked to cytoplasmic, nuclear, or mitochondrial proteins, as one of the main internal nutrient sensors of the cell (4). The substrate of O-GlcNAcylation is UDP-GlcNAc, the product of the hexosamine biosynthetic pathway, whose availability at the cellular level depends not only on sugar metabolism, but also on lipid, amino acid, and nucleic acid metabolisms. Although textbooks have only recently begun to report this protein modification, its role in many cellular processes (as survival and proliferation) and pathologies (cancer and diabetes) is well known. Therefore, it was conceivable that such an important nutrient sensor could also have a role in autophagy (5).
Autophagy takes place in the cytosol, but, surprisingly, the extracellular proteoglycan decorin (DCN) can also regulate autophagy. Proteoglycans are extracellular or cell-surface proteins that are highly glycosylated with peculiar polysaccharide chains, called glycosaminoglycans, unbranched polysaccharides consisting of a repeating disaccharide unit that is covalently bound to proteoglycan core protein. Proteoglycans are active players in the cell microenvironment controlling mechanical properties of tissues, as well as regulating the effects of extracellular signaling molecules as cytokines and growth factors.
DCN is a member of the small leucine-rich extracellular matrix proteoglycans; its name derives from the ability of this protein to interact with (or “decorate”) collagen, and it has also been dubbed the “guardian from the matrix” because it facilitates tissue homeostasis and protects against tumor development (6). However, DCN is also involved in several other processes, mainly in the regulation of proliferation and angiogenesis in cancer. The research group headed by Prof. Iozzo has been working on DCN for decades and discovered the molecular mechanism that is at the basis of the tumor-suppressing properties of DCN. DCN, via its specific interaction with receptor tyrosine kinases (i.e. vascular endothelial growth factor receptor 2 (VEGFR2)) and paternally expressed 3 (PEG3) (7), modulates autophagy in cancer, highlighting that alteration of a cell's microenvironment can deeply affect cell behavior and recovery and survival after stress.
Although caloric restriction or fasting reduces cholesterol levels and intake of saturated fats, these interventions can damage the heart. Surprisingly, Gubbiotti and colleagues (8) discovered that DCN in the heart is necessary to trigger autophagy in response to nutrient deprivation and to preserve heart physiology regulating the blood ejection fraction. In this work, the authors found that Dcn−/− mice were unable to induce autophagy in the heart during fasting. By using a metabolomics approach, the authors discovered that DCN deficiency alters hexosamine biosynthetic pathway intermediate levels, which, in turn, induce abnormal O-GlcNAcylation. DCN core protein treatment clearly restored O-GlcNAcylation and the physiological autophagic response. These findings indicate that DCN can be considered a new extracellular nutrient sensor able to shunt glucose from the normal oxidative pathway to the hexosamine biosynthetic pathway and to control O-GlcNAcylation.
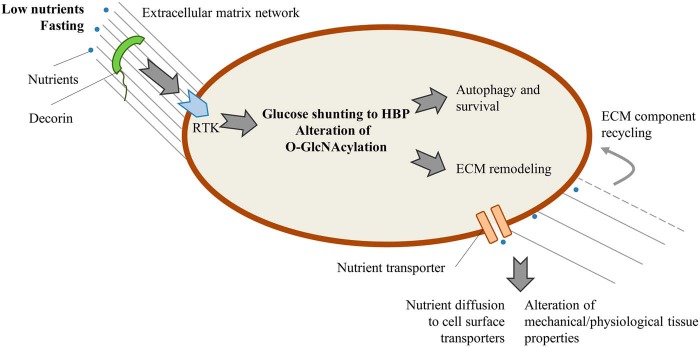
The presence of a nutrient sensor in the extracellular matrix is intriguing. As summarized in Fig. 1, DCN, via receptor tyrosine kinase, can signal nutrient shortage to the cell, which, in turn, activates autophagy via O-GlcNAcylation. We speculate that two scenarios could account for the role of extracellular matrix (ECM) components on nutrient availability. In response to a lack of nutrients, cells could alter the ECM architecture to favor nutrient diffusion toward cell-surface transporters. Further, ECM remodeling could supply the cell with new building blocks derived from the recycling of extracellular macromolecules. Eventually, an altered ECM could explain the modification of the mechanical and physiological properties of tissues as highlighted for the heart by Gubbiotti et al. (8). In support of this idea, it has been reported that the synthesis of hyaluronan, a ubiquitous glycosaminoglycan present in the ECM, is strictly regulated by the canonical nutrient sensors AMPK and O-GlcNAcylation (9), suggesting a role for hyaluronan in autophagy and stress response.
Figure 1.
Decorin is an extracellular nutrient sensor that, via receptor tyrosine kinase (RTK), modulates O-GlcNAcylation, controls mechanisms for cell survival, and regulates ECM modification, facilitating nutrient diffusion and ECM recycling to fuel the cells. Further, ECM alteration can lead to modification of the mechanical and physiological properties of tissues. Gray lines outline ECM remodeling after fasting. HBP, hexosamine biosynthetic pathway.
The discovery that DCN represents a first-line sentinel that alerts the cell to alterations in nutrient availability opens exciting questions regarding the capability of the ECM to change in response to nutrient shortage or surplus, suggesting a still more active role of the ECM in emerging pathologies such as diabetes or obesity that will represent scourges for the future.
This work was supported by financial support from the University of Insubria through FAR (to D. V. and A. P.) and a postdoctoral fellowship (to I. C.). This work was also supported by the EU H2020 Marie Skłodowska-Curie Grant 645756 “GLYCANC” (to A. P.). The authors declare that they have no conflicts of interest with the contents of this article.
- AMPK
- adenosine monophosphate kinase
- DCN
- decorin
- O-GlcNAcylation
- O-β-N-acetylglycosylation
- ECM
- extracellular matrix.
References
- 1. Jiang P., and Mizushima N. (2014) Autophagy and human diseases. Cell Res. 24, 69–79 10.1038/cr.2013.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mizushima N. (2007) Autophagy: Process and function. Genes Dev. 21, 2861–2873 10.1101/gad.1599207 [DOI] [PubMed] [Google Scholar]
- 3. Yuan H. X., Xiong Y., and Guan K. L. (2013) Nutrient sensing, metabolism, and cell growth control. Mol. Cell 49, 379–387 10.1016/j.molcel.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bond M. R., and Hanover J. A. (2015) A little sugar goes a long way: The cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880 10.1083/jcb.201501101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan H. B., Ma Y., Torres S., Zhang B., Feriod C., Heck R. M., Qian K., Fu M., Li X., Nathanson M. H., Bennett A. M., Nie Y., Ehrlich B. E., and Yang X. (2017) Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 31, 1655–1665 10.1101/gad.305441.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neill T., Schaefer L., and Iozzo R. V. (2012) Decorin: A guardian from the matrix. Am. J. Pathol. 181, 380–387 10.1016/j.ajpath.2012.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gubbiotti M. A., Neill T., Frey H., Schaefer L., and Iozzo R. V. (2015) Decorin is an autophagy inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol. 48, 14–25 10.1016/j.matbio.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gubbiotti M. A., Seifert E., Rodeck U., Hoek J. B., and Iozzo R. V. (2018) Metabolic reprogramming of murine cardiomyocytes during autophagy requires the extracellular nutrient sensor decorin. J. Biol. Chem. 293, 16940–16950 10.1074/jbc.RA118.004563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vigetti D., Karousou E., Viola M., Deleonibus S., De Luca G., and Passi A. (2014) Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta 1840, 2452–2459 10.1016/j.bbagen.2014.02.001 [DOI] [PubMed] [Google Scholar]